Abstract
Endocytic sorting of signaling receptors between recycling and degradative pathways is a key cellular process controlling the surface complement of receptors and, accordingly, the cell’s ability to respond to specific extracellular stimuli. The beta-2 adrenergic receptor (β2AR) is a prototypical seven-transmembrane signaling receptor that recycles rapidly and efficiently to the plasma membrane after ligand-induced endocytosis. β2AR recycling is dependent on the receptor’s C-terminal PDZ ligand and Rab41,2. This active sorting process is required for functional resensitization of β2AR-mediated signaling3,4. Here we show that sequence-directed sorting occurs at the level of entry into retromer tubules and that retromer tubules are associated with Rab4. Further, we show that sorting nexin 27 (SNX27) serves as an essential adapter protein linking β2ARs to the retromer tubule. SNX27 does not appear to directly interact with the retromer core complex, but does interact with the retromer associated Wiskott-Aldrich Syndrome Protein and SCAR Homolog (WASH) complex. The present results identify a role for retromer in endocytic trafficking of signaling receptors, in regulating a receptor-linked signaling pathway, and in mediating direct endosome-to-plasma membrane traffic.
After treatment with agonist such as isoproterenol, β2ARs trigger a signaling cascade and undergo clathrin mediated endocytosis. β2ARs are then rapidly recycled from the early endosome antigen 1 (EEA1) compartment (Fig 1a, b, 4a) to the plasma membrane (Fig 2c), resensitizing the cell5. Internalized transmembrane proteins are generally thought to leave the endosome through tubules6. In the case of β2AR, receptor-containing tubular endosomal protrusions can be visualized in living cells (Fig 1a, c)7. β2AR recycling is sequence-dependent, requiring a C-terminal PDZ ligand2. When this ligand is occluded by a HA tag (β2AR-HA), mutant receptors fail to recycle efficiently and are not seen in endosomal tubules (Fig 1c)2. Therefore, these tubules likely represent the structure responsible for sequence-dependent recycling of β2AR.
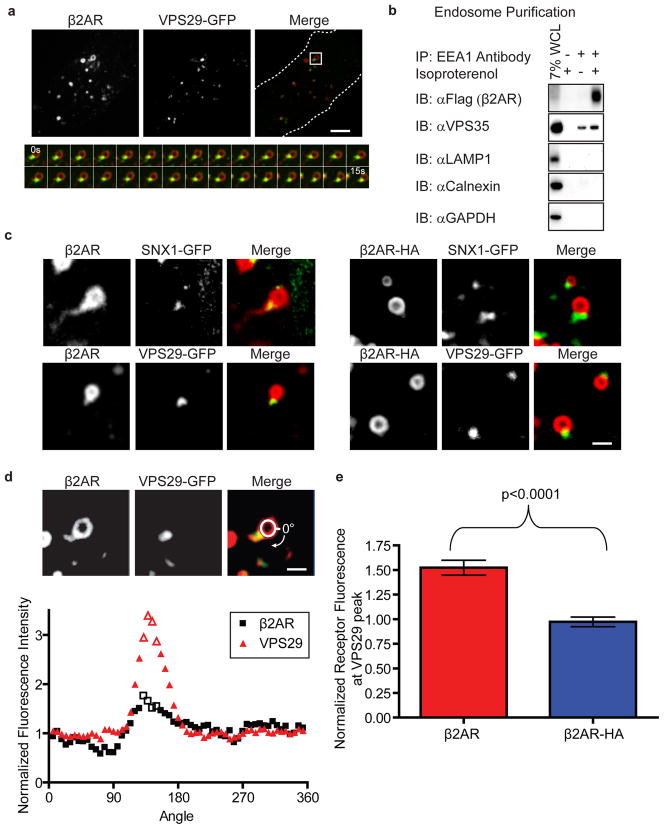
Figure 1. Rapid recycling β2ARs selectively enter retromer-associated endosomal tubules.
(a) Representative images from a movie of a cell expressing β2AR (red) and VPS29-GFP (green). Below, the inset box is shown across multiple frames of the movie. The edges of the cell are shown as dotted lines in the merged image. The scale bar represents 4 μm. (b) Representative immunoblots are shown from endosomes that were immuno-purified using antibody against the early endosome component EEA1 (n=3). (c) Representative images of endosomes containing wild type β2AR or recycling-defective β2AR-HA receptors (red) and SNX1-GFP or VPS29-GFP (green). Images were acquired by confocal microscopy of living cells after stimulating receptor endocytosis with isoproterenol. Wild type β2ARs, but not recycling-defective β2AR-HA mutant receptors, were visible in the VPS29-associated tubule extending from the endosome body. The scale bar represents 1 μm. (d) Fluorescence intensity tracing of labeled β2AR (black squares) and VPS29 (red triangles) around the edge of the endosome. Each point represents average fluorescence over a six degree arc of the endosome circumference. The four points of greatest VPS29-GFP fluorescence (open triangles) were used to mark the tubule. β2AR fluorescence values were background-corrected and normalized to the average fluorescence of a portion (240o) of the endosome, excluding the 120o of circumference centered at the tubule base. The scale bar represents 1 μm. (e) Relative receptor enrichment (average of open squares in panel b) of β2AR (red bar) or β2AR-HA (blue bar) at the tubule base. 20 endosomes (4 independent experiments), extending a single retromer-associated tubule, per receptor type were analyzed. Data points are the mean ± standard error of the mean (SEM).
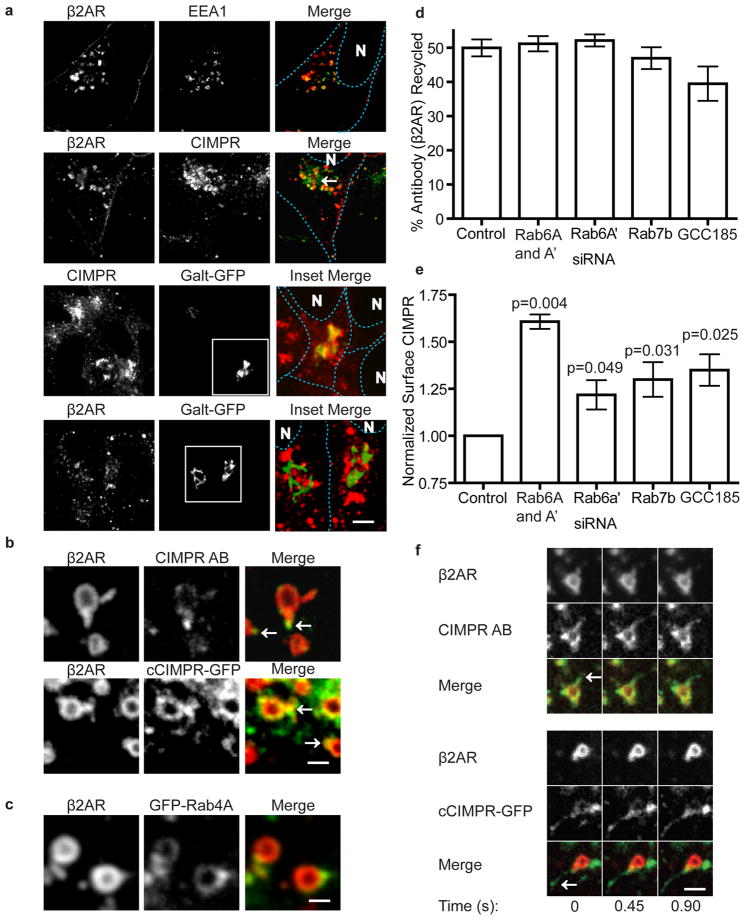
Figure 4. β2AR and CIMPR follow divergent trafficking paths upon exit from the same retromer-associated tubule.
(a) Comparative localization of CIMPR and β2AR (red) with early endosome marker EEA1 or trans-Golgi marker GalT-GFP (green). Cells were fixed and imaged following incubation with isoproterenol for 30 min to drive β2AR to steady state. Plasma membrane and nuclei (N) have been outlined with dotted lines. The arrow indicates an example of a trans-Golgi region labeled for CIMPR but not β2AR. The scale bar represents 6 μm. (b) Representative endosome images from live cell confocal microscopy showing β2AR (red) localization in tubules with endogenous (top panels) or recombinant (bottom panels) CIMPR (green). Arrows indicate tubules containing both β2AR and CIMPR. The scale bar represents 1 μm. (c) Images, as in (b), of β2AR (red) and GFP-Rab4A (green) localization on the endosome. (d and e) Various components of the endosome to TGN transport pathway were depleted and β2AR recycling (panel d) or CIMPR surface immunoreactivity (panel e) was quantified by flow cytometry (n=4 experiments) to assess integrity of β2AR and CIMPR trafficking. (f) Representative images from live confocal imaging showing differential enrichment of CIMPR (green) relative to β2AR (red) localization on distal regions of the same endosomal tubules. Each column represents a frame from a continuous time lapse movie (450 ms/frame). Arrows indicate distal regions of the indicated tubules on which CIMPR labeling was visibly enriched relative to β2AR. Multiple frames are shown to demonstrate that the observed difference in lateral enrichment did not occur as an artifact of tubule movement. The scale bar represents 1 μm. Data points are the mean ± SEM.
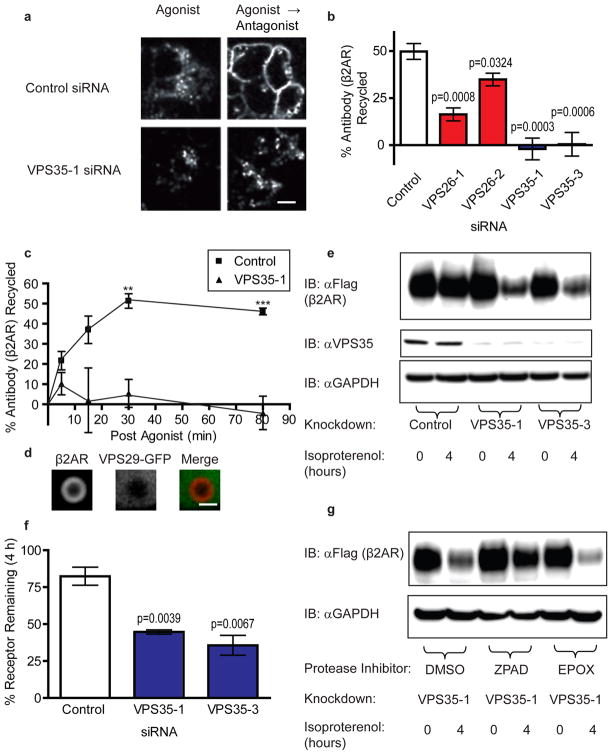
Figure 2. Knockdown of retromer by RNAi inhibits β2AR recycling and misroutes internalized β2ARs to lysosomes.
(a) Representative images from a visual assay for β2AR trafficking are shown. Stably transfected HEK 293 cells expressing FLAG-β2AR were transfected with either control siRNA or siRNA targeting the retromer component VPS35. In the “Agonist” condition, cells were incubated in the presence of the β2AR agonist isoproterenol (10 μM) and Alexa-conjugated M1 anti-FLAG for 25 min. In the “Agonist → Antagonist” condition, cells were incubated with isoproterenol for 25 min and then for an additional 45 min in the absence of isoproterenol (and in the presence of 10 μM of the β2AR antagonist alprenelol to prevent effects of any residual agonist). The scale bar represents 20 μm. (b) Flow cytometric analysis of β2AR recycling by uptake and efflux of bound M1 anti-FLAG antibody (n=4). (c) A time-course of recycling is shown for β2AR. The experiment was performed as in (b) but the duration after agonist washout was varied (n=4). (d) Representative confocal image from live cell imaging showing an endosome from a VPS35-1 siRNA treated cell expressing FLAG-β2AR (red) and VPS29-GFP (green). The scale bar represents 1 μm. (e) A representative immunoblot assay of agonist induced FLAG-β2AR degradation. Detergent extracts were prepared from HEK 293 cells expressing FLAG-β2AR incubated in the absence of agonist or in the continuous presence of 10 μM isoproterenol for 4 h. Knockdown was verified by VPS35 immunoblot of the same lysates (middle panel), and equal loading was verified by immunoblotting for GAPDH (bottom panel). (f) FLAG-β2AR immunoblots were quantified by scanning densitometry across multiple experiments (n=3), and the percent receptor remaining after 4 h isoproterenol exposure was calculated. (g) Representative immunoblot showing isoproterenol induced β2AR degradation in cells depleted of VPS35 and treated with the vehicle dimethyl sulfoxide (DMSO), the lysosomal cathepsin inhibitor N-CBZ-L-phenylalanyl-L-alanine-diazomethylketone (ZPAD), or the proteosomal inhibitor epoxomicin (EPOX) (n=3). Data points are the mean ± SEM.
Cargo that are capable of recycling efficiently with bulk membrane flux, such as the transferrin receptor (TFR), exit endosomes via multiple dynamic tubules8. The β2AR was previously shown to enter a specific subset of these tubules7. To investigate the hypothesis that β2AR-containing tubules are biochemically distinct, we took a candidate-based approach to identify specific tubule components.
Because both recycling (β2AR) and non-recycling (β2AR-HA) receptors were localized to similar endosomes but appeared to differ in lateral distribution near the highly curved neck region of endosomal tubules (Fig 1c), we asked whether curvature sensing/inducing Bin–Amphiphysin–Rvs (BAR) domain-containing proteins might be localized there. We first considered sorting nexins, because several contain a BAR domain and an endosome-associating PX domain9,10. We looked at four of these BAR domain-containing sorting nexins (SNX1, SNX4, SNX5, and SNX9). We noticed striking, and nearly complete, overlap of SNX1 and SNX5 with β2AR-containing tubules (Fig 1c; data not shown for SNX5). Quantification across multiple cells verified that the vast majority (92.5%; n = 40 tubules) of endosomal tubules containing internalized β2ARs co-localized with a concentrated region of GFP-SNX18.
SNX1 and SNX5 have been previously associated with retromer. Retromer is composed of two distinct multi-protein subcomplexes, one containing sorting nexins and the other containing vacuolar protein sorting (VPS) proteins VPS26, VPS29, and VPS3511,12. Recruitment of the VPS26/29/35 subcomplex to the endosome requires previous recruitment of the SNX-containing subcomplex and Rab713. Therefore, we next visualized a component of the VPS26/29/35 retromer subcomplex to determine whether the complete retromer complex was forming on these tubules.
VPS29-GFP was also concentrated on β2AR-containing tubules (Fig 1c, Supplementary Movie); quantification across multiple examples indicated that 96.5% (n=57 tubules) of β2AR-containing endosome tubules were decorated with VPS29-GFP. These VPS29 foci extended off the edge of the endosome limiting membrane and co-localized with tubular β2AR staining. In rapid (2Hz) image series, β2AR could be seen extending distally beyond the SNX1 or VPS29 marked portion of the tubule (Fig 1c, d, Supplementary Movie). Essentially all VPS29-GFP puncta co-localized with β2AR on vesicles (95.4%, n=177 spots) (Fig 1a). Additionally, 31.1% of these VPS29-GFP spots were tubular protrusions from an endosome with a resolvable lumen. Consistent with this co-localization, biochemical purification of EEA1 endosomes co-purified β2AR and retromer component VPS35 (Fig 1b; uncut blots for all figures are in Supplementary Figure 2). Together, this data suggests that β2ARs access the retromer marked tubular endosomal network14.
In contrast to β2AR, recycling-defective β2AR-HA did not extend into retromer tubules (Fig 1c). This distinction between β2AR and β2AR-HA localization was quantified by circumferential line scan analysis around the endosome limiting membrane. Whereas β2AR was enriched ~50% at the base of VPS29-GFP coated tubules relative to the rest of the endosome membrane, β2AR-HA was not (Fig 1d, e). These results indicate that β2ARs specifically enter retromer-associated tubules following endocytosis, and suggest that tubule entry is the primary sorting step required for β2AR recycling to the plasma membrane.
To investigate if retromer is required for β2AR recycling, we depleted retromer components by RNAi. We first assessed trafficking effects using fluorescence microscopy to visualize ligand-dependent redistribution of surface-labeled receptors between the plasma membrane and intracellular membranes. The β2AR agonist isoproterenol induced pronounced redistribution of antibody-labeled β2ARs from the plasma membrane to intracellular puncta, indicative of ligand-induced endocytosis. This process was not detectably inhibited by siRNA-mediated knockdown of VPS35 (Fig 2a). Subsequent recycling of receptors to the plasma membrane after agonist removal was obviously reduced in VPS35-depleted cells (Fig 2a).
We then quantified this effect of retromer depletion using a flow cytometry assay measuring changes in the internalized pool of receptors. β2AR recycling, as measured by antibody efflux following agonist removal, was strongly inhibited by multiple siRNA sequences targeting either VPS26 or VPS35 (Fig 2b). Further, retromer depletion inhibited β2AR recycling at all time points examined (Fig 2c). Consistent with this, VPS35 depletion, which prevents endosome association of VPS29-GFP, resulted in endosomes devoid of β2AR tubules (Fig 2d). Additionally, we verified that retromer is required for β2AR recycling in physiologically relevant A10 cells, a rat atrial-derived vascular smooth muscle cell line (Supplementary Figure 1).
Retromer depletion leads β2ARs to be misrouted to the lysosome and degraded (Fig 2e-g). This effect of retromer depletion on receptor degradation is fully consistent with the observed inhibition of β2AR recycling, and similar to that of disrupting the receptor’s C-terminal PDZ ligand2.
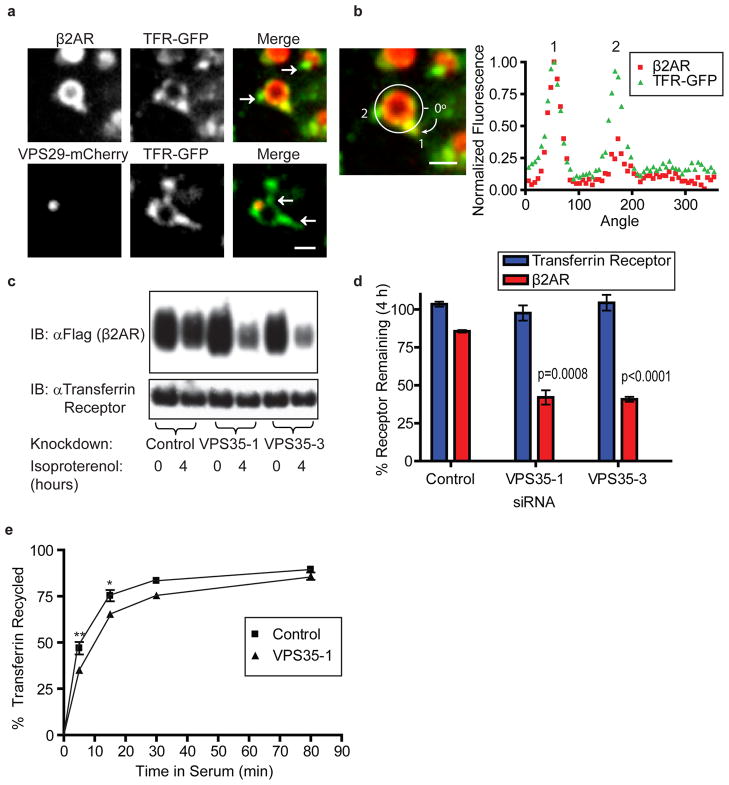
To examine the specificity of retromer depletion effects, we tested another protein that traffics through the same endosome as β2AR. For this we used TFR, a constitutively endocytosed nutrient receptor that can recycle with bulk membrane15. When TFR-GFP or labeled Transferrin was imaged alongside internalized β2AR in living cells, β2AR only appeared in a subset of the TFR containing tubules projecting from the limiting membrane of individual early endosomes (Fig 3a, b), as shown previously7. Additionally, only a subset of TFR tubules labeled with VPS29-mCherry (Fig 3a). As TFR can access non-retromer recycling tubules, it should be able to exit the endosome in the absence of retromer tubules. Consistent with this, retromer depletion had no effect on degradation of endogenous TFR (Fig 3c, d) and produced only a small kinetic delay in the recycling of TFR back to the plasma membrane (Fig 3e). This second observation is consistent with the ability of a visible fraction of TFR to enter retromer tubules and further supports the ability of these tubules to mediate rapid plasma membrane recycling.
Figure 3. Retromer depletion preferentially affects β2ARs over TFRs traversing the same endosomes.
(a) Top panels show representative images of endosomes containing β2AR (red) and TFR-GFP (green). Bottom panels show localization of VPS29-mCherry (red) relative to TFR-GFP (green). Arrows point to TFR tubules lacking β2AR in the top image and lacking VPS29 labeling in the bottom image. The scale bar represents 1 μm. (b) Fluorescence intensity scan of labeled β2AR (red squares) and TFR (green triangles) on a circumferential path set outside of the representative endosome shown in panel (a) but crossing both TFR-labeled tubules projecting from the endosome body, as indicated on the detail merged image. Positions of the tubules are marked by the numbers 1 and 2. The scale bar represents 1 μm. (c) Biochemical analysis comparing degradation of surface-biotinylated β2AR (top panel) and TFR (bottom panel) in cells maintained in the absence or continuous presence of isoproterenol for 4 h. A representative immunoblot is shown. Knockdown of VPS35 using two independent siRNA targets selectively destabilized β2AR. (d) Receptor immunoreactivity from the experiment shown in panel (c) was quantified by scanning densitometry and the percent receptor remaining after 4 h was calculated by dividing the 4 h agonist treatment by the untreated control. Recovery of TFR (blue bars) and β2AR (red bars) was averaged across experiments (n=3). (e) The graph shows efflux of labeled transferrin in HEK 293 cells treated with siRNA against a control sequence or VPS35 (n=3). Percent transferrin recycled was calculated from these flow cytometry experiments as in Fig 2b. Data points are the mean ± SEM.
As retromer complex has not previously been implicated in recycling from endosomes to the plasma membrane, we next sought to compare β2AR localization and trafficking to that of a membrane cargo which exhibits retromer-dependent trafficking to the TGN. For this purpose we focused on the cation-independent mannose phosphate receptor (CIMPR)16,17. We first evaluated the localization of β2AR relative to CIMPR in fixed cells. β2AR was induced to reach a steady-state of internalization and recycling so that it populated various intermediates in endocytic and recycling pathways. Under this condition, β2AR was localized primarily in EEA1 marked endosomes and at the plasma membrane (Fig 1b, 4a)18,19. CIMPR co-localized with β2AR in a large fraction of early endosomes but was also prominently localized in perinuclear endomembranes not containing β2AR (Fig 4a). This is consistent with steady state localization of CIMPR in the TGN and late endosomes20. Supporting this, the localization of perinuclear CIMPR was similar to, or closely adjacent to, that of the trans-Golgi/TGN marker galactosyltransferase-GFP (GalT-GFP) 21,22. We also note that retromer cargo that traverse the TGN on the way to indirect plasma membrane delivery have shown significant colocalization with markers of the TGN region23. Importantly, and in marked contrast, the β2AR localization observed in these cells was clearly distinct. β2ARs localized to peripheral early endosomes but not in the perinuclear distribution characteristic of CIMPRs (Fig 4a).
Given that β2AR and CIMPR achieve different steady state localization patterns, we next used live imaging to ask if these distinct membrane cargoes localize to the same endosomes and, if so, if they localize to the same retromer tubules. Confocal microscopy revealed that both FITC-conjugated anti-CIMPR antibody (CIMPR AB), used to detect endogenous receptor, and a GFP-tagged CIMPR construct (cCIMPR-GFP) clearly co-localized with β2AR in the same endosomes and in the same tubules (Fig 4b, f). This overlap was extensive, as >90% of the β2AR-positive tubules resolved also contained GFP-tagged CIMPR.
We next asked how the retromer tubule could support rapid endosome-to- plasma membrane traffic, evidently independent of traversing the TGN. Knowing that the small GTPase Rab4A had previously been implicated in this process we tested if it associated with β2AR containing endosomes. GFP-Rab4A localized to these compartments and was visibly enriched on the β2AR-containing retromer tubules (Fig 4c)7. Further, as expected, depletion of Rab4A by siRNA inhibited recycling of β2AR (data not shown)1. In contrast, depletion of several components that function in endosome-to-TGN trafficking of CIMPR (Rab6A’, Rab7b (a distinct gene from Rab7), and GCC185) failed to significantly affect β2AR recycling24–28 (Fig 4d). As a control for depletion, we established in parallel experiments that these siRNAs caused a pronounced increase in cell surface expression of CIMPR, consistent with net disruption of normal endosome-TGN cycling of CIMPRs established previously (Fig 4e)16.
Despite the co-occurrence of both membrane cargoes in a large fraction of endosomal tubules, β2AR and CIMPR were not identically localized in individual tubules imaged at high temporal resolution. Specifically, CIMPR was typically enriched near the distal end of tubules or in protrusions that extended beyond the β2AR labeled areas (Fig 4f). Apparent separation of cargo was visualized for both endogenous CIMPR and the GFP CIMPR transgenic protein (Fig 4f). These results support the previously proposed idea that an elaborated tubular endosomal network, capable of mediating the trafficking of cargoes to multiple destinations, is fed by the retromer tubule14. Further work will be required to investigate this, as there is also evidence that a significant fraction of CIMPRs can traffic to the plasma membrane following endosome exit20.
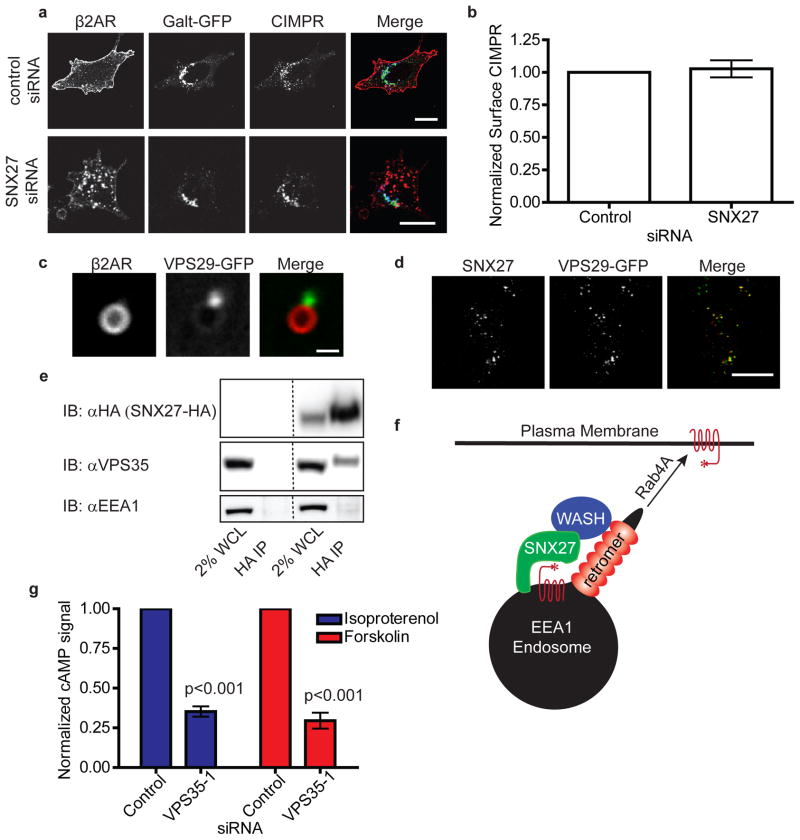
We next investigated the mechanistic basis for the role of retromer in β2AR recycling. For CIMPR, it is proposed that a direct interaction between the cytoplasmic tail and retromer complex is required for proper trafficking29. β2AR trafficking is dependent on a PDZ motif present in the cytoplasmic tail that is both necessary and sufficient to mediate its plasma membrane recycling, but the core retromer complex is devoid of any recognizable PDZ domain. SNX27 contains a PDZ domain that binds the β2AR tail, and has recently been shown to be essential for PDZ-directed recycling of the β2AR30. Verifying this, knockdown of SNX27 robustly inhibited recycling of β2ARs (Fig 5a). Despite this pronounced decrease in β2AR recycling, CIMPR distribution in the same cells appeared unaffected. Furthermore, SNX27 depletion did not alter CIMPR surface expression (Fig 5b) or CIMPR turnover in the presence of cyclohexamide (data not shown).
Figure 5. SNX27 serves as an adapter for β2AR, sorting it into the retromer tubule.
(a) Comparative localization of β2AR (red) with the trans-Golgi marker GalT-GFP (green) and CIMPR (blue). Cells were fed anti-FLAG antibody in the presence of isoproterenol for 25 minutes prior to agonist washout with alprenelol for 45 minutes. Successful knockdown of SNX27 was judged by failure of β2AR to recycle to the plasma membrane. The scale bar represents 20 μm. (b) CIMPR surface immunoreactivity was quantified by fluorescence flow cytometry (n=6) to assess the integrity of CIMPR trafficking when SNX27 is depleted with siRNA. Successful depletion of SNX27 was confirmed by looking at visual recycling assays of β2AR as in (a). (c) Representative confocal image from live cell imaging showing an endosome from a SNX27-4 siRNA treated cell expressing FLAG-β2AR (red) and VPS29-GFP (green). The scale bar represents 1 μm. (d) Confocal image of transiently transfected SNX27-HA (red) and VPS29-GFP (green) in fixed cells. The scale bar represents 20 μm. (e) Representative immunoblot showing co-immunoprecipitation of endogenous VPS35 with transiently transfected SNX27-HA. Lanes 1 and 3 show 2% whole cell lysate for the mock IP at left and the experiment at right. (f) SNX27 mediates plasma membrane recycling of PDZ motif containing cargo by linking to the retromer through an interaction with the WASH complex. SNX27 also interacts with the endosome directly through its lipid binding PX domain. (g) Isoproterenol or forskolin induced cAMP formation was measured in HEK 293 cells stably expressing β2AR, in the absence of IBMX, 20 minutes after agonist addition (n=6). Data points are the mean ± SEM.
Live imaging of SNX27 depleted cells revealed that VPS29-GFP puncta still associate with endosomes but that β2ARs no longer appear in these tubular structures (Fig 5c). Depletion of SNX27 also decreased the aggregation of β2ARs at the retromer tubule. In control siRNA treated cells there was a relative receptor density of 1.51±.061 (n=16 endosomes) at the base of the VPS29 tubule, while in SNX27 depleted cells this dropped significantly to 1.15±.055 (n=19 endosomes, p=.0002), phenocopying disruption of the PDZ motif (β2AR-HA). Together, this data suggests that SNX27 acts as a cargo adaptor to retromer, but is not a core component.
To investigate further how SNX27 was serving as a cargo adaptor to the retromer tubule, we used an affinity tagging/purification-mass spectrometry approach to determine which other proteins physically associate with it. In all 5 independent SNX27 purifications, components of the WASH actin nucleation complex were predominant hits (Supplementary Table). However, WASH complex was not detected in control purifications or in those of other bait molecules run at the same time. WASH has been shown to physically link to the retromer complex31. Therefore this association with SNX27 supports the adaptor hypothesis by establishing protein connectivity.
Association of SNX27 with the retromer tubule was further supported by extensive colocalization between SNX27 and VPS29-GFP in fixed cells (Fig 5d). Moreover, SNX27 co-immunoprecipated endogenous VPS35 but not EEA1, an abundant protein that localizes to the same endosomes. Interestingly, VPS35 co-immunoprecipitated with SNX27 only under crosslinking conditions, in contrast to WASH complex components that co-purified with SNX27 in the absence of crosslinking (Fig 5e). These observations suggest that SNX27 does not interact with the core retromer complex directly, but that its association with the retromer tubule is mediated by other interaction(s) including through the WASH complex. Together, these results indicate that SNX27 acts, through novel connectivity involving the WASH complex, as a specific adapter to promote PDZ-directed plasma membrane sorting through the retromer tubule (Fig 5f).
To probe the generality of the SNX27/retromer -mediated recycling pathway we next examined two other GPCRs that are known to recycle rapidly to the plasma membrane after endocytosis. Efficient recycling of the beta-1 adrenergic receptor (β1AR) requires a class 1 PDZ motif present in its distal cytoplasmic tail, but this motif differs in primary structure and binding properties from the PDZ motif present in the β2AR tail32,33. The D1 dopamine receptor (D1R) recycles efficiently after endocytosis but does not contain a C-terminal PDZ motif, and truncation of its distal cytoplasmic tail does not disrupt receptor recycling34. We observed a significant inhibition of β1AR recycling following knockdown of either VPS35 (33.7%±0.1 inhibition, n=9, p= 0.0013) or SNX27 (19.5%±0.1 inhibition, n=9, p= 0.0293). Recycling of FLAG-D1Rs, in contrast, was not significantly inhibited by either manipulation. Thus, retromer-dependent recycling is not unique to the β2AR and can be specified by distinct PDZ motifs. This suggests that many other membrane cargoes which contain C-terminal PDZ ligands, including a large group of G-protein coupled receptors (GPCRs), may traffic via this SNX27/retromer pathway35.
Retromer has been shown to function in trafficking of various cargo from endosomes to the TGN, and to function in transcytosis in polarized cells12,36. Recently several retromer cargo have been identified that traffic to the plasma membrane, though it is unclear whether they do so directly from the endosome or via the TGN37–39. In this paper we identify an essential role of retromer in mediating rapid recycling of a prototypical GPCR apparently directly to the plasma membrane. We also establish that β2ARs can enter the same retromer tubules as a canonical TGN-directed cargo, and identify a distinct adaptor protein that links signaling receptors specifically to the retromer-dependent plasma membrane recycling pathway. Our results support a revised view of retromer tubules as a multi-functional exit port supporting diverse membrane itineraries, including direct endosome-to-plasma membrane trafficking.
Our results have fundamental implications in the field of cell signaling because they indicate that retromer plays a critical role in mediating the sorting of a prototypical GPCR between the functionally opposing pathways of resensitization and down-regulation, the distinct physiological consequences of which are well established4,40. Consistent with this, depletion of VPS35 significantly decreased isoproterenol mediated signaling from the β2AR, as measured by cellular cAMP accumulation (Fig 5g). Interestingly, retromer depletion also affected receptor-independent activation of adenylyl cyclase by forskolin, suggesting that retromer tubules have other role(s) in cell signaling.
Methods
Cell Culture, cDNA constructs, and Transfection
HEK 293 and A10 cells were grown in Dulbecco’s modified Eagles medium supplemented with 10% fetal bovine serum (UCSF Cell Culture Facility, San Francisco, CA). Stably transfected HEK 293 cell clones expressing the indicated receptor constructs were created using the previously described FLAG-tagged β2AR and β2AR-HA and G418 selection at 500 μg/ml (Invitrogen)2.
GFP-SNX1 and VPS29-GFP were generous gifts from J. Bonifacino25. Vps29-mCherry was created by PCR-mediated amplification of VPS29 from the VPS29-GFP construct, inserting it into pENTR using the pENTR directional cloning kit (Invitrogen), and then into a pcDNA3 Dest53 mCherry vector using the LR clonase kit (Invitrogen). Galt-GFP was a gift from J. Lippincott-Schwartz22. cCIMPR-GFP was created by amplifying the transmembrane and cytoplasmic domains of CIMPR from a cDNA library with the primers TATACAAAACCGGTCTGTCAGAACGGAGCCAGGCAGTCGGC and TTTGTATAGGATATCCCCCTGCAGGCACTGCGGAGTCAGATG and cloning this fragment into a N-terminal signal sequence and GFP-containing pcDNA3 vector using Age1 and EcoRV. GFP-Rab4A was a gift from S. Ferguson41. Snx27-HA30, FLAG-β1AR42, and FLAG-D1R34 constructs were previously described.
For transient expression of constructs, cells were transfected using Lipofectamine 2000 (Invitrogen) according to manufacturers’ instructions. Cells expressing FLAG-tagged receptors were harvested with PBS EDTA and plated in 12 well plates at 80% confluence before transfection with plasmid DNA. Cells were cultured for a further 48 h before experimentation. Target sequences for knockdown were hVPS26 (1: CTGCATAATGTTGATTATAAA, 2:CACCAAGGAATTAGAATTGAA), hVPS35 (1: CTGGACATATTTATCAATATA, 3: CAGGAAATGCATAATTAT), RnVPS35 (ACCAGGTAGATTCCATAATGA), hRab4A (AATGCAGGAACTGGCAAATCT), hRab6A (CCCACTTATTGTCACCTTGTA), hRab6A’(AACAGCTGTAGTAGTTTACGA)28, hRab7B (AAGTAGCTCAAGGCTGGTGTA)26, hGCC185 (AAGGAGTTGGAACAATCACAT)27, hSnx27-430, and control (1027281, Qiagen). 25 picomoles of duplex RNA (Qiagen) were transfected into 30% confluent cells in a 12 well dish with Lipofectamine RNAi Max (Invitrogen) 36 h prior to experimentation.
Live Receptor Imaging and Quantification
Live imaging of FLAG-tagged receptors and the indicated GFP-labeled protein was performed using a previously described antibody feeding method7. Briefly, cells expressing both constructs were plated onto glass coverslips and surface receptors were labeled by the addition of M1 anti-FLAG antibody (Sigma) conjugated to Alexa 555 (A10470, Invitrogen) to the media for 30 min. Isoproterenol was added (Sigma) and cells were imaged on a Nikon TE-2000E inverted microscope with a 100×1.49 NA TIRF objective (Nikon) and a Yokagawa CSU22 Spinning Disk confocal head (Solamere, Salt Lake City, UT). A 488nm Ar laser and a 568nm Ar/Kr laser (Melles Griot) were used as light sources for imaging GFP and FLAG signals, respectively. Movies of endosomes were taken between 5 and 30 min after agonist addition and exported as TIF files. Each frame corresponds to 450 ms.
Analysis of receptor co-enrichment with VPS29-GFP was performed using the ImageJ (http://rsb.info.nih.gov/ij/) and the “Oval Profile” plugin. Endosomes with single VPS29-GFP spots were outlined using the oval tool. Oval Profile was used to perform a linear fluorescence intensity scan in 60 subsections of both VPS29-GFP and β2AR signals as well as background signals from an endosome free region. The data was then exported to Excel (Microsoft) where the background was subtracted and the top four consecutive points of VPS29-GFP fluorescence were determined. To determine the enrichment of receptor at the tubule as compared to the rest of the endosome, the average intensity of the four β2AR points corresponding to the four peak VPS29 points was divided by the average receptor intensity of the rest of the endosome except for the 120o centered at the VPS29 peak.
Recycling Assays
Visualizationof FLAG receptors was carried out using fluorescence microscopy of stably transfected HEK 293 cells or transiently transfected A10 cells that had been plated on glass coverslips. Surface receptorswere labeled by exposing intact cells to M1 anti-FLAG antibody conjugated to Alexa 555(Sigma) for 25 min at 37 °C in the presence of 10 μM isoproterenol to promote endocytosis of labeled receptors. Cells werethen either fixed or washed and further incubated in media for 45 min in the presence of the antagonist 10 μM alprenolol (Sigma) to allow for recycling. Cells werefixed using 4% formaldehyde freshly dissolved in PBS and imaged on the aforementioned spinning disk microscope.
In the flow cytometry based recycling assay, cells were plated in 12 well plates and treated as in the visual assay except that instead of fixation, HEK 293 cells were lifted in PBS EDTA, which strips the calcium dependent M1 antibody from the cell surface. When surface CIMPR was measure, CD222-FITC (BioLegend) was added to the PBS-EDTA at 1:250. Lifting of A10 cells required addition of trypsin. Fluorescence intensity profiles of cell populations (5,000 cells/sample for HEK 293 and >1500 cells/sample for A10)were measured using a FACS-Calibur instrument (BD Biosciences).
Transferrin recycling experiments involved washing cells 3 times with PBS, applying Alexa-488 labeled transferrin (Invitrogen) in serum free media for 25 minutes, and then rapid transferrin efflux occurred by switching cells to media that contained serum and no labeled transferrin. 10% serum was added to the PBS-EDTA when cells were lifted to strip all surface transferrin.
In each experiment, triplicate treatments were analyzed for eachcondition. All experiments were carried out on at least three separate days (number indicated in figure legends), and values reported werederived from the mean determination across experiments. Thepercentage of antibody recycled was calculated from internal fluorescence values as follows: % recycling = 1-(“agonist → antagonist signal”/“agonist signal”)
Fixed Cell Imaging
HEK 293 cells stably expressing β2AR plated onto cover slips were treated with the agonist isoproterenol for 30 min prior to fixation with 4% formaldehyde in PBS and processed for immunocytochemical staining. Staining of β2AR was done using Rabbit anti-FLAG (Sigma) and the secondary goat anti-rabbit Alexa 594 (Invitrogen). Mouse antibodies against EEA1 (BD Biosciences) and CIMPR (Biolegend) were used in combination with donkey anti-mouse Alexa 488 (Invitrogen). Fixed cells were imaged using the aforementioned spinning disk microscope.
Degradation Assays and Western Blotting
Briefly, stably transfected HEK 293 cells expressing FLAG-β2AR were transfected with siRNA VPS35-1 or VPS35-3 as described above, and stimulated with 10 μM isoproterenol for four h before washing three times in ice-cold PBS and lysed in extraction buffer (0.2% Triton X-100, 50 mM NaCl, 5 mM EDTA, 50 mM Tris pH 7.4, Complete EDTA free protease inhibitor cocktail (Roche)). Extracts were clarified by centrifugation (21,000 × g for 10 min), and then mixed with SDS sample buffer for denaturation. The proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed for FLAG-tagged β2AR receptor (M1 antibody, Sigma), VPS35 (generously provided by C. Haft and J. Bonafacino), or GAPDH (Chemicon) by immunoblotting using horseradish peroxidase-conjugated sheep anti-mouse IgG or donkey anti-rabbit IgG (Amersham Biosciences), and SuperSignal extended duration detection reagent (Pierce). Band intensities of unsaturated immunoblots were analyzed and quantified by densitometry using a 12-bit cooled CCD camera and FluorChem 2.0 software (AlphaInnotech Corp.). The amount of FLAG-β2AR remaining at each time point was first expressed as a percentage of the amount of FLAG-β2AR in the identically transfected unstimulated cells. The lysosomal protease inhibitor ZPAD (Bachem) or the proteosomal inhibitor epoxomicin (Sigma) were added to cells 40 minutes prior to addition of isoproterenol when used at 200μM and 2μM respectively.
To compare degradation of endogenous TFR to FLAG-β2AR, stably transfected HEK 293 cells were grown in 6 well dishes, washed with ice-cold PBS and incubated with 300μg/ml sulfo-N-hydroxysuccinimide-biotin (Pierce) in PBS for 30 min at 4°C to biotinylate surface proteins. Following washing with Tris buffered saline to quench unreacted biotin, cells were returned to 37°C for incubation in media, with or without agonist, before extraction as described above. Biotinylated proteins were isolated from cell extract by immobilization on streptavidin-conjugated sepharose beads (Amersham) and washed three times with extraction buffer. Washed beads were eluted with SDS sample buffer before resolving by SDS-PAGE as above. Blotting was performed for β2AR and TFR (13–6800, Invitrogen). In each experiment, triplicate treatments were analyzed for eachcondition. All experiments were carried out at least in triplicate (number indicated in figure legends), and values reported werederived from the mean determination across experiments.
Immunopurification of SNX27-HA was done using HEK 293 cells three days after transient transfection. Cells were cross-linked using 0.3mM DSP (Pierce) for 30 min prior to lysis in extraction buffer and addition of mouse HA11 antibody (Covance). SNX27-HA complexes were isolated using protein A/G beads (Pierce), washed four times with extraction buffer, and eluted with Nupage LDS buffer (Invitrogen).
Mass Spectrometry
Immunopurification and mass spectrometry of SNX27-3×FLAG was performed as previously described43.
Endosome Purification
Endosome purification was performed as previously published44. In addition to previously mentioned antibodies, Calnexin (ab22595, Abcam) and LAMP1 (H4A3, Santa Cruz) antibodies were used.
Signaling
HEK 293 cells stably expressing β2AR were treated with 10 μm isoproterenol or 1 μm forskolin (Sigma) for 20 minutes before signaling assays were performed according the manual of the Direct cAMP EIA Kit (Enzo Life Sciences, Plymouth Meeting, PA). cAMP levels were normalized to protein level using Coomasie Plus Protein Quantification Reagent (Thermo Scientific).
Statistics
Results are presented as mean ± SEM. based on data averaged across multiple independent experiments. The n value of an experiment represents experiments done on different days unless otherwise noted. To assign significance, results were compared to control experiments with an unpaired t-test using Prism (v4.03, GraphPad, La Jolla, CA). Only p-values below 0.05 are shown. Statistical comparison of knockdown effect on CD222 surface labeling (Fig 4e and 5d) and of knockdown effect on cAMP accumulation (Fig 5f) was performed using the one sampled t-test against the normalized value of one or 100%. Two-way ANOVA with Bonferonni posttest was performed on the recycling time-courses of β2AR and TFR. In these graphs * represents a p<0.05, ** represents p<0.01, and *** represents p<0.001 as no value is given by the program.
Supplementary Material
(a) A representative image of endosomes exhibiting retromer tubules is shown. A10 cells were transiently transfected with FLAG-β2AR (red) and VPS29-GFP (green). Cells were imaged live by spinning disk confocal microscopy as described in Fig. 1a. The scale bar represents 1 μm. (b) A10 cells transiently transfected with FLAG-β2AR were assayed by flow cytometry for their ability to recycle Alexa conjugated M1 anti-FLAG antibody. This experiment was performed as in Fig. 2b (n=3). Data points are the mean ± SEM.
SNX27-3×FLAG was immunopurified from HEK 293 cells and copurifying proteins were analyzed by mass spectrometry. Components of the WASH complex were highly represented and are shown in the table. Reproducibility refers to the number of independent experiments, out of five, in which the indicated component was identified.
Representative movie of Alexa-labeled β2AR (left) and GFP-tagged VPS29 (middle) in live cells, imaged at 2Hz and played back at 10Hz (5x real time). The merged image (β2AR in red and VPS29 in green) is at right. Asterisk indicates an example of retromer tubule with a dynamic β2AR-containing membrane structure protruding distally. This tubule moves close to the plasma membrane.
Acknowledgments
We thank B. Padilla, J. Bonifacino, Y. Prabhu, N. Gulbahce, S. Duleh, M. Welch, R. Rojas, J. Hislop, M. Puthenveedu, K. Mostov, J. Weissman, K. Thorn, the Nikon Imaging Center, A. Burlingame, and the UCSF Mass Spectrometry Facility (supported by P41RR001614) for reagents, advice, technical training, and support. Lastly, we thank H. Bourne and M. Ray for critical readings of the manuscript. This work was supported by research grants from the National Institutes of Health. PT was supported by the National Science Foundation. NJK is a Searle and Keck Young Investigator Fellow.
Footnotes
Author Contributions
PT and MvZ conceived the project and wrote the manuscript. PT performed most of the experiments with additional contributions from BL. Mass-Spectrometry was performed by SJ, analyzed by PC, in the lab of NJK.
Competing Interest Statement
The authors of this paper declare no competing financial interests.
Reference List
- 1.Seachrist JL, Anborgh PH, Ferguson SS. beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem. 2000;275:27221–8. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- 2.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–90. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Lauffer B, Von Zastrow M, Kobilka BK, Xiang Y. N-ethylmaleimide-sensitive factor regulates beta2 adrenoceptor trafficking and signaling in cardiomyocytes. Mol Pharmacol. 2007;72:429–39. doi: 10.1124/mol.107.037747. [DOI] [PubMed] [Google Scholar]
- 4.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–68. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 5.Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47:666–76. [PubMed] [Google Scholar]
- 6.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 7.Puthenveedu MA, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143:761–73. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–14. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlton JG, Cullen PJ. Sorting nexins. Curr Biol. 2005;15:R819–20. doi: 10.1016/j.cub.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Carlton J, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 11.Mari M, et al. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic. 2008;9:380–93. doi: 10.1111/j.1600-0854.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–36. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas R, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–26. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–79. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 15.Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–69. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–22. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore RH, Millman EE, Alpizar-Foster E, Dai W, Knoll BJ. Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. J Cell Sci. 2004;117:3107–17. doi: 10.1242/jcs.01168. [DOI] [PubMed] [Google Scholar]
- 19.von Zastrow M, Kobilka BK. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992;267:3530–8. [PubMed] [Google Scholar]
- 20.Lin SX, Mallet WG, Huang AY, Maxfield FR. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol Biol Cell. 2004;15:721–33. doi: 10.1091/mbc.E03-07-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth J, Berger EG. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982;93:223–9. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole NB, et al. Diffusional mobility of Golgi proteins in membranes of living cells. Science. 1996;273:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- 23.Franch-Marro X, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–7. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallard F, et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–64. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganley IG, Espinosa E, Pfeffer SR. A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells. J Cell Biol. 2008;180:159–72. doi: 10.1083/jcb.200707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Progida C, et al. Rab7b controls trafficking from endosomes to the TGN. J Cell Sci. 2010;123:1480–91. doi: 10.1242/jcs.051474. [DOI] [PubMed] [Google Scholar]
- 27.Derby MC, et al. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic. 2007;8:758–73. doi: 10.1111/j.1600-0854.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 28.Utskarpen A, Slagsvold HH, Iversen TG, Walchli S, Sandvig K. Transport of ricin from endosomes to the Golgi apparatus is regulated by Rab6A and Rab6A’. Traffic. 2006;7:663–72. doi: 10.1111/j.1600-0854.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 29.Seaman MN. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci. 2007;120:2378–89. doi: 10.1242/jcs.009654. [DOI] [PubMed] [Google Scholar]
- 30.Lauffer BE, et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190:565–74. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, et al. Proteomic analysis of beta1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem. 2006;281:2820–7. doi: 10.1074/jbc.M509503200. [DOI] [PubMed] [Google Scholar]
- 33.Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G Protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J Biol Chem. 2005;280:3305–13. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- 34.Vargas GA, Von Zastrow M. Identification of a novel endocytic recycling signal in the D1 dopamine receptor. J Biol Chem. 2004;279:37461–9. doi: 10.1074/jbc.M401034200. [DOI] [PubMed] [Google Scholar]
- 35.Heydorn A, et al. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279:54291–303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 36.Verges M, et al. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2004;6:763–9. doi: 10.1038/ncb1153. [DOI] [PubMed] [Google Scholar]
- 37.Tabuchi M, Yanatori I, Kawai Y, Kishi F. Retromer-mediated direct sorting is required for proper endosomal recycling of the mammalian iron transporter DMT1. J Cell Sci. 2010;123:756–66. doi: 10.1242/jcs.060574. [DOI] [PubMed] [Google Scholar]
- 38.Kleine-Vehn J, et al. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177:115–25. doi: 10.1083/jcb.200609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–29. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–49. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, et al. Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the beta1-adrenergic receptor. Proc Natl Acad Sci U S A. 1999;96:12559–64. doi: 10.1073/pnas.96.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jager S, et al. Purification and characterization of HIV-human protein complexes. Methods. 53:13–9. doi: 10.1016/j.ymeth.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cottrell GS, et al. Endosomal endothelin-converting enzyme-1: a regulator of beta-arrestin-dependent ERK signaling. J Biol Chem. 2009;284:22411–25. doi: 10.1074/jbc.M109.026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) A representative image of endosomes exhibiting retromer tubules is shown. A10 cells were transiently transfected with FLAG-β2AR (red) and VPS29-GFP (green). Cells were imaged live by spinning disk confocal microscopy as described in Fig. 1a. The scale bar represents 1 μm. (b) A10 cells transiently transfected with FLAG-β2AR were assayed by flow cytometry for their ability to recycle Alexa conjugated M1 anti-FLAG antibody. This experiment was performed as in Fig. 2b (n=3). Data points are the mean ± SEM.
SNX27-3×FLAG was immunopurified from HEK 293 cells and copurifying proteins were analyzed by mass spectrometry. Components of the WASH complex were highly represented and are shown in the table. Reproducibility refers to the number of independent experiments, out of five, in which the indicated component was identified.
Representative movie of Alexa-labeled β2AR (left) and GFP-tagged VPS29 (middle) in live cells, imaged at 2Hz and played back at 10Hz (5x real time). The merged image (β2AR in red and VPS29 in green) is at right. Asterisk indicates an example of retromer tubule with a dynamic β2AR-containing membrane structure protruding distally. This tubule moves close to the plasma membrane.