Abstract
Tetanus with hiatal hernia was diagnosed in a four-month-old female sheepdog pup. The animal was treated with tetanus antitoxin, antibiotics, fluids and intensive nursing care for three weeks and subsequently made a full recovery.
Keywords: Dog, Tetanus, Hiatal hernia
Introduction
Tetanus is a relatively uncommon disease in dogs and cats because of their natural resistance to the toxin; nonetheless, the prognosis has to be very guarded, especially if there are concurrent problems such as hiatal hernia or aspiration pneumonia. Oesophageal hiatal hernia is an uncommon complication of tetanus. The prognosis in these cases appears to be extremely poor although only a few cases have been reported [14,5,15]. In this communication we present a brief review of tetanus in the dog and report on the full recovery of a dog from tetanus and concurrent hiatal hernia.
Review: tetanus in the dog
Animals affected with generalised tetanus have rigidity of the limb, neck and tail muscles and characteristic facial muscle abnormalities (risus sardonicus, trismus, prolapsed third eyelids). Intracranial signs, gastrointestinal signs, respiratory signs and arrhythmias have also been reported. The signs of tetanus are caused by a neurotoxin produced by the vegetative form of Clostridium tetani, an obligate anaerobic, sporeforming Gram-positive bacillus. Resistant spores, commonly found in the environment, will vegetate in response to anaerobic conditions such as in a deep penetrating wound or in teething animals. Three toxins are produced: tetanospasmin, tetanolysin and nonspasmogenic toxin. Tetanospasmin is the most important toxin and is responsible for the principal clinical signs. This toxin inhibits neurotransmitter release at inhibitory interneurons of the spinal cord and brain resulting in muscle hyperextension. Tetanolysin causes haemolysis and enhances the multiplication of anaerobic bacteria by increasing tissue necrosis. The role of nonspasmogenic toxin is poorly understood; it is thought to cause paralysis of the peripheral nervous system [2,8,11].
Following germination of the spores in an anaerobic environment, the toxins are released and tetanospasmin can gain access to the central nervous system by uptake into axonal terminals and retrograde intra-axonal transport. The toxin is also transferred by lymph to the blood stream and it will bind to axon terminals at distant sites. The cranial nerves are commonly affected as the nerve axons are relatively short and the toxin quickly reaches the membranes of presynaptic vesicles where it exerts its toxic action. Dogs and cats have a natural resistance to the toxin. The average incubation period in dogs is five to ten days and signs may be generalised or localised. The localised form is more common in cats than in dogs as cats are more resistant to the toxin [11,8]. The interspecies difference in resistance is related to the relative difficulty for the toxin to penetrate and bind to nervous tissue in different species. The horse is the most susceptible animal, somewhat more so than the human species; poultry are most resistant.
Tetanospasmin has an affinity for the gangliosides in the grey matter of the central nervous system and can cause cerebral signs. It can also act at the level of the neuromuscular junction where it may induce direct neuromuscular facilitation before migration to the central nervous system [8]. Increased sympathetic and parasympathetic activity may result in tachyarrhythmia or bradyarrhythmia [13].
A diagnosis of tetanus is usually based on the clinical signs. In some cases a wound may be detected. Blood samples for complete blood count (CBC) and biochemistry will often be unhelpful although an elevation in creatine kinase may be noted. However, these investigations may help in ruling out other possible causes of muscle rigidity.
Isolation of C. tetani can be attempted but this requires anaerobic conditions and special culture media. Growth is slow and will often take longer than 12 days. If a wound is present, Gram stain may demonstrate typical rods and spherical endospores in a smear [11,8].
Treatment of generalised tetanus consists of intravenous antitoxin, antibiotics, wound debridement, sedation/muscle relaxation, and nursing care (feeding, maintaining hydration, preventing soiling, etc). Untreated cases are usually fatal due to respiratory complications.
Antitoxin neutralizes any free or unbound toxin and should be given as soon as possible after the onset of clinical signs. Intravenous administration of antitoxin is associated with a high prevalence of anaphylaxis. Wheal formation after intradermal injection may indicate the likelihood of a systemic reaction to occur. Intrathecal (intracisternal) antitoxin administration has been recommended in severely affected animals, as the antitoxin does not penetrate the blood-brain barrier. However, due to its potential toxicity in the subarachnoid space, it should be used in severely affected cases only [11,8].
Wound debridement and the use of topical hydrogen peroxide is important to remove necrotic tissue and inhibit further bacterial growth by increasing oxygen tension [2,11].
Antibiotic therapy is indicated to eliminate vegetative C. tetani organisms and prevent further toxin formation. Penicillin has long been thought to be the drug of choice but a study by Ahmadsyah and Salim [1] compared the response in dogs with tetanus to penicillin and to metronidazole. This study revealed that the dogs receiving metronidazole had an improved response to treatment, with a significantly lower mortality rate and a shorter stay in the hospital. Metronidazole is more active against anaerobes and will achieve high concentrations in anaerobic tissues [1].
Muscle relaxants and sedatives are indicated in cases with marked muscle rigidity and hyperexcitability. Diazepam can be used as a muscle relaxant and it can be used when seizures occur. Phenothiazine derivatives (e.g., acepromazine) have a sedative effect and a weak anticholinergic action [11,8].
Supportive nursing care is important as dogs are often hyperthermic, anorexic or dysphagic, hyperexcitable and prone to pressure sores and urine/faecal soiling. Some cases will require tube feeding if dysphagia or regurgitation is severe and even tracheostomy if laryngeal spasms develop. Physiotherapy is important to improve blood supply to and lymphatic drainage from the muscles. It also helps relax spastic muscles and relieve discomfort [7,11,8].
Case report: Concurrent tetanus with hiatal hernia
Case history
A four-month-old female sheepdog was presented with a fiveday history of lethargy, difficulty in eating, and recumbency. She had previously been healthy and had been fully vaccinated and treated for endoparasites. The referring veterinary surgeon reported generalised muscle hypertonia and pyrexia. There had been no improvement after treatment with antibiotics and dexamethasone. The animal was referred to the University Veterinary Hospital for further investigations and treatment.
On physical examination, the dog was alert and in good body condition. She was recumbent, the muscles were contracted with the limbs, neck and tail hyperextended. The ears were erect and facial muscles were contracted with retracted lips, protruding nictitating membrane and enophthalmos (Figure 1). The rectal temperature was 40.1°C. Other vital signs were normal; although panting was observed initially, this stopped after hospitalisation. There were several pressure ulcers on the limbs but a putative causative wound was not found.
Figure 1.
The pup on day 1. She was recumbent, the muscles were contracted with the limbs, neck and tail hyperextended. The ears were erect and facial muscles were contracted with retracted lips, protruding nictitating membrane and enophthalmos.
Case assessment and management
Blood samples were collected on day 1 for complete blood count (CBC) and serum biochemistry profile (Table 1). Significant abnormalities were not found.
Table 1.
Complete blood count and serum biochemistry concentrations at days 1 and 7 (reference range)
| HAEMATOLOGY | DAY 1 | DAY 7 |
|---|---|---|
| PCV | 0.37 L/L (0.37-0.55) | 0.37 |
| Hb | 120 g/L (120-180) | 123 |
| RBC | 5.1 × 1012/L (5.5-8.5) | 5 |
| MCHC | 323 g/L (320-360) | 333 |
| MCV | 73.2 f1 (60-77) | 74.5 |
| MCH | 23.7 rg (19-25) | 24.8 |
| Platelets | 487 × 109/L (200-500) | 989 |
| Plasma proteins | 51.6 g/L (58-75) | NM |
| WBC | 10.9 × 109/L (7-17) | 16.8 |
| Mature neutrophils | 6.1 × 109/L (3.0-11.5) | 11.6 |
| Lymphocytes | 2.8 × 109/L (1.0-4.8) | 2.0 |
| Monocytes | 1.7 × 109/L (0.2-1.3) | 2.1 |
| Eosinophils | 0.2 × 109/L (0-1.3) | 0.0 |
| Basophils | 0 × 109/L (0-0) | 0 |
| BIOCHEMISTRY | ||
| Creatinine | 67 μmol/L (20-130) | NM |
| Urea | 2.4 mmol/L (3.5-8.6) | NM |
| ALT | 27 U/L (5-20) | NM |
| AP | 116 U/L (5-50) | NM |
| Albumin | 25.3 g/L (27-38) | NM |
| Globulin | 26.3 g/L (28-42) | NM |
| The plasma proteins, AP, albumin and globulin are considered within the reference range for a young dog. There is a mild mature neutrophilia on day 7. | ||
| PCV: | packed cell volume; | |
| Hb: | haemoglobin; | |
| RBC: | red blood cells; | |
| MCHC: | mean corpuscular haemoglobin concentration; | |
| MCV: | mean corpuscular volume; | |
| MCH: | mean corpuscular haemoglobin; | |
| WBC: | white blood cells; | |
| ALT: | alanine aminotransferase; | |
| AP: | alkaline phosphatase | |
| NM: | not measured | |
A presumptive diagnosis of tetanus was made based on the characteristic clinical signs. Tetanus antitoxin (Boehring, Intervet) was administered intradermally at a dose of 100 international units (IU). As this did not elicit a cutaneous reaction, the recommended maximum dose of 20,000 IU antitoxin was given by slow intravenous injection.
The dog was hospitalised in a darkened and quiet kennel. Over the next seven days parenteral penicillin G 25 mg/kg (Crystapen, Schering-Plough) intravenously q8 h and fluid therapy (compound sodium lactate at 2 to 4 ml/kg/h, depending on hydration status and presence of hyperthermia) were administered. To control excitability and spasticity, diazepam 0.15 mg/kg q8 h (Valium, Roche) and acepromazine 0.01 mg/kg q4-6 h (ACP, Novartis) were given intravenously.
Nursing care consisted of careful hand-feeding (Hills a/d) to prevent aspiration pneumonia, treatment of decubital ulcers with antibiotic ointment (Fuciderm, Leo), physiotherapy three times daily, and monitoring urination and defecation. Vital signs were monitored every 12 hours and were normal except for short and intermittent episodes of panting, presumably due to excitement and muscle hypertonicity. She was well-hydrated and could take small amounts of food when hand-fed.
The muscle rigidity was still very marked after the first week of hospitalisation. She was unable to rise and could not eat or drink unassisted. Heart rate and rectal temperature were normal but there was permanent tachypnoea with an average respiratory rate of 120 breaths per minute.
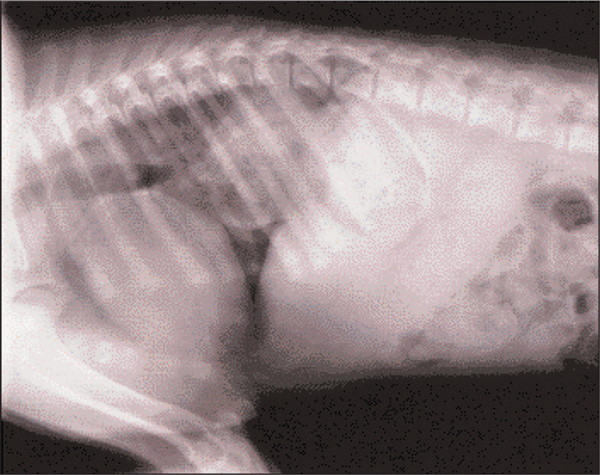
With the difficulties in feeding the dog, aspiration pneumonia was considered a possible diagnosis for the tachypnoea. Blood samples for CBC showed a mild mature neutrophilia (Table 1). Thoracic radiographs revealed hiatal hernia with herniation of the proximal stomach through the oesophageal hiatus of the diaphragm. There was no evidence of aspiration pneumonia (Figure 2).
Figure 2.
Day 7: Lateral thoracic radiography with a soft tissue opacity, which is the proximal stomach, in the caudal thorax.
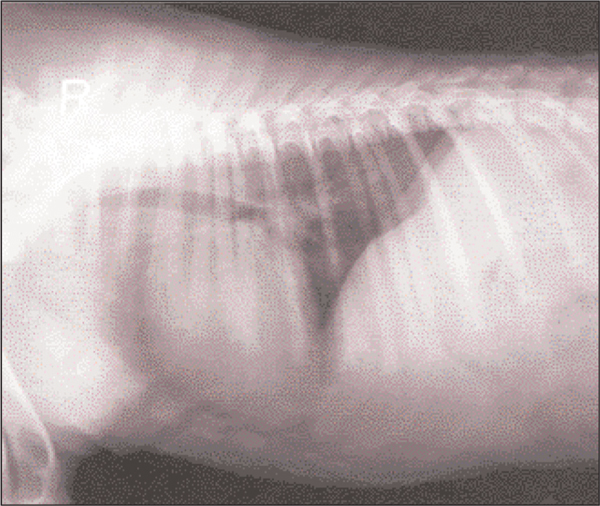
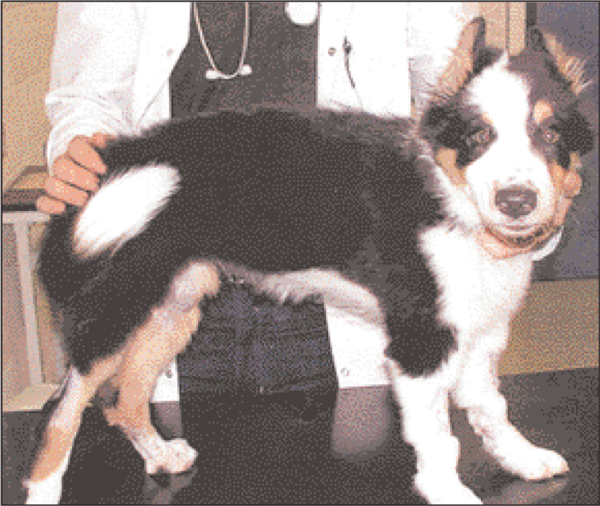
Careful hand-feeding was continued, from an elevated platform to prevent regurgitation and aspiration of food. Also, she was placed with the front limbs and thorax elevated. After a further week there was improvement, she was more relaxed and vital signs returned to normal. Fluid therapy and intravenous antibiotics were continued and combined with metronidazole (Flagyl, May and Baker) per os (20 mg/kg q12 h) when she started to eat unassisted. Vital signs remained normal and the muscle rigidity improved. Radiographs were repeated on day 20 and there were no signs of hiatal hernia (Figure 3). After three weeks of hospitalisation the clinical signs had almost completely resolved. The ears were still erect and there was a mild lameness of the right hind limb (Figure 4). She was discharged and made a full recovery.
Figure 3.
Day 20: Lateral thoracic radiograph with no evidence of hiatal hernia.
Figure 4.
The pup after three weeks of intensive treatment. After three weeks of hospitalisation the clinical signs had almost completely resolved. the ears were still erect and there was a mild lameness of the right hind limb.
Discussion
The clinical signs were typical of generalised tetanus: 'risus sardonicus' with wrinkled forehead, erect ears and retracted lips, protruding nictitating membranes, excessive jaw tone (trismus), enophthalmus and hyperextension of the limbs [3,9,12,10,6]. These signs resolved over a period of three weeks following treatment with the recommended maximum dose of 20,000 IU tetanus antitoxin (which was not repeated as therapeutic concentrations persist for two weeks), penicillin G intravenously at 20 mg/kg q8 h, diazepam and acepromazine. When the dog's appetite improved, metronidazole per os (15 mg/kg q12 h) was administered concurrently with the penicillin.
There have been few reports of hiatal hernia associated with tetanus [14,5,15]. Concurrent megaoesophagus was present in two of the cases [5]. Hiatal hernia is usually congenital but can also occur secondary to trauma, diaphragmatic hernia or increased respiratory effort (e.g., laryngeal paralysis). It is characterised by herniation of the most distal part of the oesophagus or proximal part of the stomach through the diaphragm [4]. In this case the hernia was suspected to have occurred as a complication to tetanus although congenital hiatal hernia could not be ruled out. Thoracic radiography was not performed when the dog was first examined, as there was no suspicion of intrathoracic disease. Many dogs with hiatal hernia are asymptomatic but some show regurgitation due to oesophagitis and reflux of gastric contents. The diagnosis can be confirmed on plain radiography, but in some animals contrast radiography, fluoroscopy and endoscopy are required. Sliding hiatal hernia is the most common form of hiatal hernia, diagnosis can be difficult and a presumed diagnosis can be made based on the demonstration of gastrooesophageal reflux and oesophagitis [4].
In the present case the proximal stomach was herniated through the oesophageal hiatus and the dog showed no signs of oesophagitis. Thoracic radiographs were taken to assess the lungs for evidence of aspiration pneumonia when the dog showed signs of persistent tachypnoea after one week of conservative treatment. Abnormalities other than the hiatal hernia were not found and the tachypnoea may have been caused by decreased lung expansion and discomfort. A diagnosis of congenital, sliding hiatal hernia cannot be eliminated but, as the respiratory problems and radiographic changes resolved after relaxation of the muscle rigidity, the probability is that gastric displacement was secondary to abnormalities of muscle tension associated with tetanus. The mechanism of hiatal hernia and megaoesophagus secondary to tetanus is not clear. Inhibition of neurotransmitter release at the motor endplate of the phrenic nerve may cause relaxation of the oesophageal hiatus. Muscular diaphragmatic spasm could cause stretching of the central tendon in the diaphragm, with concurrent oesophageal shortening which may result in hiatal hernia. There may be decreased oesophageal motor function and oesophageal paralysis by binding of tetanospasmin in the brain stem nucleus ambiguous, resulting in megaoesophagus [5]. Treatment of hiatal hernia consists of administration of cholinergic agonists such as metoclopramide to increase the gastro-oesophageal sphincter tone, administration of antacids, dietary therapy and feeding from an elevated platform. Surgical correction may be indicated if there is no improvement with medical therapy [4]. Medical treatment, tube feeding or surgical correction of the hiatal hernia were not performed in this case, as the presumed underlying cause was tetanus. The prognosis of cases with tetanus is very guarded, especially if there are concurrent problems such as hiatal hernia or aspiration pneumonia. Recovery is slow and it may take several weeks before the animal becomes ambulatory [11,8]. This puppy started to improve after two weeks and made a full recovery within one month.
Acknowledgements
The authors thank the referring practice and all staff at the University Veterinary Hospital involved with management of the case for their help.
References
- Ahmadsyah I, Salim A. Treatment of tetanus: an open study to compare the efficacy of procaine penicillin and metronidazole. British Medical Journal. 1985;291:648–650. doi: 10.1136/bmj.291.6496.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MM, Matros LE. Tetanus. Compendium on Continuing Education for the Practicing Veterinarian. 1982;4:S473–S478. [Google Scholar]
- Bark H. A case report: Canine tetanus. Canine Practice. 1980;7:25–43. [Google Scholar]
- Bright RM, Sackman JE, DeNovo C, Toal C. Hiatal hernia in the dog and cat: A retrospective study of 16 cases. Journal of Small Animal Practice. 1990;31:244–250. doi: 10.1111/j.1748-5827.1990.tb00795.x. [DOI] [Google Scholar]
- Dieringer TM, Wolf AM. Esophageal hiatal hernia and megaoesphagus complicating tetanus in two dogs. Journal of the American Veterinary Medical Association. 1991;199:87–89. [PubMed] [Google Scholar]
- Edwards GT. Tetanus in the dog. Veterinary Record. 1989;125:117. doi: 10.1136/vr.125.5.117-a. [DOI] [PubMed] [Google Scholar]
- Fleming EJ, Hill B. Nursing the patient through canine tetanus. Veterinary Medicine and the Small Animal Veterinary Clinician. 1984;79:1357–1361. [Google Scholar]
- Greene CE. Infectious Diseases of the Dog and Cat. Second. CE Greene. Philadelphia: Saunders; 1998. Tetanus; pp. 267–273. [Google Scholar]
- Hanson CJ. Tetanus in a dog: a case report. Veterinary Record. 1982;110:336–337. doi: 10.1136/vr.110.14.336. [DOI] [PubMed] [Google Scholar]
- Matthews BR, Forbes DC. Case report: Tetanus in a dog. Canadian Veterinary Journal. 1985;26:159–161. [PMC free article] [PubMed] [Google Scholar]
- Merrett DJ. Canine tetanus. Veterinary Annual. 1993;33:209–219. [Google Scholar]
- Merrithew MA, Newsome DJ, Devor R. Tetanus in a four-month-old pup. Veterinary Medicine and the Small Animal Veterinary Clinician. 1983;78:1241–1244. [Google Scholar]
- Panciera DL, Baldwin CJ, Keene BW. Electrocardiographic abnormalities associated with tetanus in two dogs. Journal of the American Veterinary Medical Association. 1988;192:225–227. [PubMed] [Google Scholar]
- van Bree H. Esophageal hiatal hernia and eventration of the diaphragm as a complication in tetanus in three dogs. Veterinary Radiology. 1982;23:83. [Google Scholar]
- van Ham L, van Bree H. Conservative treatment of tetanus associated with hiatus hernia and gastro-oesophageal reflux. Journal of Small Animal Practice. 1992;33:289–294. doi: 10.1111/j.1748-5827.1992.tb01146.x. [DOI] [Google Scholar]