Summary
High-frequency stimulation transiently increases spontaneous synaptic transmission and the amplitude of evoked synaptic transmission (known as post-tetanic potentiation, PTP). Here we examine the roles of the calcium-dependent protein kinase C isoforms PKCα and PKCβ in PTP at the calyx of Held synapse. In PKCα/β double knockouts 80 % of PTP is eliminated, whereas basal synaptic properties are unaffected. PKCα and PKCβ produce PTP by increasing the size of the readily-releasable pool of vesicles evoked by high-frequency stimulation, and by increasing the fraction of this pool released by the first stimulus. PKCα and PKCβ do not facilitate presynaptic calcium currents. The small PTP remaining in double knockouts is mediated partly by an increase in mEPSC amplitude, and partly by a mechanism involving myosin light chain kinase. These experiments establish that PKCα and PKCβ are crucial for PTP, and suggest that long-lasting presynaptic calcium increases produced by tetanic stimulation may activate these isoforms to produce PTP.
Introduction
At many synapses, a period of high-frequency (tetanic) stimulation can evoke a transient increase in synaptic strength known as post-tetanic potentiation (PTP) (Feng, 1941; Griffith, 1990; Magleby, 1987; Magleby and Zengel, 1975; Zucker and Lara-Estrella, 1983; Zucker and Regehr, 2002). PTP is thought to provide an important means of synaptic regulation that can contribute to working memory and information processing (Abbott and Regehr, 2004; Silva et al., 1996). Many high-frequency stimuli are needed to induce PTP, and the frequency and duration of tetanic stimulation regulate the magnitude and duration of the enhancement (which lasts tens of seconds to minutes) (Habets and Borst, 2005, 2007; Korogod et al., 2005; Lev-Tov and Rahamimoff, 1980; Magleby, 1979; Zucker, 1989). Tetanic stimulation also increases both the frequency and the magnitude of spontaneous miniature excitatory postsynaptic currents (mEPSCs) at many (Castillo and Katz, 1954; Delaney and Tank, 1994; Eliot et al., 1994; Habets and Borst, 2005; He et al., 2009; Korogod et al., 2005, 2007; Magleby, 1987), but not all (Brager et al., 2003) synapses. It is not known whether increases in the frequency and amplitude of spontaneous transmission and the increase in evoked release share a common presynaptic mechanism.
Numerous mechanisms could contribute to PTP. According to the leading hypothesis, known as the residual calcium hypothesis, tetanic stimulation leads to an accumulation of calcium in the presynaptic terminal, and an accompanying increase in the probability of release that persists for tens of seconds (Brager et al., 2003; Delaney and Tank, 1994; Delaney et al., 1989; Regehr et al., 1994; Zucker and Regehr, 2002). Other possibilities include an increase in the size of the readily releasable pool of vesicles (Habets and Borst, 2005; Lee et al., 2008), an increase in the size of mEPSCs as a result of vesicles fusing with each other before ultimately fusing with the plasma membrane (He et al., 2009), a change in action potential waveform (Eccles and Krnjevic, 1959; Habets and Borst, 2005) and an increase in calcium entry (Habets and Borst, 2005, 2006).
Pharmacological studies have implicated protein kinase C (PKC) in PTP. Phorbol esters, activators of PKC (Newton, 2001), increase the amplitude of evoked release and occlude PTP (Hori et al., 1999; Korogod et al., 2007; Lou et al., 2008; Lou et al., 2005; Malenka et al., 1986; Oleskevich and Walmsley, 2000; Rhee et al., 2002; Shapira et al., 1987; Virmani et al., 2005; Wierda et al., 2007). Phorbol esters also increase the frequency of mEPSCs (Hori et al., 1999; Lou et al., 2008; Lou et al., 2005; Oleskevich and Walmsley, 2000; Parfitt and Madison, 1993). In addition, PKC inhibitors reduce the magnitude of PTP at many synapses (Alle et al., 2001; Beierlein et al., 2007; Brager et al., 2003; Korogod et al., 2007; Lee et al., 2007). Studies of the role of PKC in PTP have been limited by the selectivity and ineffectiveness of pharmacological tools available to inhibit and activate PKC (e.g., Lee et al., 2008) (for review see Brose and Rosenmund, 2002). Phorbol esters activate other synaptic proteins, including Munc13 (Lou et al., 2008; Rhee et al., 2002; Wierda et al., 2007). PKC inhibitors have highly variable effects on PTP: at the same synapse, different PKC inhibitors disrupt PTP to very different extents (Lee et al., 2008) (also Fioravante and Regehr, unpublished); at some synapses, PKC inhibitors do not affect PTP (Eliot et al., 1994; Lee et al., 2008; Reymann et al., 1988a; Reymann et al., 1988b); and in some cases PKC inhibitors and their inactive analogues have similar effects on PTP (Lee et al., 2008). In addition, other proteins have been implicated in PTP, including Munc13 (Junge et al., 2004; Shin et al., 2010), calmodulin and CamKII (Chapman et al., 1995; Fiumara et al., 2007; Junge et al., 2004; Khoutorsky and Spira, 2009; Reymann et al., 1988a; Wang and Maler, 1998) and myosin light chain kinase (Lee et al., 2008). These findings have cast doubt on the involvement of PKC in PTP.
If PKC is involved in PTP, identifying which PKC isoform mediates PTP is of fundamental importance. Is it a classical, calcium-sensitive PKC isoform such as PKCα, PKCβ or PKCγ, or one of the eight calcium-insensitive isoforms? The involvement of calcium-sensitive PKCs would be compatible with PKC being a sensor of calcium according to the residual calcium hypothesis, whereas if calcium-insensitive PKC isoforms regulate PTP, tetanic stimulation would have to elevate presynaptic DAG or act through some unidentified pathway, and another presynaptic calcium sensor would need to respond to residual calcium. At the calyx of Held, calcium-insensitive PKCs have been implicated (Saitoh et al., 2001). These results suggest that if PKC plays a role in PTP, it does not serve as a calcium sensor.
Here we use knockout animals to examine the roles of PKCα and PKCβ in tetanus-induced enhancement of evoked and spontaneous transmission, and phorbol-ester-mediated enhancement at the calyx of Held synapse, where these forms of plasticity have been thoroughly characterized in wild-type animals (Habets and Borst, 2005, 2006, 2007; He et al., 2009; Hori et al., 1999; Korogod et al., 2005, 2007; Lee et al., 2008; Lou et al., 2008; Lou et al., 2005; Wu and Wu, 2001). We find that PKCα and PKCβ are both present at the calyx of Held. In PKCα/β double knockout animals, the calyx of Held is devoid of all calcium-dependent PKCs, as PKCγ is not present at this synapse (Saitoh et al., 2001). In PKCα/β double knockouts, basal properties of synaptic transmission are normal but 80 % of PTP is eliminated. PKCα and PKCβ produce PTP primarily by increasing the size of the readily releasable pool of vesicles that fuse in response to brief high-frequency stimulation; they do not facilitate presynaptic calcium currents. The small PTP remaining in double knockout animals is mediated in part by an increase in mEPSC amplitude and in part by a mechanism involving myosin light chain kinase (MLCK). In contrast to PTP, the increase in the mEPSC frequency following tetanic stimulation does not depend on PKCα/β, suggesting that tetanic enhancement of evoked and spontaneous release are mediated by different mechanisms. Finally, phorbol ester-dependent enhancement is greatly reduced in slices from double knockout animals. These findings establish the crucial role of calcium-sensitive PKCs in the enhancement of evoked synaptic responses induced by either tetanic stimulation or phorbol esters.
Results
Calcium-dependent PKC isoforms in the calyx of Held
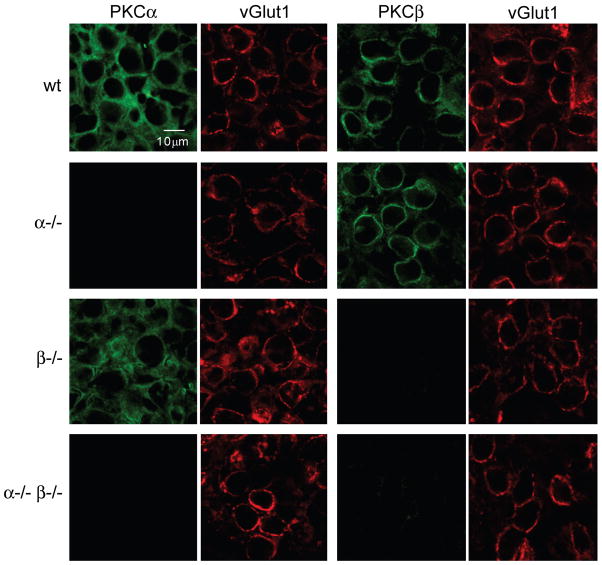
We used immunohistochemistry to determine the localization of PKCα and PKCβ within the medial nucleus of the trapezoid body (MNTB) (Figure 1). Slices were co-labeled with antibodies to either PKCα or PKCβ (green) and to the vesicular glutamate transporter vGlut1 to label glutamate-containing synaptic vesicles within presynaptic terminals (red). vGlut1 labeling was used to identify the calyces of Held, the large presynaptic terminals that provide a synaptic contact between globular bushy cells in the anteroventral cochlear nucleus and the principle neurons in the MNTB. In slices from wild-type mice, antibody labeling for PKCα and PKCβ overlapped with vGlut1 labeling, consistent with presynaptic localization of these kinases at the calyx of Held synapse. No labeling was detected in MNTB primary neurons. Anti-PKCα antibody labeling appeared less restricted than anti-PKCβ antibody labeling, suggesting that PKCα might also be present in other structures, in addition to the presynaptic terminals.
Figure 1. Immunohistochemical localization of PKCα and PKCβ in the medial nucleus of the trapezoid body (MNTB).
Brain slices containing the MNTB were labeled with antibodies to either PKCα or PKCβ (green), and co-labeled with an antibody to the presynaptic marker vGlut1 (red). Closely spaced pairs of images correspond to the same field of view imaged through the green and red channels. Representative images for PKCα/vGlut1 and PKCβ/vGlut1 are shown for slices from wild-type (wt), PKCα knockout (α−/−), PKCβ knockout (β−/−), and double knockout (α−/− β−/−) animals.
In PKCα−/− mice, labeling with antibodies to PKCβ and vGlut1 was similar to that observed in wild-type animals, but labeling with antibodies to PKCα was absent. Similarly, in PKCβ−/− mice labeling with antibodies to PKCα and vGlut1 was similar to that observed in wild-type animals, but labeling associated with antibodies to PKCβ was absent. In PKCα−/−β−/− double knockout mice no labeling was observed with antibodies to PKCα or PKCβ, but vGlut1 labeling was similar to that observed in wild-type littermate animals. These findings indicate that the targeted PKC isoforms are eliminated in knockout animals without noticeably affecting the synaptic distribution of vGlut1. They also suggest that the presence of one isoform is not required for the proper subcellular localization of the other.
Calcium-dependent PKC isoforms contribute to PTP
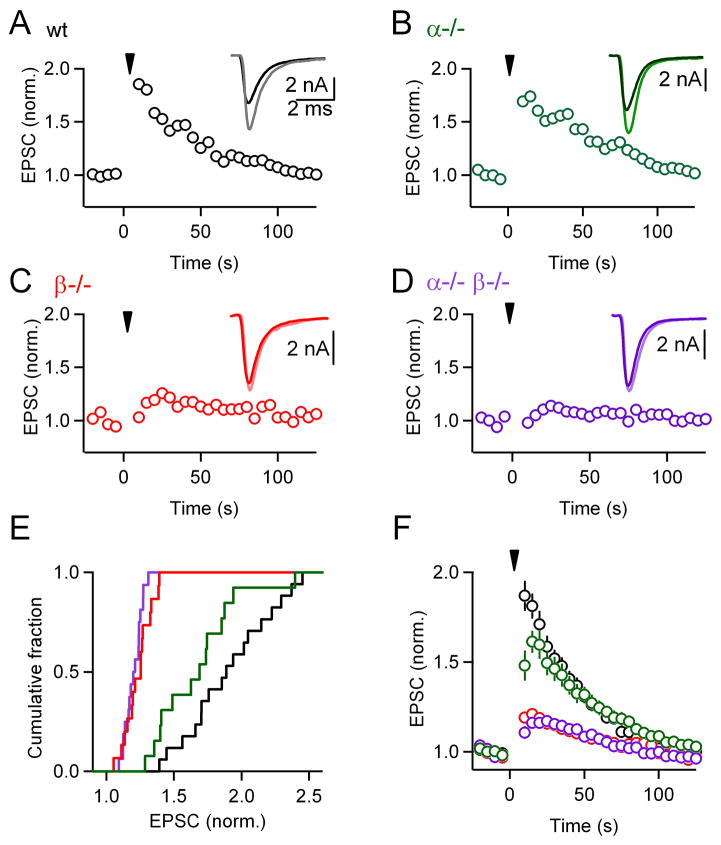
We investigated the role of calcium-dependent PKCs in PTP by activating single inputs at 100 Hz for 4 s. Robust PTP was observed in wild-type mice. In a representative experiment, the amplitude of PTP was 1.9-fold and the time constant of decay was ~ 40 s (Figure 2A). Representative experiments show that somewhat smaller PTP was observed in PKCα−/− animals (Figure 2B), and PTP was greatly reduced in PKCβ−/− (Figure 2C) and PKCα−/−β−/− mice (Figure 2D). The varying extent of PTP within each group is shown in the cumulative histograms (Figure 2E). Significant differences between groups in the extent of PTP were apparent both in the cumulative histograms (Figure 2E) and in the summary plot of average potentiation (Figure 2F). In wild-type animals, the amplitude of enhancement ranged from 1.4-fold to 2.5-fold and averaged 1.81 ± 0.07-fold (n = 17), which is similar to what has been described previously (Korogod et al., 2005, 2007; Lee et al., 2008). The extent of PTP in slices from PKCα−/− animals (1.61 ± 0.06-fold, n = 13) was smaller than for wild-type animals (p < 0.01), and PTP was greatly reduced in PKCβ−/− (1.21 ± 0.02-fold, n = 15, p < 0.01) and PKCα−/−β−/− mice (1.16 ± 0.02-fold, n = 16, p < 0.01). These results suggest an important role for calcium-dependent PKCs in PTP.
Figure 2. Calcium-dependent isoforms of PKC are necessary for post-tetanic potentiation (PTP) at the calyx of Held.
(A through D), Plots of normalized EPSC amplitudes as a function of time from representative experiments. At t = 0 s, a train of 400 stimuli at 100 Hz (inverted triangles) was delivered to induce PTP. Insets show traces of the second potentiated response (light traces) superimposed to the average baseline response (bold traces). PTP elicited in PKCα knockouts (B), PKCβ knockouts (C), or PKCα/β double knockouts (D) was compared to wild-type littermate controls (A). These genetic manipulations significantly affected PTP amplitude (p < 0.01). (E), Cumulative histograms of normalized amplitudes of the 2nd potentiated response for wild-type (black), PKCα (green), PKCβ (red), and double knockout (purple) groups. (F), Average EPSC amplitudes (± SEM) for control and PKC knockout groups, plotted as a function of time. EPSCs were normalized to baseline before averaging.
Properties of baseline synaptic transmission in slices from PKC knockout animals
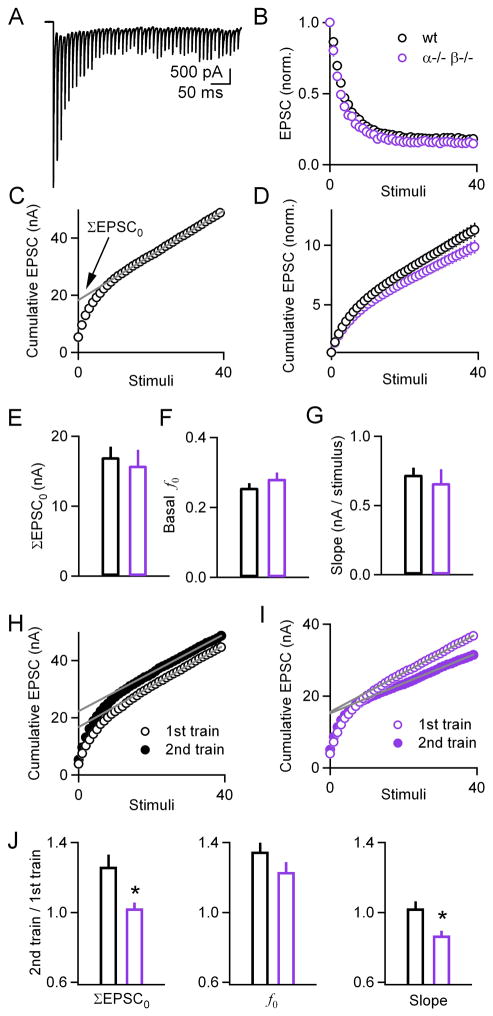
To determine whether deletion of PKCα/β selectively impairs PTP or whether other aspects of transmission are also altered, we examined the properties of basal synaptic transmission in slices from wild-type and double knockout animals. The amplitude and frequency of mEPSCs was the same in wild-types and PKC knockouts (Supplementary Figures 1A–B). We also measured the properties of use-dependent plasticity because changes in the initial probability of release alter the extent of use-dependent plasticity during high-frequency trains. These experiments were performed in the presence of kynurenate (1 mM) and cyclothiazide (0.1 mM) to reduce AMPA receptor saturation and desensitization, respectively, which can obscure changes in use-dependent plasticity. In Figure 3A, an example of EPSCs during 100 Hz train in a wild-type slice is shown. The average normalized EPSC amplitudes (Figure 3B) were similar in wild-type (black) (n=22) and PKCα−/−β−/− (purple) (n=18) animals. There was no significant difference in the use-dependent plasticity in wild-type and in PKCα−/−β−/− mice (p = 0.24 for the second stimulus, p = 0.13 for the third stimulus, p = 0.08 for the average of the 31st to 40th stimuli) (Figure 3B).
Figure 3. Deletion of PKCα/β impairs PTP-induced increases in the size of the readily releasable pool and release probability without affecting basal release properties.
(A), Representative example of EPSCs elicited by 100 Hz train of stimuli. (B), Plot of normalized EPSC amplitude as a function of stimulus number during a 100 Hz, 0.4 s train delivered to slices from either wild-type (black) or double knockout (purple) animals. (C), Cumulative EPSC plotted as a function of stimulus number. The linear fit (gray line) to the last 15 points, back-extrapolated to the y-intercept, is used to obtain ΣEPSC0, and provide a measure of the size of the readily releasable pool exocytosed by a train of stimuli (RRPtrain). (D), Plot of normalized cumulative EPSCs for wild-type (black) and double knockout (purple) groups. (E through G), Basal ΣEPSC0 (E), f0 (the fraction of the RRPtrain released by the first stimulus) (F), and slope (G) measurements for wild-type (black) and double knockouts (purple) groups. (H through I), Cumulative EPSCs in response to the first 40 stimuli of a PTP-inducing train (1st train; 100 Hz, 4 s; open symbols), and a 2nd train (100 Hz, 0.4 s) delivered 10 s later (closed symbols), for wild-type (black) (H) and double knockout (purple) (I) groups. (J), plots of ratios for ΣEPSC0, f0 and slope for wild-type (black) and double knockout (purple) groups. Deletion of PKCα/β significantly impaired the increase in RRPtrain as well as the slope, and to a lesser extent the increase in f0. * p < 0.01.
Synaptic currents evoked by stimulus trains can also be used to quantify the size of the vesicle pool that is readily released by a train (RRPtrain), as in Figure 3C. In this approach, the amplitudes of the EPSCs are measured and integrated. In the plot of the cumulative EPSC, after approximately the first 10 EPSCs, RRPtrain is depleted, and the remaining steady-state EPSC is thought to reflect replenishment of RRPtrain. The cumulative EPSC (ΣEPSC0) can then be determined by extrapolating back to the first EPSC in the train, as in Figure 3C. ΣEPSC0 is proportional to RRPtrain [RRPtrain = ΣEPSC0/(average mEPSC size)]. The fraction of vesicles (f0) within RRPtrain that is liberated by the first action potential in a train can then be determined (f0 = EPSC0/ΣEPSC0). There was no significant difference in the size of RRPtrain for wild-type and double knockout animals, because there was no difference in either ΣEPSC0 (Figures 3D–E, p = 0.26) or in basal mEPSC size (Supplementary Figure 1). There was also no significant difference in basal f0 (p = 0.66) (Figure 3F), or in the slope of the cumulative EPSC (p = 0.59) (Figure 3G). These findings indicate that the basal properties of synaptic transmission are similar in wild-type and double knockout animals.
Tetanically-Induced Changes in RRPtrain and f0
Our studies indicate that calcium-dependent PKCs play a crucial role in PTP, but questions remain as to the mechanisms underlying this enhancement. One approach would be to determine the extent to which the size of the readily releasable pool (RRP), or the probability of releasing a vesicle (p) increases. Once RRP was determined, p would be calculated by dividing the number of vesicles that contribute to an evoked EPSC by the number of vesicles in the RRP. However, different measures of the RRP do not agree: non-specific PKC activators cause little or no increase in the size of the readily releasable pool (RRP) as determined by a strong and prolonged depolarization (Lou et al., 2005; Wu and Wu, 2001), but produce large increases in RRPtrain (Lou et al., 2008). It is unlikely that differences between RRP and RRPtrain can be accounted for by the stimulus frequency used to determine RRPtrain (100 Hz trains in Lou et al. 2008 and in our study), because 300 Hz trains lead to only slightly larger estimates of RRPtrain (Sakaba, 2006). One explanation for the differential effects of non-specific PKC activators on RRP and RRPtrain is that the RRP consists of different pools of vesicles, some that are located near calcium channels, and some that are located further from calcium channels (Neher and Sakaba, 2008). While prolonged depolarization or large presynaptic calcium signals can release the entire RRP, presynaptic action potentials produce brief and local calcium transients that trigger fusion of vesicles near calcium channels, but are not effective at triggering the fusion of more distant vesicles. Increasing the size of the calcium transient, as when external calcium levels are elevated, can increase RRPtrain by extending the spread of calcium entering through calcium channels to influence vesicle release. Alternatively, PKC could similarly extend the influence of calcium entering through calcium channels and increase RRPtrain by increasing the calcium sensitivity of release (lowering the calcium cooperativity) (Lou et al., 2008). Thus, if activation of calcium-dependent PKCs produces PTP by increasing the calcium sensitivity of vesicles, it could lead to both an increase in RRPtrain and an increase in the fraction of those vesicles that are liberated by the first action potential in a train (f0).
We tested this possibility by measuring the effect of tetanic stimulation on ΣEPSC0 and f0. Experiments were performed in the presence of CTZ and kynurenate to prevent receptor desensitization and saturation. Kynurenate and CTZ did not affect the magnitude of PTP in slices from either wild-type (1.79 ± 0.11, n = 9, p = 0.85) (Supplementary Figure 2A) or double knockout mice (1.14 ± 0.08, n = 7, p = 0.78) (Supplementary Figure 2B). To assess the effects of tetanization on ΣEPSC0, synaptic currents were evoked by stimulating at 100 Hz for 4 seconds, followed by a brief train (100 Hz, 0.4 s) 10 s later. Plots of the cumulative EPSC were obtained for both trains, and used to calculate ΣEPSC0 and f0. As shown in representative experiments, tetanic stimulation increased ΣEPSC0 in wild-type (Figure 3H), but not in double knockout mice (Figure 3I). Tetanic stimulation increased ΣEPSC0 by 26 ± 0.7 % and 2 ± 3 % (Figure 3J, left; p < 0.01) and f0 by 34 ± 5 % and 23 ± 6 % (Figure 3J, middle; p = 0.14), in wild-type and double knockout animals, respectively. Thus, the reduced PTP in double knockout mice arises primarily from decreases in the ΣEPSC0 and perhaps f0 (although the effect on f0 is not statistically significant). This finding is consistent with calcium-dependent PKCs increasing the probability of release of vesicles located both near and far from calcium channels (see Discussion). Moreover, in wild-type animals the slope of the cumulative EPSC vs. stimulus number was unaffected by tetanization (Figures 3H, 3J, right), but was reduced in double knockout animals (Figures 3I–J, right, p < 0.01). Impairment in the replenishment of the RRPtrain or a decrease in steady-state release probability during the tetanus could contribute to decreased slope.
Previous studies suggest that myosin light chain kinase (MLCK) contributes to PTP through a mechanism that is distinct from calcium-dependent PKCs, raising the possibility that the PTP remaining in double knockout animals could be mediated by MLCK. This kinase is thought to be responsible for an activity-dependent increase in the RRPtrain that follows tetanic stimulation, but not the calcium-dependent increase in the probability of release (Lee et al., 2010; Lee et al., 2008). The time course of the action of MLCK has not been thoroughly characterized, although it is thought to be independent of the slow mitochondrial-dependent decay of presynaptic calcium following tetanic stimulation (Lee et al., 2008). According to a current model, calcium increases during tetanic stimulation activate calmodulin and MLCK, which contribute to PTP by increasing RRPtrain without affecting the overall RRP (Lee et al., 2010).
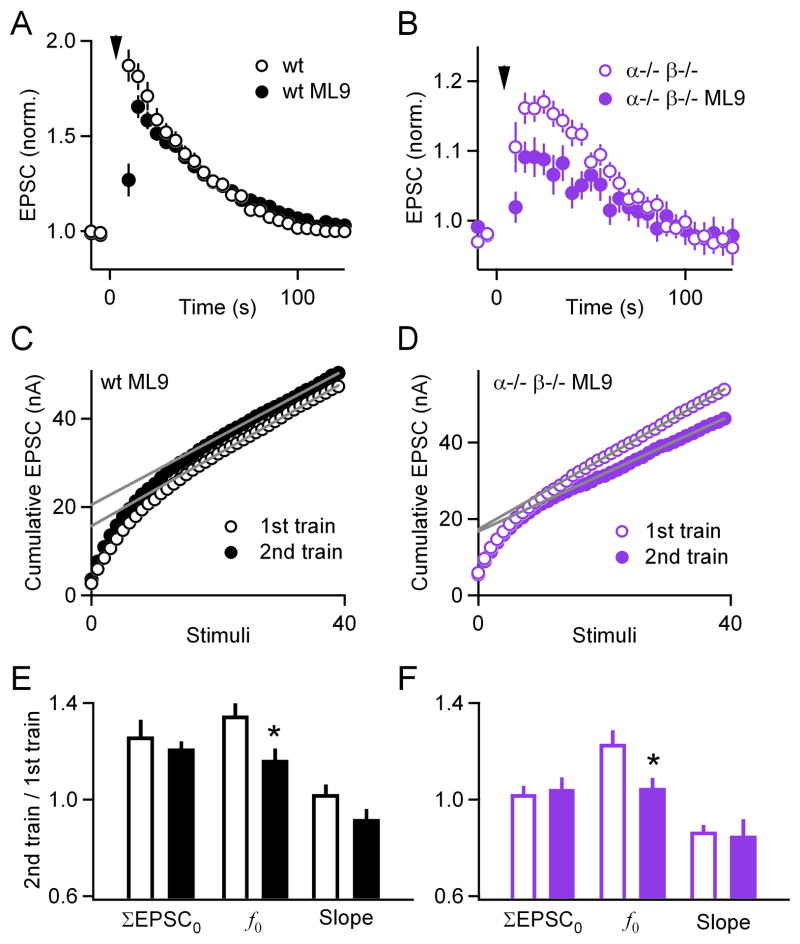
We tested this model by examining the contribution of MLCK to PTP in both wild-type and double knockout mice. In wild-type mice, the MLCK inhibitor ML9 reduced PTP from 87 ± 2 % (n = 17) to 26 ± 8 % (n = 10, p < 0.0001) 5 s after the train, and from 81 ± 2 % to 69 ± 2 % (p = 0.21) 10 s after the train (Figure 4A). These findings confirm that MLCK contributes to PTP. They also indicate that the contribution of MLCK is short-lived compared to overall PTP, and that by 10 s after stimulation the contribution is much smaller than that of calcium-dependent PKCs. In double knockout animals, ML9 also influenced the magnitude of PTP, which at 10 s was reduced from 16 ± 2 % (n = 16) to 9 ± 2 % (n = 11, p < 0.05, Figure 4B). These findings indicate that MLCK-dependent mechanisms account for some, but not all, of the PTP remaining in double knockout mice.
Figure 4. Limited contribution of myosin light chain kinase to PTP.
(A through B), Plots of normalized EPSC amplitude as a function of time before and after PTP-inducing tetanization (inverted triangles) in slices from wild-type (black) and double knockout (purple) animals. Incubation with the MLCK inhibitor ML9 (filled symbols) partially affected PTP. (C through D), Plots of cumulative EPSCs as a function of stimulus number in slices from wild-type (C) and double knockout (D) animals, incubated with ML9. Gray lines are linear fits to the last 15 points. (E through F), Plots of ratios (2nd train/1st train) for ΣEPSC0, f0, and the slope of the linear fits. Incubation with ML9 (filled bars) significantly decreased f0 in both wild-types (black) and double knockouts (purple). * p < 0.05.
In order to compare the mechanisms of action of MLCK and calcium-dependent PKCs in PTP, we examined the effect of MLCK inhibitors on f0 and ΣEPSC0 in both wild-type and double knockout mice. Inhibiting MLCK did not significantly change the basal properties of synaptic transmission in either wild-type or double knockout mice (Supplementary Figure 3). In wild-type mice, inhibiting MLCK did not alter the tetanus-induced increases in ΣEPSC0 (26 ± 7 %, n = 13, compared to 21 ± 3 % in the presence of ML9, n = 10, p = 0.51, Figures 4C,E), it reduced the increase in f0 (35 ± 5 % compared to 16 ± 5 %, p < 0.05), and the slope was not statistically different (2 ± 4 % compared to −8 ± 4 %, p = 0.09). Similarly, in double knockout mice ML9 did not affect the tetanus-induced increase in ΣEPSC0 (2.2 ± 4.4 % n = 11 compared to 4.3 ± 4.9 % n = 11 in ML9, p = 0.72, Figures 4D, F), but it reduced the increase in f0 (23 ± 6 % compared to 5 ± 4 %, p < 0.05), and the slope was not statistically different (−13 ± 3 % compared to −15 ± 7 %, p = 0.82). Therefore, under our experimental conditions, MLCK did not contribute to PTP-induced increases in RRPtrain but did contribute to increases in f0. This finding indicates that calcium-dependent PKCs contribute to PTP through mechanisms that are distinct from MLCK.
Tetanus-induced presynaptic calcium signals
To further elucidate the manner in which calcium-dependent PKCs contribute to PTP, we examined the role of presynaptic calcium signaling in PTP. Previous studies suggest that presynaptic calcium could be involved in PTP in different ways. First, PTP could involve the small but long-lasting presynaptic residual calcium (Cares) signals that follow tetanic stimulation (Zucker and Regehr, 2002). At the calyx of Held, PTP and Cares decay with similar time courses, and a roughly linear relationship between Cares and PTP has been suggested (Habets and Borst, 2005; Korogod et al., 2005). This is consistent with the hypothesis that Cares activates proteins that respond to modest Cares levels to increase synaptic efficacy. Calcium-dependent PKCs could be the Cares sensor that produces PTP. Another possibility is that tetanic stimulation increases presynaptic calcium entry by modulating calcium channels (Catterall and Few, 2008). At the calyx of Held, prolonged tetanic activation can increase presynaptic calcium entry under some circumstances (Habets and Borst, 2006). Indeed, tetanic stimulation for 4 s at 100 Hz can result in a 15 % increase in presynaptic calcium entry (Korogod et al., 2007). Calcium-dependent PKCs could mediate this calcium channel facilitation. There is controversy over the relative importance of Cares and calcium channel facilitation, but there is agreement that presynaptic calcium signaling plays an important role in PTP.
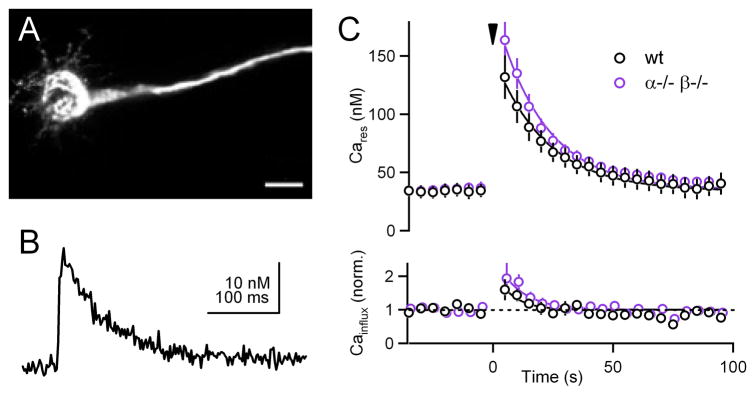
We therefore examined calcium signaling in calyces of Held from wild-type and double knockout animals. We introduced a low concentration of a calcium indicator (Calcium Green-1 dextran, KD = 326 nM) into the calyx of Held, as described previously for other synapses (Beierlein et al., 2004), and quantified calcium signals using established methodology (Brenowitz et al., 2006; Maravall et al., 2000). Brief loading times were used so that a small amount of indicator was introduced in order to minimize perturbations of presynaptic calcium signaling. A red dye (Alexa 594 - dextran) was also used to allow visualization of calyces (Figure 5A), because basal Calcium Green-1 fluorescence is faint. As shown for an example experiment, single stimuli evoked fluorescence transients that decayed rapidly (Figure 5B). Single stimuli produced calcium increases of 20 ± 3 nM (n = 10) and 18 ± 2 nM (n = 10) in wild-type and double knockout animals, respectively (p = 0.53). Following tetanic stimulation of 100 Hz for 4 seconds, Cares in wild-type was 132 ± 19 nM 5 s after the end of stimulation, and 164 ± 16 nM in double knockout animals (p = 0.21). Cares decayed with a time constant of ~ 22 s in both groups (Figure 5C top). This indicates that diminished PTP in double knockout animals is not a result of perturbed Cares signals following tetanic stimulation.
Figure 5. Presynaptic residual calcium signals and calcium entry in wild-type and PKCα−/− β−/− animals.
(A), 2-photon image of a calyx filled with Alexa 594-dextran and Calcium Green-1 dextran. Scale bar: 20 μm. (B), Fluorescence calcium transient evoked by a single stimulus. (C), Plots of residual calcium (Cares, top) and calcium influx (bottom) in slices from wild-type (black) and double knockout (purple) animals. Inverted triangle indicates 100 Hz, 4 s tetanus delivered to induce PTP.
We tested whether calcium channel facilitation contributes to PTP by measuring the effect of tetanic stimulation on increases in calcium transients evoked by single stimuli (Figure 5C bottom). In wild-type animals tetanic stimulation elevated the calcium increases evoked by single stimuli by 60 ± 31 % (n = 10; 5 s post-tetanus), but the enhancement was short-lived (τ ~ 10 s). This suggests that under our experimental conditions, tetanus-induced increases in calcium influx make only a short-lived contribution to PTP. In double knockout animals the calcium increases evoked by single stimuli (93 ± 46 % of baseline at 5 s post-tetanus; n = 10; p = 0.55 between wild-type and double knockout) show a similar short-lived increase following tetanic stimulation (τ ~ 7 s). This suggests that the impairment of PTP in double knockout animals is not due to impaired facilitation of calcium currents in response to tetanic stimulation.
Activity-dependent increases in mEPSC frequency
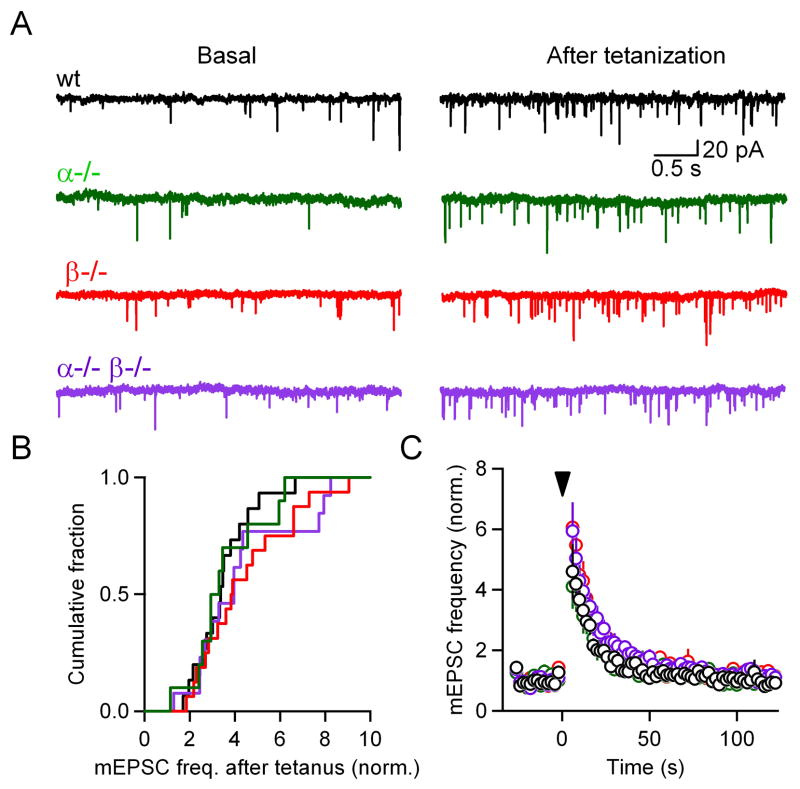
In addition to enhancing the amplitude of evoked synaptic transmission, tetanic stimulation also enhances the frequency of spontaneous release (Castillo and Katz, 1954; Eliot et al., 1994; Habets and Borst, 2005; Korogod et al., 2005, 2007; Magleby, 1987). We tested whether PKCα and PKCβ mediate this activity-dependent increase in mEPSC frequency. In these experiments the same tetanic stimulation was used as in our PTP experiments (4 s, 100 Hz), but without test stimuli. This allowed us to monitor mEPSCs before and after tetanic stimulation. Our tetanic stimulation protocol resulted in a large increase in mEPSC frequency, which is illustrated in a representative experiment in which the mEPSC frequency before and 2–6 s after stimulation increased from 1.0 to 3.5 Hz (Figure 6A, black). The extent of enhancement ranged from 1.7-fold to 6.7-fold (Figure 6B, black, the ratio of mEPSC frequency 2 - 6 s following tetanic stimulation to basal frequency). In wild-type animals the time course of mEPSC frequency enhancement decayed with a time constant of ~12 s (Figure 6C). Tetanic stimulation also increased the frequency of spontaneous events in PKCα−/−, PKCβ−/−, and PKCα−/−β−/− mice, as shown in representative experiments in which enhancement was 4.2-fold (increased from 0.67 to 2.8 Hz), 3.7-fold (0.74 to 2.77 Hz), and 3.9-fold (0.80 to 3.1 Hz), respectively (Figure 6A). There was no significant difference in the enhancement of mEPSC frequency among wild-type (3.5 ± 0.3, n = 15), PKCα−/− (3.5 ± 0.5, n = 10), PKCβ−/− (4.4 ± 0.5, n = 16) and PKCα−/−β−/− groups (4.3 ± 0.6, n = 13) (p = 0.43) (Figures 6B–C). These results suggest that at the calyx of Held synapse, PTP and the enhancement of spontaneous release arise from different mechanisms. Calcium-dependent PKCs are crucial to PTP, but they do not mediate tetanus-evoked increases in mEPSC frequency.
Figure 6. The increase in spontaneous transmission after tetanization is independent of PKCα and PKCβ.
(A), Representative recordings of spontaneous transmission before (basal; left) and after (right) tetanization from slices from wild-type (black), PKCα (green), PKCβ (red), and double knockout (purple) animals. (B), Cumulative histograms of average mEPSC frequency after tetanization, normalized to that before the tetanus. (C), Plot of normalized average mEPSC frequency as a function of time for wild-type (○), PKCα (○), PKCβ (○) and double knockout (○) groups. At t = 0 s, a 4 s, 100 Hz tetanus was delivered (inverted triangle). No statistical difference was observed among the 4 groups.
Activity-dependent increases in mEPSC amplitude
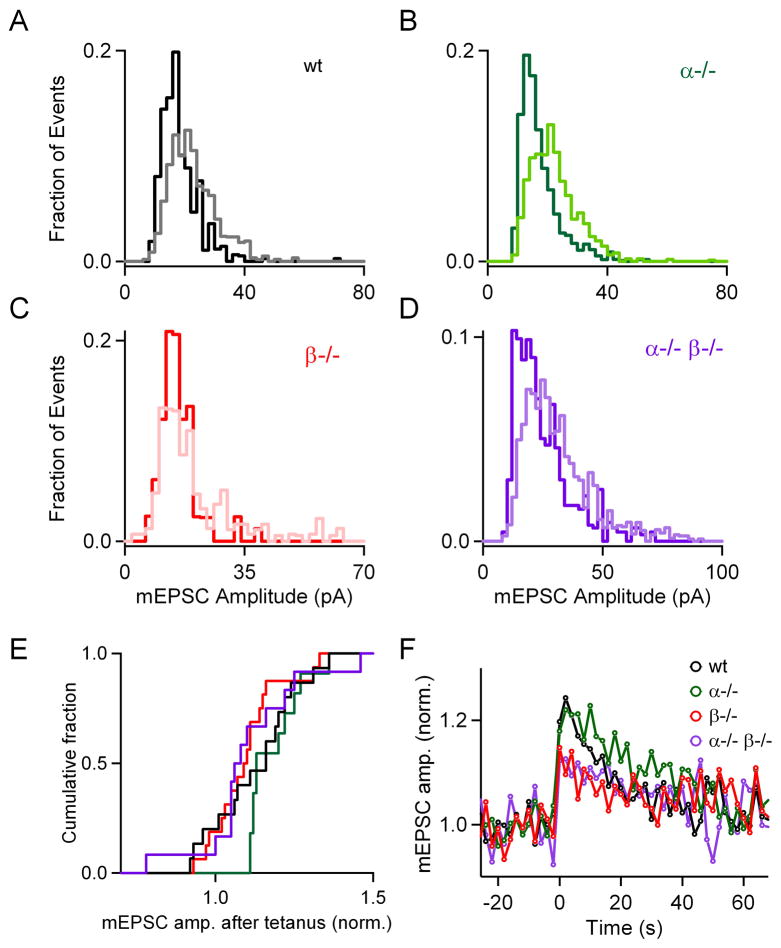
We tested whether PKCα and PKCβ mediate the increase in mEPSC amplitude that follows tetanic stimulation (He et al., 2009). In wild-type mice, tetanic stimulation altered the distribution of mEPSC sizes, and after tetanic stimulation the fraction of small mEPSCs was reduced and the fraction of large mEPSCs increased, as shown in a representative experiment (Figure 7A). In slices from PKC knockout animals, tetanic stimulation also increased mEPSC amplitude and produced similar effects on the mEPSC distributions, as illustrated in representative experiments from slices from PKCα−/− (Figure 7B), PKCβ−/− (Figure 7C), and PKCα−/−β−/− (Figure 7D) mice. As shown in the cumulative histograms (Figure 7E), tetanic stimulation significantly increased the mEPSC amplitude in slices from wild-type, PKCα−/−, PKCβ−/−, and PKCα−/− β−/− mice compared to their respective baseline (p < 0.05 for all paired comparisons). On average, enhancement was somewhat smaller in PKCβ−/− (10.1 ± 2.8 %), and PKCα−/−β−/− (10.9 ± 4.7 %) compared to wild-type (13.1 ± 3.5 %) and PKCα−/− (18.7 ± 2.5 %), but these differences were not statistically significant (p = 0.34). The time courses of the enhancement of mEPSC amplitude in the different genotypes (Figure 7F) can be approximated by single exponential decays with time constants of 47 ± 9 s, 39 ± 4 s, 67 ± 17 s and 35 ± 8 s for wild-type, PKCα−/−, PKCβ−/− and PKCα−/−β−/− groups, respectively.
Figure 7. The increase in mEPSC size following tetanic stimulation is independent of PKCα and PKCβ.
(A through D), Representative distributions of mEPSC amplitudes recorded before (light traces, 25 s interval prior to stimulation) and after a 4 s, 100 Hz tetanus (bold traces, 2–12 s after tetanus) are shown for wild-type (A, black), PKCα knockout (B, green), PKCβ knockout (C, red) and double knockout (D, purple) groups. To facilitate comparison of the distributions, the mEPSC distributions are expressed as fractional contribution of each 2-pA bin to the total number of events detected. (E), Cumulative histograms of average mEPSC amplitude after tetanization, normalized to that before the tetanus. (F), Plot of normalized average mEPSC frequency as a function of time for wild-type (○), PKCα (○), PKCβ (○) and double knockout (○) groups.
Synaptic enhancement mediated by phorbol esters
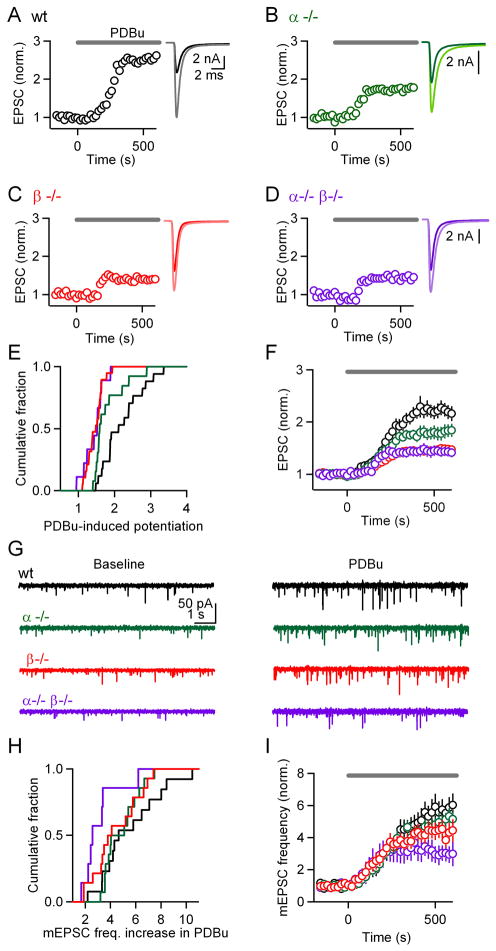
Phorbol esters activate PKC by binding to the diacylglycerol (DAG) binding site (Newton, 2001), leading to large synaptic enhancement that mimics and occludes PTP (Korogod et al., 2007; Malenka et al., 1986). Synaptic enhancement by phorbol esters has been studied extensively to provide insight into the mechanism mediating PTP. We examined the role of PKCα and PKCβ in phorbol ester-induced enhancement. As shown in representative experiments, the phorbol ester PDBu (1 μM) enhanced EPSC amplitude in slices from wild-type (Figure 8A, 2.5-fold), PKCα−/− (Figure 8B, 1.7-fold), PKCβ−/− (Figure 8C, 1.4-fold), and PKCα−/−β−/− (Figure 8D, 1.4-fold) mice, but the degree of enhancement was smaller in the knockout groups. Although there was variability in the extent of enhancement in the different genotypes (Figure 8E), the average extent of enhancement was clearly reduced in the PKC knockout groups (Figure 8F), and there was a significant difference in the PDBu-dependent enhancement between wild-type (2.22 ± 0.14, n = 17) and PKCα−/− (1.80 ± 0.12, n = 13, p < 0.05), PKCβ−/− (1.46 ± 0.05, n = 13, p < 0.01), and PKCα−/− β−/− (1.44 ± 0.09, n = 9, p < 0.01) groups. These experiments establish that calcium-dependent PKCs play an important role in phorbol ester-dependent enhancement at the calyx of Held. Compared to baseline, there is still significant enhancement remaining in slices from PKCα−/−β−/− mice (p < 0.01), which indicates that other target(s) of phorbol esters (Brose and Rosenmund, 2002; Lou et al., 2008; Rhee et al., 2002; Wierda et al., 2007) are engaged at this synapse.
Figure 8. The contribution of calcium-dependent isoforms of PKC to synaptic plasticity mediated by phorbol esters.
The phorbol ester PDBu (1 μM) was bath-applied at t = 0 s and the effect on the amplitudes of evoked EPSCs (A through F) and mEPSC frequencies (G through I) were monitored. Experiments corresponding to different genotypes are displayed in different colors: wild-type (black), PKCα (green), PKCβ (red), and double knockout (purple) groups. Representative experiments show the effects on the evoked EPSCs for wild-type (A), PKCα knockouts (B), PKCβ knockouts (C), and PKCα/β double knockouts (D). (E), Cumulative histograms of EPSC enhancement in PDBu. (F), Average EPSC amplitudes (± SEM) plotted as a function of time. (G), Representative experiments show mEPSCs before (left) and during (right) the application of PDBu. (H), Cumulative histograms of mEPSC enhancement in PDBu. (I), Average mEPSC frequency (± SEM) plotted as a function of time.
In addition to enhancing the amplitude of evoked EPSCs, phorbol esters increase mEPSC frequency. This is illustrated in a representative experiment by comparing spontaneous mEPSCs recorded in control conditions and in the presence of PDBu (Figure 8G, black). We tested whether PKCα and PKCβ also contribute to this enhancement of mEPSC frequency. As shown in the representative experiments, PDBu increased the mEPSC frequency in slices from PKCα−/− (Figure 8G, green), PKCβ−/− (Figure 8G, red), and PKCα−/−β−/− (Figure 8G, purple) mice. The range of mEPSC frequency enhancement was quite broad in all genotypes (Figure 8H). The average enhancement was 5.6 ± 0.7 in wild-type (Figure 8I, black, n = 13), 4.9 ± 0.4 in PKCα−/− (Figure 8I, green, n = 14), 4.4 ± 0.5 in PKCβ−/− (Figure 8I, red, n = 14), and 3.1 ± 0.6 in PKCα−/−β−/− (Figure 8I, purple, n = 7) groups. Although there was a trend suggesting that PKCα and PKCβ contributed to the phorbol ester-dependent enhancement in mEPSC frequency, the differences did not reach statistical significance (p = 0.054), despite the relatively large sample sizes. However, a pair-wise comparison using a Kolmogorov-Smirnoff 2-sample test indicated that the mEPSC frequency distributions for wild-type and double knockout groups were significantly different (p < 0.05).
Summary of the properties of synaptic transmission in wild-type and knockout animals
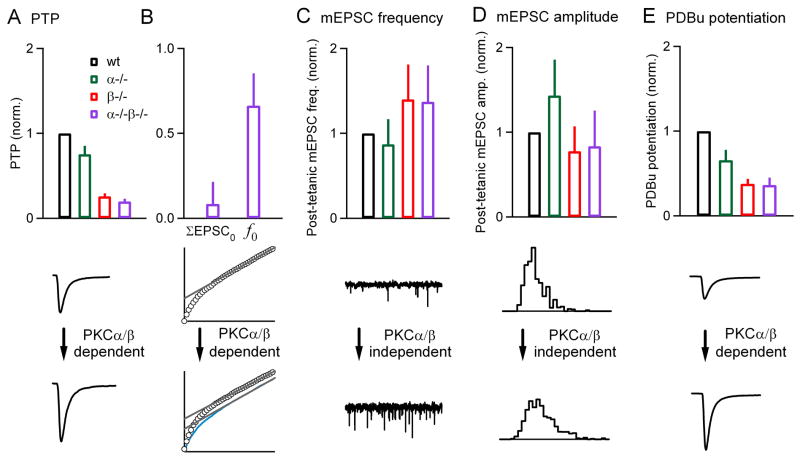
Our findings indicate that PKCα and PKCβ play important roles in synaptic transmission at the calyx of Held synapse. Although there are no discernable effects on basal properties of synaptic transmission, there are profound differences in synaptic plasticity, with various synaptic properties affected differentially. This is illustrated in Figure 9, where the plasticity in different genotypes is expressed relative to the plasticity in the wild-type group. The largest effect was on PTP. In PKCα−/−, PKCβ−/− and PKCα−/−β−/− groups PTP was 75 ± 10 %, 26 ± 4 % and 20 ± 3 %, respectively of that observed in the wild-type group (Figure 9A, top). The primary effect of PKCα/β was on ΣEPSC0, which in double knockouts was reduced to 8 ± 13 % of wild-type (Figure 9B, top). Deletion of PKCα/β also modulated f0, which was reduced to 66 ± 19 % of wild-type. The increases in mEPSC frequencies following tetanic stimulation in PKCα−/−, PKCβ−/−, and PKCα−/−β−/− were 87 ± 30 %, 140 ± 41 % and 137 ± 43 %, respectively of that observed in the wild-type group (Figure 9C, top). Thus, the same stimulus train profoundly reduced PTP without reducing the frequency of spontaneous mEPSCs. Furthermore, the amplitude of mEPSC was also not significantly affected by the absence of calcium-sensitive PKCs (Figure 9D, top). The mEPSC amplitude changes in PKCα−/−, PKCβ−/−, and PKCα−/−β−/− after the tetanus were 143 ± 42 %, 77 ± 29 % and 83 ± 42 % respectively, of the wild-type group. Following application of PDBu, the increase in the amplitude of evoked synaptic responses in PKCα−/−, PKCβ−/−, and PKCα−/−β−/− was 66 ± 12 %, 38 ± 6 % and 36 ± 9 %, respectively, of that observed in the wild-type group (Figure 9E, top). In the PKCα−/−β−/− group a higher percentage of enhancement remains for PDBu-dependent enhancement (36 %) than for PTP (20 %).
Figure 9. Summary of the properties of synaptic transmission in slices from wild-type and PKC knockout animals.
(A through E, top), The tetanus-induced (A through D, top) and PDBu-induced (E, top) changes in the properties of synaptic transmission are compared for wild-type (black), PKCα knockouts (green), PKCβ knockouts (red), and double knockouts (purple). The synaptic enhancement in the different genotypes was normalized to that in wild-type animals. PTP and the enhancement of mEPSC frequency and amplitude were evoked by 4 s, 100 Hz stimulation. (A through E, bottom), Examples of the different types of synaptic modulation are shown, and their dependence on PKCα and PKCβ is indicated.
Discussion
Here we report that in the absence of both PKCα and PKCβ, PTP is 20 % of that observed in wild-type animals, indicating that calcium-dependent PKCs mediate most of PTP at the calyx of Held synapse. The remaining PTP appears to be mediated in part by an MLCK-dependent mechanism and in part by an increase in mEPSC size. Calcium-dependent PKCs enhance transmission primarily by increasing RRPtrain, and to a lesser extent by increasing the fraction of vesicles released in response to a stimulus; they also influence replenishment of RRPtrain following tetanic stimulation. Similar to PTP, phorbol ester-dependent enhancement was greatly reduced in slices from double knockout animals. The differential effects of PKCα and PKCβ on evoked and spontaneous synaptic transmission are summarized in Figure 9 (top: group averages, bottom: individual examples).
PTP is mediated primarily by PKCα and PKCβ
Our finding that PTP is greatly reduced in the absence of PKCα and PKCβ establishes an important role for these kinase isoforms in PTP at the calyx of Held. Our results resolve a long-standing controversy over whether PKC plays a role in PTP. Previous observations that phorbol esters occlude PTP (Korogod et al., 2007; Malenka et al., 1986) were thought to support a role for PKC in PTP until it was realized that in addition to activating PKC, phorbol esters activate other proteins such as Munc13 (Brose and Rosenmund, 2002; Lou et al., 2008; Rhee et al., 2002; Wierda et al., 2007). Similarly, the finding that PKC inhibitors reduce the magnitude of PTP (Alle et al., 2001; Beierlein et al., 2007; Brager et al., 2003; Korogod et al., 2007; Lee et al., 2007) supported a role for PKC in PTP, but the observation that inactive analogues have similar effects on PTP (Lee et al., 2008) and that some PKC inhibitors do not affect PTP at all (Eliot et al., 1994; Lee et al., 2008; Reymann et al., 1988a; Reymann et al., 1988b) have blurred the role of PKC in PTP. Using a molecular genetic approach allowed us to overcome the limitations associated with the lack of selectivity of PKC inhibitors and activators and establish that PKC plays a crucial role in PTP.
Our results establish that calcium-dependent PKC isoforms mediate most of the PTP at the calyx of Held, with PKCβ playing a more prominent role than PKCα (Figure 9A, top). This challenges the previously-held view that a calcium-independent PKC isoform mediates PTP (Saitoh et al., 2001). Previous studies at the calyx of Held found that phorbol esters induce translocation of PKCε and suggested that this calcium-independent isoform mediates PKC-dependent plasticity at this synapse (Saitoh et al., 2001). Moreover, different PKC inhibitors were found to have very different effects on PTP. A broad-spectrum inhibitor (bisindolymalemide, BIS) and one that preferentially targets calcium-independent isoforms (Ro31-8220) reduced PTP (expressed as fraction of PTP in control conditions) to approximately 40 % and 20 %, respectively (Korogod et al., 2007). One interpretation of these results is that PTP involves calcium-independent PKCs, which might be activated by a tetanus-dependent elevation of DAG rather than by calcium. This interpretation is, however, complicated, because PKC inhibitors do not readily penetrate brain slices, and slices must be soaked in high concentrations of the inhibitors for long periods of time prior to the experiment. In some cases, broad-spectrum inhibitors (chelerythrine) do not reduce the magnitude of PTP (Lee et al., 2008). Limitations associated with the use of PKC inhibitors in slice preparation raise the possibility that the differential efficacy of PKC inhibitors may reflect their ability to penetrate the slice, rather than their isoform selectivity (Brose and Rosenmund, 2002). This seems to be a plausible explanation for the differential effects of PKC inhibitors, in light of our observation that the calcium-dependent isoforms PKCα and PKCβ account for most of the PTP.
Mechanisms of PTP mediated by PKCα and PKCβ
Our experiments provide new insight into the mechanisms underlying PTP. Calcium measurements suggest that although calcium channels are briefly facilitated, this facilitation makes a short-lived contribution to PTP (Figure 5C). Facilitated calcium entry is still present in double knockout animals, indicating that it is not mediated by calcium-dependent PKCs. PTP at cultured superior cervical ganglion neurons is also mediated primarily by mechanisms that are independent of calcium channel facilitation (Mochida et al., 2008).
The effect of tetanic stimulation on responses evoked by brief 100 Hz stimulus trains was revealing with respect to how calcium-dependent PKCs contribute to PTP. The use-dependent increase in the size of RRPtrain is absent in double knockout animals (Figure 3I–J), suggesting that PKCα and PKCβ mediate the increase in the size of the pool of vesicles following tetanic activation. This appears to be the primary mechanism by which calcium-dependent PKCs produce PTP, although they also appear to be partially responsible for the increase in the fraction of vesicles exocytosed by an action potential. The substantial increase in RRPtrain is compatible with the observation that phorbol esters have only minor effects on the overall RRP size, provided the properties of different vesicle pools at the calyx of Held are considered (Lou et al., 2008). When an action potential invades a presynaptic bouton, vesicles that are located near calcium channels are exposed to a larger calcium signal than more distant vesicles (Neher and Sakaba, 2008). Any increase in the sensitivity of a vesicle to calcium could increase both the size of the vesicle pool that can be exocytosed by a train of action potentials, and the fraction of the vesicles that are released by the first action potential (Lou et al., 2008). The relative contributions of these two mechanisms depend upon the detailed ultrastructure of the synapse, the spatiotemporal calcium signal, and the calcium sensitivity of the vesicles (Branco and Staras, 2009; Neher and Sakaba, 2008). In the case of PTP, our findings suggest that PKCα/β act primarily to increase the size of the readily releasable pool.
The involvement of calcium-dependent PKC isoforms in PTP raises the question: are PKCα and PKCβ the calcium sensors that, according to the residual calcium hypothesis of PTP, detect presynaptic calcium signals evoked by tetanic stimulation to phosphorylate downstream targets thus increasing the probability of release? We find that Cares decays (τ ~ 22 s) more quickly than PTP (τ ~ 45 s), suggesting that for our experimental conditions PTP is longer-lived than Cares at the calyx of Held, as is the case at hippocampal and cerebellar synapses (Beierlein et al., 2007; Brager et al., 2003). Furthermore, we find that PTP is produced by tetanic stimulation that increases Cares by several hundred nanomolar. Can calcium-dependent PKCs respond to such small calcium increases? In the absence of lipid membranes, the Ca+2-binding affinities for PKCα and PKCβ are ~ 40 μM (Kohout et al., 2002), which is much higher than the observed residual calcium signals. However, in the presence of phosphatidylserine and/or PIP2-containing membranes or in model systems, cooperative Ca+2 binding is observed for both isoforms, and calcium affinities range from 0.1 to 5 μM (Corbalan-Garcia et al., 1999; Corbin et al., 2007; Guerrero-Valero et al., 2007; Kohout et al., 2002). It is also possible that factors in the intracellular milieu raise the binding affinity of PKCs for calcium, as is the case for calmodulin (Xia and Storm, 2005). Thus, it is plausible that PKCα and PKCβ could be sufficiently sensitive to detect residual calcium. Alternatively, PKCs could be initially activated by the calcium signals during the train and then, because of positive cooperative binding, become sensitive to residual calcium. Once activated, PKC could phosphorylate proteins such as Munc18 to increase the probability of release (Wierda et al., 2007). Further studies are needed to determine if PKCα and PKCβ are indeed the calcium sensors in PTP, and if they increase the probability of release by phosphorylating Munc18.
Increases in mEPSC frequency and amplitude evoked by tetanic stimulation
Tetanic stimulation increases the frequency of mEPSCs several-fold at the calyx of Held synapse and at other synapses (Figure 6) (Castillo and Katz, 1954; Eliot et al., 1994; Groffen et al., 2010; Habets and Borst, 2005; Korogod et al., 2005, 2007; Magleby, 1987). The increase in the frequency of spontaneous release and PTP are both dependent on presynaptic calcium increases (Bao et al., 1997; Korogod et al., 2005; Nussinovitch and Rahamimoff, 1988; Zucker and Lara-Estrella, 1983), suggesting that they share a common mechanism. However, the elevation in mEPSC frequency does not last as long as the enhancement of evoked EPSCs (τ ~ 12 s and 45 s respectively) (see also Korogod et al., 2007). In addition, pharmacological inhibitors of PKC that reduce the increase in evoked EPSC amplitude do not prevent the increase in mEPSC frequency at calyx of Held synapses (Korogod et al., 2007). Here, using a genetic approach, we also find that the frequency of mEPSC and the amplitude of evoked EPSCs are regulated independently. Indeed, potentiation of evoked EPSCs is reduced by 80 % in slices from PKCα−/−β−/− double knockout animals compared to controls (Figure 9A) whereas the increase in mEPSC frequency is largely unaffected (Figure 9C). Therefore, the activity-dependent regulation of mEPSC frequency is not mediated by PKCs, and is likely regulated by other calcium-sensitive proteins in the presynaptic terminal, such as Doc2a and Doc2b (Groffen et al., 2010).
Tetanic stimulation also results in increased mEPSC amplitude in slices from wild-type animals (Figure 7). Although modest, this increase has a time course (τ ~ 47 s) that is similar to that of PTP (τ~ 45 s, compare Figures 2F and 7F), and it is thought to contribute to PTP (He et al., 2009). The increase in mEPSC amplitude appears to reflect the fusion of vesicles with each other prior to ultimate fusion with the plasma membrane (He et al., 2009). We find that the increase in mEPSC amplitude persists in the absence of PKCα, PKCβ or both isoforms (Figure 7). This suggests that calcium-dependent isoforms of PKC do not regulate vesicle-to-vesicle fusion within the calyx of Held, in contrast to their prominent role in regulating vesicle fusion with the plasma membrane that underlies evoked EPSCs (Figure 2). The 10 % increase in mEPSC amplitude that remains in PKCα/β double knockout animals could account for some of the remaining PTP observed in this group (Figure 9A).
Synaptic enhancement by phorbol esters
Previous studies have supported a role for PKCs in the phorbol ester-induced enhancement of evoked EPSCs at the calyx of Held (Hori et al., 1999; Lou et al., 2008), but the isoform responsible for this enhancement was not known. A broad-spectrum PKC inhibitor, though with questionable selectivity (Lee et al., 2008), reduced enhancement by PDBu to approximately 40 % of control, and inclusion of a more selective PKC blocking peptide in the presynaptic terminal reduced the enhancement to less than 20 % of that observed in control conditions (Hori et al., 1999). Previous studies suggested that the calcium-insensitive isoform PKCε mediates this enhancement because it is present at the calyx of Held and is activated by phorbol esters (Saitoh et al., 2001). However, our observation that phorbol ester-induced potentiation of evoked EPSCs is reduced by ~ 70 % in PKCα/β double knockouts compared to controls indicate that these two isoforms account for the bulk of the contribution of PKCs to EPSC enhancement by phorbol esters. Moreover, our results are consistent with the observation that ~ 50 % of phorbol ester-induced potentiation in the hippocampus is impaired in PKCβ knockout mice (Weeber et al., 2000). The component of phorbol ester-induced enhancement that is not mediated by PKCs is likely mediated by the synaptic protein Munc13, either as a result of phorbol esters directly activating Munc13, or as a result of phorbol ester binding to the N-terminal domain of Doc2α, thereby allowing it to interact with Munc13 (Hori et al., 1999; Lou et al., 2008).
Phorbol esters enhance mEPSC frequency ~ 6-fold in wild-type animals (Figure 8). In the absence of PKCα and PKCβ, this enhancement is reduced by ~ 50 % (compare black and purple traces in Figure 8I). This result agrees with previous observations using pharmacology (Lou et al., 2008; Oleskevich and Walmsley, 2000) and suggests that PKC plays a less important role in potentiating spontaneous release compared to evoked release. In double knockout animals, the impairment of the phorbol ester-induced increase in mEPSC frequency (Figure 8I), although moderate, contrasts with the lack of effect on tetanus-induced increase in mEPSC frequency (Figure 9C). Further studies are needed to understand this potential difference in the regulation of spontaneous activity.
Experimental Procedures
Animals
PKCα (in 129S2 genetic background) and PKCβ (in C57BL/6J genetic background) single knockout animals, generated by M. Leitges (Leitges et al., 2002; Leitges et al., 1996), were bred together to obtain offspring heterozygous for both genes (het-het animals). Crosses of het-het animals generated α+/+ β+/+ (wt), α−/− β+/+ (αKO), α+/+ β−/− (βKO), and α−/− β−/− (double knockout) animals with a frequency of 1:16 each. All animal handling and procedures abided by the guidelines of the Harvard Medical Area Standing Committee on Animals.
Preparation of brain slices
Transverse 190- to 200-μm-thick brainstem slices containing the region of the medial nucleus of the trapezoid body (MNTB) were made with a vibratome slicer (VT1000S, Leica) from postnatal day P11-14 mice deeply anesthetized with isoflurane. Brains were dissected and sliced at 4 °C in cutting solution consisting of the following (in mM): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 0.1 CaCl2, 3 MgCl2, 25 glucose, 3 myo-inositol, 2 Na-pyruvate, 0.4 ascorbic acid, continuously bubbled with 95 % O2/5 % CO2 (pH 7.4). Slices were incubated at 32 °C for at least 30 min in a bicarbonate-buffered solution composed of the following (in mM): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 glucose, 3 myo-inositol, 2 Na-pyruvate, 0.4 ascorbic acid, continuously bubbled with 95 % O2/5 % CO2 (pH 7.4).
Electrophysiology
Slices were transferred to a recording chamber at room temperature (21–24 °C) in an upright microscope (Olympus, Center Valley, PA) equipped with a 60X, 0.9 N.A. objective. During recordings, the standard perfusion solution consisted of the bicarbonate-buffered solution (see above) with 1 μM strychnine and 25 μM bicuculline to block inhibitory synaptic transmission. Slices were superfused at 1–3 ml/min with this external solution. Whole-cell postsynaptic patch-clamp recordings were made from visually identified cells in the MNTB region using glass pipettes of 2–3 MΩ resistance, filled with an internal recording solution of the following (in mM): 20 CsCl, 140 Cs-gluconate, 20 TEA-Cl, 10 HEPES, 5 EGTA, 5 Na2-phophocreatine, 4 ATP-Mg, 0.3 GTP-Na, pH: 7.3, 315–320 mOsm. Series resistance (Rs) was compensated by up to 70 % and the membrane potential was held at −70 mV.
Excitatory postsynaptic potentials (EPSCs) were evoked by stimulating presynaptic axons with a bipolar stimulating electrode (custom-made or from FHC, Bowdoin ME) placed midway between the medial border of the MNTB and the midline of the brainstem. Multiclamp 700A and 700B (Axon Instruments/Molecular Devices, Union City, CA) amplifiers were used. Recordings were digitized at 20 KHz with an ITC-18 A/D converter (Instrutech Corp., Port Washington, NY) using custom macros (written by M.A. Xu-Friedman) in Igor Pro (Wavemetrics, Lake Oswego, OR) and filtered at 8 kHz.
The protocol for inducing PTP was as follows: an estimate of baseline synaptic strength was obtained through low-frequency stimulation at 0.2 Hz for 25 s. PTP was induced with a 4-s stimulus train at 100 Hz, followed by low-frequency stimulation to test for PTP. Changes in miniature EPSCs (mEPSCs) were measured by delivering the same PTP-inducing train, but without the low-frequency stimulation. For phorbol ester experiments, basal synaptic strength was evaluated by paired (50 ms interval) stimuli, repeated every 20 s. During the intertrial intervals, 10 s stretches of postsynaptic current were recorded to assess the frequency and amplitude of mEPSCs. For all recordings, the access resistance and leak current were monitored, and experiments were rejected if either of these parameters changed significantly.
Presynaptic calcium measurements
Alexa 594 dextran and Calcium Green-1 dextran (10 kDa, Invitrogen) were loaded into presynaptic boutons as described previously (Beierlein et al., 2004). Loading times were 3–5 min and the loading solution contained 0.025–0.1 % Alexa 594 dextran and 0.5 % Calcium Green-1 dextran. Fluorescence transients from calyces were monitored with a 2-photon microscope as described previously (Brenowitz et al., 2006). Fluorescence signals were converted to calcium by determining the Fmax/Fmin ratio (Fmax/Fmin = 5.5) in a cuvette, determining Fmax using high frequency stimulation according to the approach presented previously (Maravall et al., 2000). In general, calyces that had bright green fluorescence at rest were found to be unsuitable for further study, either because they had elevated resting calcium levels, or they were overloaded with calcium indicator and the calcium transients were slowed.
Data analysis
Data analysis was performed using routines written in IgorPro (WaveMetrics, Lake Oswego, OR). PTP magnitude was calculated as the ratio of EPSC amplitude 10 s after the 100 Hz train over the average baseline. mEPSCs were detected using a threshold (average peak-to-peak noise in the baseline) of the first derivative of the raw current trace, and confirmed visually. mEPSC frequency measurements were made during the baseline (25 s before PTP induction) and starting 6 s after PTP induction. The observed increases in mEPSC size cannot be attributed to the near synchronous fusion of 2 vesicles because, assuming a Poisson distribution and a peak mEPSC frequency (ν) of 12 events/s (as observed following tetanic stimulation), we estimate that only (1-exp(-Δt*ν))= 2.4 % of mEPSCs occur within 2 ms of each other following tetanic stimulation (a conservative upper bound for the timing of 2 closely spaced mEPSCs that can be both detected). Statistical analyses were done using one-way ANOVA tests for multiple group comparisons followed by Tukey post-hoc analysis. Pair-wise comparisons were performed with Student’s paired t-tests or Wilcoxon signed rank tests. Level of significance was set at p < 0.05.
Immunohistochemistry
150 μm-thick transverse brainstem slices were prepared from P12 animals as described above and fixed with 4 % paraformaldehyde for 2 h at 4 °C. At the end of fixation, slices were transferred to phosphate buffer (Sigma-Aldrich, St Louis, MO) and stored at 4 °C until further processing. Slices were then incubated in blocking solution [phosphate buffered solution + 0.25 % Triton X-100 (PBST) + 10 % normal goat serum] for 1 h at room temperature. Slices were incubated with primary antibodies in PBST overnight at 4 °C, followed by incubation with secondary antibodies in PBST for 2 h at room temperature. Slices were mounted to Superfrost glass slides (VWR, West Chester PA) and air-dried for 30 min. Following application of DAPI-containing Prolong anti-fade medium (Invitrogen, Carlsbad CA), slices were covered with a top glass coverslip (VWR) and allowed to dry for 24 h prior to imaging. The following antibodies were used: anti-vGlut1guinea pig polyclonal (Synaptic Systems, Göettingen Germany), anti-PKCα rabbit monoclonal (Abcam, Cambridge, MA), anti-PKCβI rabbit polyclonal (Santa Cruz Biotechnology, Santa Cruz CA), goat anti-guinea pig rhodamine-conjugated and goat anti-rabbit FITC-conjugated secondaries (Santa Cruz Biotechnology, Santa Cruz CA). All antibodies were used at 1:500 dilution.
Images were acquired with a Zeiss 510 Meta confocal microscope using a Plan-apochromat 63X 1.4 N.A. oil lens. Excitation was set at 543 nm for rhodamine (vGlut1) and 488 nm for FITC (PKCs). Emission filters were LP560 for vGlut1 and BP505-530 for PKCs. An optical zoom of 2 was used. Single optical sections at 1024×1024 (Kalman average of 4 scans) were obtained sequentially for the different channels. Experiments with slices from different animals of all genotypes were repeated 3 times.
Supplementary Material
Acknowledgments
We thank Evangelos Antzoulatos, Miklos Antal, Aaron Best, John Crowley, Lindsey Glickfeld, Court Hull, Michael Myoga, Todd Pressler, and Monica Thanawala for comments on a previous version of the manuscript. We thank Kimberly McDaniels for help with genotyping. This work was supported by NIH grant R37 NS032405 to WGR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci U S A. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao JX, Kandel ER, Hawkins RD. Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 1997;275:969–973. doi: 10.1126/science.275.5302.969. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Fioravante D, Regehr WG. Differential expression of posttetanic potentiation and retrograde signaling mediate target-dependent short-term synaptic plasticity. Neuron. 2007;54:949–959. doi: 10.1016/j.neuron.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gee KR, Martin VV, Regehr WG. Presynaptic calcium measurements at physiological temperatures using a new class of dextran-conjugated indicators. J Neurophysiol. 2004;92:591–599. doi: 10.1152/jn.00057.2004. [DOI] [PubMed] [Google Scholar]
- Brager DH, Cai X, Thompson SM. Activity-dependent activation of presynaptic protein kinase C mediates post-tetanic potentiation. Nat Neurosci. 2003;6:551–552. doi: 10.1038/nn1067. [DOI] [PubMed] [Google Scholar]
- Branco T, Staras K. The probability of neurotransmitter release: variability and feedback control at single synapses. Nat Rev Neurosci. 2009;10:373–383. doi: 10.1038/nrn2634. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Best AR, Regehr WG. Sustained elevation of dendritic calcium evokes widespread endocannabinoid release and suppression of synapses onto cerebellar Purkinje cells. J Neurosci. 2006;26:6841–6850. doi: 10.1523/JNEUROSCI.1280-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Rosenmund C. Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- Castillo JD, Katz B. Statistical factors involved in neuromuscular facilitation and depression. J Physiology. 1954;124:574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. The alpha-Ca2+/calmodulin kinase II: a bidirectional modulator of presynaptic plasticity. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Rodriguez-Alfaro JA, Gomez-Fernandez JC. Determination of the calcium-binding sites of the C2 domain of protein kinase Calpha that are critical for its translocation to the plasma membrane. Biochem J. 1999;337(Pt 3):513–521. [PMC free article] [PubMed] [Google Scholar]
- Corbin JA, Evans JH, Landgraf KE, Falke JJ. Mechanism of specific membrane targeting by C2 domains: localized pools of target lipids enhance Ca2+ affinity. Biochemistry. 2007;46:4322–4336. doi: 10.1021/bi062140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KR, Tank DW. A quantitative measurement of the dependence of short-term synaptic enhancement on presynaptic residual calcium. J Neurosci. 1994;14:5885–5902. doi: 10.1523/JNEUROSCI.14-10-05885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KR, Zucker RS, Tank DW. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989;9:3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Krnjevic K. Presynaptic changes associated with post-tetanic potentiation in the spinal cord. J Physiol. 1959;149:274–287. doi: 10.1113/jphysiol.1959.sp006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot LS, Kandel ER, Hawkins RD. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J Neurosci. 1994;14:3280–3292. doi: 10.1523/JNEUROSCI.14-05-03280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng TP. Studies on the neuromuscular junction XXVI. The changes of the end-plate potential during and after prolonged stimulation. Chinese Journal of Physiology. 1941;16:341–372. [Google Scholar]
- Fiumara F, Milanese C, Corradi A, Giovedi S, Leitinger G, Menegon A, Montarolo PG, Benfenati F, Ghirardi M. Phosphorylation of synapsin domain A is required for post-tetanic potentiation. J Cell Sci. 2007;120:3228–3237. doi: 10.1242/jcs.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith WH. Voltage-clamp analysis of posttetanic potentiation of the mossy fiber to CA3 synapse in hippocampus. J Neurophysiol. 1990;63:491–501. doi: 10.1152/jn.1990.63.3.491. [DOI] [PubMed] [Google Scholar]
- Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Valero M, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. The C2 domains of classical PKCs are specific PtdIns(4,5)P2-sensing domains with different affinities for membrane binding. J Mol Biol. 2007;371:608–621. doi: 10.1016/j.jmb.2007.05.086. [DOI] [PubMed] [Google Scholar]
- Habets RL, Borst JG. Post-tetanic potentiation in the rat calyx of Held synapse. J Physiol. 2005;564:173–187. doi: 10.1113/jphysiol.2004.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets RL, Borst JG. An increase in calcium influx contributes to post-tetanic potentiation at the rat calyx of Held synapse. J Neurophysiol. 2006;96:2868–2876. doi: 10.1152/jn.00427.2006. [DOI] [PubMed] [Google Scholar]
- Habets RL, Borst JG. Dynamics of the readily releasable pool during post-tetanic potentiation in the rat calyx of Held synapse. J Physiol. 2007;581:467–478. doi: 10.1113/jphysiol.2006.127365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Xue L, Xu J, McNeil BD, Bai L, Melicoff E, Adachi R, Wu LG. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature. 2009;459:93–97. doi: 10.1038/nature07860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Takai Y, Takahashi T. Presynaptic mechanism for phorbol ester-induced synaptic potentiation. J Neurosci. 1999;19:7262–7267. doi: 10.1523/JNEUROSCI.19-17-07262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Khoutorsky A, Spira ME. Activity-dependent calpain activation plays a critical role in synaptic facilitation and post-tetanic potentiation. Learn Mem. 2009;16:129–141. doi: 10.1101/lm.1275709. [DOI] [PubMed] [Google Scholar]
- Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ. C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry. 2002;41:11411–11424. doi: 10.1021/bi026041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korogod N, Lou X, Schneggenburger R. Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held. J Neurosci. 2005;25:5127–5137. doi: 10.1523/JNEUROSCI.1295-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korogod N, Lou X, Schneggenburger R. Posttetanic potentiation critically depends on an enhanced Ca(2+) sensitivity of vesicle fusion mediated by presynaptic PKC. Proc Natl Acad Sci U S A. 2007;104:15923–15928. doi: 10.1073/pnas.0704603104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Lee KH, Ho WK, Lee SH. Target cell-specific involvement of presynaptic mitochondria in post-tetanic potentiation at hippocampal mossy fiber synapses. J Neurosci. 2007;27:13603–13613. doi: 10.1523/JNEUROSCI.3985-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ho WK, Lee SH. Post-tetanic increase in the fast-releasing synaptic vesicle pool at the expense of the slowly releasing pool. J Gen Physiol. 2010;136:259–272. doi: 10.1085/jgp.201010437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim MH, Ho WK, Lee SH. Presynaptic release probability and readily releasable pool size are regulated by two independent mechanisms during posttetanic potentiation at the calyx of Held synapse. J Neurosci. 2008;28:7945–7953. doi: 10.1523/JNEUROSCI.2165-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–858. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Rahamimoff R. A study of tetanic and post-tetanic potentiation of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1980;309:247–273. doi: 10.1113/jphysiol.1980.sp013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Korogod N, Brose N, Schneggenburger R. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28:8257–8267. doi: 10.1523/JNEUROSCI.0550-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- Magleby K. Short-term changes in synaptic efficacy. In: Edelman, Gall, Cowan, editors. Synaptic function. New York: Wiley; 1987. [Google Scholar]
- Magleby KL. Facilitation, augmentation, and potentiation of transmitter release. Prog Brain Res. 1979;49:175–182. doi: 10.1016/S0079-6123(08)64631-2. [DOI] [PubMed] [Google Scholar]
- Magleby KL, Zengel JE. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975;245:183–208. doi: 10.1113/jphysiol.1975.sp010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986;321:175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Maravall M, Mainen ZF, Sabatini BL, Svoboda K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic Ca(V)2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron. 2008;57:210–216. doi: 10.1016/j.neuron.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Newton A. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Nussinovitch I, Rahamimoff R. Ionic basis of tetanic and post-tetanic potentiation at a mammalian neuromuscular junction. J Physiol. 1988;396:435–455. doi: 10.1113/jphysiol.1988.sp016971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Walmsley B. Phosphorylation regulates spontaneous and evoked transmitter release at a giant terminal in the rat auditory brainstem. J Physiol. 2000;526(Pt 2):349–357. doi: 10.1111/j.1469-7793.2000.t01-1-00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt KD, Madison DV. Phorbol esters enhance synaptic transmission by a presynaptic, calcium-dependent mechanism in rat hippocampus. J Physiol. 1993;471:245–268. doi: 10.1113/jphysiol.1993.sp019900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymann KG, Brodemann R, Kase H, Matthies H. Inhibitors of calmodulin and protein kinase C block different phases of hippocampal long-term potentiation. Brain Res. 1988a;461:388–392. doi: 10.1016/0006-8993(88)90274-0. [DOI] [PubMed] [Google Scholar]
- Reymann KG, Frey U, Jork R, Matthies H. Polymyxin B, an inhibitor of protein kinase C, prevents the maintenance of synaptic long-term potentiation in hippocampal CA1 neurons. Brain Res. 1988b;440:305–314. doi: 10.1016/0006-8993(88)91000-1. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Saitoh N, Hori T, Takahashi T. Activation of the epsilon isoform of protein kinase C in the mammalian nerve terminal. Proc Natl Acad Sci U S A. 2001;98:14017–14021. doi: 10.1073/pnas.241333598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of held. J Neurosci. 2006;26:5863–5871. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R, Silberberg SD, Ginsburg S, Rahamimoff R. Activation of protein kinase C augments evoked transmitter release. Nature. 1987;325:58–60. doi: 10.1038/325058a0. [DOI] [PubMed] [Google Scholar]
- Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Sudhof TC, Bourtchuladze R. Impaired learning in mice with abnormal short-lived plasticity. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Virmani T, Ertunc M, Sara Y, Mozhayeva M, Kavalali ET. Phorbol esters target the activity-dependent recycling pool and spare spontaneous vesicle recycling. J Neurosci. 2005;25:10922–10929. doi: 10.1523/JNEUROSCI.3766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Maler L. Differential roles of Ca2+/calmodulin-dependent kinases in posttetanic potentiation at input selective glutamatergic pathways. Proc Natl Acad Sci U S A. 1998;95:7133–7138. doi: 10.1073/pnas.95.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wu XS, Wu LG. Protein kinase c increases the apparent affinity of the release machinery to Ca2+ by enhancing the release machinery downstream of the Ca2+ sensor. J Neurosci. 2001;21:7928–7936. doi: 10.1523/JNEUROSCI.21-20-07928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Lara-Estrella LO. Post-tetanic decay of evoked and spontaneous transmitter release and a residual-calcium model of synaptic facilitation at crayfish neuromuscular junctions. J Gen Physiol. 1983;81:355–372. doi: 10.1085/jgp.81.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.