Abstract
Low level laser therapy (LLLT) is frequently used in the treatment of wounds, soft tissue injury and in pain management. The exact penetration depth of LLLT in human tissue remains unspecified. Similar uncertainty regarding penetration depth arises in treating animals. This study was designed to test the hypothesis that transmission of LLLT in horses is increased by clipping the hair and/or by cleaning the area to be treated with alcohol, but is unaffected by coat colour. A LLLT probe (810 nm, 500 mW) was applied to the medial aspect of the superficial flexor tendon of seventeen equine forelimbs in vivo. A light sensor was applied to the lateral aspect, directly opposite the laser probe to measure the amount of light transmitted. Light transmission was not affected by individual horse, coat colour or leg. However, it was associated with leg condition (F = 4.42, p = 0.0032). Tendons clipped dry and clipped and cleaned with alcohol, were both associated with greater transmission of light than the unprepared state. Use of alcohol without clipping was not associated with an increase in light transmission. These results suggest that, when applying laser to a subcutaneous structure in the horse, the area should be clipped and cleaned beforehand.
Keywords: low, level, laser, therapy, equine, tendon
Introduction
Low level laser therapy (LLLT) is a popular modality in the physiotherapeutic management of human and animal patients. LASER ('light amplification by the stimulated emission of radiation') differs from ordinary light in its unique properties of monochromacity (single wavelength), coherence (waves in phase) and collimation (waves in parallel). As a result, it can deliver large amounts of energy to a small region over a short period of time [20,23]. The use of low powered laser (<500 mW, <35 J/cm2, typical wavelengths of 600-1300 nm) in the management of pain, wound healing and soft tissue injury was developed in the 1960s and has grown steadily since [4].
Low level laser therapy is believed to have photochemical rather than thermal effects because low irradiation levels are used and no appreciable temperature rise takes place [20]. The photochemical theory, which is not yet universally accepted, postulates that the absorbed light interacts with chromophores (organic molecules) which in turn modulate cellular activities [7,11,3].
Research, therefore, centres on the cellular effects of laser as well as the effects that lasers have on wound healing, pain and musculoskeletal conditions. Cellular research has demonstrated that LLLT reduced experimentally induced inflammation by 20-30% and that wavelengths of 660, 820 and 870 nm encouraged macrophages to stimulate fibroblast proliferation [35,18].
Clinically, LLLT is perceived as effective in the acceleration of wound healing [6]. A meta-analysis [7] and a Cochrane review [14] drew no conclusions regarding the efficacy of LLLT in skin disorders and venous leg ulcers respectively. Controlled trials on animal wounds treated with laser revealed varying degrees of success [19,15,24,29]. Two recent studies reported pain relief in both RA (rheumatoid arthritis) and fibromyalgia patients post irradiation with LLLT while a meta-analysis established no effect [16,9,17]. In the equine model, Martin and Kilde [26] demonstrated the alleviation of back pain in a non-controlled study. Finally, a Cochrane review of the use of 904 nm laser in musculoskeletal conditions concluded that LLLT appeared effective for pain relief and quicker functional recovery [12]. Sharma et al. [33] displayed an ultrasound assessed decrease in tendon diameter of tenosynovitis diagnosed tendons, after laser application. Laser was no more effective than glycosaminoglycan or conservative management in treating superficial flexor tendon injuries in racehorses [25].
It is difficult to compare results of the various studies into laser therapy as parameters, when fully recorded, often vary [20,4]. Different conditions are treated, different dosages utilised and different outcome measures are recorded. Investigative findings are ambiguous. For practically every supportive trial, there is a contradictory counterpart. Laser appears a popular therapy lacking sufficient supportive research [34,16,32]. However, as Basford [1] states, there exists enough evidence to justify the continued investigation of this mode of therapy. Practising physiotherapists surveyed corroborated the beneficial clinical effects of LLLT [6]. The recent extension of FDA (Federal Drug Agency, USA) approval to LLLT also illustrates this [5].
As previously mentioned, penetration depth is one of the uncertainties pertaining to laser parameters. It is accepted and proven that penetration depth is a wavelength dependent property. Longer wavelengths will penetrate further [21,22,13]. As a result, visible red laser light is used to treat wounds while infrared laser light is used for deeper musculoskeletal conditions. Nevertheless, energy density applied to the target tissues is unknown as the result of uncertainties pertaining to penetration depth [23]. Irradiation levels, in the form of energy density (J/cm2) at the surface applied, are commonly recorded by the clinician [7,3]. When LLLT is used in veterinary medicine, depth of penetration through hair and pigment is a major issue [26,8,25,29,31]. Currently, it is recommended that the irradiated area should be clipped and cleaned with an alcohol wipe before treatment [8,23].
In conclusion, many questions remain unanswered regarding LLLT, including optimal dosimetry, mode of action, and number of treatments required etc. This study was undertaken in an attempt to address one of the above questions i.e., to what extent laser penetration in the equine model is affected by cleaning and clipping the area of application.
Materials and methods
Subjects were recruited from the resident equine population at the Hawkshead campus of the Royal Veterinary College, London. Permission was obtained from the relevant animal guardian. Horses with a history of, or obvious evidence of, forelimb tendon pathology were excluded. In all, seventeen sets of data were collected from the forelimbs of nine horses (one set of incomplete data was excluded). Three of the horses were grey, while the rest were bay. Five of the horses were thoroughbred (TB) while the rest were Irish Draught/TB crosses.
The laser therapy system used was a Thor DDII unit (Thor International Ltd, Amersham, Bucks, UK). A single point GaAlAs (gallium aluminium arsenide) laser probe, emitting a continuous beam of wavelength 810 nm and power 500 mW, was used. In compliance with safety measures, the investigator and assistant wore protective goggles to prevent inadvertent ocular damage [10,11]. The light sensor used was a M3000 (FOE Ltd, Argyll, UK). This sensor was capable of detecting light waves in the 600-1300 nm wavelength and gave readings in microwatts × 10 (uW × 10). This was pre-calibrated by the manufacturer. A standard vernier caliper (Mitutoyo (UK) Ltd, Hampshire, UK) was used. These calipers possess an accuracy of 0-200 mm +/-0.03 mm and a graduation of 0.02 mm/0.001.
Alcohol solution was used to clean the horses' coats. It was also used to clean the horses' skin after clipping. Electric clippers (number 30 blades) were used to clip the horses' skin. A preliminary study tested light penetration through the tendons of six subjects. These were tendons of varying thickness and coat colour. Results indicated that the light sensor was sensitive enough to detect changes in penetration of light through various equine tendons. It also suggested that the readings should be taken after sunset to minimise ambient light. Each horse was restrained with a head collar and lead rope in a loose box with an attendant and some loose hay. Measurements were conducted with the animal either standing on a rubber mat or a shallow bed of shavings and with the animal standing as 'square' as possible i.e., equal weight bearing on all four legs. Tendon lateromedial dimensions were gauged using the vernier calipers. This was measured at the junction of the distal third and proximal two-thirds of the superficial digital flexor tendon (SDF) as this is an easily identifiable site and is a frequent site of tendon injury [19]. The therapeutic laser probe was applied at the medial aspect of the SDF of the horse's forelimb, at the junction of the distal third and proximal two thirds. This was done using the contact technique: firmly, perpendicular to the skin with slight indentation of the skin and by the same investigator each time [2]. The light sensor was then held in contact, directly opposite the laser probe, on the lateral aspect of the SDF. The two were maintained in position until the sensor delivered a reading. This was repeated three times and the mean measurement recorded.
Following findings from the preliminary study, a particular sequence was followed with each subject:
1. Measurement with the leg untreated (i.e., dry and dirty).
2. Measurement after the leg (i.e., that portion of the leg) was cleaned with alcohol solution and was still wet.
3. Measurement after the leg was cleaned with alcohol solution and had dried.
4. Measurement with the surrounding hair clipped.
5. Measurement with the clipped leg cleaned with alcohol solution and still wet.
Results were presented using tables and graphs. Analysis was by multiway analysis of variance with 'light transmitted' as the dependent variable and colour (grey or bay), horse, leg (right or left) and condition (dry, alcohol wet, alcohol dry, clipped dry or clipped wet) as the independent variables. The variable 'leg' was nested in the variable 'horse' and the variable 'horse' was nested in the variable 'colour'. All significance tests were two sided [30].
Results
The results of the tests performed have been summarised in Tables 1 and 2.
Table 1.
Lateromedial dimensions (diameter) of tendons used in study and average light transmitted through tendon (uW × 10, n = 9)
| Horse no. 1 | Horse no. 2 | Horse no. 3 | Horse no. 4 | Horse no. 5 | Horse no. 6 | Horse no. 7 | Horse no. 8 | Horse no. 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horse colour and sex | Grey gelding | Grey gelding | Grey gelding | Bay gelding | Bay gelding | Bay mare | Bay mare | Bay gelding | Bay gelding | ||||||||
| CASE (R = right leg, L = left leg) | 1 (R) | 2(R) | 3(L) | 4(R) | 5(L) | 6(R) | 7(L) | 8(R) | 9(L) | 10(R) | 11(L) | 12(R) | 13(L) | 14(R) | 15(L) | 16(R) | 17(L) |
| Diameter (cm) | 2.8 | 3.5 | 3.7 | 2.9 | 3.5 | 3 | 3.1 | 2 | 2 | 2 | 2 | 2.2 | 2.4 | 2.2 | 2 | 2.3 | 2.4 |
| Average light transmitted (uW × 10) | 0.28 | 0.3 | 0.23 | 0.53 | 1.81 | 0.24 | 0.21 | 0.14 | 0.14 | 0.25 | 0.28 | 0.03 | 0.02 | 0.05 | 0.06 | 0 | 0.10 |
Table 2.
Mean amount of light (uWx10) transmitted through the tendon: grey subjects compared with bay subjects (n = 9)
| Mean amount of light transmitted (uWx10) | ||
|---|---|---|
| Grey (n = 5) Mean lateromedial dimensions (diameter) 3.28 cm | Bay (n = 12) Mean lateromedial dimensions (diameter) 2.3 cm | |
| Dry i.e., untreated | 0.01 | 0 |
| Cleaned with alcohol (wet) | 0.30 | 0.01 |
| Cleaned with alcohol (dry) | 0.25 | 0.04 |
| Clipped | 1.24 | 0.18 |
| Clipped and cleaned with alcohol | 1.12 | 0.28 |
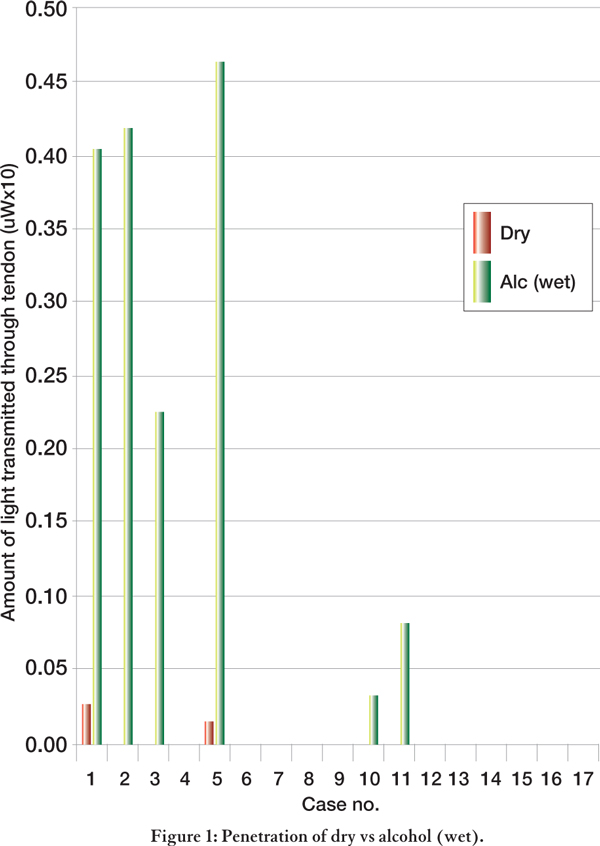
Simple cleaning of the hair and skin surrounding the tendon with alcohol, caused a general increase in penetration of light through the tendons (p = 0.5762) (Figure 1, see p277). Readings which failed to register on the light sensor (i.e., those which produced a reading of 0) would indicate that the laser light introduced had been completely absorbed by the tendon and that none of this light had penetrated to the other side.
Figure 1.
Penetration of dry vs alcohol (wet)
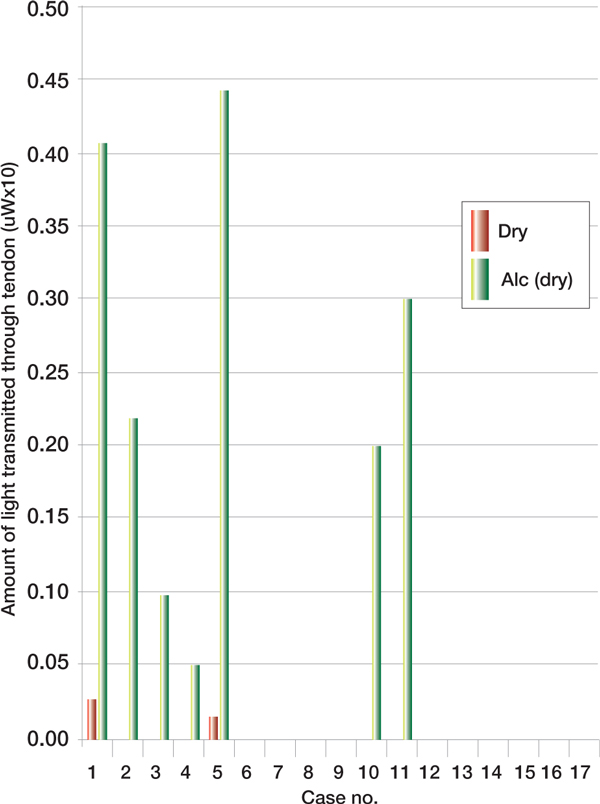
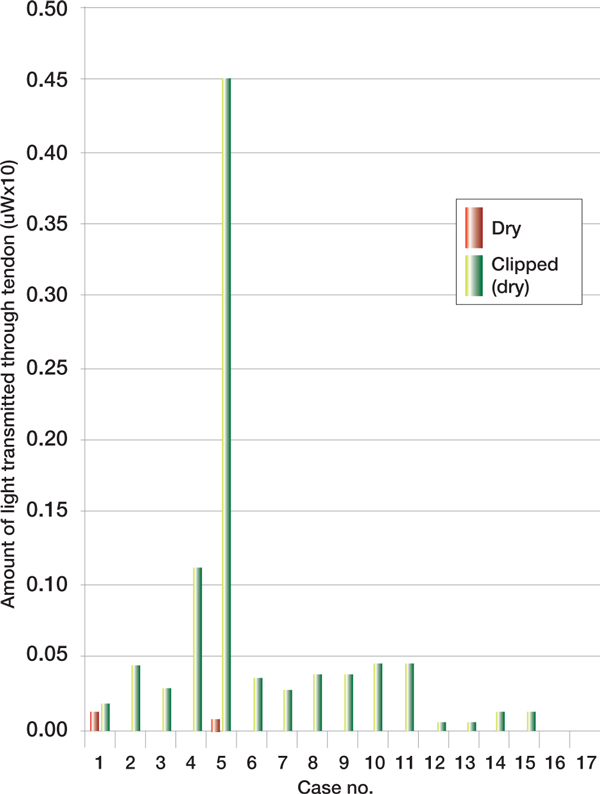
There was a similar non-significant increase in the degree of penetration of light when the same tendon was re-measured after the alcohol solution was allowed to dry (p = 0.5428) (Figure 2, see p277). Clipping of the hair covering the tendon increased light transmission through the tendon in all cases (p = 0.0043) (Figure 3, see p277).
Figure 2.
Penetration of dry vs alcohol (dry)
Figure 3.
Penetration of dry vs clipped (dry)
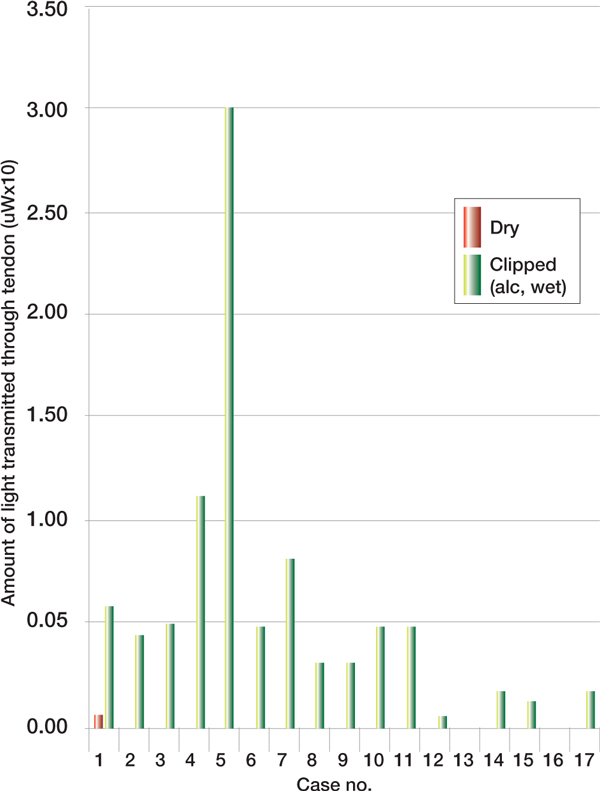
The most pronounced increase in light penetration through the tendon was noted when the exposed skin of the clipped leg was cleaned with alcohol solution (clipped [+wet alco-hol]), (p = 0.0023) (Figure 4, see p277).
Figure 4.
Penetration of dry vs clipped (alcohol, wet).
Discussion
The current mode of application of laser therapy is through the animal's coat, without any preparation [27,26,25]. However, results of this study suggest this to be the most inefficient way of applying laser. In the treatment of wounds, depth of penetration is not a major issue as the target tissue is superficial and exposed. However, LLLT is also advocated in the treatment of pain, soft tissue injuries and trigger points [8]. In order for the laser to penetrate to the target tissue e.g., the centre of the superficial flexor tendon for a core lesion, it is necessary for the light to penetrate through hair, skin, subcutaneous tissue and the tendon.
With the majority of cases, cleaning the area of application with alcohol solution caused an increase in the amount of light penetrating through. However, when analysed, a non-significant p value of 0.5428 is returned. Low and Reed [23] recommend that the skin of the (human) patient should be cleaned with an alcohol wipe prior to treatment. Subsequent to this, the surface of the tendon was clipped. This was in compliance with the recommendation of Bromiley [8] that the area to be treated should be shaved beforehand. There was an increase in the depth of penetration of laser through all clipped tendons, compared with the untreated tendons (p = 0.0043). This would indicate that clipping has a greater impact on laser penetration than cleaning with alcohol solution alone. Following this, the clipped tendon was cleaned with alcohol solution and a cotton swab. Again there was an increase in penetration, compared with the untreated tendon (p = 0.0023).
On average, a greater amount of light was transmitted through the tendons of the grey subjects compared with the bay subjects. This was notwithstanding the difference in lateromedial dimensions, with an average grey tendon dimension of 3.28 cm compared with 2.30 cm for the bay horses. However, these results were not significant. The results of this study would appear to indicate that clipping the area to be irradiated before laser treatment would increase the depth of penetration of the light. This is most relevant in the treatment of pain and musculoskeletal conditions e.g., treating a muscle injury, where one is aiming to influence deeply seated structures. Furthermore, the results would suggest that even cleaning the coat and skin over the area to be irradiated with alcohol solution would have a positive effect on light penetration. This may sometimes be indicated where clipping is out of the question (e.g., show animals). None of these recommendations are novel. Cleaning of the area to be treated has already been advised [23]. Furthermore, it has been stipulated that the hair covering the area to be treated should be clipped [8]. However this does not appear to be adhered to clinically [25]. In addition, this area does not appear to have been investigated previously.
In conclusion, based on the results of this research, it is to be recommended that the practitioner clips and cleans the area to be irradiated prior to treatment with LLLT.
Acknowledgements
The authors wish to acknowledge the assistance of Thor Lasers, Dr N.F. Horgan, RCSI, and staff and personnel at the Royal Veterinary College Hawkshead campus.
References
- Basford JR. Low energy laser therapy: Controversies and new research findings. Lasers in Surgery and Medicine. 1989;9:1–5. doi: 10.1002/lsm.1900090103. [DOI] [PubMed] [Google Scholar]
- Baxter GD. Therapeutic Lasers, Theory and Practice. 1. New York: Churchill Livingstone; 1994. [Google Scholar]
- Baxter GD. Laser therapy seminar, Royal College of Surgeons in Ireland. 1999.
- Baxter GD. In: Electrotherapy: Evidence-Based Practice. 11. Kitchen S, Bazin S, editor. London: Churchill Livingstone; 2002. Low-intensity laser therapy; pp. 171–190. [Google Scholar]
- Baxter GD. Personal communication. 2004.
- Baxter GD, Bell AJ, Allen JM, Ravey J. Low level laser therapy: Current clinical practice in Northern Ireland. Physiotherapy. 1991;77(3):171–178. doi: 10.1016/S0031-9406(10)61696-3. [DOI] [Google Scholar]
- Beckerman H, de Bie RA, Bouter LM, De Cuyper HJ, Oostendorp RA. The efficacy of laser therapy for musculoskeletal and skin disorders: A criteria based meta-analysis of randomised clinical trials. Physical Therapy. 1992;72(7):483–491. doi: 10.1093/ptj/72.7.483. [DOI] [PubMed] [Google Scholar]
- Bromiley M. Equine Injury, Therapy and Rehabilitation. 2. Oxford: Blackwell Science; 1993. [Google Scholar]
- Brousseau L, Welch V, Wells G, deBie R, Gam A, Harman K, Morin M, Shea B, Tugwell P. The Cochrane Library. Vol. 4. Chichester: John Wiley and Sons; 2003. Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis (Cochrane review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartered Society of Physiotherapy. Guidelines for the safe use of lasers in physiotherapy. Physiotherapy. 1991;77(3):169–171. doi: 10.1016/S0031-9406(10)61695-1. [DOI] [Google Scholar]
- Chartered Society of Physiotherapy. Standards for the use of electrophysical modalities and guidance in their application. Chartered Society of Physiotherapy Information Resource Centre; 1998. Laser therapy; pp. 27–30. [Google Scholar]
- De Bie R, Verhagen A, Lenssen T, de Vet R, van den Wildenberg F. The Cochrane Library. Chichester: John Wiley and Sons; 1996. Oral presentation: Efficacy of 904 nm laser therapy in musculoskeletal disorders: a systematic review. [Google Scholar]
- Enwemeka CS. Attenuation and penetration of visible 632.8 nm and invisible infra-red 904 nm light in soft tissues. Laser Therapy Journal. 2003;13:16. [Google Scholar]
- Flemming K, Cullum N. The Cochrane Library. Vol. 4. Chichester, UK: John Wiley and Sons; 2003. Laser therapy for venous leg ulcers (Cochrane methodology review) [Google Scholar]
- Fretz PB, Li Z. Low energy laser irradiation treatment for second intention wound healing in horses. Canadian Veterinary Journal. 1992;33:650–653. [PMC free article] [PubMed] [Google Scholar]
- Gam AN, Thorsen H, Lonnberg F. The effect of low level laser therapy on musculoskeletal pain: a meta-analysis. Pain. 1993;53:63–66. doi: 10.1016/0304-3959(93)90114-5. [DOI] [PubMed] [Google Scholar]
- Gur A, Cosut A, Sarac AJ, Cevik R, Nas K, Uyar A. Efficacy of different therapy regimes of low power laser in painful osteoarthritis of the knee: a double blind and randomised controlled trial. Lasers in Surgery and Medicine. 2003;33(5):330–338. doi: 10.1002/lsm.10236. [DOI] [PubMed] [Google Scholar]
- Honmura A, Yanase M, Obata J, Haruki E. Therapeutic effect of Ga-Al-As diode laser irradiation on experimentally induced inflammation in rats. Lasers in Surgery and Medicine. 1992;12:441–444. doi: 10.1002/lsm.1900120414. [DOI] [PubMed] [Google Scholar]
- Kaneps AJ, Hultgren BD, Riebold TW, Shires GMH. Laser therapy in the horse: Histopathological response. American Journal of Veterinary Research. 1984;45(3):581–582. [PubMed] [Google Scholar]
- Kitchen SS, Partridge CJ. A review of low level laser therapy. Physiotherapy. 1991;77(3):161–168. doi: 10.1016/S0031-9406(10)61694-X. [DOI] [Google Scholar]
- Kolari PJ. Penetration of unfocused laser light into the skin. Archives of Dermatological Research. 1985;277:342–344. doi: 10.1007/BF00509097. [DOI] [PubMed] [Google Scholar]
- Kolarova H, Ditrichova D, Wagner J. Penetration of the laser light into the skin in vitro. Lasers in Surgery and Medicine. 1999;24:231–235. doi: 10.1002/(SICI)1096-9101(1999)24:3<231::AID-LSM8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Low J, Reed A. Electrotherapy Explained: principles and practice. 3. Oxford: Butterworth Heinemann; 2000. [Google Scholar]
- Lucroy MD, Edwards BF, Madewell BR. Low-intensity laser light-induced closure of a chronic wound in a dog. Veterinary Surgery. 1999;28:292–295. doi: 10.1053/jvet.1999.0292. [DOI] [PubMed] [Google Scholar]
- Marr CM, Love JS, Boyd Q, McKellar Q. Factors affecting the clinical outcome of injuries to the superficial digital flexor tendon in National Hunt and point-to-point racehorses. The Veterinary Record. 1993;132:476–479. doi: 10.1136/vr.132.19.476. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Kilde AM. Treatment of chronic back pain in horses: Stimulation of acupuncture points with a low powered infrared laser. Veterinary Surgery. 1987;16(1):106–110. doi: 10.1111/j.1532-950X.1987.tb00919.x. [DOI] [PubMed] [Google Scholar]
- McKibbin LS, Paraschak D. Use of laser light to treat certain lesions in standardbreds. Modern Veterinary Practice. 1984;65(3):210–213. [PubMed] [Google Scholar]
- Mester AF, Mester A. Wound healing. Laser Therapy. 1989;1(1):7–15. [Google Scholar]
- Petersen SL, Botes C, Olivier A, Guthrie AJ. The effect of low level laser therapy (LLLT) on wound healing in horses. Equine Veterinary Journal. 1999;31(3):228–231. doi: 10.1111/j.2042-3306.1999.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Petrie A, Sabin C. Medical Statistics at a glance. Oxford: Blackwell Scientific Publications; 2000. [Google Scholar]
- Ramey DW, Basford JR. Laser therapy in horses. Compendium on Continuing Education for the Practicing Veterinarian. 2000;22(3):263–272. [Google Scholar]
- Saunders L. The efficacy of low level laser therapy in supraspinatus tendonitis. Clinical Rehabilitation. 1995;9:126–134. doi: 10.1177/026921559500900207. [DOI] [Google Scholar]
- Sharma R, Thukral A, Kumar S, Bhargava SK. Effect of low level lasers in de Quervains tenosynovitis - a prospective study with ultrasonographic assessment. Physiotherapy. 2002;88(12):714–775. doi: 10.1016/S0031-9406(05)60716-X. [DOI] [Google Scholar]
- Vasseljen O, Hoeg N, Kjeldstad B, Johnsson A, Larsen S. Low level laser versus placebo in the treatment of tennis elbow. Scandinavian Journal of Rehabilitation in Medicine. 1992;24:37–42. [PubMed] [Google Scholar]
- Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C. Macrophage responsiveness to light therapy. Lasers in Surgery and Medicine. 1989;9:497–505. doi: 10.1002/lsm.1900090513. [DOI] [PubMed] [Google Scholar]