Abstract
Early life experience affects behavior and brain mechanisms. Handling rats during the first three weeks in life can slow age-related cognitive decline (as measured by a hippocampal-dependent spatial learning task) and reduce age-related hippocampal neuron loss. It is not clear, however, whether this early environmental influence on learning is selective for old age or is more general, affecting cognitive development during infancy and young adulthood as well. We briefly exposed neonatal rats to a novel non-home environment for 3 min daily during the first three weeks of life (as a component of the handling method). We found that this brief early environmental manipulation resulted in enhanced hippocampal-dependent learning immediately after weaning and that this learning enhancement persisted into adulthood. These results suggest that subtle early life events can affect cognitive development in all developmental stages and that changes in neural mechanisms other than neuron number are likely to mediate the learning enhancement at multiple developmental stages.
Early life environment is important for the development of cognitive functions and their underlying neural mechanisms (Weiler et al. 1995; Rosenzweig 1996). Neonatal handling, one particular early environmental manipulation, has been shown to enhance learning performance along with other changes in growth and development, emotional reactivity, and stress responses (for review, see Levine 1960; Denenberg 1964, 1970, 1977; Meaney et al. 1991, 1996). The handling method as a means for investigating early environmental influence was studied extensively 30–40 years ago and received critical scrutiny with regard to how robust the handling effects are (Russell 1971; Daly 1973) and how viable it is to attribute specific aspects of this behavioral manipulation to specific changes in behavior (Levine 1957; Smotherman and Bell 1980; Denenberg 1999). In the late 1970s, it became clear that the hippocampus plays a critical role in learning and memory (O'Keefe and Nadel 1978), and the Morris water task was established as the gold standard for evaluating hippocampal-dependent learning (Morris et al. 1982; Sutherland et al. 1982). Early environmental manipulation through the handling procedure was found to lead to retardation of cognitive aging as measured by performance on this hippocampal-dependent task and to cause changes in neural circuitry within the hippocampus, such as reduced neuronal loss during aging (Meaney et al. 1988). It has been suggested that this early environmental modulation of cognitive aging may be mediated by prevention of age-related neuronal loss (Meaney et al. 1988).
We present two series of studies with dual purposes. The primary goal of these experiments was to investigate whether early environmental manipulation can lead to enhancement in hippocampal-dependent learning during early development and young adulthood, long before aging. The secondary goal was to improve the handling design by reducing the number of factors involved in the handling procedure step by step to better relate one particular factor to subsequent changes in learning. Combining a neonatal novelty-exposure procedure with a moving-platform version of the water task (Whishaw 1985) (aversive and spatial learning) and a two-odor-discrimination task (appetitive and nonspatial learning), we found that early environmental manipulations, as subtle as a brief 3-min daily exposure to a novel environment, can enhance hippocampal-dependent memory function. Furthermore, this memory enhancement can be detected during both infancy and adulthood, long before senescence, and is generalized from aversive to appetitive learning and from spatial to nonspatial learning.
RESULTS
We used a one-trial learning score as a measure for learning in the water task. As this measure is sensitive to trial-to-trial variability, we averaged one-trial learning scores for the initial and the last three days of a seven-day initial training to reduce the variability. We measured odor discrimination learning by computing a daily percentage of correct trials and the amount of forgetting due to the six-day delay (percent correct on the last training day minus percent correct on the day of retest). We measured stress response or emotional reactivity by computing the average freeze duration and average distance traveled across all eight trials. As there was no significant litter or gender effect, data were pooled across litter and gender. Two-sample t-tests were performed on the above scores. Based on the literature, we expected better learning and reduced emotional reactivity in the experimental rats. Therefore, directional statistical tests were performed.
Modified Neonatal Handling
For rats that experienced the modified neonatal handling during the first three weeks of life, we report data from two tasks.
Open Field Task/P22
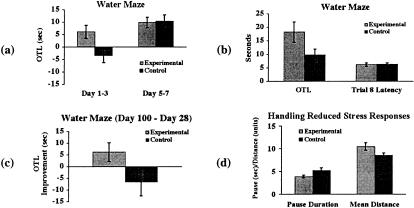
Immediately after weaning, on postnatal day 22 (P22), the experimental (E) group showed significantly shorter pause/freeze duration and traveled greater distances in the open field compared to the control (C) group (freeze duration: t = 2.125, P ≤ 0.025; distance traveled: t = 1.939, P < 0.05, Fig. 1d).
Figure 1.
Modified handling procedure enhanced spatial memory during infancy and adulthood—removal of maternal separation as a confounding factor (N = 40, results expressed as mean±SEM). (a) Left: One-trial learning (OTL: trial 1 latency − trial 2 latency) in the experimental (E) rats was significantly greater than that in the control (C) rats during the first three days of training. Right: Motor, sensory, and motivational factors were ruled out by similar performances by the E and C groups after overtraining (last three days). (b) Left: Handling significantly increased one-trial learning on a single day retest at ∼100 d of age (new platform location). Right: Motor, sensory, and motivational confounding factors were ruled out by the similar performance on the last trial (trial 8) performance on retest. (c) E rats showed a significantly greater increase in one-trial learning from infancy to adulthood compared to the C rats. (d) Handling significantly decreased the initial freeze duration and increased the distance traveled.
Water Task/P22–28
During the first three days of training, the one-trial learning scores of the experimental (E) rats were significantly greater than those of the control (C) rats (t = 2.481, P < 0.01, Fig. 1a). As training proceeded, both groups were able to reach the same level of performance at the end of the seven-day initial training, indicated by a lack of significant difference in one-trial learning scores based on an average of the last three days of training between the E and C groups (Fig. 1a). This similar level of performance after overtraining serves as a control to rule out sensory, motor, or motivational factors as confounds.
Water Task/P100 Retest
Upon entering adulthood (∼100 days of age), the E group continued to show greater one-trial learning on the single day of retest (t = 1.848, P < 0.05, Fig. 1b). As learning continued across trials on this single day of retest, both groups of rats were able to reach the same level of performance on trial 8, as there was no significant difference on the last trial swim latency (trial 8 latency) between the two groups (Fig. 1b). This lack of difference after overtraining indicates that the difference in one-trial learning scores on the retest day was not due to differences in sensory, motor, or motivational functions. We also computed an improvement score for one-trial learning from infancy to adulthood—the difference between the scores at ∼100 days (retest) and at four weeks of age (last day of the initial training). Although there was no difference in swim latency at the end of the seven-day initial learning, the E group showed significantly greater improvement compared to the C group over the two-month delay (t = 1.834, P < 0.05, Fig. 1c). Whereas one-trial learning in the E rats increased over the intervening 12 weeks, one-trial learning in the C rats decreased.
Although one-trial learning scores could differ because of a difference in the first trial latencies, we found no significant differences in the first trial latency between the E and C rats. The behavior of the animals during trial 1 did not appear to be influenced by the previous day's platform location, as they did not head for the quadrant in which the hidden platform was located on the previous day more than chance would predict. The swim paths taken by the two groups do not show the drastic contrast between normal and hippocampal-lesioned rats.
Neonatal Novelty Procedure
For rats that experienced the neonatal novelty exposure during the first three weeks of life, we report data from three tasks.
Water Task/P22–28
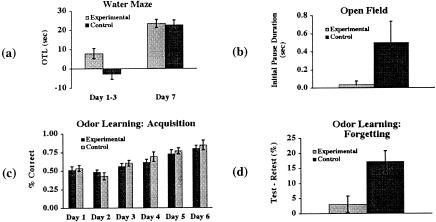
In 26 rats, we found results similar to those from the modified handling study. During the first three days of training, one-trial learning scores of the novelty-exposed (E) rats were significantly greater than those of the control (C) rats (t = 2.152, P < 0.02, Fig. 2a). The two groups were able to reach the same level of performance at the end of the seven-day training, indicated by a lack of significant difference in one-trial learning score between the E and C groups based on an average of the last three days of training (Fig. 2a). This lack of difference after overtraining rules out differences in sensory, motor, and motivation functions as possible confounding factors.
Figure 2.
Novelty exposure enhanced spatial and nonspatial memory and reduced emotional reactivity—further removal of experimenter handling as a confounding factor (all data are shown as mean±SEM). (a) Left: The experimental (E) group showed a significantly greater one-trial learning (OTL: trial 1 latency − trial 2 latency) compared to the control (C) group (average of the first three days). Right: Motor, sensory, and motivational confounding factors were ruled out by a lack of difference in performance after extended training (N = 26). (b) The E group showed significantly shorter initial freeze duration compared to the C group (N = 52); (c, d) The E group did not differ significantly from the C group during the acquisition but showed significantly less forgetting than the C group after a six-day delay (N = 26).
Open Field Task/P42
For the remaining 52 rats that experienced the neonatal novelty exposure, the E group again showed a significantly shorter freeze duration compared to the control group at six weeks of age (t = 1.923, P < 0.03, Fig. 2b) but did not differ from the controls in the distance traveled (data not shown).
Odor Task/P180–192
At six months of age, 26 of the 52 rats were further tested on the odor discrimination task. Both E and C animals acquired the task within a week, reaching a criterion of 85% correct responses. During this initial acquisition period, there was no significant difference between the E and C groups (Fig. 2c), indicating that both groups were capable of discriminating between the two odors. In contrast, after a six-day delay between this initial training and a retest, the experimental group showed a greater retention (less forgetting) at the retest, measured as a smaller reduction in the percentage of correct responses from the last day of the initial training to the retest (Fig. 2d, t = 3.934, P < 0.006).
DISCUSSION
Through two series of experiments and multiple replications involving 118 animals, we showed that simple, brief, and transient neonatal environmental manipulations can lead to enhancement in learning and memory. We introduced several innovations to the classic neonatal handling manipulation, including a split-litter design procedure, the use of a moving platform version of the Morris water task with a one-trial learning measure, and an odor-discrimination task designed for in-home (cage) testing. We were able to demonstrate cognitive enhancement as a result of early life environmental manipulation and to address a number of issues concerning the nature, properties, and mechanisms of this enhancement. These issues include: whether brief and transient neonatal novelty exposures (one of the four components in the handling method) are sufficient for inducing longlasting cognitive enhancement; whether the component of maternal separation in the original handling study is necessary for cognitive enhancement; whether the component of experimenter handling per se is necessary for cognitive enhancement; whether this enhancement is limited to senescence (if not, how early the enhancement can be detected; if detectable during infancy, whether the early enhancement can persist into adulthood); whether age-related neuronal death is the likely mediating mechanism; whether this enhancement is limited to spatial tasks; whether this enhancement is limited to aversive tasks; whether this enhancement is due to simple priming by prior experimenter touch; and whether this enhancement is necessarily expressed in the acquisition of the task.
Subtle Early Environmental Manipulation Enhanced Memory Function During Both Infancy and Adulthood
Previous studies of neonatal handling and hippocampal-dependent learning revealed a relationship between early life stimulation and learning differences during aging (Meaney et al. 1988). It remained an open question whether neonatal stimulation selectively affects cognitive aging or affects learning during both early development and aging. By using the moving platform version of the Morris water task and a one-trial learning measure, we were able to detect subtle learning enhancement very early during development (immediately after weaning) following the modified neonatal handling procedure (Fig. 1a). We were able to replicate this finding with even subtler environmental manipulation using the neonatal novelty exposure procedure (Fig. 2a). These results suggest that early life environmental manipulation can result in enhanced hippocampal-dependent learning not only during aging but during infancy as well. Our observed significant difference in one-trial learning from the water task retest conducted at adulthood (100 days of age) (Fig. 1b) further indicates that the early life stimulation-induced learning enhancement also persists into adulthood. This long-lasting memory enhancement was further replicated by the enhanced memory retention of odor-discrimination measured at adulthood (six months of age) (Fig. 2d). Together, these findings significantly broaden the window of impact that early life environment can have on cognitive development. The finding that subtle early life environmental manipulation is capable of affecting cognitive development throughout life (as opposed to only during aging) has implications for the design of educational and social programs that aim to facilitate cognitive development in children.
Mediating Mechanisms: Difference in Neuronal Death Versus Difference in Receptor Sensitivity
As previous neonatal handling studies revealed no difference in hippocampal neuron number before senescence between neonatally stimulated and nonstimulated rats (Meaney et al. 1988), and as the neonatal stimulation procedures described herein resulted in enhanced hippocampal-dependent learning throughout early development and adulthood, it is clear that change in hippocampal neuron number is not necessary for enhancement in hippocampal-dependent learning. Partial hippocampal lesion studies showed that removal of 40% of the hippocampal tissue did not affect new learning, nor did it affect a task that was acquired postoperatively (Moser and Moser 1998). This finding further indicates that change in neuron number is unlikely to mediate the neonatal stimulation-induced enhancement we observed in hippocampal-dependent learning during early development. In fact, evidence of an alternative mechanism, that is, an increase in glucocorticoid receptor (GR) binding in handled young and adult rats was found in the same neonatal handling study (Meaney et al. 1988). This difference in GR binding between neonatally stimulated and nonstimulated rats would predict that the stress hormone corticosterone could exert a differential influence on neuronal excitability and plasticity as well as on hippocampal-dependent learning through a difference in GR receptor sensitivity (Dekloet et al. 1999). In agreement with this notion, by performing separate in vitro electrophysiological experiments, we found that corticosterone has a greater modulatory effect on synaptic transmission and plasticity in the CA1 region of the hippocampus in novelty-exposed rats (Zou et al. 2001). Coupled with this change in GR sensitivity, we also found that long-term potentiation (LTP) was enhanced in novelty-exposed rats (7 and 14 mo of age) (Tang and Zou 2001). Together, the above findings suggest that changes in basic cellular mechanisms that mediate and modulate neural plasticity offer an alternative, more parsimonious explanation for both the early enhancement in hippocampal-dependent learning observed in the present study and the retardation of cognitive aging reported in the Meaney et al. (1988) study.
Implications of Methodological Improvement
We have departed from the handling method, an early environmental manipulation, which had over four decades of history (Levine 1957; Denenberg 1964). As multiple factors are involved in the handling method, including maternal separation, maternal stress, experimenter handing, and novelty exposure, one can only conclude from the past handling studies that the cause of any learning enhancement could be one of the four or any combination of these four components. It was impossible to determine which of the four components is sufficient for producing the handling effects. In our first set of experiments, by adopting a split-litter design, we were able to remove maternal disturbance and separation as confounding factors because both groups were separated from the dams. Our results showed that neonatal handling in the absence of maternal separation and maternal stress as confounding factors was sufficient to produce an enhancement in water task learning (Fig. 1a). In the second set of experiments, by further matching the amount of tactile stimulation by the experimenter between the experimental and control rats, we were able to further remove experimenter handling per se as a confounding factor. We replicated the early learning enhancement in the water task (Fig. 2a) and the persistence of memory enhancement into adulthood in the retention of odor discrimination task (Fig. 2d). These results provide direct evidence that novelty exposure is sufficient to produce the observed memory enhancement and that neither maternal separation and maternal stress nor handling per se is necessary for memory enhancement. It is important to point out that our results do not preclude the contribution of maternal care. Neonatal novelty exposure should be considered a triggering event that not only provides direct stimulation to pups early in life, but also initiates a chain of events in the subsequent pup-to-pup and dam-to-pup interactions (Liu et al. 1997; Denenberg 1999; Francis et al. 1999; Liu et al. 2000) that can further interact with the initial treatment effects.
Neonatal Novelty Exposure Enhanced Episodic Memory
The standard fixed hidden platform task requires the rat to remember a single platform location that is invariant across days. Therefore, this task measures reference memory, which is relatively stable over a long period of time and can be viewed as general knowledge. In contrast, the moving platform version of the water task requires the rat to learn a new platform location each day. The rat must remember where the hidden platform was located on that given day and distinguish it from platform locations on the previous days of training. The one-trial learning score—the reduction in swim latency—measures how much information the animal has acquired through a single-trial exposure to the spatial location of the hidden platform. Therefore, the one-trial learning score from a moving platform version of the Morris water task measures episodic memory–memory for a specific episode of past experience. Our finding that one-trial learning differed significantly between the experimental and control groups indicates that neonatal novelty exposure enhanced not only hippocampal-dependent learning in general but specifically improved episodic memory (Figs. 1a and 2a).
An Aversively Motivated Task is Not Necessary for the Expression of Memory Enhancement
Because it involves food as rewards, the odor discrimination task is an appetitive task, in contrast to aversive tasks (e.g., escape to terminate a shock, or swim to find the platform to escape the cold water). We found no differences in acquisition of the odor discrimination task between the E and C groups (Fig. 2c). Similarly, several early handling studies also showed a lack of effect on the acquisition of appetitive learning (Griffiths and Stringer 1952; Hymovitch 1952; Schaefer 1963; Salama and Hunt 1964; Wong 1966). In contrast, the effects of neonatal handling on aversive learning have always been more readily demonstrated than those on the appetitive learning in the past handling literature (Daly 1973). Based on these early studies, it may appear that aversive tasks are necessary for the expression of the neonatal stimulation-induced memory enhancement. However, by further evaluating the animals beyond the acquisition phase, we were able to show that odor memory retention was enhanced by the neonatal novelty manipulation (Fig. 2d). This finding clearly indicates that aversive tasks are not necessary for the expression of memory enhancement.
Neonatal Stimulation Resulted in Reduced Fear Responses
Reduced fear responses in the open field task among handled rats has been considered the behavioral hallmark of neonatal handling (Denenberg 1964, 1999). We used the open field task as a comparison task to ensure that the modified handling and neonatal novelty procedure result in changes that are qualitatively similar to those induced by the classical handling procedure. Open field freeze duration was reduced as a result of both early environmental manipulations (i.e., the modified handling and the neonatal novelty procedures) (Figs. 1d and 2b). These observations are consistent with the handling literature, suggesting that the effects of our neonatal stimulation procedures are similar to those induced by the handling method, in that both methods resulted in reduced fear responses to novelty. The freeze durations observed among the neonatal novelty exposed rats seemed to be much shorter (Fig. 2b). This is most likely due to the differences between the ages at testing (modified handling: immediate after weaning; neonatal novelty exposure: 6 weeks of age). The second open field test (Fig. 2b) was performed two weeks after weaning instead of on the first day of weaning, when the animals were likely to be more fearful.
Memory Enhancement is Not Due to Habituation of a Specific Fear
It has been suggested that the effect of neonatal stimulation is merely a specific habituation of fearful reactions to human handling or human presence (Ader 1965; Goldman 1965; Abel 1971). The reduction in this specific fear can in turn lead to other changes in various task performances. In nearly all of the past neonatal handling studies and in our first set of experiments, the experimental group received more human contact than the control group. It would be a relatively trivial finding to note that prior experience of being handled by a human experimenter reduces the stress of being handled during subsequent learning tasks. The memory enhancement observed even when experimenter handling was completely matched between the experimental and control groups (Figs. 2a, d) suggests that a reduction of specific fear in response to experimenter handling or presence cannot be an adequate explanation for the observed learning enhancement.
Conclusions
We found that simple and brief environmental manipulations can enhance hippocampal-dependent spatial learning throughout life, from as early as immediately after weaning to adulthood, thus extending the time window during which early life environmental manipulation can have its impact on cognitive function. We contend that this learning enhancement is more parsimoniously explained by a change in the basic cellular mechanisms that mediate and modulate neural excitability and plasticity than by a change in neuron number and that maternal separation, maternal stress, and experimenter handling per se are not necessary for learning enhancement. We show that the memory enhancement is not limited to spatial learning and aversive learning (water task), but includes nonspatial and appetitive learning (odor discrimination) as well. We contend that this learning and memory enhancement is not due to specific priming by experimenter handling. This enhancement can be expressed in nonaversive appetitive learning tasks and in the retention but not necessarily in the acquisition of the tasks.
These findings and interpretations broaden the impact of neonatal exposure to a novel environment in several important dimensions, including developmental stages (early development and adulthood versus senescence), types of mediating mechanisms (change in neuron number versus change in receptor-mediated dynamic modulation of synaptic transmission and plasticity), and types of learning and memory tasks (aversive versus appetitive). Although the present findings show that neonatal novelty exposure is sufficient for enhancing hippocampal-dependent learning, the contribution of postmanipulation maternal behavior should not be ruled out. On the contrary, we believe that both the stimulating effects from novelty exposure and the subsequent effects of maternal care (Francis et al. 1999; Liu et al. 2000) on the stress responses system can synergetically program the stress response system early in life (Dallman 2000), and that this early programming can have a broad impact on multiple types of memory processing.
Exposing animals to an enriched environment has also been shown to enhance hippocampal-dependent learning (van Praag et al. 1999; Duffy et al. 2001). In comparison to the enrichment method, which usually takes several weeks of continuous exposure (Rosenzweig 1996), the neonatal handling method and our neonatal novelty exposure are early life environmental manipulations that are brief, requiring only a few minutes per day, and transient, applied typically during the first few weeks of life. Yet, they produce changes that persist long after the initial environmental manipulation, in both behavior and neural mechanisms. In comparison to genetic manipulations that have been shown to enhance learning and memory at one particular point during development (Tang et al. 1999), the neonatal novelty exposure offers a simple behavioral method that results in long-term learning and memory enhancement from early development to adulthood.
MATERIALS AND METHODS
Animals
Eighteen pregnant Long-Evans hooded rats arrived in the Department of Psychology vivarium 7–11 days prior to giving birth. The litters were culled to maintain a litter size of approximately eight (range 5–9 pups). A total of 118 pups (six females) born of the these dams were used. Pups were housed with the dams until weaning at postnatal day 21. The dams and postweaning pups were housed separately in translucent plastic cages (51 cm × 25cm × 22 cm) with a 7am–7pm light/dark cycle and water and food ad libitum.
Split-Litter Design
A split-litter design starting on postnatal day 1 was not commonly used in prior neonatal handling studies because it requires daily identification of the experimental (E) and control (C) pups from birth until weaning. In place of ear-marking, we applied a toe-marking procedure using the hindpaws to indicate the pups' group identity on postnatal day 1. To avoid interference with a lateralized effect (Denenberg et al. 1986; Tang and Verstynen 2001; Verstynen et al. 2001), we marked both the left and right hindpaws, with the E and C pups indicated by different digit combinations (E, left little finger and right thumb; C, right little finger and left thumb). The combinations were counterbalanced between the E and C groups. This procedure did not seem to produce any noticeable behavioral deficits or any prolonged distress.
Neonatal Environmental Manipulation
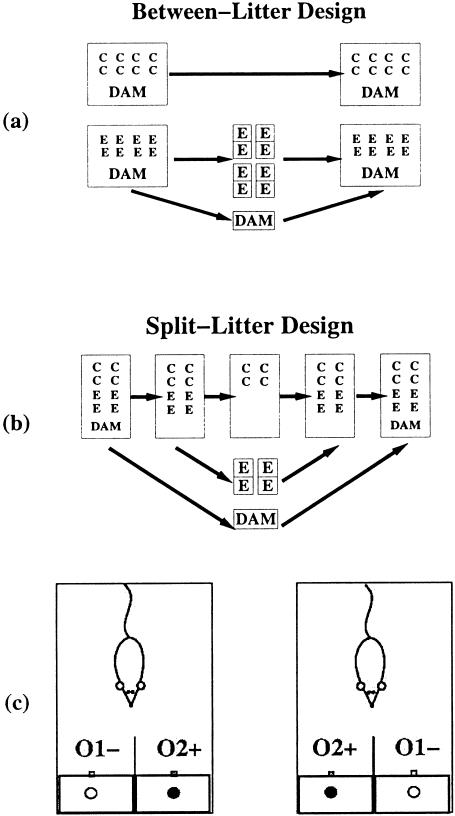
As a result of a between-litter design, the handling method (Levine 1957) actually involves multiple factors between the treatment and control groups (Fig. 3a): (1) disturbance to the dams of the handled litters; (2) separation of the handled pups from the dam; (3) experience of handling by the experimenter; and (4) exposure to a novel environment. This handling procedure, therefore, is not only handling, but handling plus maternal disturbance, maternal separation, and novelty exposure. In designing our neonatal manipulation, we aimed to reduce the number of factors involved in this classic “handling” procedure.
Figure 3.
Neonatal stimulation procedures. (a) The classical handling procedure with a between-litter design introduces four behavioral differences between the handled (E) and nonhandled (C) pups: (1) maternal separation of the E pups, (2) maternal stress due to separation, (3) experimenter handling of the E pups during transfer to individual cages, and (4) novelty exposure of the E pups in the individual non-home cage. (b) The modified handling procedure and novelty exposure procedure. In both procedures, a split-litter design was used to remove the maternal separation and maternal stress as confounding factors (both E and C groups were separated from the mother). In the modified handling procedure, E pups were exposed to the novel individual cage and were hand-brushed (longer experimenter handling). In the novelty exposure procedure, the effect of novelty exposure was further isolated from the experimenter handling effect by removing the hand-brushing and by matching each touch of the E pups with a touch of the C pups. (c) Odor discrimination learning setup. The odor port consisted of two sponges separated by a piece of Plexiglas, with two odor tubes (O+ and O−) plugged in each sponge, facing the back of the cage, from which the rat approached the sponges. On the top of each sponge, a small 1.5 cm × 1.5 cm square well was cut ∼1 cm deep where the food reward could be hidden from the animal's sight as it approached the sponge. Both wells were speckled with crushed powdered FrootLoop to prevent the odor of FrootLoop from becoming a predictor for where the one-half FrootLoop was placed. Only the well on the side of O+ contained a piece of FrootLoop hidden from the rat's sight (filled circles). The relative locations of the food and odor were such that the animal had to first experience the odor stimulus before it could see or obtain the food reward. The location of O+ and the reward appeared on the left or right randomly and counter-balanced.
In the first series of experiments, we equalized the amount of maternal disturbance and maternal separation between the handled and nonhandled animals by using the above described split-litter design (Fig. 3b), in which both the experimental pups and the control pups were separated from the dam. This modified neonatal handling procedure allows the isolation of the combined effects of novelty exposure and experimenter handling. Because removing the between-group differences in maternal separation and maternal disturbance represents a rather dramatic reduction in the differential treatment of the experimental and control pups, we augmented experimenter handling manipulation to increase our chance of detecting the treatment effect by adding a 1-min hand-brushing with a soft paintbrush to the handling manipulation (which we removed in the second series of experiments).
In the second series of experiments, we further equalized the amount of experimenter handling by removing the hand-brushing and by matching each experimenter–pup contact between the experimental and control pups. This matching is accomplished by picking up a matching control pup and replacing it at its original location within the home cage every time an experimental pup is picked up during the transfer to and from the novel non-home cage. This ensured that the handling of the pups per se would not be a cause of between-group differences. The only treatment difference between the experimental and control pups is the exposure to the novel cage. This neonatal novelty procedure allows the isolation of the effect due to novelty exposure.
Morris Water Task With a Moving Platform
Past neonatal handling studies did not detect any learning difference before senescence in the standard fixed platform version of the Morris water task using a learning measure based on daily average swim latencies (Meaney et al. 1988). Two possible explanations are that the standard Morris water task is too easy and/or the average performance measure was not sufficiently sensitive to distinguish two groups of normal rats without gross brain lesions. To increase the task difficulty, the water task we used required the rats to learn a new platform location each day (Whishaw 1985). The platform location was randomly selected from one of the four quadrants and six distances from the center of the pool, without repetition. The swimming pool, 120 cm in diameter and 52 cm in depth, was filled with tap water (22°C) mixed with one cup of powdered milk. Several conspicuous distal cues were available. On each day, single rats entered the pool from four different locations (N, S, E, W) in a pseudorandom sequence in two blocks of four trials. Once the rat reached the platform, or was placed on the platform by the experimenter after failing to find the platform within 60 sec, the rat was allowed to stay on the platform for 10 sec. To increase the sensitivity of the testing instrument, we adopted a one-trial learning measure (Whishaw 1985), defined as the swim latency difference between the first two trials (T1–T2). Since new platform locations were used daily, the one-trial learning score measured how much the rat learned after a single-trial exposure to the spatial location of the platform.
Odor Discrimination Task
Four days before the odor discrimination training, animals were put on food restriction and their weights were brought to and maintained at 90–95% of their baseline weights. All rats were preexposed to the two odors (peppermint (O+) and jasmine (O−)) and the food reward (FrootLoops breakfast cereal) prior to training. During training, animals were tested on eight trials daily for seven days. After a six-day delay, a retention test was performed on the 14th day. Trials were conducted in the animal's home cage, involving no direct experimenter–animal contact. At the beginning of each trial, the experimenter placed a small, custom-designed odor apparatus into the front of the animal's home cage (Fig. 3c). O+ was always paired with the presence of a FrootLoop, and O− was always paired with the absence of the FrootLoop. Except on the first trial, in which two minutes were allowed, a 1-min approach latency was allowed in each trial before the trial was terminated. When the animal removed the reward from the odor apparatus and began to consume it, the trial was considered successful. If the animal approached and touched the sponge on the side where O− was placed, the trial was immediately terminated. At the end of each day's test, both sponges were removed from the odor port (Fig. 3c) and washed with hot water and baking soda to remove odors left by the animals. Because this task involves simultaneous discrimination between two odors, it can be viewed as a hippocampal-dependent task (Eichenbaum et al. 1989).
Open Field Task
Rats were tested in eight 20-sec trials in the open field apparatus, a square cardboard box (60 cm × 60 cm × 20 cm). In each trial, the rat was placed in the center of the box and under a small cardboard cover. At the beginning of the trial, the cover was lifted and the animal was free to move about. At the end of 20 sec, the experimenter returned the animal to its home cage to wait for the next trial. To minimize interference with the animal's ongoing behavior, the experimenter remained at the same location in the room throughout the testing.
Procedures
Two series of experiments were performed using two groups of rats: The first group experienced the modified neonatal handling procedure, and the second group experienced the neonatal novelty exposure procedure.
Forty rats were used in the first set of experiments in which the modified neonatal handling procedure was carried out daily from postnatal day 1 to day 21 (P1–P21). The open field task was performed on P22 to measure handling-induced differences in stress response or emotional reactivity. The water task was performed from P22 to P28 to evaluate the effect of modified handling on learning during early development. The water task retest was performed when the animals entered adulthood, at approximately P100, to evaluate whether the effect observed during infancy persists into adulthood.
Seventy-eight rats participated in the second series of experiments, in which the novelty exposure procedure was carried out daily from P1 to P21 to further isolate the effect of neonatal novelty exposure on learning and emotional reactivity. Twenty-six of these rats were tested on the water task during the period from P 22 to P28 (to replicate the enhanced learning effect during infancy observed in the first series of experiments). Fifty-two of the 78 rats were tested on the open field task at six weeks of age (P42) to replicate the reduced emotionality and stress responses observed in the first series of experiments. Twenty-six of these 52 rats were further tested at six months of age (P180–194) on the odor discrimination task to extend the impact of the neonatal manipulation from spatial to nonspatial learning, and from aversive learning to appetitive learning, and to replicate the long-lasting effect on adult memory function.
Acknowledgments
I thank L. Alvarado, J. Proietti, L. Zamora, T. Urbanki, T. Verstynen, and J. Jones for their assistance, V. Denenberg, C. Stevens, R. Sutherland, and M. Dallman for discussions, and G. Cowan (Santa Fe Institute) for his generous gift.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL akaysha@unm.edu.; FAX (505) 277-4946.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.43101.
REFERENCES
- Abel E. Habituation as a factor in early handling. J Comp Physiol Psychol. 1971;74:219–221. doi: 10.1037/h0030337. [DOI] [PubMed] [Google Scholar]
- Ader R. Effects of early experience and differential housing on behavior and susceptibility to gastric erosions in the rat. J Comp Physiol Psychol. 1965;60:233–238. doi: 10.1037/h0022313. [DOI] [PubMed] [Google Scholar]
- Dallman M. Moments in time: The neonatal rat hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141:1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- Daly M. Early stimulation of rodents: A critical review of present interpretations. Br J Psychol. 1973;64:435–460. doi: 10.1111/j.2044-8295.1973.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Dekloet E, Oitzl M, Joels M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Denenberg V. Critical periods, stimulus input, and emotional reactivity: A theory of infantile stimulation. Psychol Rev. 1964;71:335–351. doi: 10.1037/h0042567. [DOI] [PubMed] [Google Scholar]
- ————— . Experimental programming of life histories and the creation of individual differences: A review. In: Jones M, editor. Miami symposium on the prediction of behavior, 1968: Effects of early experience. University of Miami Press; 1970. , Coral Gables. [Google Scholar]
- ————— . Assessing the effects of early experience. In: Myers R, editor. Methods in psychobiology. 1977. , III: 127–147. Academic Press. [Google Scholar]
- ————— Hemispheric laterality in animals and the effects of early experience. Behav Brain Sci. 1981;4:1–21. [Google Scholar]
- ————— Commentary: Is maternal stimulation the mediator of the handling effect in infancy? Dev Psychobiol. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Denenberg V, Garbanati J, Sherman G, Yutzey D, Kaplan R. Infantile stimulation induces brain lateralization in rats. Science. 1978;201:1150–1152. doi: 10.1126/science.684436. [DOI] [PubMed] [Google Scholar]
- Duffy S, Craddock K, Abel T, Nguyen P. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Mathews P, Cohen N. Further studies of hippocampal representation during odor discrimination-learning. Behav Neurosci. 1989;103:1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- Francis D, Champagne F, Liu D, Meaney M. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann NY Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Goldman P. Conditioned emotionality in the rat as a function of stress in infancy. Anim Behav. 1965;13:434–442. doi: 10.1016/0003-3472(65)90103-x. [DOI] [PubMed] [Google Scholar]
- Griffiths W, Stringer W. The effects of intense stimulation experienced during infancy on adult behavior in the rat. J Comp Physiol Psychol. 1952;45:301–306. doi: 10.1037/h0054146. [DOI] [PubMed] [Google Scholar]
- Hymovitch B. The effects of experimental variations on problem solving in the rat. J Comp Physiol Psychol. 1952;45:313–321. doi: 10.1037/h0061535. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Stimulation in infancy. Sci Am. 1960;202:80–86. [PubMed] [Google Scholar]
- Liu D, Diorio J, Day J, Francis DD, Meaney M. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky P, Meaney M. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Meaney M, Aiken D, Bhatnager S, Vanberkel C, Sapolsky R. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–769. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Meaney M, Diorio J, Francis D, Widdowson J, Laplante P, Caldji C, Sharma S, Seckl J, Plotsky P. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meaney M, Mitchell J, Aitken D, Bhatnagar S, Bodnoff S, Iny L, Sarrieau A. The effects of neonatal handling on the development of the adrenocortical-response to stress : Implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Morris R, Garrud G, Rawlings J, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser M, Moser E. Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford, England: Oxford Press; 1978. [Google Scholar]
- Rosenzweig M. Aspects of the search for neural mechanisms of memory. Annu Rev Psychol. 1996;47:1–32. doi: 10.1146/annurev.psych.47.1.1. [DOI] [PubMed] [Google Scholar]
- Russell P. Infantile stimulation in rodents a consideration of possible mechanisms. Psychol Bull. 1971;75:192–202. [Google Scholar]
- Salama, Hunt JM. Fixation in the rat as a function of infantile shocking, handling, and gentling. J Genet Psychol. 1964;105:131–162. doi: 10.1080/00221325.1964.10533653. [DOI] [PubMed] [Google Scholar]
- Smotherman W, Bell R. Maternal influences and early behavior. New York: Spectrum Publications; 1980. Maternal mediation of early experience; pp. 282–296. [Google Scholar]
- Sutherland R, Kolb B, Whishaw I. Spatial mapping: Definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Schaefer JT. Early experience and its effects on later behavioral processes in rats. II: A critical factor in the early handling phenomenon. Trans NY Acad Sci. 1963;25:871–889. doi: 10.1111/j.2164-0947.1963.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Tang, A.C. and Verstynen, T. 2001. Early life exposure to a novel environment modulates “handedness” in rats. Behav. Brain Res. In press. [DOI] [PubMed]
- Tang, A.C. and Zou, B.D. 2001. Neonatal exposure to novel environment enhances long-term potentiation in CA1 of the rat hippocampus. (in press) [DOI] [PubMed]
- Tang Y, Shimizu E, Dube G, Rampon C, Kerchner G, Zhuo M, Liu G, Tsien J. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie B, Sejnowski T, Gage F. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen, T., Tierney, R., Urbanski, T., and Tang, A.C. 2001. Neonatal novelty exposure modulates hippocampal volumetric asymmetry in the rat. Neuroreport. (in press) [DOI] [PubMed]
- Weiler I, Hawrylak N, Greenough W. Morphogenesis in memory formation: Synaptic and cellular mechanisms. Behav Brain Res. 1995;66:1–6. doi: 10.1016/0166-4328(94)00116-w. [DOI] [PubMed] [Google Scholar]
- Whishaw I. Formation of a place learning-set by the rat: A new paradigm for neurobehavioral studies. Physiol Behav. 1985;35:139–143. doi: 10.1016/0031-9384(85)90186-6. [DOI] [PubMed] [Google Scholar]
- Wong R. Infantile handling and performance in the T-maze. Psychol Sci. 1966;5:203–204. [Google Scholar]
- Zou, B.D., Golarai, G., Connor, J.A., and Tang, A. 2001. Neonatal exposure to a novel environment enhances the effects of corticosterone on neuronal excitability and plasticity in adult hippocampus. Dev. Brain. Res. (in press). [DOI] [PubMed]