Abstract
Two tests of auditory recognition memory were given to four patients with bilateral hippocampal damage (H+) and three patients with large medial temporal lobe lesions and additional variable damage to lateral temporal cortex (MTL+). When single stimuli were presented, performance was normal across delays as long as 30 sec, presumably because information could be maintained in working memory through rehearsal. When lists of 10 stimuli were presented, performance was impaired after a 5-min delay. Patients with MTL+ lesions performed marginally worse than patients with H+ lesions, consistent with findings for recognition memory in other modalities. The findings show that auditory recognition, like recognition memory in other sensory modalities, is dependent on the medial temporal lobe.
Bilateral medial temporal lobe lesions in humans produce severe, multimodal memory impairment (Scoville and Milner 1957; Stefanacci et al. 2000). In one study, the noted patient H.M. failed tests of recognition memory when either visual stimuli (shades of red, lights flashes, or nonsense patterns) or auditory stimuli (tones or clicks) were presented, and delays of 60 sec were interposed between study and test (Milner 1972). After the development of one-trial tests of recognition memory for the monkey (Gaffan 1974; Mishkin and Delacour 1975), impaired recognition memory after medial temporal lobe lesions has been observed in the visual (Mishkin 1978; Zola-Morgan et al. 1982) and tactile (Murray and Mishkin 1984; Suzuki et al. 1993) modalities. Impaired recognition memory also has been observed in rats in the visual and olfactory modalities, when tests were given that were similar to those used with the monkey (Clark et al. 2000, 2001; Otto and Eichenbaum 1992).
Aside from early studies of patient H.M., the effects of medial temporal lobe lesions on auditory recognition memory have not been well studied. Patients with left or right unilateral temporal lobe excisions, which included the amygdala, temporal pole, anterior neocortex, and variable extents of hippocampus and parahippocampal gyrus, were impaired at recognizing novel melodies (Samson and Zatorre 1992). However, it is unclear to what extent this impairment depended on damage specifically to the medial temporal lobe.
Preliminary studies in the monkey suggested that large medial temporal lobe lesions do impair auditory recognition memory (Fritz et al. 1999). However, smaller lesions limited to perirhinal and entorhinal cortex had little effect (Saunders et al. 1998), in sharp contrast with the deficit in both visual and tactile recognition memory that is found in monkeys after perirhinal and entorhinal lesions (Meunier et al. 1993; Buffalo et al. 1999) . In addition, studies of auditory recognition memory have been conducted in dogs, with delays of up to 90 sec (Kowalskaet al. 2001). Neither lesions of the hippocampus (H), lesions of perirhinal and entorhinal cortex (Rh), nor combined lesions (H + Rh) had a detectable effect on performance. These findings raise questions about the importance of the medial temporal lobe for auditory recognition memory.
To evaluate the effect of large medial temporal lobe lesions and more limited lesions on auditory recognition memory, we tested four patients with bilateral hippocampal damage (H+) and three patients with large medial temporal lobe lesions and additional variable damage to lateral temporal cortex (MTL+). In one test, auditory recognition memory for single stimuli was assessed across delays of 2, 10, and 30 sec. In a second test, auditory recognition memory was assessed by presenting 10 different stimuli and then 5 min later testing with the 10 old stimuli intermixed with 10 new ones.
RESULTS
Delays
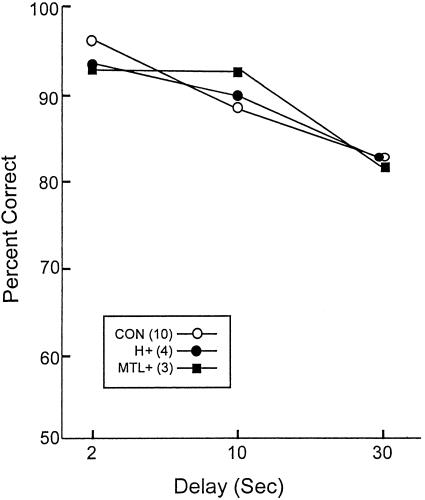
Figure 1 shows percentage correct scores for each of the three delays for the controls (CON), hippocampal patients (H+), and medial temporal lobe patients (MTL+), averaging across trials with targets and trials with foils. A two-way repeated-measures ANOVA comparing the performance of the three groups across the three delays (2, 10, and 30 sec) revealed a significant effect of delay [F(2,28) = 15.7, P < 0.001] and no effect of group.
Figure 1.
Delays. Percentage correct scores for controls (CON), patients with hippocampal damage (H+), and patients with large medial temporal lobe lesions (MTL+) when single sounds were presented and yes/no recognition was tested after a variable delay (2–30 sec). The standard errors of the mean ranged from 1.5 to 5.7.
A second ANOVA was conducted to assess performance on trials when a target was presented and on trials when a foil was presented. This analysis found no effect of trial type [F(1,14) < 1.0] and an interaction of delay x trial type. Specifically, within each group performance on target trials tended to decrease as a function of delay, whereas performance on foil trials tended to remain constant across the delays.
Lists
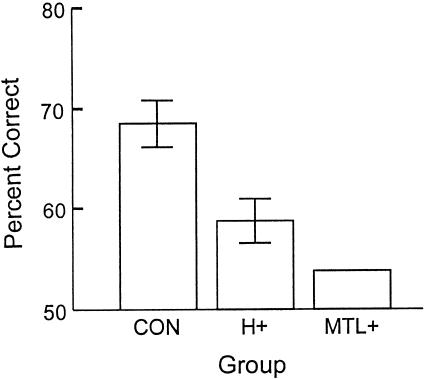
Figure 2 shows percentage correct scores (averaging across target trials and foil trials and across the three tests) for the controls (CON), hippocampal patients (H+), and medial temporal lobe patients (MTL+). Because the three patients in the MTL+ group scored within 1% of each other, nonparametric statistics were used for the first analysis and the related pairwise comparisons. A Kruskal-Wallis ANOVA revealed a significant effect of group (P < .02). Pairwise comparisons revealed that the MTL+ group was impaired relative to the CON group (P < .05) and marginally impaired relative to the H+ group (P < .06). In addition, the H+ group was marginally impaired relative to the CON group (P < .06). The two patients thought to have the most restricted damage within the medial temporal lobe (A.B. and L.J.) both performed poorly (A.B., 56% correct; L.J., 59% correct). Finally, although both patient groups performed poorly, they did score above chance (t > 3.9; P > .05). A second (two-way) ANOVA to assess performance as a function of trial type (targets vs. foils) revealed no effect of trial type [F(1,32) = 1.9; P > 0.10] and no interaction of trial type x group [F(2,32) < 1.0].
Figure 2.
Lists. Percentage correct scores for controls (CON), patients with hippocampal damage (H+), and patients with large medial temporal lobe lesions (MTL+) when 10 sounds were presented for study and yes/no recognition was tested 5 min later. Brackets show standard errors of the mean. The three patients in the MTL+ group scored within 1% of each other.
DISCUSSION
Two tests of auditory recognition memory were given to four patients with hippocampal damage, and three patients with large medial temporal lobe lesions and additional variable damage to lateral cortex. In test 1, participants listened to a sound and then after a delay of 2, 10, or 30 sec judged whether a second sound was the same as or different from the first sound. Both patient groups performed normally. In test 2, 10 sounds were presented and then after a 5-min delay participants judged whether each of the 20 sounds had just been presented. Both patients groups were impaired.
The finding of good performance in test 1 when single sounds needed to be remembered across a delay likely reflects the fact that single sounds could be held effectively in working memory during the relatively short delays (2–30 sec) that were tested. In these conditions, performance might be supported by auditory association cortex, perhaps in cooperation with prefrontal cortex. Patient H.M. did show impaired recognition on a similar test for single auditory stimuli, but performance was rather good after a 30-sec delay (about two errors in 12 trials, whereas controls averaged only one error), and performance was severely impaired only at a 60-sec delay (∼4.5 errors in 12 trials; Milner 1972). Furthermore, for the tests given to H.M., five different values were assigned to each type of stimulus, to reduce the possibility that rehearsal could be used to bridge the retention interval. In contrast, in the tests we used each stimulus was unique, there was a minimum of intrastimulus interference, and verbal rehearsal could have helped bridge the delay intervals. Nevertheless, it is possible that an impairment would have emerged if testing had been extended to 60 sec.
The finding of poor recognition performance in test 2 confirms what was described originally for patient H.M., namely, that auditory recognition memory depends on the integrity of the medial temporal lobe. In addition, the present findings show that damage limited to the hippocampal region is sufficient to impair auditory recognition. It is also noteworthy that the effects of large medial temporal lobe lesions were even greater than the effects of hippocampal lesions. This pattern of results has been observed repeatedly in studies of recognition memory in monkeys, in which the deficit associated with restricted hippocampal lesions is exacerbated by additional damage to adjacent cortex (Zola-Morgan et al. 1994). Similarly, in studies of human amnesia, large medial temporal lobe lesions consistently produce a more severe recognition memory impairment than restricted hippocampal lesions (Hamann and Squire 1997; Buffalo et al. 1998; Stark and Squire 2000).
Studies in monkeys (Saunders et al. 1997) and dogs (Kowalska et al. 2001) suggested that lesions limited to the perirhinal and entorhinal cortex of the medial temporal lobe spare auditory recognition memory. This finding may be understandable in the light of the neuroanatomy of auditory processing. Auditory cortical areas, for example, the superior temporal gyrus, reach the medial temporal lobe mainly by way of projections to area TH of the parahippocampal cortex and, except for its polar portion (area 36d), reach perirhinal cortex only indirectly (Suzuki and Amaral 1994). Suzuki and Amaral (1994) note the strong projections from auditory association areas of the superior temporal gyrus to area TH and suggest that area TH particularly may be involved in auditory memory function. Thus, lesions of more anterior medial temporal lobe cortex that spare parahippocampal cortex might be expected to spare auditory recognition. In this respect, the anatomy of auditory recognition memory differs from the anatomy of visual recognition memory. Visual cortical areas project especially to perirhinal cortex (Suzuki and Amaral 1994), and damage to perirhinal cortex impairs visual recognition memory (Meunier et al. 1993; Buffalo et al. 1998).
The perirhinal and parahippocampal cortices originate projections to the entorhinal cortex and the hippocampus. The present results show that damage to the hippocampal region, which is at the end of this processing hierarchy, also impairs recognition memory. Restricted hippocampal lesions have been found to impair recognition memory in adult-onset amnesia (Reed and Squire 1998; Manns and Squire 1999), in monkeys (Zola and Squire 2000), and in rodents (Clark et al. 2000; for discussion of these results as well as negative findings, see Brown and Aggleton 2001; Manns and Squire 2001a). In the case of auditory recognition memory, damage limited to the hippocampal region in dogs had no effect on auditory recognition (Kowalska et al. 2001). However, as the authors point out, recognition was evaluated only at relatively short delays (up to 90 sec). It is possible that testing after longer delays or after the presentation of lists of sounds (as in the present study) would have revealed an impairment.
In summary, the present study shows the importance of both the hippocampal region and adjacent cortical structures for auditory recognition memory. These findings thereby strengthen the conclusion that the memory impairment associated with medial temporal lobe amnesia is multimodal. They also extend to the auditory modality the finding that lesions limited to the hippocampal region impair recognition memory.
MATERIALS AND METHODS
Patients
Two groups of patients were studied (Table 1). The first group consisted of three males (E.P., G.P., and G.T.), who developed profound anterograde and retrograde amnesia after herpes simplex encephalitis. They have extensive bilateral temporal lobe lesions as well as variable damage to anterolateral temporal cortex (MTL+). Within the medial temporal lobe, the lesions included, bilaterally, the hippocampal region (CA fields, dentate gyrus, and subiculum), the entorhinal cortex, the perirhinal cortex, the parahippocampal cortex, and the amygdaloid complex (see Figs. 1–3, Schmolck et al. 2000; Stark and Squire 2000).
Table 1.
Characteristics of Amnesic Patients
| Patient
|
Lesion
|
Date of birth
|
WAISIII IQ
|
Years of education
|
WMS-R
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Attention
|
Verbal
|
Visual
|
General
|
Delay
|
|||||
| E.P. | MTL+ | 1922 | 98 | 12 | 94 | 59 | 92 | 68 | 56 |
| G.P. | MTL+ | 1946 | 98 | 16 | 102 | 79 | 62 | 66 | <50 |
| G.T. | MTL+ | 1936 | 84 | 12 | 120 | 57 | <50 | <50 | <50 |
| A.B. | H+ | 1937 | 107 | 20 | 87 | 62 | 75 | 54 | <50 |
| H.C. | H+ | 1961 | 98 | 20 | 96 | 83 | 53 | 68 | 51 |
| L.J. | H+ | 1937 | 101 | 12 | 105 | 83 | 60 | 69 | <50 |
| P.H. | H+ | 1922 | 105 | 19 | 117 | 67 | 83 | 70 | 57 |
| Mean (n = 7) | 98.7 | 15.8 | 103 | 70 | 67.8 | 63.6 | 52 | ||
The WAIS-III and the WMS-R indices yield a mean score of 100 in the normal population with a standard deviation of 15. The WMS-R does not provide scores for individuals who score below 50. Therefore, the six scores below 50 were scored as 50 for calculating the group mean.
WAIS-III, Wechsler Adult Intelligence Scale–III (Wechsler 1981); WMS-R, Wechsler Memory Scale–Revised. MTL+, patients with large medial temporal lobe lesions and variable damage to the anterolateral temporal lobe; H+, patients with damage limited to the hippocampal region and, for H.C. and P.H., some additional damage to parahippocampal gyrus.
The second group (H+) consisted of four patients (A.B., H.C., L.J., and P.H.). The damage in this group involved the hippocampal region and for H.C. and P.H. some additional damage to the parahippocampal gyrus (for more detail, see Manns and Squire 2001b). A.B. became amnesic after an anoxic episode in 1976. He is unable to participate in magnetic resonance imaging (MRI) studies but is presumed to have circumscribed hippocampal damage as a result of this etiology (Cummings et al. 1984; Rempel-Clower et al. 1996). H.C. underwent a right parietal craniotomy to evacuate a right occipital and parietal hematoma after a ruptured arteriovenous malformation. MRI showed reduced size of the hippocampal region (27%) and parahippocampal gyrus (25%), which is thought to have occurred as a result of the ischemia associated with the rupture. L.J., the only female, became amnesic with no known precipitating event during a six-month period beginning in late 1988. Her memory impairment has remained stable since that time. MRI has identified that the hippocampal region is reduced in area 46% bilaterally. P.H. had a six-year premorbid history of 1–2-min attacks (presumably of epileptic origin) in association with gastric symptoms and transient memory impairment. In 1989, he suffered a series of small attacks that resulted in marked and persisting memory impairment. MRI shows a 30% reduction in the size of the hippocampal region bilaterally and a 30% reduction in the size of the parahippocampal gyrus.
Controls
The participants in the control group were employees or volunteers at the San Diego Veterans Affairs Medical Center, or were recruited from the retirement community of the University of California, San Diego or through an ad in the local newspaper. In test 1 (Delays), 10 participants (four males and six females) were matched to the patient groups with respect to age and education (mean age, 65.0 yr; range, 55–76 yr; mean education, 15.3 yr, range, 12–18 yr). In test 2 (Lists), the same 10 participants from test 1 and two additional participants (a total of six males and six females) were matched to both patient groups with respect to age and education (mean age, 64.6; range, 55–76 yr; mean education, 15.4 yr; range, 12–18 yr).
Materials
The materials consisted of 216 sounds, drawn from a large set graciously provided by J. Fritz and M. Mishkin (NIMH). The sounds were synthetic, including single tones, short novel melodies, harmonies, gurgling sounds, and chimes. Each sound was 2–3 sec in length. All participants were seated in front of a laptop computer, and the sounds were presented through a set of headphones. The volume was adjusted individually so that each participant could comfortably hear the sounds.
Procedure
Test 1 (Delays)
The test session consisted of 144 trials. On each trial, participants heard a sound and then heard a second sound 2, 10, or 30 sec later. Half of the time, the second sound had just been presented, and half of the time it was new. The computer screen indicated the phase of the trial, that is, whether the participant should be listening to the first sound, waiting during the delay, or listening to the second sound. When the second sound was presented, the participant pressed one of two keys to indicate whether the sound was the same as or different from the first sound. Across participants, sounds were about equally likely to appear in each delay condition and about equally likely to appear as a target or a foil. The test session consisted of 18 eight-trial blocks. All eight trials within each block were assigned to the same delay condition (2, 10, or 30 sec). Thus, six blocks (48 trials) were assigned to each delay. Blocks of trials at each delay were intermixed such that no two delay conditions were presented consecutively. The experimental session took ∼1 hr.
Test 2 (Lists)
Test 2 was administered in a similar way to test 1, except that participants listened to 10 sounds, separated by a 500-msec interval. After a 5-min delay, testing proceeded with 20 sounds, 10 targets, and 10 foils. For each sound, participants pressed a key to indicate whether the sound had been presented earlier. Controls completed three separate study-test sequences within a single session, for a total of 60 test trials (30 with targets and 30 with foils). The patients completed two sessions and received six study-test sequences for a total of 120 test trials (60 with targets and 60 with foils). Finally, across participants, the sounds were equally likely to appear as a target or a foil. The sounds were taken from the same set of sounds used in test 1. The experimental session took approximately one-half hour.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs, NIMH Grant 24600, the Metropolitan Life Foundation, and DFG (Deutsche Forshungsgeimeinschaft) Grant VO 770/1–1 (H.S.). We thank Joyce Zouzounis for assistance.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL lsquire@ucsd.edu; FAX (858) 552-7457.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.42001.
REFERENCES
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire L, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem. 1999;6:572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–186. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Tomiyasu U, Read S, Benson DF. Amnesia with hippocampal lesions after cardiopulmonary arrest. Neurology. 1984;34:679–681. doi: 10.1212/wnl.34.5.679. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Becker D, Mishkin M, Saunders RC. A comparison of the effects of medial temporal and cortical lesions on auditory recognition memory in the rhesus monkey. Abstr Soc Neurosci. 1999;25:789. [Google Scholar]
- Gaffan D. Recognition impaired and association intact in the memory of monkeys after transection of the fornix. J Comp Physiol Psychol. 1974;86:1100–1109. doi: 10.1037/h0037649. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Squire LR. Intact perceptual memory in the absence of conscious memory. Behav Neurosci. 1997;111:850–854. doi: 10.1037//0735-7044.111.4.850. [DOI] [PubMed] [Google Scholar]
- Kowalska DM, Kusmierek P, Mishkin M. Neither perirhinal/entorhinal nor hippocampal lesions impair short-term auditory recognition memory in dogs. Neuroscience. 2001;104:965–978. doi: 10.1016/s0306-4522(01)00140-3. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR. Impaired recognition memory on the Doors and People Test after damage limited to the hippocampal region. Hippocampus. 1999;9:495–499. doi: 10.1002/(SICI)1098-1063(1999)9:5<495::AID-HIPO2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR. The medial temporal lobe and memory for facts and events. In: Baddeley A, Wilson B, Kopelman M, editors. Handbook of memory disorders. 2nd ed. London: Wiley Press; 2001a. (in press). [Google Scholar]
- ———. 2001b. Perceptual learning, awareness, and the hippocampus. Hippocampus (in press). [DOI] [PubMed]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;12:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Delacour J. An analysis of short-term visual memory in the monkey. J Exp Psychol Anim Behav Process. 1975;4:326–334. doi: 10.1037//0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res. 1984;1:67–83. doi: 10.1016/0166-4328(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Polich J, Squire LR. P300 from amnesic patients with bilateral hippocampal lesions. Electroencephalogr Clin Neurophysiol. 1993;6:408–417. doi: 10.1016/0013-4694(93)90136-j. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: Findings from four new cases. J Neurosci. 1998;10:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson S, Zatorre RJ. Learning and retention of melodic and verbal information after unilateral temporal lobectomy. Neuropsychologia. 1992;30:815–826. doi: 10.1016/0028-3932(92)90085-z. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Fritz JB, Mishkin M. The effects of rhinal cortical lesions on auditory short-term memory (STM) in the rhesus monkey. Abstr Soc Neurosci. 1998;24:1907. [Google Scholar]
- Schmolck H, Stefanacci L, Squire LR. Detection and explanation of sentence ambiguity are unaffected by hippocampal lesions but are impaired by larger temporal lobe lesions. Hippocampus. 2000;10:759–770. doi: 10.1002/1098-1063(2000)10:6<759::AID-HIPO1013>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiat. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Recognition memory and familiarity judgments in severe amnesia: No evidence for a contribution of repetition priming. Behav Neurosci. 2000;114:459–467. doi: 10.1037//0735-7044.114.3.459. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Buffalo BA, Schmolck H, Squire LR. Profound amnesia after damage to the medial temporal lobe: A neuroanatomical and neuropsychological profile of patient E.P. J Neurosci. 2000;20:7024–7036. doi: 10.1523/JNEUROSCI.20-18-07024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the Macaque monkey: Cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci. 1993;6:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR. The medial temporal lobe and the hippocampus. In: In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. New York: Oxford; 2000. pp. 501–520. [Google Scholar]
- Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: Amygdala-hippocampus versus temporal stem. Science. 1982;218:1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]