Abstract
Fungal exposure may elicit a number of pulmonary diseases in man, including allergic asthma. Fungal sensitization is linked to asthma severity, although the basis for this increased pathology remains ambiguous. To recapitulate environmental fungal allergen exposure in a human, a nose-only inhalation delivery of Aspergillus fumigatus conidia was employed in mice previously sensitized to Aspergillus antigen extract. BALB/c mice were immunized with subcutaneous and intraperitoneal injections of soluble A. fumigatus extract in alum, followed by 3 intranasal inoculations of the same fungal antigens dissolved in saline to elicit global sensitization in a manner similar to other published models. The animals were then challenged with a 10-min inhaled dose of live conidia blown directly from the surface of a mature A. fumigatus culture. After a single challenge with inhaled A. fumigatus conidia, allergic pulmonary inflammation and airway hyperresponsiveness were significantly increased above that of either naïve animals or animals that had been sensitized to A. fumigatus antigens but not challenged with conidia. The architecture of the lung was changed by inhalation of conidia with epithelial thickness, goblet cell metaplasia, and peribronchial collagen deposition significantly increased when compared to controls. Additionally, α-smooth muscle actin staining of histological sections showed visual evidence of increased peribronchial smooth muscle mass after fungal challenge. In summary, the inhalation of live A. fumigatus spores to the sensitized airways of BALB/c mice advances the study of the pulmonary response to fungus by providing a more natural route of exposure and, for the first time, demonstrates the consistent development of fibrosis and smooth muscle changes accompanying exposure to inhaled fungal spores in a mouse model.
Keywords: Asthma, Allergy, Fungi, Aspergillus, Murine model
Introduction
Asthma is characterized by reversible airway obstruction due to smooth muscle constriction, peribronchial inflammation, and increased mucus in the airways. In contrast, architectural remodeling of the airway wall, which is marked by increased peribronchial fibrosis and smooth muscle hyperplasia, signifies chronic, irreversible lung obstruction. Seventy percent of asthma patients have attendant atopy [1], and in many of these allergic asthmatics’ respiratory symptoms are triggered by the inhalation of airborne allergens. Asthmatic patients with mold sensitivities represent a particularly hard to treat clinical population [2-4]. Sensitization to common molds has been linked with increased need for medication in asthmatic children [5] and increased numbers of hospital stays in patients 16-60 years of age [6]. Although the connection between fungal sensitization and asthma is becoming better recognized, our understanding of host-mold interactions is still emerging.
Fungal allergy models typically use intratracheal inoculation of spores [7] or fungal extracts [8-10] to the sensitized airways of mice to study asthma-like immune responses. When germinating Aspergillus spores are suspended in liquid, as they are in intratracheal, intranasal ‘pulmonary’, or nebulized inhalation methods, the spore rapidly undergoes structural changes including the continual loss of the hydrophobins [11] and differential β-glucan display [12]. Inhalation delivery directly from the fungal culture has the advantage of preserving the antigenic character and the hydrophobicity of the spore coat. We hypothesized that this more natural route of inhalation exposure would provide a practicable model for human allergic asthma. The intent of this study was to verify that Aspergillus spores could be inhaled into murine lungs and that the inhalation of these spores into allergen-sensitized lungs would reproducibly elicit parameters of fungus-induced lung disease.
Methods
Mice
Specific pathogen-free BALB/c mice (6-8 wk of age) were purchased from Jackson Labs (Bar Harbor, ME) and bred and maintained in a specific pathogen-free facility for the duration of this study. Animals were fed and given water ad libitum throughout the study and housed on Alpha-dri™ paper bedding (Shepherd Specialty Papers, Watertown, TN) in micro filter topped cages (Ancare, Bellmore, NY). Prior approval for these studies was obtained from the Institutional Animal Care and Use Committee of North Dakota State University (Fargo, ND).
Fungal antigen, conidia, and culture
Soluble Aspergillus fumigatus antigen was purchased from Greer Laboratories (Lenoir, NC) and fungal culture stock (strain NIH 5233) was purchased from American Type Culture Collection (Manassas, VA). All experimental procedures utilizing A. fumigatus were conducted with prior approval of the Institutional Biological Safety Office of North Dakota State University.
A single lyophilized A. fumigatus culture was reconstituted per ATCC recommendations, and 60-μl aliquots of the suspension were stored at 4°C until use. To ensure that each culture used for the inhalation experiments yielded a similar number of mature conidia, ten 25-cm2 Sabouraud Dextrose Agar (SDA) culture flasks were inoculated with individual aliquots of the stock fungal suspension, grown for 8 days at 37°C, harvested in 20 ml of PBS with 0.1% Tween® 80, and the spores were counted using a hemacytometer. A mean of 6.5 × 10 9 (± 0.28 × 10 9) spores were recovered from each culture, demonstrating the reproducibility of the inocula used for the inhalation challenge.
Controls
One group of naïve animals, which were neither sensitized nor challenged, was assessed to determine baseline values in the BALB/c strain. A second group of animals that was sensitized (as described below) but not challenged with conidia was used to assess changes in the lung caused solely by sensitization in comparison to sensitized animals that were challenged with live conidia (day 0 time point).
Allergen sensitization
To elicit global sensitization, mice were injected subcutaneously (SC) and intraperitoneally (IP) with a total of 10 μg of soluble A. fumigatus antigen (Greer Laboratories) dissolved in 0.1 ml PBS and 0.1 ml Imject® Alum (Pierce, Rockford, IL). Two weeks later, mice started a series of three, weekly 20μg intranasal (IN) sensitizations consisting of soluble A. fumigatus antigen (Greer Laboratories) dissolved in 20 μl of normal saline [7].
Inhalation fungal challenge
Treatment animals were challenged with airborne A. fumigatus conidia one week after the final intranasal sensitization. An inoculation chamber with ports for 3 mice was fitted with an in-line 25-cm2 culture flask in which a mature (8-day-old), A. fumigatus culture had been grown on SDA. Airflow through the culture flask was delivered at 2 psi to liberate the hydrophobic spores and allow their delivery through the inoculation chamber. The inoculation chamber was housed in a Class II biological safety cabinet for inhalation experiments and spores in the exhaust air were collected into a series of two sporicidal traps. Before the first group of 3 animals was treated, the inoculation chamber’s ports were plugged, and fungal spores were blown through the chamber for 10 min to coat the interior. A sample of the fungus that was deposited on the inner wall of the inoculating chamber was removed and visualized under oil immersion to verify that the content of the inoculums was resting conidia. No evidence of spore swelling or hyphal fragments was detected. The ports were then unplugged. Each anesthetized animal was placed supine with its nose in one of the three inoculation ports for 10 min with airborne fungal spores blowing through the chamber. The fungal culture was changed with each new set of mice. Figure 1 shows a graphic representation of the experimental model.
Figure 1.
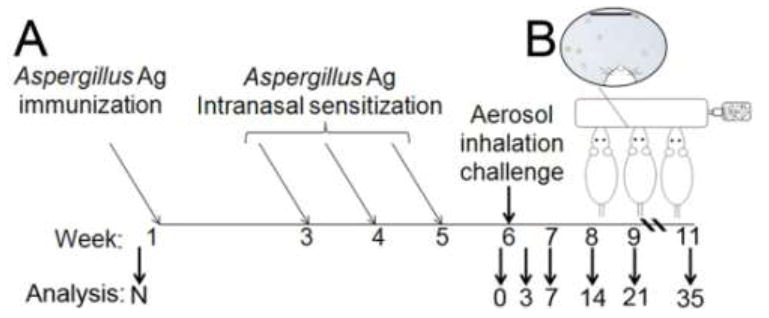
The Aspergillus fumigatus inhalation model: sensitization with soluble antigens followed by allergen challenge with airborne conidia. (A) Naïve BALB/c mice (N) received neither sensitization nor allergen challenge. Mice were sensitized with immunizations of soluble Aspergillus Ag in alum and 3 intranasal inoculations with soluble Ag. The “Day 0” control group was sensitized, but not challenged. For allergen challenge, each of 5 treatment groups was exposed to resting, airborne A. fumigatus conidia for 10 min, and parameters of allergic airways disease were assessed at days 3, 7, 14, 21, and 35 after conidia. (B) Samples from the inoculation chamber were assessed under oil immersion microscopy to determine that the conidia were in the resting phase. Scale bar = 20 μm.
Assessment of A. fumigatus conidia in murine airways after inhalation
To evaluate the spatial delivery of the spores to the airways, naïve BALB/c mice were anesthetized and treated with a 5-min dose of conidia. Whole left lungs were immediately removed, inflated with 10% neutral buffered formalin, and processed for histological assessment. Gomori methanamine silver (GMS) staining was used to visualize spores in the lung.
To calculate the number of viable spores that were delivered to the lungs, naïve BALB/c mice were anesthetized and treated with a 10-min dose of conidia. Whole lungs were immediately removed, minced in PBS, and serial dilutions of lung homogenates were made and plated on SDA. 6.6 × 10 5 (± 0.43 × 10 5) CFU were counted after incubation at 37°C for 24h. Although the mouse in the first port received more conidia, the range of difference among the 3 ports was less than 15,000 spores. In addition, when port of delivery was linked to the pathology resulting from conidia inhalation, no differences were detected between the ports (data not shown).
Measurement of airway hyperresponsiveness and tissue collection
Airway hyperresponsiveness (AHR) to intravenous methacholine (480 μg/kg) was assessed at days 0, 3, 7, 14, 21, and 35 after conidia challenge as previously described [7]. This dose of methacholine had been previously shown to double the baseline airway resistance in naïve animals. For these studies, experimental airway responses significantly higher than the 2X baseline of naïve animals were considered hyperresponsive and responses significantly different (p<0.05) from those of the day-0 time point are indicated with an asterisk in the results section. Sodium pentobarbital (0.1 mg/10 g of mouse body weight; Butler, Columbus, OH) was used to anesthetize each mouse before intubation and ventilation with a Harvard pump ventilator (Harvard Apparatus, Reno, NV). Baseline airway response was assessed before methacholine injection and a peak airway resistance was measured post injection using Buxco plethysmography (Buxco, Troy, NY). After airway assessment, 500 μl of blood was removed from each mouse and centrifuged at 12,000 Xg for 10 min to yield serum. Lungs were dissected in a deflated state from each mouse and either snap frozen in liquid N2 for protein analysis or inflated ex vivo with 1.0 ml of 10% neutral buffered formalin and fixed for 18 h until processed for histology.
Morphometric analysis of leukocytes in bronchoalveolar lavage (BAL) samples
After AHR measurement, 1.0 ml of sterile normal saline was used to lavage the bronchoalveolar space. BAL cells were pelleted and resuspended in 200 μl of PBS. Morphometric analysis of macrophages/monocytes, neutrophils, eosinophils, and lymphocytes was performed on BAL samples that had been cytospun (Shandon Scientific, Runcorn, UK) onto glass microscope slides and stained with Quik-Dip differential stain (Mercedes Medical; Sarasota, FL). The mean number of each cell type per high powered field (hpf, 1000X) was determined in allergen-challenged lungs after counting a total of at least 300 cells per slide in random fields.
Cytokine analysis from whole lung homogenate and serum IgE levels
Levels of murine IL-4 and IFN-γ were determined in 100-μl samples from whole lung homogenates using commercially available ELISAs (BD Biosciences Pharmingen, San Diego, CA). Cytokine levels were normalized to total lung protein using a Bradford assay (Bio-Rad, Hercules, CA). IgE levels were analyzed by ELISA on serum samples using complementary capture and detection antibody pairs for the immunoglobulin (BD Biosciences Pharmingen) according to the manufacturer’s protocol.
Histological and morphometric analysis
Formalin-fixed whole left lungs were paraffin-embedded and sectioned. Resulting 5-μm serial sections were affixed to glass microscope slides and stained with periodic acid-Schiff’s (PAS) stain, hematoxylin and eosin (H&E) stain, or Gomori’s trichrome stain. Other sections were used for immunohistochemical analysis of smooth muscle (primary Ab anti-mouse α-smooth muscle actin, 1:500, Abcam, Cambridge, MA; R&D Cell and Tissue Staining Kit, Minneapolis, MN for all other IHC reagents).
PAS-stained tissue sections were used to visualize mucus-producing goblet cells which were counted along ten randomly selected 100-μm segments of basement membrane for each mouse lung. The ratio of goblet cells to total cells per 1.0 mm of basement membrane was used to determine the percentage of goblet cells at each time point.
H&E-stained histological sections were used to assess the thickness of the epithelial/goblet cell lining of the airways in control and treatment mice after allergen challenge. For each sample, at least 50 discrete points were measured at 50-μm intervals along the second (L2) or third (L3) lateral bronchial branch for each section. Using Olympus’s MicroSuite™ Biological Suite photometric analysis package, a perpendicular line was drawn from each point on the basement membrane through the full thickness of the epithelium. Care was taken to avoid branches or areas where oblique angles would confound the measurement and only areas where contiguous cells were attached to the basement membrane were measured. The mean epithelial thickness was reported for each group.
Gomori’s trichrome-stained histological sections were used to assess subepithelial collagen deposition in control and sensitized and challenged mice. For each sample, the full thickness of peribronchial collagen (blue staining) was measured at 100 discrete points at 50-μm intervals along the sides of the L2 or L3 bronchial branch with care to avoid areas of branching that would inappropriately increase the measurement. Results were expressed as the mean of the means of the each tissue section’s measurements.
Statistical analysis
The presented data are from a single experiment, and represent comparable findings from at least 4 independent repeats of the model. InStat software (GraphPad Software, Inc., La Jolla, CA) was used to calculate statistics; differences between groups were tested with a one-way analysis of variance (ANOVA) and Dunnett’s multiple comparisons post test. p<0.05 (*) as compared to day-0 controls was considered significant and p<0.01 (**) very significant, dashed lines on graphs represent the mean value from naïve mice. n = 3-5/group. All results are expressed as the mean ± SEM.
Results
Resting Aspergillus spores are inhaled into the distal airways of murine lungs
A sample of airborne fungal material that was retrieved from the inside of the inoculation chamber showed that the inoculum delivered to the airways of the anesthetized mice were conidia in the resting phase (Fig 1B). The distribution of conidia to the airways of the murine lung was assessed in tissue sections from naïve lungs immediately after a 5min dose of inhaled spores. The small size (1-2 μm) and compact oval shape of the spores facilitated their inhalation to the distal airways of the lung (Fig 2).
Figure 2.
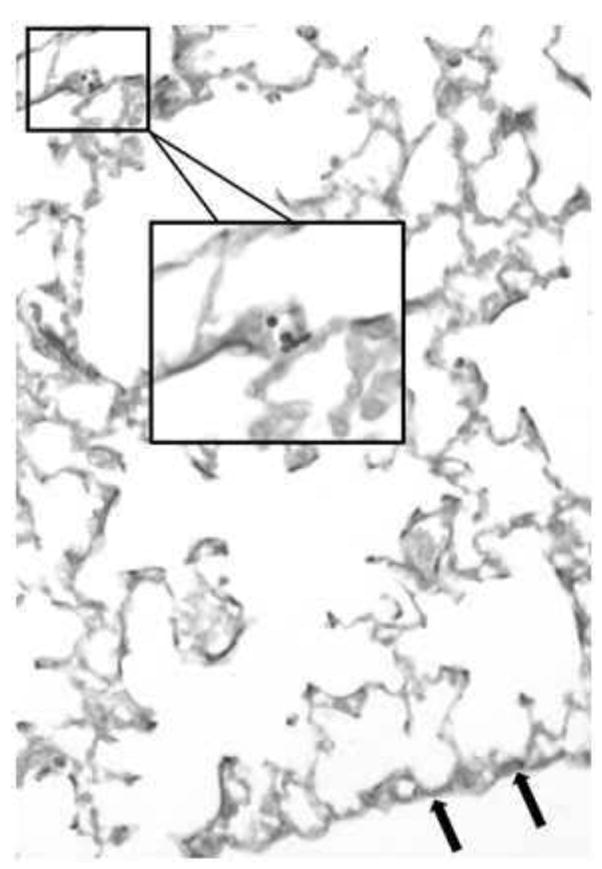
Aspergillus fumigatus conidia are found in the distal airways of BALB/c mice after inhalation. GMS staining of lung sections from naïve BALB/c mice immediately after treatment with a 5min dose of airborne A. fumigatus spores. Spores (black) were noted in the small airways of the distal lung (arrows point to the visceral pleura).
Airway epithelial cells undergo metaplasia to goblet cells after inhalation of Aspergillus conidia
To assess the response of the allergen-sensitized lung to the inhalation of airborne spores, histological sections were differentially stained to assess goblet cell metaplasia (Fig 3A) and epithelial thickening (Fig 3B). Nearly 70% of the cells lining the large, conducting airways were mucus-producing goblet cells at day 7 after allergen challenge. This corresponded to the peak thickness of the epithelium for this study (Fig 3B). Goblet cell numbers decreased steadily after day 7 and were replaced by new ciliated, epithelial cells. By day 35 after allergen challenge goblet cells were no longer apparent by PAS staining (Fig 3A); however, the epithelium remained significantly thicker in sensitized and challenged mice as compared to unchallenged controls (Fig 3B).
Figure 3.
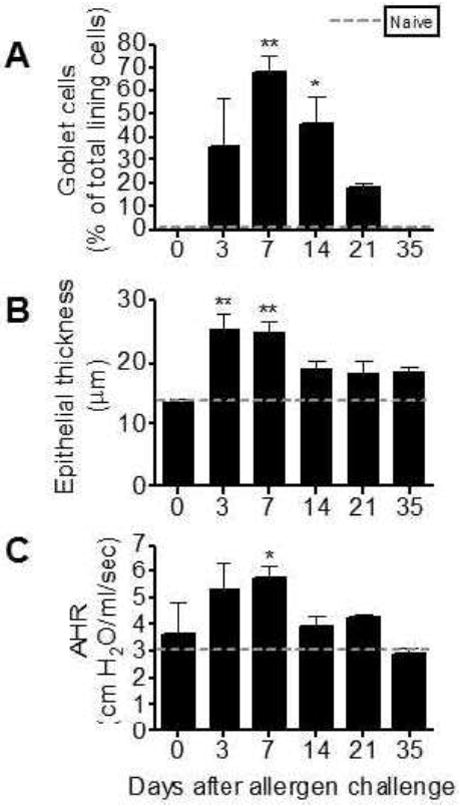
Airway epithelial changes and hyperresponsiveness in allergen sensitized BALB/c mice after inhalation allergen challenge with live Aspergillus fumigatus conidia. Goblet cell numbers were reported as the percent of total epithelial cells along segments of airway epithelium lining the large lateral branches of the bronchi (A). Mean epithelial thickness was measured at 50 μm intervals along the basement membrane of the large lateral branches of the bronchi through the full thickness of the epithelium (B). Fifty measurements were collected per sample and the group’s mean is reported. Baseline airway resistance in all groups was similar prior to methacholine provocation (1.52 ± 0.061 cm H2O/ml/s). Peak increases in airway resistance were recorded after i.v. methacholine injection (480 μg/kg). Naïve values are represented with a dashed line: naïve goblet cell percentage (A), naïve epithelial thickness (B), and airway response after methacholine injection (C). Bars represent the mean ± SEM, n = 35 mice/group, * = p<0.05 as compared to day0 controls.
Inhalation fungal challenge results in airway hyperresponsiveness after systemic and local sensitization with A. fumigatus antigens
Airway responses from naïve animals after methacholine injection were used to determine the threshold for airways hyperresponsiveness (Fig 3C, dashed line). When compared to naïve airway responses, AHR was significantly elevated at days 3, 7, 14, and 21 after conidia (Fig 3C). However, when compared to the day-0 control animals, AHR was significantly elevated at day 7 only (Fig 3C). By day 35 after conidia challenge, AHR had returned to that observed in naïve BALB/c animals after methacholine provocation and remained comparable to naïve levels through at least day 49, the last time point that animals have been tested with a single allergen challenge (data not shown).
Leukocytes are recruited to the allergic airways after inhalation conidia challenge
Temporal and spatial recruitment of leukocytes was assessed in tissue sections and BAL counts of sensitized, unchallenged animals (Fig 4A&B) as well as allergic lungs (Fig 4C-J) at various time points after allergen challenge. Perivascular and peribronchial tissue inflammation was absent in sensitized lungs before allergen challenge (Fig 4A&B), which was comparable to naive lungs (data not shown). In contrast, recruited inflammatory cells were noted at day 3 in allergen-challenged animals (Fig 4C&D) when leukocytes extravasated from the vasculature and moved to the peribronchial space of the conducting airways. Inflammation localized around the large airways at day 7 (Fig 4E&F) and when observed under high magnification appeared to consist of a mixed leukocytic population. By day 14, a distinct lymphocytic infiltrate occupied the peribronchial space (Fig 4G&H). Persistent peribronchial inflammation was evident until at least day 35 (Fig 4I&J) after allergen challenge.
Figure 4.
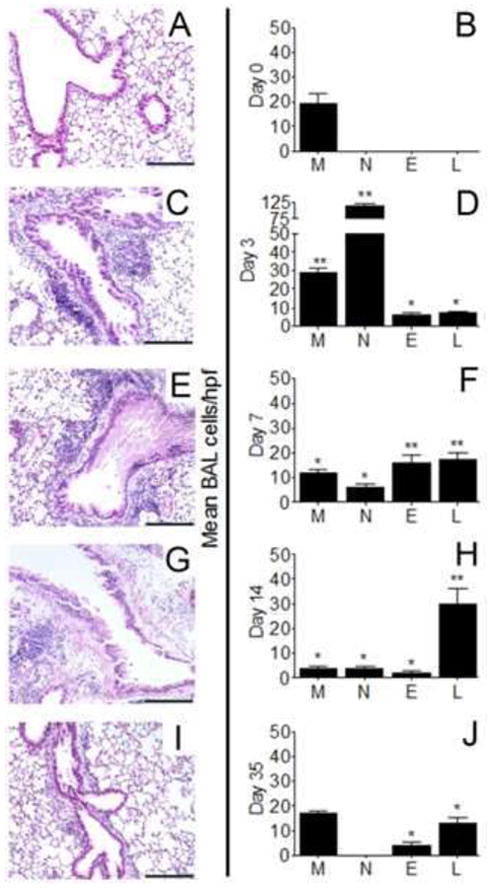
Inflammation in and around the conducting airways of sensitized BALB/c mice before and after airborne conidia challenge. Lungs from sensitized BALB/c mice (A) or sensitized lungs removed at day 3 (C), 7 (E), 14 (G), or 35 (I) after a 10min inhalation dose of mature A. fumigatus conidia were stained with hematoxylin and eosin to reveal peribronchial inflammation, bar = 200 μm. Macrophage (M), neutrophil (N), eosinophil (E), or lymphocyte (L) cells washed from the airways of sensitized mice (B) or at days 335 after conidia (D,F,H,J) were cytospun onto microscope slides and differentiated by morphometric and staining characteristics. Histology and BAL cell counts for naïve mice were similar to day0 controls (not shown). Data are expressed as the mean number of cells per hpf ± SEM, n = 35 mice/group. *, p<0.05; **, p<0.01 as compared to day0 controls.
Morphometric identification of monocyte/macrophage lineage cells (M), neutrophils (N), eosinophils (E), and lymphocytes (L) was used to assess leukocyte egress to the airway lumen. Macrophages were the only cell type routinely counted in either naïve (data not shown) or day-0 control lungs (Fig 4B). Neutrophils dominated the early inflammation at day 3 after allergen challenge (Fig 4D). Both eosinophils and lymphocytes were prominent inflammatory components by day 7 after allergen challenge (Fig 4F). BAL cells consisted mostly of lymphocytes by day 14 after challenge (Fig 4H). Few cells were noted in the day-21 BAL samples and consisted of scarce macrophages, eosinophils, and ~5 lymphocytes per hpf (data not shown). By day 35 after allergen challenge, a return to the normal number of macrophages was observed in the BAL compartment, a low number of eosinophils remained persistent in the airways, and lymphocytes were noted at this late time point (Fig 4J).
Allergic sensitization and inhalation challenge are accompanied by elevations in mediators associated with inflammatory and allergic responses
As compared to either naïve (dashed line) or day-0 controls, inhalation of conidia significantly elevated serum IgE levels at all time points at day 3 (Fig 5A). IL-4 production was induced during sensitization of the lung, as shown by the increase in its production at day 0 before inhalation challenge (Fig 5B). Additionally, its production was significantly enhanced above the day-0 control at day 3 after inhalation challenge (Fig 5B). The canonical Th1type cytokine, IFN-γ, was also elevated at day 3 after challenge (Fig 5C).
Figure 5.
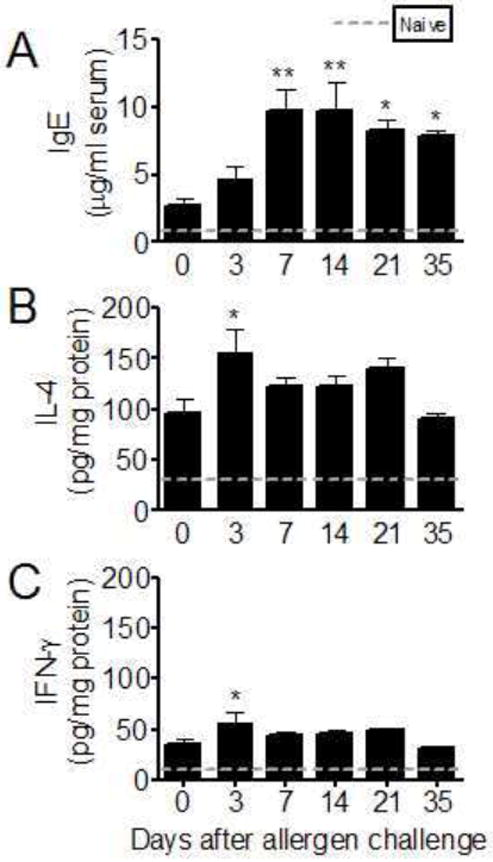
Total IgE from serum and IL4 and IFNγ from whole lung homogenates after allergen challenge with airborne Aspergillus fumigatus conidia in BALB/c mice. Total serum IgE (A), lung IL4 (B), and lung IFNγ (C) levels were measured using commercially available ELISAs. Specimens were collected 3, 7, 14, 21, and 35 days after allergen challenge with conidia and compared to sensitized, but unchallenged, (day 0) levels. Mean values for naïve animals are represented by the dashed line for comparison. All results are expressed as the mean ± SEM, n = 35/group. *, p<0.05; **, p<0.01 as compared to day0 controls.
Inhalation fungal challenge increases signs of airway wall remodeling around the airways of BALB/c mice sensitized to A. fumigatus antigens
Smooth muscle was assessed using α-SMA IHC in naïve controls, in mice that had been sensitized to A. fumigatus antigens but not challenged, and in animals that had been both sensitized to and challenged with fungal spores. In naïve animals (Fig 6A) or in those that had been sensitized with Aspergillus antigens but not challenged with conidia (Fig 6B), peribronchial α-SMA staining was scant beneath the basement membrane. In animals that had been sensitized to fungal antigens and challenged with airborne conidia, the peribronchial smooth muscle appeared distinctly more prominent and muscle cells appeared to be organized into small bundles (Fig 6C).
Figure 6.
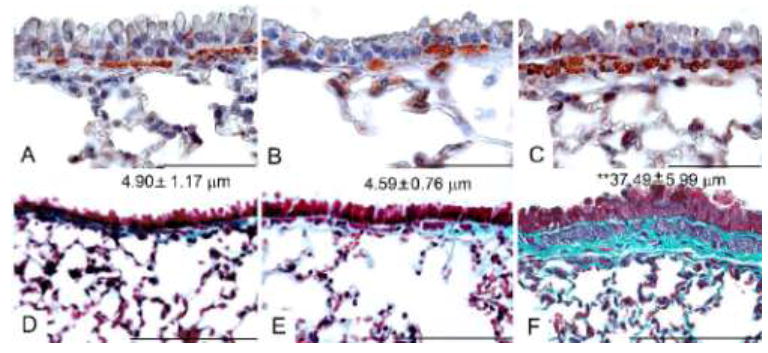
Smooth muscle and fibrotic changes signifying airway wall remodeling after airborne conidia allergen challenge. αSMA staining (red) was used to identify peribronchial smooth muscle changes in histological sections from naïve BALB/c mice (A), mice sensitized to Aspergillus antigens (B), or sensitized mice that had been challenged with a 10min dose of inhaled conidia 35 days before (C). Gomori’s trichrome stain was used to show the accumulation of peribronchial collagen (blue) on histological sections from naïve mice (D), mice sensitized to Aspergillus antigens (E), or sensitized mice that had been challenged with a 10min dose of inhaled conidia 35 days before (F). Mean thickness of collagen is indicated for each group ± SEM. **, p<0.01. Bar in A, B, C = 50 μm, D, E,F = 100 μm.
Marked collagen deposition was observed in every sensitized lung that had been treated with airborne conidia and was significantly increased over that of naive lungs. Visually, naive mice (Fig 6D) exhibited a uniform, thin layer of collagen in subepithelial peribronchial areas, and this phenotype was characteristic of the sensitized animals as well (Fig 6E). After inhalation challenge, collagen matrix was markedly and significantly increased in the peribronchial space (Fig 6F). Dense fibers were evident in the subepithelial space above and below the peribronchial smooth muscle layer, and appeared to delineate smooth muscle bundles. Collagen was also noted around peribronchial inflammatory cells in this area.
Discussion
The size, shape, and surface hydrophobicity of A. fumigatus’ spores provide an efficient vehicle for their inhalation into the lungs of humans, but murine alveolar spaces may be as small as 10 μm in diameter—20 times smaller than human’s [13]. To mimic human inhalation exposure, we first showed that the spores of the A. fumigatus fungus are distributed to the small airways of the murine lung when inhaled. We were unable to find reports of Aspergillus models using the inhalation of live spores for asthma research, but a number of invasive aspergillosis (IA) models have been published. Native spore delivery methods are largely variations on the early work of Sidransky and Friedman (pumping lyophilized spores into a closed chamber) or Piggott and Emmons (blowing air over a fungal culture grown in the bottom of an Erlenmeyer flask) [14,15]. Piggott and Emmons’ live culture method was improved by Burrell’s use of a box in which fungal culture plates could be exchanged [16]. Our introduction of an in-line fungal culture flask eliminates the exposure of the operator to the fungus, increases the animal’s nose-only exposure to the mold, allows a controlled and reproducible flow of air over the culture, and facilitates a more natural pulmonary movement for the murine subject.
Structural and resident immune cells of the lung interact with inhaled resting conidia. A. fumigatus spores change quickly and dramatically upon germination [11], and the immune response targeting the conidia change along with the metamorphosis of the fungal cell’s coat properties [12,17]. In the present study, resting conidia were distributed throughout the large and small airways of the lung, contacting a large surface area. Interestingly, even with fewer viable spores than any other published model of asthma, Allergic Bronchopulmonary Aspergillosis (ABPA), or IA (Table 1); the resulting pathology was clearly and consistently present.
Table 1.
Models of pulmonary Aspergillus fumigatus exposure.
| Model | Challenge antigen / route / host | AHR | IL-4 | IgE | Eos | GCH | SMH | Fib |
|---|---|---|---|---|---|---|---|---|
| Acute fungal sinusitis [33] | 1 × 106 conidia in NS IN Neutropenic host | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| ABPA [7] | 5 × 106 conidia in 0.1% tween 80 IT Normal host | + | + | + | + | + | - | +/- |
| ABPA [34-36] | 100-200 μg of fungal extract antigens IN, IP, or IV Normal host | n.d. | + | + | + | - | - | - |
| Allergic fungal asthma [37] | 6-9 protease units in PBS IN Normal host | + | + | + | + | + | n.d. | n.d. |
| IA [38] | 2 × 107 conidia in NS IN Genetically manipulated host | n.d. | + | + | n.d. | n.d. | n.d. | n.d. |
| IA [39] | 5-10 × 105 conidia in 0.025% tween 20 IV Normal host | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Airborne delivery | 7 × 104 native conidia, airborne Inhalation Normal host | + | + | + | + | + | + | + |
AHR= airway hyperresponsiveness, Eos=eosinophilia, GCH=goblet cell hyperplasia, SMH=smooth muscle hyperplasia, Fib= fibrosis, IN=intranasal, IT=intratracheal, IP=intraperitoneal, IV=intravenous, ABPA=Allergic Bronchopulmonary Aspergillosis, IA=Invasive Aspergillosis, +=present, =not present, +/*=shown only at the highest doses tested, n.d.=not described
While serious morbidity is not noted in every case of long-term clinical asthma, fibrosis is noted with enough regularity that the NHLBI has included it in the diagnostic scope of the disease [18]. The orchestration of the allergic response, including aspects of airway wall remodeling, has been attributed primarily to the cytokine production of T cells [19-24] and eosinophils [25,26], both of which were plentiful after fungal inhalation challenge. Hogaboam’s model of Allergic Bronchopulmonary Aspergillosis (ABPA), using an intratracheal conidia challenge, is the only fungal model to show evidence of collagen deposition around the airways after inoculation with an intact spore [7]. It was an interest in the initiation and maintenance of fibrotic remodeling that led us to attempt to improve upon this method by eliminating the surgical component, reducing the potential for alterations of the immune and wound healing responses. Other models have demonstrated fibrotic remodeling only after a significant antigenic load [27], and none have demonstrated smooth muscle changes with consistency (Table 1). Not only does the current model provide a means for native, live spores to be delivered in a nose-only fashion, it also enables multiple fungal exposures to be employed without invasive surgery, which is necessary in intratracheal inoculations. The inhalation delivery method reliably results in peribronchial fibrosis and increased smooth muscle mass around the large airways of the asthmatic lung, signaling the structural changes associated with chronic airway wall remodeling [18,28-30].
Both primate experimental models [31] and human asthma [32] show increased smooth muscle mass as a consequence of disease. Smooth muscle around the airways may reduce the caliber of the lumen and add to its contractility. It may also function to recruit eosinophils and enhance their survival [33]. In this study, α-SMA staining revealed a layer of myocytes below the basement membrane of the large airways in naive samples. After allergen challenge, this cell layer was more easily seen on H&E-stained sections, and α-SMA staining showed an apparent increase in cell mass around the airways. Peribronchial collagen deposition was noted above, below, and between these cells, suggesting a potential source of extracellular collagen production.
Genetically altered animals provide useful tools in the understanding of complex biological process. Many of these animals are generated on a C57BL/6 background. The current model shows a robust inflammatory and remodeling phenotype in C57BL/6 mice (manuscript submitted) and is anticipated to be useful in these applications.
The processes which comprise the syndrome of clinical allergic asthma are complex. Models that more closely mimic the types and mechanisms of human allergen exposure are helpful to examine the interplay among the multiple cell and tissue types that are involved in pulmonary allergic responses. We have developed a model of fungal allergic airways disease in a mouse system that will permit the study of the mechanisms that initiate and perpetuate changes in the airway associated with allergic fungal asthma. In particular, this in vivo system will allow the examination of temporal and spatial interaction of cells and their mediators in the changing physiology of the allergic lung.
Acknowledgments
The authors would like to thank Kayla Serie for data collection for this study. Funding for this project was supported NIH/NCRR 2P20 RR015566 (including the use of Core Biology Facilities), NIH/NIAID 1R15AI69061, and through a faculty development grant from the NDSU Advance FORWARD program (NSF HRD-0811239).
Footnotes
Conflict of interest. None.
References
- 1.WHO. Global surveillance, prevention and control of chronic respiratory diseases: A comprehensive approach, 2007. World Health Organization; 2007. [Google Scholar]
- 2.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: A summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, O’Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The fungal asthma sensitization trial (fast) study. Am J Respir Crit Care Med. 2009;179:11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 4.Portnoy JM, Barnes CS, Kennedy K. Importance of mold allergy in asthma. Curr Allergy Asthma Rep. 2008;8:71–78. doi: 10.1007/s11882-008-0013-y. [DOI] [PubMed] [Google Scholar]
- 5.Schwindt CD, Tjoa T, Floro JN, McLaren C, Delfino RJ. Association of atopy to asthma severity and medication use in children. J Asthma. 2006;43:439–446. doi: 10.1080/02770900600758234. [DOI] [PubMed] [Google Scholar]
- 6.O’Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005;5:4. doi: 10.1186/1471-2466-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogaboam CM, Blease K, Mehrad B, et al. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol. 2000;156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haczku A, Atochina EN, Tomer Y, et al. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in balb/c mice. Am J Respir Cell Mol Biol. 2001;25:45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]
- 9.Singh SP, Barrett EG, Kalra R, et al. Prenatal cigarette smoke decreases lung camp and increases airway hyperresponsiveness. Am J Respir Crit Care Med. 2003;168:342–347. doi: 10.1164/rccm.200211-1262OC. [DOI] [PubMed] [Google Scholar]
- 10.Corry DB, Grunig G, Hadeiba H, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 11.Dague E, Alsteens D, Latge JP, Dufrene YF. High-resolution cell surface dynamics of germinating Aspergillus fumigatus conidia. Biophys J. 2008;94:656–660. doi: 10.1529/biophysj.107.116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohl TM, Van Epps HL, Rivera A, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochs M, Nyengaard JR, Jung A, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–124. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 14.Piggott WR, Emmons CW. Device for inhalation exposure of animals to spores. Proc Soc Exp Biol Med. 1960;103:805–806. doi: 10.3181/00379727-103-25677. [DOI] [PubMed] [Google Scholar]
- 15.Sidransky H, Friedman L. The effect of cortisone and antibiotic agents on experimental pulmonary aspergillosis. Am J Pathol. 1959;35:169–183. [PMC free article] [PubMed] [Google Scholar]
- 16.Burrell R. Improved device for the administration of fungal spores to small animals via the respiratory route. Appl Microbiol. 1970;20:984–985. doi: 10.1128/am.20.6.984-985.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera A, Van Epps HL, Hohl TM, Rizzuto G, Pamer EG. Distinct cd4+-t-cell responses to live and heat-inactivated aspergillus fumigatus conidia. Infect Immun. 2005;73:7170–7179. doi: 10.1128/IAI.73.11.7170-7179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busse W, Banks-Schlegel S, Noel P, et al. Future research directions in asthma: An NHLBI working group report. Am J Respir Crit Care Med. 2004;170:683–690. doi: 10.1164/rccm.200311-1539WS. [DOI] [PubMed] [Google Scholar]
- 19.Xiong L, Fang ZY, Tao XN, Bai M, Feng G. Effect and mechanism of ligustrazine on Th1/Th2 cytokines in a rat asthma model. Am J Chin Med. 2007;35:1011–1020. doi: 10.1142/S0192415X07005478. [DOI] [PubMed] [Google Scholar]
- 20.Anthoni M, Wang G, Leino MS, et al. Smad3 -signaling and Th2 cytokines in normal mouse airways and in a mouse model of asthma. Int J Biol Sci. 2007;3:477–485. doi: 10.7150/ijbs.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt PG, Sly PD. Th2 cytokines in the asthma late-phase response. Lancet. 2007;370:1396–1398. doi: 10.1016/S0140-6736(07)61587-6. [DOI] [PubMed] [Google Scholar]
- 22.Jaffar Z, Ferrini ME, Buford MC, Fitzgerald GA, Roberts K. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J Immunol. 2007;179:6193–6203. doi: 10.4049/jimmunol.179.9.6193. [DOI] [PubMed] [Google Scholar]
- 23.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Lee CM, Jeong YI, et al. D-pinitol regulates Th1/Th2 balance via suppressing Th2 immune response in ovalbumin-induced asthma. FEBS Lett. 2007;581:57–64. doi: 10.1016/j.febslet.2006.11.077. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen EA, Ochkur SI, Pero RS, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: Singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119:1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 28.Bousquet J, Vignola AM, Chanez P, et al. Airways remodeling in asthma: No doubt, no more? Int Arch Allergy Immunol. 1995;107:211–214. doi: 10.1159/000236980. [DOI] [PubMed] [Google Scholar]
- 29.Busse W, Elias J, Sheppard D, Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med. 1999;160:1035–1042. doi: 10.1164/ajrccm.160.3.9902064. [DOI] [PubMed] [Google Scholar]
- 30.Tran MU, Weir AJ, Fanucchi MV, et al. Smooth muscle hypertrophy in distal airways of sensitized infant rhesus monkeys exposed to house dust mite allergen. Clin Exp Allergy. 2004;34:1627–1633. doi: 10.1111/j.1365-2222.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 31.Woodruff PG, Dolganov GM, Ferrando RE, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 32.Fauchet F, Jadoul M, Franssen JD, Zhang J, De Groote D. Characterization of monoclonal antibodies against human interleukin-12 and their use in an ELISA for the measurement of this cytokine. Ann N Y Acad Sci. 1996;795:334–336. doi: 10.1111/j.1749-6632.1996.tb52685.x. [DOI] [PubMed] [Google Scholar]
- 33.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurup VP, Mauze S, Choi H, Seymour BW, Coffman RL. A murine model of allergic bronchopulmonary aspergillosis with elevated eosinophils and IgE. J Immunol. 1992;148:3783–3788. [PubMed] [Google Scholar]
- 35.Grunig G, Corry DB, Leach MW, et al. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J Exp Med. 1997;185:1089–1099. doi: 10.1084/jem.185.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JM, Denis M, Fournier M, Laviolette M. Experimental allergic bronchopulmonary aspergillosis in the mouse: Immunological and histological features. Scand J Immunol. 1994;39:19–26. doi: 10.1111/j.1365-3083.1994.tb03334.x. [DOI] [PubMed] [Google Scholar]
- 37.Koth LL, Rodriguez MW, Bernstein XL, et al. Aspergillus antigen induces robust Th2 cytokine production, inflammation, airway hyperreactivity and fibrosis in the absence of MCP-1 or CCR2. Respir Res. 2004;5:12. doi: 10.1186/1465-9921-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kheradmand F, Kiss A, Xu J, et al. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 39.Cenci E, Mencacci A, Del Sero G, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180:1957–1968. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 40.Van Epps HL, Feldmesser M, Pamer EG. Voriconazole inhibits fungal growth without impairing antigen presentation or T-cell activation. Antimicrob Agents Chemother. 2003;47:1818–1823. doi: 10.1128/AAC.47.6.1818-1823.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


