Abstract
Plasticity in dendritic spines may underlie learning and memory. Spinophilin, a protein enriched in dendritic spines, has the properties of a scaffolding protein and is believed to regulate actin cytoskeletal dynamics affecting dendritic spine morphology. It also binds protein phosphatase-1 (PP-1), an enzyme that regulates dendritic spine physiology. In this study, we tested the role of spinophilin in conditioned taste aversion learning (CTA) using transgenic spinophilin knockout mice. CTA is a form of associative learning in which an animal rejects a food that has been paired previously with a toxic effect (e.g., a sucrose solution paired with a malaise-inducing injection of lithium chloride). Acquisition and extinction of CTA was tested in spinophilin knockout and wild-type mice using taste solutions (sucrose or sodium chloride) or flavors (Kool-Aid) paired with moderate or high doses of LiCl (0.15 M, 20 or 40 mL/kg). When sucrose or NaCl solutions were paired with a moderate dose of LiCl, spinophilin knockout mice were unable to learn a CTA. At the higher dose, knockout mice acquired a CTA but extinguished more rapidly than wild-type mice. A more salient flavor stimulus (taste plus odor) revealed similar CTA learning at both doses of LiCl in both knockouts and wild types. Sensory processing in the knockouts appeared normal because knockout mice and wild-type mice expressed identical unconditioned taste preferences in two-bottle tests, and identical lying-on-belly responses to acute LiCl. We conclude that spinophilin is a candidate molecule required for normal CTA learning.
Dendritic spines, dynamic protrusions on the surface of dendrites, have long been implicated in synaptic-based plasticity (for review, see Shepard 1996 ). Dendritic spines receive ∼90% of excitatory synaptic contacts (Gray 1959; Harris and Kater 1994) and are functional elements involved in learning and memory (Fifkova and Van Harreveld 1977; Patel et al. 1988; Stewart et al. 1992; Andersen and Trommald 1995). Dendritic spines change shape and density in response to factors such as electrical stimulation (Fifkova and Van Harreveld 1977; Fifkova and Anderson 1981), stress (Coss and Perkel 1985), enriched environment (Globus et al. 1973), hormonal fluctuations (Gould et al. 1990; Woolley and McEwen 1992; Murphy and Segal 1996), and learning (Horn et al. 1985; Lowndes and Stewart 1994; Moser et al. 1994). Additionally, dendritic spines provide biochemical compartmentalization (by virtue of their shape and small volume) for interaction between components of second messenger systems and separation of responses to stimuli (Koch and Zador 1993).
One molecule that could participate in functional plasticity in dendritic spines is the protein spinophilin (also called neurabin II). Spinophilin, originally isolated as a protein phosphatase-1 (PP-1)-binding protein, is highly enriched in dendritic spines (Allen et al. 1997). The protein contains one PDZ domain, a coiled–coil region, and an F-actin-binding domain at its N-terminal region, indicative of a scaffolding protein (Allen et al. 1997; Satoh et al. 1998). Spinophilin may coordinate signaling events and the dynamics of spine restructuring by anchoring PP-1 close to a number of its substrates at the postsynaptic density (Allen et al. 1997), including AMPA- and NMDA-type glutamate receptors (Wang et al. 1994; Blank et al. 1997; Yan et al. 1999), calcium/calmodulin dependent protein kinase II (Strack et al. 1997a,b), protein kinase C (Keranen et al. 1995), and actin itself (Malchiodi-Albedi et al. 1997; Hsieh-Wilson et al. 1999; Stephens and Banting 2000). PP-1 plays a functional role in synaptic plasticity, because PP-1 is required for long-term depression (LTD), although not for long-term potentiation (LTP) (Mulkey et al. 1993, 1994; Blitzer et al. 1998).
Recently, spinophilin knockout mice have been developed that express some deficits relevant to synaptic plasticity (Feng et al. 2000). PP-1 regulation of glutamatergic AMPA and NMDA channels was altered in the knockouts, and hippocampal slices from the knockouts showed decreased LTD but normal LTP. Despite considerable biochemical and anatomical evidence consistent with spinophilin's role in synaptic plasticity, there are little data on its role in learning and memory in whole animals. We examined the ability of the spinophilin knockout mice (Feng et al. 2000) to learn conditioned taste aversions (CTA). CTA is a form of associative learning in which an animal learns to reject a food after the food has been paired with a malaise-inducing effect. CTA is a distinctive type of learning because: (1) it can be robustly acquired after a single taste-toxin pairing (Garcia et al. 1966; Garcia and Koelling 1967); (2) it supports long delays between taste and toxin (e.g., intervals up to 12 h; Smith and Roll 1967); and (3) it is exceptionally long-lasting (months to years) after a small number of learning trials (Houpt et al. 1996). We assessed spinophilin knockout mice in a series of CTA tests in which novel palatable tastes or flavors were paired with toxic injections of lithium chloride (LiCl). To determine if sensory processing was normal, knockout mice were also tested for unconditioned taste preferences and behavioral sensitivity to the toxic effects of LiCl.
RESULTS
Conditioned Taste Aversion
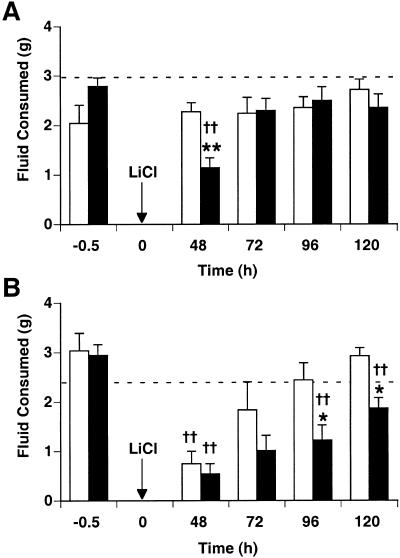
Two days after sucrose or salt solutions were paired with LiCl (0.15 M, 20 mL/kg, i.p.), wild-type, but not spinophilin knockout, mice expressed a significant taste aversion that was extinguished by the next day (Fig. 1A). Two-way ANOVA on LiCl postinjection intakes revealed a significant interaction of genotype and test time (F[1,3] = 3.1, P < 0.05). Wild-type mice had significantly lower intakes than spinophilin knockout mice and NaCl-injected controls for only the initial testing period (48 h following taste-toxin pairing; P < 0.01). The intakes of spinophilin knockout mice did not differ significantly from NaCl-injected controls at any time point.
Figure 1.
Conditioned taste aversion at low and high doses of LiCl. Taste solutions of 5% sucrose and 75 mM NaCl were offered for 30 min and mice were injected with either malaise-inducing LiCl or NaCl as control (n = 6–10/group). Beginning 48 h after pairing, CTA was tested in single bottle, 30 min intake tests at 24 h intervals. Arrows indicate time at which LiCl was injected (0.15 M, 20 mL/kg or 40 mL/kg, i.p.). Dashed lines represent average intakes of NaCl-injected controls (knockout pooled with wild type). (A) CTA after sucrose and saline paired with 20 mL/kg LiCl. Spinophilin knockout mice (white bars) did not express CTA at all; wild-type mice (black bars) express CTA 48 h postinjection, which is rapidly extinguished after just 24 h. (B) CTA after sucrose and saline paired with 40 mL/kg LiCl. Both spinophilin knockouts and wild types express CTA at 48 h. CTA was extinguished rapidly in knockout mice, returning to control levels 24 h later. Wild-type CTA extinguished more slowly, significantly differing from knockouts at 96 h and 120 h. (*) P < 0.05 vs. wild type; (**) P < 0.01 vs. wild type; (†) P < 0.05 vs. NaCl-injected controls; (††) P < 0.01 vs. NaCl-injected controls.
At 40 mL/kg LiCl, both spinophilin knockout and wild-type mice expressed a significant taste aversion (Fig. 1B). CTA expression in wild-type mice extinguished slowly over the course of four extinction tests, but CTA extinguished more rapidly in knockout mice, returning to control levels by the second postinjection trial. LiCl postinjection intakes were affected significantly by genotype (P < 0.01) and test time (P < 0.0001) but no significant interaction was found. Beginning at 96 h after pairing, spinophilin knockout intakes were greater than intakes of wild-type mice (P < 0.05).
Conditioned Flavor Aversion
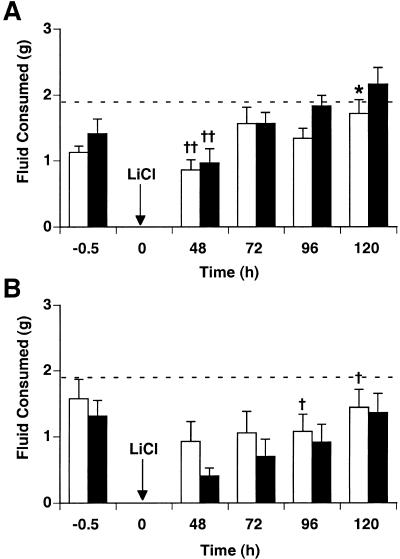
When a complex flavor stimulus (saccharin in grape or cherry Kool-Aid) was paired with a 20 mL/kg LiCl injection (Fig. 2A), both spinophilin knockout and wild-type mice expressed conditioned flavor aversions (CFAs) that were rapidly extinguished. Both genotypes had significantly lower intakes than NaCl-injected controls at 48 h postinjection only (P < 0.01). LiCl postinjection intakes were affected significantly by test time (F[1,3] = 10.0, P < 0.0001) but no interaction between genotype and test time together was found; spinophilin knockout intakes were significantly lower at 120 h postinjection only (P < 0.05).
Figure 2.
Conditioned flavor aversion at low and high doses of LiCl. Taste solutions of grape Kool-Aid and cherry Kool-Aid, sweetened with 0.2% saccharin, were offered for 30 min and mice were injected with either malaise-inducing LiCl or NaCl as control (n = 6–10/group). Beginning 48 h after pairing, CFA was tested in single bottle, 30 min intake tests at 24 h intervals. Arrows indicate time at which LiCl was injected (0.15 M, 20 mL/kg or 40 mL/kg, i.p.). Dashed lines represent average intakes of NaCl-injected controls (knockout together with wild type). (A) CFA after pairing grape and cherry Kool-Aid with 20 mL/kg LiCl. Both spinophilin knockout mice (white bars) and wild-type mice (black bars) express CFA at 48 h, which is rapidly extinguished. Knockouts had significantly lower intakes at 120 h when compared to wild type intakes. (B) CFA after pairing grape and cherry Kool-Aid with 40 mL/kg LiCl. There was a significant overall effect of drug, but not of test time. (*) P < 0.05 vs. wild type; (†) P < 0.05 vs. NaCl-injected controls; (††) P < 0.01 vs. NaCl-injected controls.
Similarly, both wild-type mice and knockout mice expressed CFA after pairing saccharin–Kool-Aid solutions with 40 mL/kg LiCl (Fig. 2B). Mean intakes of spinophilin knockout mice after LiCl injection were higher than intakes of wild-type mice, but intakes for both genotypes did not significantly differ from one another at any time point. Two-way ANOVA revealed a significant drug effect (KO: F[1,3] = 16.8, P < 0.0001; WT: F[1,3] = 10.5, P < 0.01) but no interaction between drug and test time was found.
Unconditioned Taste Preferences
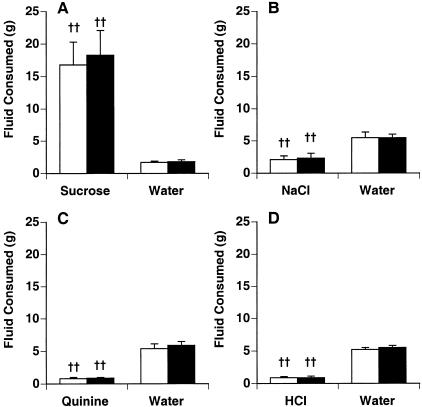
Average 48 h intakes of four taste solutions (sweet, salty, bitter, and acidic) were compared against intakes of water in two-bottle unconditioned taste preference tests (Fig. 3). Spinophilin knockout mice and wild-type mice showed normal taste preferences, with no effect of genotype for any test. Both genotypes preferred the 5% sucrose to water (F[1,20] = 26.4, P < 0.0001 ; Fig. 3A), preferred water to 75 mM NaCl (F[1,20] = 23.4, P = 0.0001; Fig. 3B), and avoided 30 μM quinine sulfate (F[1,22] = 127, P < 0.0001; Fig. 3 C) and 0.01 M HCl solutions (F[1,22] = 127, P < 0.0001; Fig. 3D).
Figure 3.
Unconditioned taste preference. Intakes for four taste solutions were compared against intakes for water (n = 5–6/group) during 48 h two-bottle tests. Spinophilin knockout mice (white bars) and wild-type mice (black bars) intakes were not significantly different from one another for any two-bottle test. Both genotypes showed a strong preference for 5% sucrose (A), preferred water slightly >75 mM NaCl (B), and avoided both 30 μM quinine sulfate (C) and 0.01 M HCl (D). (††) P < 0.01 vs. water.
LiCl Toxicity
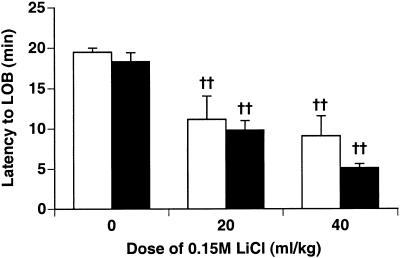
Knockout and wild-type mice showed identical unconditioned responses to the toxic effect of LiCl injection (Fig. 4). At 20 mL/kg and 40 mL/kg LiCl or NaCl, a significant effect of drug on latency to “lying-on-belly” (LOB) was seen (P < 0.001). Mice injected with LiCl display LOB after 10 min, whereas NaCl-injected mice rarely, if ever, display LOB.
Figure 4.
LiCl toxicity test. Knockout mice and wild-type mice were injected with either LiCl or NaCl, and latency to lying on belly (LOB) was recorded for 20 min (n = 12 at 0 mL/kg, n = 6 at 20 mL/kg and 40 mL/kg LiCl). Mice that did not LOB within 20 min were assigned a latency of 20 min. Knockout (white bars) and wild-type mice (black bars) showed the same reaction to LiCl toxicity. Both genotypes receiving LiCl-injections differed significantly from NaCl-injected controls. (††) P < 0.01 vs. NaCl-injected controls.
DISCUSSION
We have found that spinophilin is necessary for normal CTA learning. Spinophilin knockout mice had intact sensory processing but a significant impairment in their associative learning ability compared to wild-type littermates. Using both a low and high dose of LiCl allowed us to discern the level of impairment in knockout CTA learning. At the low dose (20 mL/kg LiCl), knockout mice did not express CTA. However, knockouts expressed CTA when given a higher dose of LiCl (40 mL/kg), although the CTA was more labile and extinguished more rapidly in knockout mice than in wild-type mice. When a more salient flavor stimulus was paired with LiCl, the spinophilin knockouts showed similar aversion learning to the wild-types at both doses. This suggests that CTA learning was not impossible for spinophilin knockout mice, but was significantly compromised.
This is the first observation that spinophilin plays a role in learning in vivo. Spinophilin does not appear to be essential for some CTA learning to take place, however. Increasing the dose of LiCl or the salience of the taste or flavor paired with LiCl allowed the mice to learn.
Spinophilin is known to bind both PP-1 and F-actin, suggesting its involvement in regulating neurotransmitter receptors and ion channels, as well as mediating dendritic spine plasticity via the actin cytoskeleton (Allen et al. 1997; Malchiodi-Albedi et al. 1997; Satoh et al. 1998; Hsieh-Wilson et al. 1999; Stephens and Banting 2000). We have found an additional role for spinophilin as a candidate molecule involved in the neural mechanism underlying CTA learning. Knockout mice showed impaired CTA learning when simple taste solutions were paired with LiCl but intact CTA learning when conditioned stimuli had both taste and odor components. This suggests that the learning deficiency most likely does not involve olfactory processing but instead, seems to involve taste processing or higher-order associative processing.
CTA acquisition and retention has been linked previously to several neural substrates: nucleus of the solitary tract in brainstem, parabrachial nucleus in pons, gustatory cortex, and central nucleus of the amygdala in the limbic system, but not hippocampus (Braun et al. 1972; Flynn et al. 1991; Houpt et al. 1994; Yamamoto et al. 1994; Schafe et al. 1998). Thus, we have found an effective behavioral paradigm that will help elucidate additional functions of spinophilin outside of hippocampal-dependent learning and memory paradigms such as spatial learning in water maze tasks (Morris et al. 1982).
Despite the lack of spinophilin in the knockouts, PP-1 remains highly enriched in dendritic spines (C. Ouimet, J. Feng, and P. Greengard, unpubl.). However, PP-1's efficacy may be attenuated because it is no longer anchored at an optimal position near its preferred substrates at the postsynaptic density (PSD) in dendritic spines. Many of the alterations seen in spinophilin knockouts are consistent with decreased efficacy of PP-1-regulated dephosphorylation (Feng et al. 2000). PP-1 regulation of glutamatergic channels was altered in the knockouts: rundown of AMPA currents was decreased, and enhancement of NMDA currents by PP-1 inhibitors was attenuated. Further investigation revealed decreased LTD but normal LTP in hippocampal slices from spinophilin knockouts, consistent with earlier studies demonstrating that PP-1 is required for LTD but not LTP (Mulkey et al. 1993, 1994; Blitzer et al. 1998). Spinophilin knockout mice also show resistance to kainate-induced seizures and apoptosis, suggesting abnormal glutamatergic receptor function. These physiological decrements are analogous to our behavioral results showing that in spinophilin knockout mice, CTA learning was attenuated rather than completely blocked.
The functions of PP-1 substrates at the PSD could be altered in the spinophilin knockout mice. Calcium/calmodulin dependent protein kinase II (CaMK-II), through phosphorylation, regulates neurotransmission and cell morphology (Braun and Schulman 1995) and is enriched at the PSD (Kelly et al. 1984). CaMK-II may no longer be dephosphorylated effectively by PP-1 in the spinophilin knockouts. Previous studies have shown that PP-1 down-regulates NMDA and AMPA-type glutamate receptors (Wang et al. 1994; Yan et al. 1999). Glutamate receptors have been implicated in LTP, a physiological plasticity believed to be related to whole animal learning (Wang et al. 1994; Blank et al. 1997; Yan et al. 1999).
NMDA receptors have been implicated as necessary for CTA learning in the gustatory area of insular cortex (Rosenblum et al. 1997). During CTA training, blockade of NMDA receptors by the antagonist APV impaired CTA memory. NMDA receptor inhibition by PP-1 in the absence of spinophilin is greatly attenuated, suggesting that PP-1 can no longer properly regulate these channels (Feng et al. 2000). Improper NMDA receptor functioning could compromise taste learning. Our findings support this notion; although PP-1 can still presumably dephosphorylate NMDA receptors in the spinophilin knockouts, it can no longer do so optimally.
Spinophilin is expressed widely throughout the rodent brain. Using immunohistochemistry, spinophilin was reported to be mostly highly expressed in the hippocampus, with less dense expression in the cerebral cortex, striatum, and thalamus (Allen et al. 1997). Further studies to verify the subcellular localization of spinophilin to spines in brain regions mediating CTA (e.g., nucleus of the solitary tract in brainstem, parabrachial nucleus in pons, gustatory cortex, and central nucleus of the amygdala in the limbic system) versus CFA should be undertaken. Given the findings of this study, possible functional deficiencies in neuronal activity within brain circuits in spinophilin knockout mice could also be explored. For example, expression of the immediate early gene c-Fos is required for encoding CTA learning (Lamprecht and Dudai 1996; Swank et al. 1996). c-Fos activation might be compromised during taste learning in the knockouts.
As in many transgenic knockout studies, it is unknown if the learning deficit found in spinophilin knockouts is caused by a developmental deficiency or to an acute lack of spinophilin at the time of acquisition or expression of learning. Thus, it would be interesting to develop inducible spinophilin repressor mice that are functionally dependent on a specific ligand to control for the onset of the spinophilin deficit, enabling the researcher to repress the gene of interest at critical stages of learning and recall. This would allow for more detailed studies to confirm a role for spinophilin in a model of behavioral learning and memory.
MATERIALS AND METHODS
Animals
Adult male C57BL6 × 129/Sv spinophilin knockout mice (−/−) and wild-type littermates (+/+) (4–6 mo old) were generated at the Rockefeller University as described previously (Feng et al. 2000). There was a significant difference in body weight between knockout (30±2.5g) and wild-type mice (35±2.2 g; P < 0.01). All mice were caged individually and kept on a 12:12 light-dark cycle at 22°C with food and water available ad libitum unless noted otherwise. Experiments were conducted during the light phase of the cycle.
Conditioned Taste Aversion
To test the ability of spinophilin knockout mice to form taste aversions, knockouts and wild-type littermates were conditioned against sucrose or NaCl paired with high and low doses of LiCl. Mice were placed on a water deprivation schedule that restricted access to water overnight for at least one week, with water returned each morning for 6–8 h (n = 6–10 for each LiCl dose). On the conditioning day, a 5% sucrose solution was available for 30 min. Drinking tubes were weighed before and after test periods to measure fluid intake. At the end of the 30 min access to sucrose, mice were injected with LiCl or NaCl at one of two doses (0.15 M; 20mL/kg or 40 mL/kg). In a preliminary experiment, the doses 20 mL/kg and 40 mL/kg were found to give a submaximal and maximal CTA in wild-type C57BL6/J mice, respectively (data not shown). Drinking water was returned after the injection for 6–8 h. The deprivation schedule resumed until 48 h postpairing when mice were given 5% sucrose for 30 min and then water for the remainder of the day. Sucrose consumption during 30 min access after overnight water deprivation was measured daily for 4–5 consecutive testing days. After another week of nightly water deprivation, mice were given access to a 75 mM NaCl solution for 30 min. At the end of the 30 min access to the NaCl solution, mice injected with LiCl in the preceding week received NaCl and mice injected with NaCl received LiCl (0.15 M, 20 mL/kg or 40 mL/kg, i.p.). Drinking water was returned after the injection for 6–8 h. The deprivation schedule resumed until 48 h postpairing when mice were given 75 mM NaCl for 30 min and then water for the remainder of the day. NaCl consumption during 30 min access after overnight water deprivation was measured daily for 4–5 consecutive testing days. Because no significant difference was found in intakes between taste solutions, the results from sucrose and NaCl conditioning at each LiCl dose were pooled.
Conditioned Flavor Aversion
CFA was also studied using Kool-Aid solutions that combined gustatory and olfactory stimuli. Mice were placed on a water deprivation schedule that restricted access to water overnight for at least one week, with water returned each morning for 6–8 h (n = 6–10 for each LiCl dose). On the conditioning day, grape Kool-Aid sweetened with 0.2% saccharin was available for 30 min. At the end of the 30 min access to grape Kool-Aid, mice were injected with LiCl or NaCl at one of two doses (0.15 M; 20mL/kg or 40 mL/kg). Drinking water was returned after the injection for 6–8 h. The deprivation schedule resumed until 48 h postpairing when mice were given grape Kool-Aid for 30 min and then water for the remainder of the day. Grape Kool-Aid consumption during 30 min access after overnight water deprivation was measured daily for 4–5 consecutive testing days. After another week of nightly water deprivation, mice were given access to cherry Kool-Aid sweetened with 0.2% saccharin for 30 min. At the end of the 30 min access to cherry Kool-Aid, mice injected with LiCl in the preceding week received NaCl and mice injected with NaCl received LiCl (0.15 M, 20 mL/kg or 40 mL/kg, i.p.). Drinking water was returned after the injection for 6–8 h. The deprivation schedule resumed until 48 h postpairing when mice were given cherry Kool-Aid for 30 min and then water for the remainder of the day. Cherry Kool-Aid consumption during 30 min access after overnight water deprivation was measured daily for 4–5 consecutive testing days. Intakes of grape and cherry Kool-Aid were pooled at each dose of LiCl or NaCl.
Unconditioned Taste Preferences
The ability of knockout mice to discriminate basic taste qualities was tested using two-bottle, 48 h intake tests. Mice were housed in cages with access to two drinking tubes. Mice were given one empty tube and one tube containing water. Every 24 h the tubes were switched to train the mice to drink from either tube position. After one week, mice were given one tube that contained one of four taste solutions (5% sucrose, 75 mM NaCl, 30 μM quinine sulfate, or 0.01 M HCl), and the other contained water. Tube position was switched after 24 h, and intake was recorded again at the end of 48 h. All mice were tested with all four taste solutions (n = 5–6 for each taste solution).
LiCl Toxicity
To test knockout and wild type response to LiCl toxicity, mice were injected with LiCl or NaCl (0.15 M, 20 mL/kg or 40 mL/kg, i.p., n = 6 at each dose). Two observers scored behavior for 20 min and recorded latency to the LOB response. LOB was characterized by periods of immobility, lack of grooming, and a flattened, stretched out posture (Parker et al. 1984; Meachum and Bernstein 1990). Mice not observed in the LOB position at any time during the testing period received a latency score of 20 min. Each mouse was observed after one LiCl injection and one NaCl injection, at each dose, with at least three days between test injections.
Statistics
Postinjection intakes during extinction trials were analyzed using two-way ANOVA and Fisher' LSD for posthoc comparisons. To detect differences between wild-type and knockout mice that received solutions paired with LiCl injection, intakes after LiCl injection were analyzed using genotype and test time as factors. To detect differences between LiCl-injected mice and NaCl-injected control mice, intakes after injection were analyzed separately for knockout mice or wild-type mice, with drug and test time as factors.
Acknowledgments
This work was supported by National Institutes of Health grants DA10044 (to P.G.), DC03198 (to T.A.H.), MH40899 (to P.G. and C.O.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL houpt@neuro.fsu.edu; FAX (850) 644-0989.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.42101.
REFERENCES
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Trommald M. Possible strategies for finding the substrate for learning-induced changes in the hippocampal cortex. J Neurobiol. 1995;26:396–402. doi: 10.1002/neu.480260311. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Teichert U, Kugler H, Behrsing H, Feinberg A, Greengard P, Spiess J. The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proc Natl Acad Sci. 1997;94:14859–14864. doi: 10.1073/pnas.94.26.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Ann Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in the learning of taste aversions. Physiol Behav. 1972;9:637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- Coss RG, Perkel DH. The function of dendritic spines: A review of theoretical issues. Behav Neural Biol. 1985;44:151–181. doi: 10.1016/s0163-1047(85)90170-0. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifkova E, Anderson CL. Stimulation-induced changes in dimensions of stalks of dendritic spines in the dentate molecular layer. Exp Neurol. 1981;74:621–627. doi: 10.1016/0014-4886(81)90197-7. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977;6:211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Schulkin J, Grill HJ, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. A comparison of aversions induced by X-rays, toxins, and drugs in the rat. Radiation Res Supl. 1967;7:439–450. [PubMed] [Google Scholar]
- Garcia J, Ervin FR, Koelling RA. Learning with prolonged delay of reinforcement. Psychonomic Sci. 1966;1966:121–122. [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley C, Frankfurt M, McEwen B. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscopic study. J Anat. 1959;83:420–433. [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: Cellular specializations imparting both stability and flexibility to synaptic function. Ann Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Horn G, Bradley P, McCabe BJ. Changes in the structure of synapses associated with learning. J Neurosci. 1985;5:3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Wessel TC, Joh TH, Smith GP. Increased c-Fos expression in the rat nucleus of the solitary tract after conditioned taste aversion formation. Neurosci Lett. 1994;172:1–5. doi: 10.1016/0304-3940(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Joh TH, Smith GP. c-Fos induction in the rat nucleus of the solitary tract correlates with the retention and forgetting of a conditioned taste aversion. Learn Mem. 1996;3:25–30. doi: 10.1101/lm.3.1.25. [DOI] [PubMed] [Google Scholar]
- Hsieh-Wilson LC, Allen PB, Watanabe T, Nairn AC, Greengard P. Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1. Biochem. 1999;38:4365–4373. doi: 10.1021/bi982900m. [DOI] [PubMed] [Google Scholar]
- Kelly PT, McGuinness TL, Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci. 1984;81:945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- Koch C, Zador A. The function of dendritic spines: Devices subserving biochemical rather than electrical compartmentalization. J Neurosci. 1993;13:413–422. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- Lowndes M, Stewart MG. Dendritic spine density in the lobus parolfactorius of the domestic chick is increased 24 h after one-trial passive avoidance training. Brain Res. 1994;654:129–136. doi: 10.1016/0006-8993(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Malchiodi-Albedi F, Petrucci T, Picconi B, Iosi F, Falchi M. Protein phosphatase inhibitors induce modification of synapse structure and tau hyperphosphorylation in cultured rat hippocampal neurons. J Neurosci Res. 1997;48:425–438. [PubMed] [Google Scholar]
- Meachum CL, Bernstein IL. Conditioned responses to a taste conditioned stimulus paired with lithium chloride administration. Behav Neurosci. 1990;104:711–715. doi: 10.1037//0735-7044.104.5.711. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Hills K, Jensen K. Behavioral CRs elicited by a lithium- or an amphetamine-paired contextual test chamber. Anim Learn Behav. 1984;12:307–315. [Google Scholar]
- Patel SN, Rose SPR, Stewart MG. Training induced dendritic spine density changes are specifically related to memory formation processes in the chick, Gallus domesticus. Brain Res. 1988;463:168–173. doi: 10.1016/0006-8993(88)90542-2. [DOI] [PubMed] [Google Scholar]
- Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci. 1997;17:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y. Neurabin-II/Spinophilin: An actin filament-binding protein with one PDZ domain localized at cadherin-based cell–cell adhesion sites. J Biol Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Thiele TE, Bernstein IL. Conditioning method dramatically alters the role of amygdala in taste aversion learning. Learn & Mem. 1998;5:481–492. [PMC free article] [PubMed] [Google Scholar]
- Sheperd G. The dendritic spine: A multifunctional unit. J Neurophysiol. 1996;75:2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- Smith JC, Roll DL. Trace conditioning with X-rays as an aversive stimulus. Psychon Sci. 1967;9:11. [Google Scholar]
- Stephens DJ, Banting G. In vivo dynamics of the F-actin binding protein neurabin-II. Biochem J. 2000;345:185–194. [PMC free article] [PubMed] [Google Scholar]
- Stewart MG, Lowdnes M, Hunter A, Doubell T. Memory storage in chicks involves an increase in dendritic spine number and synaptic density. Brain Dysfunct. 1992;5:50–64. [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997a;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J Biol Chem. 1997b;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Swank MW, Ellis AE, Chochran BN. c-Fos antisense blocks acquisition and extinction of conditioned taste aversion in mice. Neuroreport. 1996;7:1866–1870. doi: 10.1097/00001756-199607290-00036. [DOI] [PubMed] [Google Scholar]
- Wang LY, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatase 1 and 2A. Nature. 1994;369:230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65:123–137. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: Regulation by DARPP-32 and spinophilin. Nature Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]