Abstract
Previously-reported evidence showed that freezing to a context previously associated with footshock is impaired by lesion of the ventral periaqueductal gray (vPAG). It has also been shown that stepwise increase in the intensity of the electrical stimulation of the dorsal periaqueductal gray (dPAG) produces alertness, then freezing, and finally escape. These aversive responses are mimicked by microinjections of GABA receptor antagonists, such as bicuculline, or blockers of the glutamic acid decarboxylase (GAD), such as semicarbazide, into the dPAG. In this work, we examined whether the expression of these defensive responses could be the result of activation of ventral portion of the periaqueductal gray. Sham- or vPAG electrolytic–lesioned rats were implanted with an electrode in the dPAG for the determination of the thresholds of freezing and escape responses. The vPAG electrolytic lesions were behaviorally verified through a context-conditioned fear paradigm. Results indicated that lesion of the vPAG disrupted conditioned freezing response to contextual cues associated with footshocks but did not change the dPAG electrical stimulation for freezing and escape responses. In a second experiment, lesion of the vPAG also did not change the amount of freezing and escape behavior produced by microinjections of semicarbazide into the dPAG. These findings indicate that freezing and escape defensive responses induced by dPAG stimulation do not depend on the integrity of the vPAG. A discussion on different neural circuitries that might underlie different inhibitory and active defensive behavioral patterns that animals display during threatening situations is presented.
When faced with a threatening situation, animals tend to present two opposite patterns of defensive reactions: one related to freezing behavior, in which the animal starts to inhibit its ongoing behavior, and the other related to vigorous escape responses such as running and jumping. Distinct regions of the periaqueductal gray (PAG) seem to play a different role on these different defensive behaviors (Fanselow 1991; Rizvi et al. 1991; Davis 1992; Carrive 1993; Bandler and Shipley 1994; Fanselow et al. 1995; De Oca et al. 1998). The ventral portion of the PAG (vPAG) seems to be involved exclusively on freezing response that gradually takes place when an animal is exposed to innate or learned aversive stimuli. Lesions of the vPAG reduced freezing responses to neutral stimuli associated with footshock as well as to the animal's natural predator (De Oca et al. 1998) but did not affect escape responses, such as running or jumping, behavior elicited by footshock (Fanselow 1991). Defensive freezing behavior seems to be modulated by afferent projection that the vPAG receives from forebrain structures, especially from the amygdaloid complex (Fanselow 1991; Carrive 1993; Bandler and Shipley 1994; Fendt and Fanselow 1999).
The dorsal portion of the PAG (dPAG) appears to mediate both active and inhibitory behavioral patterns of defensive responses. For example, lesions of the dPAG enhanced conditioned freezing (DeOca et al. 1998) and reduced escape reactions to electrical footshock (Fanselow 1991). Moreover, studies with electrical stimulation in the dPAG have shown that increasing the electrical current in a stepwise fashion elicits a freezing response and then vigorous escape reactions (Schenberg et al. 1990; Coimbra e Brandão 1993). Also, it has been reported that glutamate microinjections in the same area produced freezing behavior (Krieger and Graeff 1985). Finally, microinjections into the dPAG of GABA receptor blockers or GABA inhibitors of glutamic acid decarboxylase (GAD), the enzyme responsible for GABA synthesis, produce fearlike behavior with a delay of action of ∼7 min (Brandão et al. 1982, 1986; Schmitt et al. 1985). Freezing, rapid running, and jumps characterize this response. The aversiveness of this behavioral reaction has been already demonstrated in studies showing that microinjections of semicarbazide into the dPAG produce conditioned place aversion (DiScala et al. 1989; Aguiar and Brandão 1994).
Because the manifestations of freezing induced by dPAG stimulation are behaviorally similar to innate and conditioned defensive freezing, it is possible that their neural substrates may partially overlap. Freezing induced by dPAG stimulation might be mediated either by local circuitry within the PAG or through ascending projections to forebrain structures (i.e., the amygdaloid complex), which in turn could activate the vPAG. Because vPAG lesions affect expression of defensive freezing induced by innate and learned aversive stimuli but had no effect on escape responses induced by footshock, it is also possible that lesioning this area can selectively disrupt freezing but not escape responses elicited by electrical or chemical (brain microinjections of semicarbazide) stimulation of the dPAG. In this regard, the present study investigated whether vPAG lesion affects freezing and escape responses elicited by dPAG chemical or electrical stimulation. As a positive control procedure, conditioned freezing was also assayed within the same animals submitted to a context fear-conditioning procedure at the end of the dPAG electrical stimulation experiment.
RESULTS
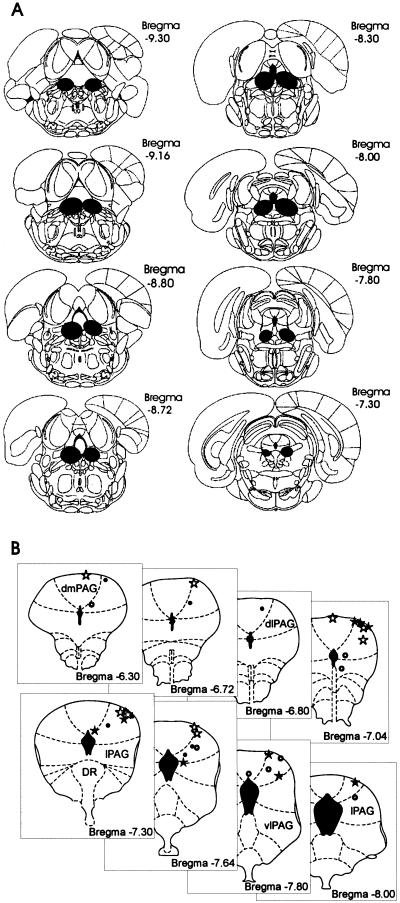
Histological analysis of the slides indicated that all rats used in this study had at least three successive plates of the atlas taken by the lesion of the vPAG. A representative lesion can be seen in Figure 1A. In general, lesions reached the vPAG bilaterally and, in some cases, damaged the dorsal raphe and extended into the lateral regions of the PAG. Figure 1B presents the internal cannula and electrode tips aimed at the dPAG. As can be observed, all stimulation sites were located within the dorsomedial and dorsolateral portion of the PAG.
Figure 1.
Schematic drawing of target brain sites. (A) Representative ventral periaqueductal gray lesion. (B) Sites of stimulation electrodes in the dorsal periaqueductal gray. Sham-lesion animals submitted to electrical (ο) or chemical stimulation (⋆). vPAG-lesion animals submitted to electrical (·) or chemical stimulation (★), respectively.
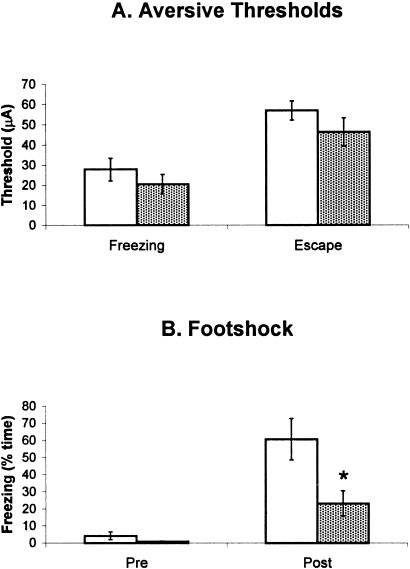
As previously shown in many works from this laboratory (Coimbra et al. 1989; Coimbra and Brandão, 1993; Brandão et al. 1994, 1999), freezing and escape behaviors occurred in a stepwise fashion as the intensity of electric current applied to dPAG was gradually increased (24.24 ± 5.22 μA for freezing and 51.56 ± 5.90 μA for escape). These aversive responses were always accompanied by at least two of the following autonomic reactions: urination, defecation, piloerection, or exhophtalmus. As shown in Figure 2A, lesion of the vPAG did not change the aversive threshold of electrical stimulation of the dPAG either for freezing responses (t = 0.95; P > 0.05; df = 13) or for escape responses (t = 1.25; P > 0.05; df = 14). In contrast, the same lesion attenuated freezing induced by the context previously paired with footshock (t = 2.65; P < 0.05; df = 14), as shown by the right pair of columns in Figure 2B. The left pair of columns of the same figure shows the amount of freezing behavior before conditioning. No difference between sham and lesioned groups was found (t = 1.45; P > 0.05; df = 14).
Figure 2.
(A) Aversive thresholds determined in rats bearing (hatched columns) or not bearing (sham-open columns) electrolytic lesion of the vPAG. (B) Percentage of time spent freezing by rats with sham (hatched columns) or vPAG (open columns) lesions during 6 min. Pre refers to the baseline period; Post, to the testing session 24 h after conditioning. N = 8 for all groups, except for the freezing threshold (lesion = 8, control = 7).
As to the quantification of the locomotor activity measured in the arena, the vPAG lesion group showed a significant increase (t = 2.82, P < 0.05; df = 14) in the number of crossings in relation to the sham lesion group (140.00 ± 15.47 in lesioned versus 85.13 ± 11.85 in sham-lesioned rats).
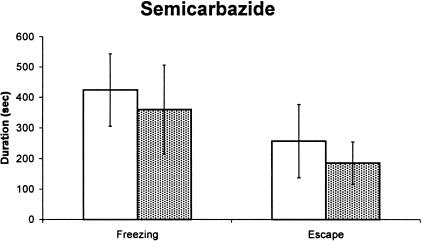
Figure 3 depicts the mean (+ SEM) of freezing and escape behavior triggered by microinfusion of semicarbazide into the dPAG among animals bearing sham or vPAG lesion. The results are clear cut and indicate that vPAG lesion did not change the defensive behavior elicited by semicarbazide stimulation of the dPAG either for freezing responses (t = 0.38; P > 0.05; df = 16) or for escape responses (t = 0.10; P > 0.05; df =16). As occurred in the first experiment, the locomotor activity measured in the arena before the behavioral activation caused by semicarbazide microinjections into the dPAG was higher in lesioned than sham-lesioned rats (t = 4.03, P < 0.05; df = 16).
Figure 3.
(A) Freezing and escape behaviors induced by microinjections of semicarbazide (8.0 μg/0.2μL) into the dPAG in rats bearing (hatched columns) or not bearing (open columns) electrolytic lesion of the vPAG. N = 9 for both groups.
DISCUSSION
The results of this study clearly show that the same vPAG lesion that markedly reduces the freezing response to a context previously associated with footshock did not change the threshold of the electrical current for eliciting freezing or escape behaviors when applied into the dPAG. Moreover, vPAG lesions were not able to affect freezing behavior induced by chemical stimulation of the dPAG with semicarbazide. The former finding is in full agreement with results previously reported by Fanselow and coworkers (1991, 1995) and highlights the critical role of the vPAG in the expression of conditioned freezing. In turn, the resistance of dPAG-induced freezing to the vPAG lesion evidences the first and major difference between the neural substrates of the two types of freezing. In fact, it has also been reported that vPAG lesion did not affect freezing response induced by electrical stimulation of the inferior colliculus, another brain structure involved in the organization of defensive reactions (Maisonnette et al. 1996). Freezing behavior generated at the dPAG is likely to represent a defense reaction distinct from the one related to innate and conditioned danger stimuli, which seem to be mediated by the vPAG (Fanselow 1991; Fanselow et al. 1995). It seems to be triggered by particular kinds of unconditioned stimuli as vPAG lesions block freezing of rats to natural predators, such as cats (De Oca et al. 1998). However, we do not know what kind of unconditioned stimuli triggers these neural mechanisms; interoceptive ones, as in panic attacks, remain one possibility. Recently, it has been reported that electrolytic lesions of the vPAG produced a deficit in conditioned freezing but had no effect on the suppression of an operant response in a conditioned emotional response paradigm (Amorapanth et al. 1999). These results together with this present report suggest that there might exist different patterns of inhibitory defensive behavior with distinct neural pathways. However, further studies are still needed to clarify this issue.
The fact that vPAG lesion did not influence freezing induced by dPAG electrical or chemical stimulation indicates that this defensive behavior is not mediated through intra-PAG connections. Alternatively, the occurrence of freezing behavior generated by dPAG stimulation might involve projections to other fear related brain structures. Fibers originating from dPAG innervate various forebrain regions, including the amygdaloid complex through the medial forebrain bundle (Rizvi et al. 1991; Cameron et al. 1995). Accordingly, Fanselow (1991) and De Oca et al. (1998) have suggested that connections between the dPAG and the amigdaloid complex might in fact modulate the occurrence of freezing behavior. Although this might be a possibility, it is important to note that the freezing induced by dPAG stimulation might not include a projection from the amygdaloid complex to the vPAG, as it appears to mediate the conditioned freezing. Finally, it has been shown that the median raphe nucleus (MRN) plays an important role in the occurrence of freezing behavior. Lesions of the MRN abolish conditioned freezing (Avanzi et al. 1998), whereas electrical stimulation of the MRN induces freezing (Graeff and Silveira-Filho 1978). Therefore, an alternative output for the freezing response induced by dPAG electrical stimulation could be through MRN, which receives direct projections from the dPAG as well as from forebrain structures involved in defensive behavior such as the amygdaloid complex (Vertes et al. 1999).
Our results also showed that active defensive responses induced by electrical stimulation of the dPAG are not mediated through the vPAG. Animals bearing electrolytic lesions of the vPAG present the same dPAG electrical stimulation threshold to trigger escape responses as sham control animals. In the same vein, vPAG lesions did not affect escape reactions induced by chemical stimulation of the dPAG. These results are in accordance with previous a report that indicates that vPAG lesions did not influence active defense responses, such as running, jumping, and vocalization, elicited by footshock (Fanselow 1991). Therefore, neural pathways independent of the vPAG might be responsible for escape reactions induced by electrical stimulation of the dPAG. In this regard, it has been proposed that GABAergic neurons from the substantia nigra pars reticulata (SNpr), which project to the midbrain tectum, mainly the dorsal layers of the superior colliculus (DiChiara et al. 1979; Chevalier et al. 1981; Kilpatrick et al. 1982), are involved on active pattern of defensive reactions. This assumption is supported by previous studies performed in one of our laboratories (M.L.B.) that show enhanced escape reaction induced by dPAG electrical stimulation following electrolytic and neurochemical lesions of SNpr with kainic acid (Coimbra et al. 1989; Coimbra and Brandão 1993). On the other hand, SNpr lesion did not change freezing induced by electrical stimulation of the midbrain tectum (Maisonnette et al. 1996), suggesting that although it seems that nigrocollicular pathways are involved in the expression of escape responses, the same does not hold true for freezing responses.
This study also presents evidence for the important role played by local GABAergic neurons in the dPAG in the regulation of the neural circuits responsible for the expression of the defensive behavior. It has been shown that dPAG has a significant amount of GABAergic interneurons on which GAD blocking agents act, reducing the tonic inhibitory control exerted by GABA on the neural circuits of defensive behavior (Brandão et al. 1986; DiScala and Sandner 1989; Aguiar and Brandão 1994). Indeed, defensive behaviors induced by the blockade of the GAD with semicarbazide were not affected by vPAG lesion. These results point to generation of freezing and escape behavior within the dPAG independently from the participation of the vPAG.
Along with the well-known participation of the dPAG on active defensive responses, this paper brings new evidence for the involvement of the dPAG neurons on freezing behavior. This finding does not discard that associative learning may still be formed using stimulation of the aversive substrates of the dPAG as already clearly shown by other investigators (DiScala et al. 1987). However, the parameters used to produce associative learning using dPAG stimulation need to be stronger that the ones used in the present experiment. A single electrical stimulation of the dPAG at the escape threshold does not lead to any context conditioning. In fact, at least two sessions of pairings of dPAG electrical stimulation environmental stimuli are required to produce contextual fear conditioning (Castilho et al. 2001).
MATERIALS AND METHODS
Animals
Male Wistar rats weighing 250–300 g from the animal house at the campus of Ribeirão Preto of the University of São Paulo were housed in a temperature-controlled (22 ± 1 °C) room and kept on a 12-h light/12-h dark cycle (lights on from 0700–1900). These animals were maintained in individual Plexiglas-walled cages and given free access to food and water throughout the experiment.
Surgical Procedures
The animals were anaesthetized with tribromoethanol (250 mg/kg, i.p.), and fixed in a David Kopf stereotaxic frame. The upper incisor bar was set at 3.3 mm below the interaural line, so that the skull was horizontal between bregma and lambda. Each animal was implanted, with a unilateral bipolar electrode (Plastics One) aimed at the dPAG. The electrodes were made of stainless-steel wire, 160 μm in diameter and insulated except at the cross-section, and were introduced at the dPAG with an angle of 16°, 1.9 mm lateral to lambda and 5.1 mm ventral to the skull. The dPAG electrode was fixed to the skull by means of acrylic resin and three stainless-steel screws. This electrode could be connected to a male pin so that it could be plugged into an amphenol socket at the end of a flexible electrical cable and used for brain stimulation. Electrolytic lesions in the vPAG were made bilaterally with a monopolar stainless-steel electrode (insect pin insulated till the exposed blunt tip) lowered at 0.8 mm posterior to lambda, 1.0 mm lateral to each side of midline, and 6.0 mm ventral to the skull. Lesions were made by passing anodal DC current (ESF-108, DelVecchio) for 60 sec (2 mA) through the electrode. The sham lesion group had identical surgery procedures except that no current was delivered through the electrode. All animals were allowed at least 7 days to recovery from surgery. For the experiment with microinjections of semicarbazide into the dPAG, each rat was implanted with a stainless-steel guide cannula (0.6 mm OD, 0.4 mm ID). The cannula was directed to the dPAG at the coordinates described above except for the depth (4.1 mm). Each cannula was fixed with polyacrylic cement anchored to the skull with three stainless-steel screws and was plugged with stainless-steel stylets. The experiments started after a 1-wk postoperative delay.
Apparatus
The dPAG electrical stimulation experiment took place in a circular arena 60 cm in diameter and 50 cm high. The arena was in a quiet experimental room illuminated with three 40-W fluorescent lamps (350 lux at the arena floor level). The floor of the circular arena was divided into 12 sections so that the general activity of the animals with sham and vPAG lesions could be quantified during the 6 min before the electrical stimulation of the dPAG began. The brain was electrically stimulated by means of a sine wave stimulator (Marseillan). Brain stimulation (AC, 60 Hz, 15 s) was presented at 1-min intervals, with the current intensity increasing by steps of 5 μA for measurements of the aversive thresholds. The stimulation current was monitored by measuring the voltage drop across a 1-K resistor with an oscilloscope (Hewlett-Packard).
Context fear conditioning was conducted in an experimental chamber (25 × 20 × 20 cm) made of stainless-steel walls with a clear acrylic door in the front. The chamber lay within a sound attenuating chest consisting of a large 50 × 54 × 56 cm plywood container with a with a hole on the door through which all behavior was observed. The chamber contained a stainless-steel rod floor, with 2.7-mm rods placed 1 cm apart, center to center. The chambers were cleaned with a 5% ammonium hydroxide solution between sessions. Scrambled electric footshock originated from an AC shock source (DelVechio). This unit was adjusted to deliver 1.0-mA shocks to the grid floor of the chamber. This equipment was in an isolated room of the laboratory that was lit by two fluorescent fixtures.
The dPAG chemical experiment took place in the same circular arena described above. Animals were gently wrapped in a cloth, and a thin dental needle (0.3 mm OD) was introduced through the guide cannula until its lower end was 1.0 mm below the guide cannula. The injection needle was linked to a 5-μL Hamilton syringe by means of polyethylene tubing. A volume of 0.2 μL was injected over 20 sec with the aid of an infusion pump (Harvard Apparatus), and the needle was held in place for an additional 10 sec. The displacement of an air bubble inside the polyethylene (PE-10) catheter connecting the syringe needle to the intracerebral needle was used to monitor the injection. Each rat received only one injection of semicarbazide (Sigma, 8 μg/0.2 μL).
Procedure
In the dPAG electrical stimulation, animals were placed in the circular arena and allowed a 6-min period of habituation. At the end of this period, brain stimulation (AC, 60 Hz, 15 s) was presented for 15 sec at 1-min intervals with the current intensity increasing by steps of 5 μA for measurements of the aversive thresholds. Freezing threshold was operationally defined as the lowest intensity producing a complete immobility, except the movements for respiration accompanied by defecation or micturition, for at least 6 sec during the period of stimulation. Only freezing that occurred during stimulation was considered. The current intensity-producing running (gallop) or jumping was considered to be the escape threshold. Animals with an escape threshold above 70 μA (peak-to-peak) were discarded from the experiment. After the determination of the aversive thresholds, the animals were taken to their home cages. After ∼24 h, all the animals were submitted to the context fear-conditioning procedure to behaviorally verify the effectiveness of the vPAG electrolytic lesions. Each animal was placed in the conditioning chamber and allowed 6 min of acclimatization and afterward received five 5-sec, 1-mA footshocks spaced for 60 sec. On the next day, the rat was replaced into the conditioning chamber and remained undisturbed for the 6-min context conditioning test. No shock was presented during this period. Freezing, defined as the absence of movement of the body and vibrissa except that required for respiration for at least 6 sec, was scored according to a time sample procedure. Every 2 sec the animal was observed and its behavior was scored as freezing or not freezing.
In the dPAG chemical experiment, sham and vPAG lesion animals received microinjections of semicarbazide into the dPAG and were placed in the circular arena for a 1-h session in the open field. The behavior of the animals was recorded by a videocamera positioned beside the open field and monitored via a closed circuit TV camera. Freezing and escape defined as described above were subsequently scored from videotape by an observer with the aid of an appropriate software (The Observer, Noldus, Wageningen, The Netherlands). The open field was thoroughly cleaned after each test with a 20% ethanol solution and dried. Each rat was tested only once.
Histology
At the end of each experiment, animals were deeply anaesthetized with urethane and perfused intracardially with saline followed by formalin solution (10%). Three days later, the brains were removed and frozen. Serial 50 μm brain sections were cut using a microtome (Cryocut 1800) to localize the stimulation electrode tips within the dPAG and the electrolytic lesions within the vPAG according to Paxinos and Watson's atlas (1986).
Analysis of Results
Time or thresholds for freezing and escape responses are presented as means ± SEM between groups with or without (sham) vPAG electrolytic lesions. Data were analyzed using Student's t test. For the dPAG electrical stimulation, eight animals were studied in each lesion and control group, except for the freezing threshold comparison, in which one animal of each group had to be excluded for not showing freezing before the escape threshold was reached. For the dPAG chemical stimulation, nine animals were used in each group (sham and lesion animals).
Acknowledgments
This work was supported by FAPESP (Proc No. 98/11187-2) and CNPq (Proc No. 94/5933-2 and 522720/95-1). F.G.G. is the recipient of a Research Fellowship from FAEPA-Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL mbrandao@usp.br; FAX 55-16-602-3632.
Article and publication are at www.learnmem.org/cgi/doi/10.1101/lm.36101.
REFERENCES
- Aguiar MS, Brandão ML. Conditioned place aversion produced by microinjections of substance P into the periaqueductal gray of rats. Behav Pharmacol. 1994;5:369–373. doi: 10.1097/00008877-199406000-00017. [DOI] [PubMed] [Google Scholar]
- Amorapanth P, Nader K, LeDoux JE. Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behavior in rats. Learn Mem. 1999;6:491–499. doi: 10.1101/lm.6.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzi V, Castilho VM, de Andrade TG, Brandão ML. Regulation of contextual conditioning by the median raphe nucleus. Brain Res. 1998;20:178–84. doi: 10.1016/s0006-8993(97)01538-2. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Brandão ML, DiScala G, Bouchet MJ, Schmitt P. Escape behavior produced by the blockade of glutamic acid decarboxilase GAD in mesencephalic central gray or medial hypothalamus. Pharmacol Biochem Behav. 1986;24:497–502. doi: 10.1016/0091-3057(86)90547-2. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Cardoso SH, Melo LL. Defense mechanisms in the inferior colliculus. Behav Brain Res. 1993;18:339–346. doi: 10.1016/0166-4328(93)90089-9. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Cardoso SH, Melo LL, Motta V, Coimbra NC. Neural substrate of defensive behavior in the midbrain tectum. Neurosci Biobehav Rev. 1994;18:339–346. doi: 10.1016/0149-7634(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Anseloni VZ, Pandóssio JE, De Araújo JE, Castilho VM. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev. 1999;23:863–875. doi: 10.1016/s0149-7634(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: A Phaseolus vulgaris-leucoagglutinin study I. Ascending projections. J Comp Neurol. 1995;351:568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: Functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Castilho VM, Brandão ML. Conditioned antinociception and freezing using electrical stimulation of the dorsal periaqueductal gray or inferior colliculus as unconditioned stimulus are differentially regulated by 5-HT2A receptors in rats. Psychopharmacology. 2001;155:154–162. doi: 10.1007/s002130100697. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Thierry AM, Shibazakii T, Feher J. Evidence for a GABAergic inhibitory nigrotectal pathway in the rat. Neurosci Lett. 1981;21:67–70. doi: 10.1016/0304-3940(81)90059-8. [DOI] [PubMed] [Google Scholar]
- Coimbra NC, Leão-Borges PC, Brandão ML. GABAergic fibers from substantia nigra pars reticulata modulate escape behaviour induced by midbrain central gray stimulation. Braz J Med Biol Res. 1989;22:111–114. [PubMed] [Google Scholar]
- Coimbra NC, Brandão ML. GABAergic nigro-collicular pathways modulate the defensive behavior elicited by midbrain tectum stimulation. Behav Brain Res. 1993;59:131–139. doi: 10.1016/0166-4328(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned fear. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction. New York: Plenum Press; 1992. pp. 255–305. [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Porceddu ML, Morelli M, Mulas ML, Gessa GL. Evidence for a GABAergic projection from substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res. 1979;176:273–284. doi: 10.1016/0006-8993(79)90983-1. [DOI] [PubMed] [Google Scholar]
- DiScala G, Mana MJ, Jacobs WJ, Phillips G. Evidence of Pavlovian conditioned fear following electrical stimulation of the periaqueductal grey in the rat. Physiol Behav. 1987;40:55–63. doi: 10.1016/0031-9384(87)90185-5. [DOI] [PubMed] [Google Scholar]
- DiScala G, Sandner G. Conditioned place aversion produced by microinjections of semicarbazide into the periaqueductal gray of the rat. Brain Res. 1989;483:91–97. doi: 10.1016/0006-8993(89)90038-3. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in responses to fear and anxiety. In: DePaulis A, Bandler R, editors. The midbrain periaqueductal grey matter: Functional, anatomical and immunohistochemical organization. New York: Plenum Publishing Corp.; 1991. pp. 151–173. [Google Scholar]
- Fanselow MS, DeCola JP, De Oca BM, Landeira-Fernandez J. Ventral and dorsolateral regions of the midbrain periaqueductal gray PAG control different stages of defensive behavior. Aggressive Behav. 1995;21:63–77. [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Silveira Filho NG. Behavioral inhibition induced by electrical stimulation of the median raphe nucleus of the rat. Physiol Behav. 1978;21:477–484. doi: 10.1016/0031-9384(78)90116-6. [DOI] [PubMed] [Google Scholar]
- Kilpatrick TC, Collingridge GL, Star MS. Evidence for the participation of nigrotectal γ-aminobutirate containing neurons in striatal and nigral derivated circling in the rat. Neuroscience. 1982;7:207–222. doi: 10.1016/0306-4522(82)90161-0. [DOI] [PubMed] [Google Scholar]
- Krieger JE, Graeff FG. Defensive behavior and hypertension induced by glutamate in the midbrain central gray of the rat. Braz J Med Biol Res. 1985;18:61–67. [PubMed] [Google Scholar]
- Maisonnette SS, Kawasaki MC, Coimbra NC, Brandão ML. Effects of lesions of amygdaloid nuclei and substantia nigra on aversive responses induced by electrical stimulation of the inferior colliculus. Brain Res Bull. 1996;40:93–98. doi: 10.1016/0361-9230(95)02136-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Schenberg LC, Costa MB, Borges PCL, Castro FS. Logistic analysis of the defense reaction induced by electrical stimulation of the rat mesencephalic tectum. Neurosci Biobehav Rev. 1990;14:473–479. doi: 10.1016/s0149-7634(05)80070-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;17:555–562. [PubMed] [Google Scholar]