Abstract
A twenty-month-old Jack Russell terrier was presented with a four-day history of thrombocytopenia, echymotic inguinal haemorrhages, coughing and reduced exercise tolerance. Clinical examination revealed several petechial haemorrhages on the gingivae and small echymotic haemorrhages in the inguinal region, along with mild bilateral epistaxis. Haematology confirmed a platelet count of 1.0 × 10/L. Thoracic radiographs revealed a wide-spread mixed alveolar-interstitial lung pattern, apparent throughout the entire lungfield, but particularly marked within the left lung lobes. A presumptive diagnosis of immune-mediated thrombocytopenia was made and the dog was treated with vincristine and immunosuppressive doses of prednisolone. Initially anaemia developed following gastrointestinal haemorrhage; however, after symptomatic treatment the dog showed a marked clinical improvement. Evaluation for an underlying cause of the disease revealed Angiostrongylus vasorum L1 larvae on faecal analysis and treatment with fenbendazole was commenced. The dog made a full clinical recovery with all treatment was withdrawn within five weeks of diagnosis. This is the second report of immune-mediated thrombocytopenia associated with Angiostrongylus vasorum infection and it is the first to be successfully managed. The report highlights that Angiostrongylus vasorum should be considered in young dogs presented with thrombocytopenia.
Keywords: Lung worm, dog, platelets
Introduction
Thrombocytopenia is the most commonly occurring platelet disorder in dogs [14], with a prevalence rate among the admissions to one US hospital of approximately 5% [20]. Thrombocytopenia may result from a variety of pathophysiological mechanisms including reduced platelet production, increased platelet sequestration, increased platelet utilisation or destruction [8].
Immune-mediated thrombocytopenia (IMTP) is a disease characterised by the premature destruction of antibody-coated platelets by macrophages [30]. This disease may occur as a primary disease, termed primary IMTP or idiopathic thrombocytopenic purpura, or as a secondary disease associated with conditions such as neoplasia, inflammation or infection [30]. The pathogenesis of primary IMTP is thought to involve the production of antibodies directed against normal host platelet-surface antigens, whereas in secondary IMTP the pathogenesis remains largely undefined [30].
Angiostrongylus vasorum is a metastrongyloid nematode that parasitises dogs and other Canidae via an indirect lifecycle involving slugs, snails and frogs that act as intermediate or paratenic hosts [2,41]. This life cycle has been extensively reviewed elsewhere [41,2]. The first report of Angiostrongylus vasorum infection in Ireland was in a Greyhound [40]. Following this, the infection became well-recognised within Greyhound kennels in Ireland [12] and the first reported case in England was a Greyhound that had been imported from Ireland [23]. More recently, Angiostrongylus vasorum infection has been reported in several pet dogs in Ireland [5]; however, the overall prevalence of the nematode in this country is unknown [5].
A wide variety of clinical signs have been associated with Angiostrongylus vasorum infection. Commonly reported clinical signs include cardiopulmonary signs such as coughing, exercise intolerance and dyspnoea [38,35,37]. In some cases progression of the signs to right-sided heart failure [21] or sudden death attributed to acute heart failure [27] has been reported. Coagulopathies have also been widely reported in association with both naturally occurring and experimental Angiostrongylus vasorum infections [12,43,44,5,6]. In addition, neurological and ocular signs have been documented [16,48,34] or more general signs such as weight loss, poor growth and weakness [12]. Asymptomatic infections have been observed in some individuals [35,37].
Thrombocytopenia has been reported in association with Angiostrongylus vasorum infection in a number of canine cases [43,37,39,18,7,5,6,50] with reported incidences in the two largest case series of four out of 20 cases [6] and two out of 35 cases [50]. In one of the previous cases, the cause of the thrombocytopenia was demonstrated to be immune-mediated [18]. In other cases the cause has been attributed to a consumptive coagulopathy resembling disseminated intravascular coagulation [43,39,7].
This case report describes the presentation, diagnosis and successful treatment of a Jack Russell Terrier with severe immune-mediated thrombocytopenia associated with Angiostrongylus vasorum infection. To the authors' knowledge, this is only the second report of IMTP and Angiostrongylus vasorum infection and is the first to be diagnosed antemortem.
Case History
A twenty-month-old male entire Jack Russell Terrier was presented to the University Veterinary Hospital (UVH), University College Dublin with a four-day history of thrombocytopenia, small inguinal echymotic haemorrhages, coughing and reduced exercise tolerance.
The dog had shown sudden onset coughing and retching that had commenced at exercise. The referring veterinarian had performed bronchoscopy on day one, which had revealed a stream of blood running ventrally along the trachea, appearing to source from the right main-stem bronchus. The bronchoscopy had been terminated at this stage and further examination had revealed small echymotic haemorrhages in the inguinal region. Treatment with Vitamin K1 (Konakion; Roche) at a dose of 10 mg twice daily per os had been commenced and a blood sample submitted to the UVH clinical pathology laboratory for haematology and a coagulation screen. The haematology results were received on day three and revealed severe thrombocytopenia (2.0 × 109/L), evidence of increased erythrocyte turnover but without anaemia (packed cell volume (PCV) 0.38 L/L), a mature neutrophilia (16.8 × 109/L) and a monocytosis (2.6 × 109/L). There was no known access to rodenticides and the dog had not received drug therapy other than vitamin K1. The coagulation screen gave results within the normal reference ranges. The dog was referred on day four. At initial presentation the dog was bright, alert and in good body condition (weight 7.3 kg). Only very occasional coughing had been observed since first presentation, although exercise intolerance was apparent. A clinical examination revealed pink mucous membranes with a normal capillary refill time. The respiratory rate was approximately 36 breaths per minute with a slight increase in lung sounds noted bilaterally on auscultation. There were several petechial haemorrhages evident on the gingivae, along with mild bilateral epistaxis and the inguinal echymoses.
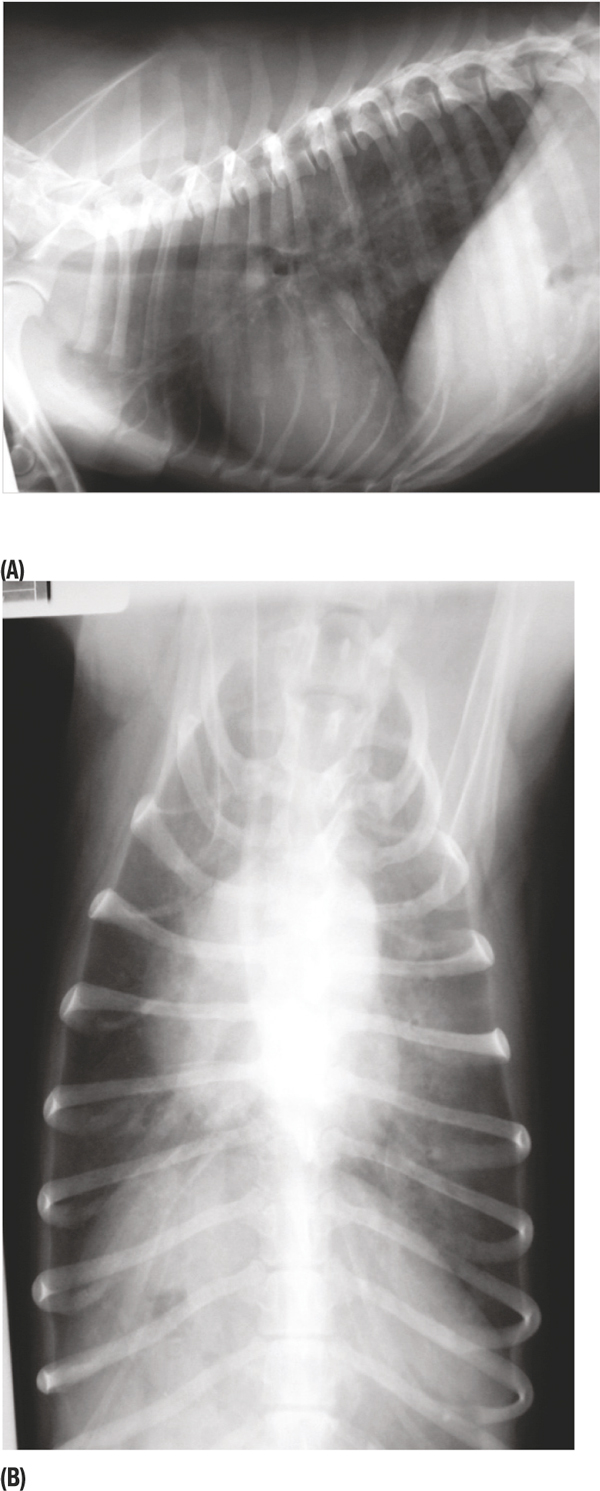
Haematology at the time of referral (day four) demonstrated severe thrombocytopenia (1.0 × 109/L machine count, confirmed as accurate on examination of a smear) accompanied by a mild, regenerative anaemia (PCV 0.27 L/L). Serum biochemistry demonstrated mild hyperglobulinaemia (45.7 g/L) and mild elevation of serum alkaline phosphatase (103 U/L). Thoracic radiography (Figure 1) revealed a widespread mixed alveolar-interstitial lung pattern. This was apparent throughout the lungfield, but was particularly marked within the left lung lobes, which contained subtle air bronchograms. Pleural fissure lines were visible between both the right middle and right caudal lobes and the accessory and left caudal lung lobes, however a significant pleural effusion was not discernable. Right lateral and dorso-ventral abdominal radiographs demonstrated no significant abnormalities.
Figure 1.
Right lateral (A) and dorsoventral (B) thoracic radiographs taken on the day of referral (day four), showing a widespread mixed alveolar-interstitial lung pattern. This is visible throughout the lungfield, but is particularly marked within the left lung lobes. Pleural fissure lines are visible between both the right middle and right caudal lobes and the accessory and left caudal lung lobes, however a significant pleural effusion is not discernable.
A presumptive diagnosis of immune-mediated thrombocytopenia was made and treatment was commenced on day four with vincristine (Vincristine; Faulding Pharmaceuticals Ltd.) at a single dose of 0.02 mg/kg i/v and prednisolone (Prednicare; Animalcare) at a dose of 2.7 mg/kg split twice daily per os (10 mg twice daily). The dog was confined to strict cage rest with minimal handling to minimise the risk of spontaneous haemorrhage.
Over the next three days of hospitalization coughing or changes in respiratory rate were not observed. Several small echymotic haemorrhages developed on the ventral abdomen on day two of hospitalisation (day six) but other signs were not observed, although his appetite was poor. Haematology was performed daily to monitor the PCV and platelet count. Significant change in the platelet count was not observed over the first three days of treatment.
On the fourth day of hospitalisation (day seven), the PCV had dropped to 0.19 L/L despite the absence of evidence of further bleeding on clinical examination. Spherocytes were not noted on the blood smear and a Coombs' test was negative. At this stage, gastrointestinal blood loss was a concern and treatment was commenced with ranitidine (Zantac; GlaxoWellcome) at a dose of 2 mg/kg every eight hours and sucralfate (Antepsin; Chugai) at a dose of 400 mg every eight hours. The dog had passed no faeces since its admission. No rectal examination was performed. In view of the sudden decline in PCV, major and minor cross matches were performed against two blood donors in case of a requirement for a blood transfusion should the dog deteriorate further. Severe thrombocytopenia (6.0 × 109/L) was still apparent following an automated platelet count, however manual counts revealed approximately five platelets per high power field, suggesting higher numbers were present and some platelet clumping was occurring. Faeces passed at the end of day seven, showed evidence of both melaena and haematochezia. Samples were submitted for a modified Baermann examination to evaluate for lungworm larvae [47].
Angiostrongylus vasorum L1 larvae were identified in the faecal sample confirming a patent infection with the parasite. These results were received on day seven of hospitalisation (day 10). Treatment was commenced with fenbendazole (Panacur; Hoechst) at a dose of 50 mg/kg daily for seven days in addition to the prednisolone, ranitidine and sucralfate. The dog continued to be bright over the next four days and showed no clinical evidence of haemorrhage or melaena. On day eleven of hospitalisation (day 14 of the history), the platelet count had risen to 24.0 × 109/L, although examination of the smear again identified platelet clumping suggesting the true count was considerably higher. A buccal mucosal bleeding time was performed to confirm adequate primary haemostasis; a normal result was obtained and the dog was discharged. Ranitidine and sucralfate treatment was discontinued. The prednisolone was continued at a dose of 1.4 mg/kg (10 mg) per os daily and the seven-day course of fenbendazole completed. After two weeks the prednisolone was reduced to 0.7 mg/kg (5 mg) per os daily.
The dog was re-examined at the UVH three weeks after the initial referral. At this stage residual clinical signs were not apparent and the platelet count was within the reference range. Lungworm larvae were not observed on faecal examination. The prednisolone dose was reduced to 0.7 mg/kg (5 mg) per os every 48 hours for one week and then 0.35 mg/kg (2.5 mg) every 48 hours for a further week before being completely withdrawn. The dog has had no recurrence of clinical signs for seventeen months.
Discussion
Thrombocytopenia may result from reduced platelet production, increased platelet sequestration, increased platelet utilisation or destruction [8]. Consumptive coagulopathies represent the most common cause of increased platelet utilisation, whereas immune-mediated thrombocytopenia, either primary or secondary, is the most common cause of increased platelet destruction [8]. IMTP may occur in association with other immunological diseases such as systemic lupus erythematosus [26] or secondary to other conditions such as neoplasia, inflammation and infection [8] or following drug administration [46]. In dogs, the infectious processes that have been reported in association with thrombocytopenia include; bacterial infections, rickettsial infections and parasitic infections [4] including both dirofilariasis [20] and angiostrongylosis [18]. These infectious agents have the potential to induce autoimmunity via a variety of immunological mechanisms, for example molecular mimicry or the induction or alteration of host antigens [9]. However, the potential role that these or other immunological processes might play in canine secondary, infection-related IMPT cases remains largely undefined.
A variety of criteria have been used to confirm a diagnosis of canine primary IMTP, including; the severity of thrombocytopenia, the presence of microthrombocytosis or platelet fragmentation, detection of anti-platelet autoantibodies, increased platelet counts following the administration of immunosuppressive doses of corticosteroids, normal to increased numbers of megakaryocytes in bone marrow and the exclusion of other aetiologies of thrombocytopenia [30]. It is widely accepted that there is no "gold standard" for diagnosis [10]. Firstly, whilst a positive test result for either platelet-bound or serum platelet-bindable IgG in a dog with thrombocytopenia implies an immune pathogenesis, it does not aid differentiation between primary and secondary IMTP [30]. Secondly, whilst direct assays for surface-bound antibodies in IMTP offer high sensitivity compared to that achieved by indirect assays detecting serum antibodies [31], these direct assays can be difficult to perform in cases with very severe thrombocytopenia due to insufficient platelet numbers [10]. Performing a megakaryocyte direct immunofluorescence assay may alleviate the latter problem, however the reported sensitivity with this test is highly variable (30% to 80%) [25,22,27]. Hence, this test has the potential to generate significant numbers of false negative results and has the additional disadvantage of requiring a bone marrow aspirate. More recently platelet-bound antibodies have been evaluated by flow cytometric assay alleviating the problems associated with low platelet numbers [11], however this technique still does not allow the differentiation between primary and secondary IMTP.
In view of the limitations of the immunological testing outlined above and the limited availability of anti-platelet antibody tests, a presumptive diagnosis of IMTP was made in the current case. This diagnosis was based on the initial severity of the thrombocytopenia, the response to immunosuppressive corticosteroid therapy and the exclusion of a consumptive coagulopathy. The initial severity of the thrombocytopenia has been suggested to be a reliable feature of IMPT cases [20,31]. A response to corticosteroid therapy provides evidence of an immune-mediated pathogenesis [31]; typically IMTP cases will have platelet counts of greater than 50 to 100 × 109/L within seven days of initiating treatment [31]. There is no definitive laboratory test to confirm a diagnosis of disseminated intravascular coagulation (DIC); however, low platelet counts, erythrocyte fragmentation on peripheral blood smears, prolonged clotting times, low levels of antithrombin, and high levels of fibrin degradation products are accepted to be helpful indicators [14,41]. In this dog there was no evidence of erythrocyte fragmentation and no prolongation of the clotting times. In addition, the thrombocytopenia was more marked than would typically be associated with DIC; thrombocytopenia at this level being much more typical of IMTP [20]. Ideally, measurement of fibrin degradation products or d-dimers would have provided further evaluation for DIC. However the FDP assay was not performed due to a lack of reagents when a sample was submitted (day four) to an external laboratory, and unfortunately the sample was not retained. A d-dimer assay was not available at the time. Despite this, in view of the lack of other suggestive signs and the response to treatment, the diagnosis of DIC was considered highly unlikely.
The majority of reported cases of angiostrongylosis have occurred in young dogs [35,37], as in this case. A wide variety of clinical signs have been associated with infection, with respiratory signs, such as coughing, widely accepted as one of the most common clinical signs [38,35,37,6], as was observed in this current case. Clinical signs relating to a haemorrhagic diathesis are also common, reported to occur in approximately one third of the cases [6]. Subcutaneous, mucosal and internal bleeding have all been reported [5,6], including haemorrhage within the central nervous system [16]. The mechanism underlying this bleeding tendency remains poorly characterized; various mechanisms have been proposed including chronic disseminated intravascular coagulation [43,39,7], acquired von Willebrand's disease [49] and immune-mediated thrombocytopenia [18].
Thrombocytopenia has been reported in association with Angiostrongylus vasorum infection in a number of canine cases [43,37,39,18,5]. In general the thrombocytopenia reported in these cases has been mild to moderate; none of the cases in the Chapman series presented with severe thrombocytopenia [6]. In a series of experimentally infected dogs [7] the mean platelet count in infected dogs was significantly lower than in the control dogs for approximately six months post infection, with the lowest mean thrombocyte count being about 100 × 109/L post infection. In one previous case, the thrombocytopenia was demonstrated to be immune-mediated [18]. On post mortem examination, this dog was additionally confirmed to have myocarditis, thrombosing arteritis, pneumonia and chronic membranoproliferative glomerulonephritis [18].
Dogs with angiostrongylosis and resultant bleeding diatheses were evaluated as part of two recent case series [5,6], yielding very variable causes for the haemorrhage. Collectively these studies examined ten such dogs; one dog was mildly thrombocytopenic, two had mild thrombocytopenia and prolonged coagulation times, six had prolonged coagulation times alone and one had no demonstrable abnormality in haemostatic function. Hence, further investigations are required in order to fully characterize the mechanisms involved in the bleeding tendency infected dogs often show. In general, although disorders of both primary and secondary haemostasis have been reported to occur with Angiostrongylus infection [43,39,7,5,6,49], thrombocytopenia as the primary cause is uncommon [6].
The current case is the first reported case in which immune-mediated thrombocytopenia was observed in association with Angiostrongylus vasorum infection and successfully treated. It has been reported that approximately 40% of primary IMTP cases show a recurrence of clinical signs [30]. It would be expected that if this current case showed IMTP secondary to Angiostrongylus vasorum infection, there would be no recurrence of the signs following treatment of the lungworm infection. This dog was weaned off corticosteroids within five weeks and has since shown no recurrence of signs in seventeen months. Hence, there is some circumstantial evidence that the Angiostrongylus vasorum infection may be linked to the IMTP; however, it cannot be confirmed.
Conclusions
This is the first case report to describe the successful treatment of immune-mediated thrombocytopenia associated with Angiostrongylus vasorum infection. IMTP in association with A. vasorum infection has been described previously [18]; however, the previous case was diagnosed post mortem. Both cases highlight the requirement to evaluate IMTP cases for an underlying cause and the potential for A. vasorum infection to trigger immune-mediated platelet destruction. The mechanism by which this destruction is triggered in the disease, whether it represents immune-complex deposition on platelets or the direct generation of anti-platelet antibodies, requires further evaluation.
Acknowledgements
The authors would like to Colin Rohu, the referring veterinarian, for referring this case to the university veterinary hospital and providing follow-up information. In addition, the help of the nursing and laboratory staff along with the final year students on rotation is gratefully acknowledged.
References
- Boag AK, Lamb CR, Chapman PS, Boswood A. Radiographic findings in 16 dogs infected with Angiostrongylus vasorum. Veterinary Record. 2004;154:426–430. doi: 10.1136/vr.154.14.426. [DOI] [PubMed] [Google Scholar]
- Bolt G, Monrad J, Koch J, Jensen AI. Canine angiostrongylosis: a review. Veterinary Record. 1994;135:536. doi: 10.1136/vr.135.19.447. [DOI] [PubMed] [Google Scholar]
- Boudreaux MK, Dillon AR, Spano JS. Enhanced platelet reactivity in heartworm-infected dogs. American Journal of Veterinary Research. 1989;50:1544–1547. [PubMed] [Google Scholar]
- Breitschwerdt EB. Infectious thrombocytopenia in dogs. Compendium on Continuing Education for the Practicing Veterinarian. 1988;10:1177–1190. [Google Scholar]
- Brennan SF, McCarthy G, McAllister H, Bassett H, Jones BR. Clinical signs, diagnosis and treatment of three dogs with angiostrongylosis in Ireland. Irish Veterinary Journal. 2004;57:103–109. doi: 10.1186/2046-0481-57-2-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PS, Boag AK, Guitian J, Boswood A. Angiostrongylus vasorum infection in 23 dogs (1999-2002) Journal of Small Animal Practice. 2004;45:435–440. doi: 10.1111/j.1748-5827.2004.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Cury MC, Lima WS, Guimarães MP, Carvalho MP. Haematological and coagulation profiles in dogs experimentally infected with Angiostrongylus vasorum (Baillet, 1866) Veterinary Parasitology. 2002;104:139–149. doi: 10.1016/S0304-4017(01)00616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport DJ, Breitschwerdt EB, Carakostas MC. Platelet disorders in the dog and cat Part I: Physiology and Pathogenesis. Journal of Continuing Education for the Practicing Veterinarian. 1982;4:762–772. [Google Scholar]
- Day MJ. In: Clinical Immunology of the Dog and Cat. Day MJ, editor. London: Manson Publishing Ltd; 1999. The basis of immune-mediated disease; pp. 61–65. [Google Scholar]
- Day MJ, Mackin AJ. In: Clinical Immunology of the Dog and Cat. Day MJ, editor. London: Manson Publishing Ltd; 1999. Immune-mediated haematological disease; pp. 79–83. [Google Scholar]
- Dircks BH, Schuberth HJ, Mischke R. Underlying diseases and clinicopathologic variables of thrombocytopenic dogs with and without platelet-bound antibodies detected by use of a flow cytometric assay: 83 cases (2004-2006) Journal of the American Veterinary Medical Association. 2009;235:960–966. doi: 10.2460/javma.235.8.960. [DOI] [PubMed] [Google Scholar]
- Dodd K. Angiostrongylus vasorum: infestation in greyhound kennels. Veterinary Record. 1973;92:195–197. doi: 10.1136/vr.92.8.195. [DOI] [PubMed] [Google Scholar]
- Drade T, Guirand C. 6th World Conference of the WSAVA. Amsterdam: Royal Netherlands Veterinary Association; 1977. Diagnosis and treatment of cardiopulmonary angiostrongylosis; pp. 141–142. [Google Scholar]
- Feldman BF, Madewell BR, O'Neill S. Disseminated intravascular coagulation: antithrombin, plasminogen, and coagulation abnormalities in 41 dogs. Journal of the American Veterinary Medical Association. 1981;179:151–154. [PubMed] [Google Scholar]
- Feldman BF, Thomason KJ, Jain NC. Quantitative platelet disorders. Veterinary Clinics of North America Small Animal Practice. 1988;18:35–49. doi: 10.1016/s0195-5616(88)50005-0. [DOI] [PubMed] [Google Scholar]
- Garosi LS, Platt SR, McConnell JF, Wrayt JD, Smith KC. Intracranial haemorrhage associated with Angiostrongylus vasorum infection in three dogs. Journal of Small Animal Practice. 2005;46:93–99. doi: 10.1111/j.1748-5827.2005.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Golden DL, Langston VC. Uses of vincristine and vinblastine in dogs and cats. Journal of the American Veterinary Medical Association. 1988;193:1114–1117. [PubMed] [Google Scholar]
- Gould SM, McInnes EL. Immune-mediated thrombocytopenia associated with Angiostrongylus vasorum infection in a dog. Journal of Small Animal Practice. 1999;40:227–232. doi: 10.1111/j.1748-5827.1999.tb03068.x. [DOI] [PubMed] [Google Scholar]
- Grau-Bassas ER, Kociba GJ, Couto CG. Vincristine impairs platelet aggregation in dogs with lymphoma. Journal of Veterinary Internal Medicine. 2000;14:81–85. doi: 10.1111/j.1939-1676.2000.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Grindem CB, Breitschwerdt EB, Corbett WT, Jans HE. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases. Veterinary Clinical Pathology. 1991;20:38–43. doi: 10.1111/j.1939-165X.1991.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Guelfi JF. Symptomes et diagnostic de la strongylose cardio-pulmonaire du chien. L'animal de Compagnie. 1976;11:65–73. [Google Scholar]
- Jackson ML, Kruth SA. Immune-mediated haemolytic anaemia and thrombocytopenia in the dog: A retrospective study of 55 cases diagnosed from 1969 through 1983 at the Western College of Veterinary Medicine. Canadian Veterinary Journal. 1985;26:245–250. [PMC free article] [PubMed] [Google Scholar]
- Jacobs DE, Prole JH. Angiostrongylus vasorum and other nematodes in British greyhounds. Veterinary Record. 1975;96:180. doi: 10.1136/vr.96.8.180. [DOI] [PubMed] [Google Scholar]
- Jain NC. In: Essentials of Veterinary Haematology. Jain NC, editor. Philadelphia: Lea and Fabiger; 1993. The platelets; pp. 105–132. [Google Scholar]
- Joshi BC, Jain NC. Detection of antiplatelet antibody in serum and on megakaryocytes of dogs with autoimmune thrombocytopenia. American Journal of Veterinary Research. 1976;37:681–685. [PubMed] [Google Scholar]
- Joshi BC, Jain NC. Experimental immunologic thrombocytopenia in dogs: a study of thrombocytopenia and megakaryocytopoiesis. Research in Veterinary Science. 1977;22:11–17. [PubMed] [Google Scholar]
- King MCA, Grose RMR, Startup G. Angiostrongylus vasorum in the anterior chamber of a dog's eye. Journal of Small Animal Practice. 1994;35:326–328. doi: 10.1111/j.1748-5827.1994.tb03297.x. [DOI] [Google Scholar]
- Kristensen AT, Weiss DJ, Klausner JS. Platelet dysfunction associated with immune-mediated thrombocytopenia in dogs. Journal of Veterinary Internal Medicine. 1994;8:323–327. doi: 10.1111/j.1939-1676.1994.tb03244.x. [DOI] [PubMed] [Google Scholar]
- Kristensen AT, Weiss DJ, Klausner JS, Laber J, Christie DJ. Detection of antiplatelet antibody with a platelet immunofluorescence assay. Journal of Veterinary Internal Medicine. 1994;8:36–39. doi: 10.1111/j.1939-1676.1994.tb03193.x. [DOI] [PubMed] [Google Scholar]
- Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. Journal of Veterinary Internal Medicine. 1996;10:207–218. doi: 10.1111/j.1939-1676.1996.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Lewis DC, Meyers KM, Callan MB, Bucheler J, Giger U. Detection of platelet-bound and serum platelet-bindable antibodies for diagnosis of idiopathic thrombocytopenic purpura in dogs. Journal of the American Veterinary Medical Association. 1995;206:47–52. [PubMed] [Google Scholar]
- Mackin AJ, Allen DG, Johnston IB. Effects of vincristine and prednisone on platelet numbers and function in clinically normal dogs. American Journal of Veterinary Research. 1995;56:100–108. [PubMed] [Google Scholar]
- Mahaffey MB, Losonsky JM, Prestwood AK, Mahaffey EA, Lewis RE. Experimental canine angylostrongylosis: II Radiographic manifestations. Journal of the American Animal Hospital Association. 1981;17:499–502. [Google Scholar]
- Manning SP. Occular examination in the diagnosis of angiostrongylosis in dogs. Veterinary Record. 2007;160:625–627. doi: 10.1136/vr.160.18.625. [DOI] [PubMed] [Google Scholar]
- Martin MWS, Ashton G, Simpson VR, Neal C. Angiostrongylosis in Cornwall: clinical presentation of eight cases. Journal of Small Animal Practice. 1993;34:20–25. doi: 10.1111/j.1748-5827.1993.tb02570.x. [DOI] [Google Scholar]
- McConnell MF. In: Manual of canine and feline haematology and transfusion medicine. Day MJ, Mackin AJ, Littlewood JD, editor. Gloucester: British Small Animal Veterinary association, Gloucester; 2000. Overview of haemostasis; pp. 165–172. [Google Scholar]
- Patteson MW, Gibbs C, Wotton PR, Day MJ. Angiostrongylus vasorum infection in seven dogs. Veterinary Record. 1993;133:565–570. [PubMed] [Google Scholar]
- Prestwood AK, Greene CE, Mahaffey EA, Burgess DE. Experimental canine angiostrongylosis: 1 Pathologic manifestations. Journal of the American Animal Hospital Association. 1981;17:491–497. [Google Scholar]
- Ramsey IK, Littlewood JD, Dunn JK, Herrtage ME. Role of chronic disseminated intravascular coagulation in a case of canine angiostrogylosis. Veterinary Record. 1996;138:360–363. doi: 10.1136/vr.138.15.360. [DOI] [PubMed] [Google Scholar]
- Roche MM, Kelliher DJ. Angiostrongylus vasorum infestation in the dog: A case report. Irish Veterinary Journal. 1968;22:108–113. [Google Scholar]
- Rosen L, Ash LR, Wallace GD. Life history of the canine lungworm Angiostrongylus vasorum. American Journal of Veterinary Research. 1970;31:451–453. [PubMed] [Google Scholar]
- Rozanski EA, Callan MB, Hughes D, Sanders N, Giger U. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune-mediated thrombocytopenia in dogs. Journal of the American Veterinary Medical Association. 2002;220:477–481. doi: 10.2460/javma.2002.220.477. [DOI] [PubMed] [Google Scholar]
- Schelling CG, Greene CE, Prestwood AK, Tsang VC. Coagulation abnormalities associated with acute Angiostrongylus vasorum infection in dogs. American Journal of Veterinary Research. 1986;47:2669–2673. [PubMed] [Google Scholar]
- Simpson VR. Angiostrongylus vasorum infection in foxes (Vulpes vulpes) in Cornwall. Veterinary Record. 1996;139:443–445. doi: 10.1136/vr.139.18.443. [DOI] [PubMed] [Google Scholar]
- Slappendel RJ. Disseminated intravascular coagulation. Veterinary Clinics of North America Small Animal Practice. 1988;18:169–184. doi: 10.1016/s0195-5616(88)50015-3. [DOI] [PubMed] [Google Scholar]
- Trepanier LA, Danhof R, Toll J, Watrous D. Clinical findings in 40 dogs with hypersensitivity associated with administration of potentiated sulfonamides. Journal of Veterinary Internal Medicine. 2003;17:647–652. doi: 10.1111/j.1939-1676.2003.tb02495.x. [DOI] [PubMed] [Google Scholar]
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. In: Veterinary Parasitology. Second. Urquhart GM, Arour J, Duncan JL, Dunn AM, Jennings FW, editor. Oxford: Blackwell Science; 1996. The laboratory diagnosis of parasitism; pp. 269–272. [Google Scholar]
- Wessmann A, Lu D, Lamb CR, Smyth B, Mantis P, Chandler K, Boag A, Cherubini GB, Cappello R. Brain and spinal cord haemorrhages associated with Angiostrongylus vasorum infection in four dogs. Veterinary Record. 2006;158:858–863. doi: 10.1136/vr.158.25.858. [DOI] [PubMed] [Google Scholar]
- Whitley NT, Corzo-Menendez N, Carmichael NG, McGary JW. Cerebral and conjunctival haemorrhages associated with von Willebrand factor deficiency and canine angiostrongylosis. Journal of Small Animal Practice. 2005;46:75–78. doi: 10.1111/j.1748-5827.2005.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Willesen JL, Jensen AL, Kristensen AT, Koch J. Haematological and biochemical changes in dogs naturally infected with Angiostrongylus vasorum before and after treatment. The Veterinary Journal. 2009;180:106–111. doi: 10.1016/j.tvjl.2007.10.018. [DOI] [PubMed] [Google Scholar]