Abstract
The objective of this study was to examine the impact of hospitalisation and antimicrobial drug administration on the prevalence of resistance in commensal faecal E. coli of horses. Faecal samples were collected from ten hospitalised horses treated with antimicrobials, ten hospitalised horses not treated with antimicrobials and nine non-hospitalised horses over a consecutive five day period and susceptibility testing was performed on isolated E. coli. Results revealed that hospitalisation alone was associated with increased prevalence of antimicrobial resistance and multidrug resistance in commensal E. coli of horses. Due to the risk of transfer of resistance between commensal and pathogenic bacteria, veterinarians need to be aware of possible resistance in commensal bacteria when treating hospitalised horses.
Keywords: Horse, antimicrobial, resistance, hospitalisation, Escherichia coli
Introduction
Since their introduction in the 1930 s, antimicrobials have revolutionised the treatment of infectious disease in human and veterinary patients, significantly enhancing treatment success and outcome. However in recent years it has become obvious that the frequent and sometimes indiscriminate use of these compounds in both human and veterinary medicine has selected for resistance among bacteria with an associated increase in morbidity and mortality from infectious disease [14,15].
Antimicrobial use, whether for therapy or prevention of bacterial diseases, will result in selection for antimicrobial resistant micro-organisms by elimination of susceptible bacteria [11]. Resistance is classified as intrinsic (naturally present) or acquired. Acquired resistance can arise because of chromosomal mutation or, more importantly, through the acquisition of transferable genetic material [7]. Following the use of antimicrobial compounds resistance emerges, not only among pathogens but also among the endogenous microflora of animals [22]. Although resistance in these commensal bacteria may be of no consequence in one host species, these organisms may cause disease in other hosts and resistant commensal bacteria can serve as reservoirs of resistance genes [14]. For example, antibiotic-resistant bacteria from animals can colonise or infect humans via occupational exposure or via the food chain and resistance genes can be transferred from bacteria of animal origin to human pathogens in the intestinal flora of humans [22]. In the past decade, infection of patients in human hospitals with antimicrobial-resistant pathogens has become a common occurrence and is now recognised to be of growing concern in veterinary hospitals also. In human medicine, hospitals are considered to be a significant source of resistant bacteria although healthy people living outside hospitals may also be important reservoirs of resistance genes [22,16]. In equine hospitals, outbreaks of disease involving multidrug-resistant bacteria have occurred [18,24] and in the USA commensal bacteria of hospitalised horses have been shown to have a higher prevalence of antimicrobial resistance when compared to commensal bacteria of horses in their normal stable or field environment [8].
Antimicrobial resistance is one of the most critical current issues in human and veterinary medicine [19], with many organisms resistant to multiple classes of antimicrobial compound [15]. In horses, multidrug resistance (MDR) has been observed in pathogenic and commensal bacteria including Salmonella Typhimurium, Staphylococcus aureus and E. coli [1,4,18,24,8]. While the identification of MDR strains of bacteria in horses has implications in the treatment of bacterial infections in this species, public health implications are also an important concern. Many horses are companion animals and some enter the food chain; therefore, the presence of antimicrobial resistance in the bacterial flora of horses poses a risk to public health.
There are limited data in Europe on antimicrobial resistance in hospitalised horses and the aim of this study was to examine the impact of hospitalisation and antimicrobial administration on the prevalence of resistance and MDR in commensal faecal E. coli of horses. This study also investigated the effects of duration of hospitalisation on the prevalence of antimicrobial resistance and susceptibility of isolates to antimicrobial drugs commonly used in horses and those normally reserved for use in humans.
Materials and methods
Study overview
This was an observational study in which faecal samples were collected over a six month period (January 2008 through July 2008) from three different groups of horses. Three faecal samples were obtained from each horse over a period of five consecutive days. E. coli recovered from each faecal sample were subsequently evaluated to determine their susceptibility to a panel of antimicrobial compounds.
Study population
Horses included in the study were those that were hospitalised at the University Veterinary Hospital (UVH) during the study period (denoted as Groups A and B) or horses that belonged to a closed herd owned by the University (denoted as Group C). Group A consisted of hospitalised horses that were treated with various systemic antimicrobial agents (penicillin, gentamicin or potentiated sulphonamides, or combinations of the former) starting within 24 hours of admission, and continuing for at least four days. Group B consisted of horses hospitalised for the same duration that did not receive antimicrobial agents. Group C horses were housed in their normal stable environment at a site remote from the hospital and were considered healthy based on their history and physical examination. Horses were excluded from the study if they were either less than two years old, had been treated with antimicrobial agents within the two weeks prior to presentation to the hospital or were presented for complaints involving the gastrointestinal system.
Sample collection
For all horses, faecal samples were collected from the top of a fresh faecal pile into a sterile 20 ml universal container up to the 20 ml mark. Samples were collected on days one, three and five of hospitalisation for groups A and B and on days one, three and five of a consecutive five day period for horses in group C. For groups A and B, the day one faecal sample was collected as close as possible to the time of hospital admission and was always within twelve hours of admission. All samples taken on days three and five were collected 48 hours after the previous sample (+/- three hours). All samples were immediately refrigerated until the time of processing.
Culture methods
All faecal samples were processed within 72 hours of collection. Standard bacterial culture was used to recover E. coli from the specimens. Briefly, a swab taken from each sample was plated directly onto a MacConkey No. 2 (Oxoid Limited) agar plate that was incubated at 37°C for 18 to 24 hours. Up to five randomly selected isolates (minimum of three) from each faecal sample, presumptively identified as E. coli on the basis of colony morphology and lactose fermentation, were subcultured onto a Harlequin TBGA agar (Lab M Ltd) plate that was incubated at 37°C for 18 to 24 hours. The presence of a blue-green colony colour on the Harlequin TBGA agar plate was considered as positive identification of E. coli.
All suspect isolates that gave inconclusive results on Harlequin TBGA agar, as well as a random selection of isolates positive on Harlequin TBGA agar, were confirmed as E. coli using API 32E (Biomerieux Inc).
Antimicrobial susceptibility testing
Susceptibility testing for 12 antimicrobials, from seven different antimicrobial classes (aminoglycosides [gentamicin], β-lactams [amoxicillin clavulanate, ampicillin, cefpodoxime, cefquinome, ceftiofur], chloramphenicol [chloramphenicol], fluoroquinolones [ciprofloxacin, marbofloxacin], polymyxin [colistin], potentiated sulphonamides [trimethoprim sulphamethoxazole], tetracyclines [tetracycline]) was performed on each isolate using the Clinical and Laboratory Standards Institute (CLSI) disc diffusion method [5]. The antimicrobials tested were selected based on their WHO and OIE classification (Table 1) and their frequency of use in horses in Ireland. Isolates were categorised as susceptible, of intermediate susceptibility or resistant to each antimicrobial (Table 1). For the purposes of statistical analysis isolates of intermediate susceptibility were considered to be sensitive. MDR was defined as resistance to three or more antimicrobial classes.
Table 1.
Disc strength, zones of inhibition, WHO and OIE rank of each antimicrobial tested.
| Antimicrobial Disc | Disc Strength | Zones of inhibition (mm) | WHO rank 2007 | OIE rank 2007 (including horses) | ||
|---|---|---|---|---|---|---|
| Resistant | Intermediate | Susceptible | ||||
| Gentamicin | 10 mcg | ≤ 12 | 13-14 | ≥ 15 | Critical | Critical |
| Amoxicillin Clavulanate | 20/10 mcg | ≤ 13 | 14-17 | ≥18 | Critical | Critical |
| Ampicillin | 10 mcg | ≤ 13 | 14-16 | ≥17 | Critical | Critical |
| Cefpodoxime | 10 mcg | ≤ 17 | 18-20 | ≥21 | Critical | |
| Ceftiofur | 30 mcg | ≤ 19 | 20-23 | ≥24 | Critical | |
| Cefquinome | 30 mcg | ≤ 19 | 20-22 | ≥23 | Critical | |
| Chloramphenicol | 30 mcg | ≤ 12 | 13-17 | ≥18 | Florphenicol listed as critical Chloramphenicol used as indicator | |
| Ciprofloxacin | 5 mcg | ≤ 15 | 16-20 | ≥21 | Critical | |
| Marbofloxacin | 5 mcg | ≤ 14 | 15-18 | ≥19 | Critical | |
| Colistin | 25 mcg | ≤ 14 | ≥15 | Critical | ||
| Trimethoprim sulphamethoxazole | (1.25/23.75) 25 mcg | ≤ 10 | 11-15 | ≥16 | Highly important | Critical |
| Tetracycline | 30 mcg | ≤ 14 | 15-18 | ≥19 | Highly important | Critical |
Data management and statistical analysis
Data were entered into and managed with Microsoft Office Excel 2007 (Microsoft Corporation) and analysed using Stata version 10 (Stata Corp). A horse was classified as demonstrating antimicrobial resistance if at least one isolate showed resistance to at least one antimicrobial. A horse was classified as demonstrating MDR if at least one isolate showed resistance to at least three classes of antimicrobials. Microsoft Office Excel was used to calculate the prevalence of resistance and MDR for each group of horses. A number of independent variables including horse, age, breed, sex, hospitalisation and treatment were assessed for each day of sampling using exact logistic regression (stata exlogistic) with the horse as the unit of interest. Due to the diversity of antimicrobial treatment protocols within group A horses, treatment was considered as a single effect for statistical analysis. The outcomes of interest were resistance status and MDR status. Univariate screening was performed so that variables with P < 0.20 at the univariate level became candidates for the model. Only hospitalisation, treatment and day of sampling were significant at P < 0.20. Multivariable analysis of the effects of treatment and day of sampling were performed using logistic generalised estimating equations (stata xtgee) to account for repeated measures within horses. As no group C (non-hospitalised) horses required treatment or developed MDR, the model was used to assess hospitalised horses only. No interaction terms were considered in the model because of the sample size.
Results
Study population
A total of 29 horses were evaluated in the study (group A, n = 10; group B, n = 10; group C, n = 9). The average age of all horses was 9 years, ranging from 2 to 31 years. The population was composed of a mix of breeds including Irish Sporthorses (n = 16), Cobs (n = 7), Thoroughbreds (n = 2), Shetland ponies (n = 2), Clydesdale (n = 1) and an Irish draught (n = 1).
Antimicrobial resistance and multidrug resistance
Descriptive analysis
A total of 412 E. coli isolates were tested for susceptibility to the selected antimicrobial agents. Of these, 28.6% (118/412) were resistant to at least one antimicrobial while 53.4% (63/118) of the resistant isolates demonstrated MDR.
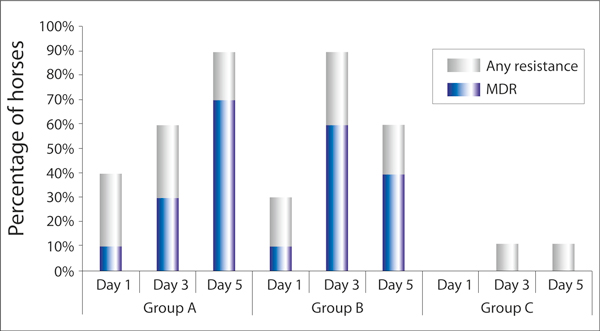
Group A - Between days one and five there was an increase in the prevalence of horses with at least one isolate resistant to at least one of the antimicrobials tested (Figure 1). A similar pattern was observed for MDR isolates in this group (Figure 1).
Figure 1.
The percentage of horses in each study group with at least one E. coli isolate showing resistance to at least one antimicrobial or multidrug resistance (MDR) on each day of sample collection (Group A (n = 10) - hospitalised horses treated with antimicrobials, Group B (n = 10) - hospitalised horses not treated with antimicrobials, Group C (n = 9) - non-hospitalised untreated horses).
Group B - The prevalence of horses with at least one isolate resistant to at least one antimicrobial tested increased between days one and three and decreased by day five (Figure 1) with a similar pattern observed for MDR isolates (Figure 1).
Group C - Antimicrobial resistance was not detected in any isolates from day one samples but was detected in one horse on day three and five (Figure 1). MDR was not detected in any isolates.
Descriptive analysis
The result of the exact logistic regression showed hospitalisation on days three and five was significantly associated with general antimicrobial resistance status (Table 2) and MDR (Table 3). The results of the multivariable analysis in hospitalised horses showed no significant effect of treatment on general antimicrobial resistance status (Table 4) and MDR (Table 5). Day of sampling was associated with increased general antimicrobial resistance status (Table 4) and MDR (Table 5). The effect of day of sampling on MDR increased on day five. Overall, hospitalisation status and day of sampling had the greatest association with the occurrence of general antimicrobial resistance and MDR.
Table 2.
Exact logistic regression results for the effect of hospitalisation on resistance to any individual antimicrobial on days 1, 3 and 5 of sampling
| Odds ratio | 95% Confidence Intervals | p-value | ||
|---|---|---|---|---|
| Day 1 | 5.88a | 0.75 | + inf | 0.099 |
| Day 3 | 20.99 | 2.05 | 1133.15 | 0.004 |
| Day 5 | 20.99 | 2.05 | 1133.15 | 0.004 |
(a) median unbiased estimates
Table 3.
Exact logistic regression results for the effect of hospitalisation on MDR status on days 1, 3 and 5 of sampling.
| Odds ratio | 95% Confidence Intervals | p-value | ||
|---|---|---|---|---|
| Day 1 | 1.12a | 0.08 | + inf | 0.936 |
| Day 3 | 8.88a | 1.16 | + inf | 0.034 |
| Day 5 | 13.05a | 1.72 | + inf | 0.010 |
(a) median unbiased estimates
Table 4.
Multivariable analysis of the effects of treatment and day of sampling on resistance to any individual antimicrobial in hospitalised horses.
| Variable | Odds ratio | 95% Confidence Intervals | p-value | |
|---|---|---|---|---|
| Treatment | 1.19 | 0.32 | 4.41 | 0.797 |
| Day 3 | 5.59 | 1.61 | 19.39 | 0.007 |
| Day 5 | 5.59 | 1.61 | 19.39 | 0.007 |
| Constant | 0.49 | 0.16 | 1.54 | 0.224 |
Table 5.
Multivariable analysis of the effects of treatment and day of sampling on MDR in hospitalised horses.
| Variable | Odds ratio | 95% Confidence Intervals | p-value | |
|---|---|---|---|---|
| Treatment | 1.00 | 0.23 | 4.27 | 1.000 |
| Day 3 | 7.36 | 1.80 | 30.07 | 0.005 |
| Day 5 | 11.00 | 2.69 | 44.93 | 0.001 |
| Constant | 0.11 | 0.02 | 0.57 | 0.008 |
Resistance to individual antimicrobials
The overall percentage of individual isolates that were resistant to each antimicrobial tested is shown in Table 6.
Table 6.
The overall percentage of individual isolates resistant to each antimicrobial (n = 412)
| Antimicrobial | Percentage of isolates resistant |
|---|---|
| Ampicillin | 20.4% |
| Amoxicillin Clavulanate | 0.5% |
| Ceftiofur | 9.2% |
| Cefquinome | 9.2% |
| Cefpodoxime | 9.5% |
| Marbofloxacin | 1.7% |
| Ciprofloxacin | 1.7% |
| Tetracycline | 17.5% |
| Colistin | 0.7% |
| Gentamicin | 0.5% |
| Trimethoprim sulphamethoxazole | 23.8% |
| Chloramphenicol | 10.7% |
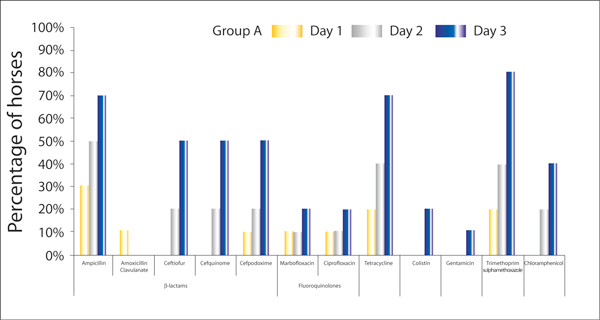
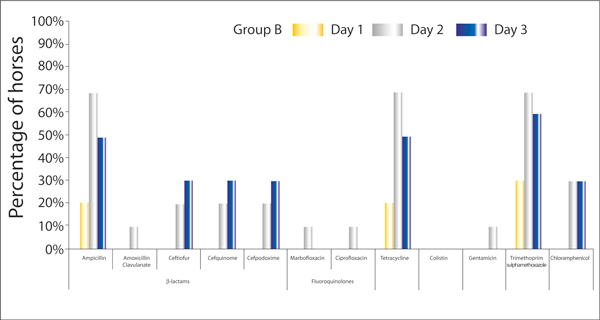
Figure 2 and 3 show the percentage of horses in group A and B respectively, that have at least one E. coli isolate showing resistance to a particular antimicrobial on days one, three or five of hospitalisation.
Figure 2.
The percentage of horses in Group A (hospitalised horses treated with antimicrobials, n = 10) with at least one E. coli isolate showing resistance to a particular antimicrobial on days one, three or five of hospitalisation.
Figure 3.
The percentage of horses in Group B (hospitalised horses not treated with antimicrobials, n = 10) with at least one E. coli isolate showing resistance to a particular antimicrobial on days one, three or five of hospitalisation.
In Group C, resistance to chloramphenicol was detected in one horse on day three and again on day five. Resistance to any other antimicrobial class was not detected (data not shown).
Discussion
In both humans and animals it is acknowledged that antimicrobial use is associated with the development of antimicrobial resistance and that resistance develops not only in pathogenic bacteria but also in commensal organisms including E. coli [17,13,10,21]. In this study it is likely that the use of antimicrobials in group A horses selected for more resistant populations of faecal E. coli during the hospitalisation period. However, increased prevalence of antimicrobial resistance in group B horses on days three and five of hospitalisation compared to the prevalence at admission cannot be attributed to antimicrobial administration and suggests that hospitalisation is associated with higher levels of resistance in commensal faecal E. coli of horses regardless of whether they have received treatment with antimicrobials. This important finding is consistent with the results of a previous study in the USA in which hospitalised horses not treated with antimicrobials for at least four days prior to sample collection were significantly more likely to have resistant faecal E. coli isolates when compared to untreated horses in the community [8]. Infections obtained from the hospital environment represent a major problem in human hospitals, with similar problems with these types of infections emerging in veterinary medicine. Epidemiological studies of small animal intensive care units have highlighted the importance of the environmental reservoir in the development of outbreaks of infections in veterinary hospitals. In one study a multiple-antimicrobial resistant strain of Acinetobacter baumannii was found to have been transferred between dogs, cats and a horse within the same hospital [3]. It is speculated that hospitalisation may have resulted in an increase in resistance in horses in group B in this study due to exposure of horses to resistant strains of bacteria in the hospital environment or transfer of resistant bacteria or antimicrobial resistance genes from antimicrobial treated horses to untreated horses by direct contact or routine husbandry procedures; however, further investigation will be necessary to identify the exact cause. An interesting observation in group B horses is the decrease in resistance prevalence seen between days three and five. No explanation could be identified for this trend and an evaluation of a larger population would be necessary to explore this finding further.
Resistance patterns of E. coli isolated from samples collected at the time of hospital admission were assumed to be representative of resistance in the general equine population. However, the prevalence of resistance was found to be higher in hospital admission samples compared to samples from control horses (group C). This difference may be due to the increased likelihood that horses being admitted to hospital for illness may have been treated with antimicrobials prior to the two weeks before hospital admission. In this study no horse had been treated with antimicrobials for at least two weeks prior to inclusion in the study but it is not known in horses how long the effect of antimicrobial administration will influence the resistance patterns of the intestinal flora. In humans the commensal microflora may return to normal following the completion of a course of antimicrobial treatment. However, selected resistant commensal bacteria may also persist for years [10]. The control horses used in this study belonged to a closed herd and it is also possible that the prevalence of antimicrobial resistance in this group was lower due to increased biosecurity, including the absence of movement of animals from this group and resulting lack of exposure to different environments or to horses outside the herd.
Infections with MDR bacteria are a major problem in human and veterinary medicine. The development of MDR results in increased morbidity and mortality in human and equine patients as well as increased cost of treatment through increased length of hospital stay, the necessity to use multiple and more expensive drugs for effective treatment and the increased costs of infection control [11,20,24]. MDR has previously been reported in bacteria isolated from horses including E. coli [8], Acinetobacter baumannii [3], S. aureus [18] and S. Typhimurium [24]. In the current study E. coli demonstrating MDR were isolated from the faeces of hospitalised horses; however, E. coli may act as opportunistic pathogens also, causing disease. The primary mechanism for the development of MDR is through genetic exchange mechanisms [20]. Enterobacteriaceae are capable of exchanging resistance genes under intestinal conditions in animals [2] and thus commensal E. coli in horses could act as a reservoir of resistance genes that could potentially be transferred to other animals or humans. The influence that antimicrobial resistance in animals has on humans is currently controversial and it is unknown how frequently bacteria from animals colonise the human gut and transfer resistance genes. Transmission of bacteria such as MRSA from horses to humans and humans to horses [18,25] has been identified both in veterinary hospitals and in the community, and the same possibilities for transmission must be considered for other bacteria.
Samples were collected over a five-day period of hospitalisation, as this was the average time that horses meeting the inclusion criteria were likely to remain in the hospital. Increasing prevalence of antimicrobial resistance and MDR was seen within two days of hospitalisation in both groups of hospitalised horses and highlights the speed at which resistant E. coli populations can develop. It has been shown that genetic transfer of determinants for drug resistance can occur rapidly in vitro, but frequency of transfer in vivo is lower [17]; therefore, it is speculated that the most likely reason for the rapid development of resistant bacterial populations in horses treated with antimicrobials is the elimination of susceptible bacterial populations, and only to a lesser extent from the genetic transfer of resistance. The mechanism for the rapid development of antimicrobial resistance in the hospitalised untreated horses is unclear; however, it is possible that resistant bacteria were acquired from the hospital environment and after a period of two days could be detected in the faeces.
In this study resistance to potentiated sulphonamide antibiotics was most commonly seen, followed by resistance to ampicillin and tetracycline. These results agree in part with those of a previous study in the USA investigating antimicrobial susceptibility patterns of commensal E. coli in horses that showed resistance to sulphonamides (not investigated in the current study) and potentiated sulphonamides was most common; however, this was followed by resistance to gentamicin and tetracycline [8]. The differences in results between the two studies may reflect geographical variations in bacterial populations or equine hospital environments, or different selection pressures due to variations in the use of antimicrobials. In human and animal studies the use of a particular antimicrobial has been shown to select for bacterial populations with resistance to that antimicrobial [17,21]; however, in the current study resistance to gentamicin in hospitalised horses was uncommon despite this being one of the most frequently used antimicrobials within the hospital.
An interesting observation in the current study is the occurrence of resistance to third and fourth generation cephalosporins. No horse in the present study underwent treatment with cephalosporin antimicrobials during the study period; however, resistance to cefquinome, ceftiofur and cefpodoxime was seen in hospitalised horses regardless of whether they were treated with antimicrobials. Resistance to cefpodoxime was detected in one horse on admission to the hospital; however, all remaining isolates showing cephalosporin resistance were isolated from samples taken on days three or five of hospitalisation. It could be speculated that this pattern of resistance suggests that cephalosporin resistant bacteria or resistance genes were transferred to horses from the hospital environment; however, further investigation would be necessary to confirm this. Resistance to ceftiofur has been reported in farm animals [12] and horses [23], and resistance to cefquinome has been reported in cattle [9]; however, to the author's knowledge this is the first report of resistance to cefquinome in E. coli of equine origin. The World Health Organisation (WHO) has defined third and fourth-generation cephalosporins as being "critically important" for use in humans [6] with certain third-generation cephalosporins being the therapy of choice in humans for treatment of infections caused by E. coli and Salmonella spp [12]. The development of resistance to these antimicrobials in animals is therefore not only a concern for treating disease in animals but also a major concern for public health. The presence of resistance to a fourth-generation cephalosporin is especially worrying at a time when fewer new antimicrobials are becoming available and highlights the need for judicious use of available antimicrobials in both humans and animals. Results of a previous study in horses showed the administration of cephalosporins was positively associated with resistance to six or more antimicrobials [8], further supporting the need for judicious use of cephalosporins.
Conclusions
The results of the current study suggest that hospitalisation is an important risk factor associated with the development of antimicrobial resistance and MDR in commensal faecal E. coli of horses. Veterinarians need to be aware of the possibility of MDR in commensal bacteria in hospitalised horses as it may have implications for treatment of disease if resistance is transferred to pathogenic bacteria. Veterinarians should also be aware of the possible influence that antimicrobial resistance in horses has on public health. Resistance to third and fourth generation cephalosporins is concerning and highlights the need for judicious use of available antimicrobials in horses. Further large scale studies will be necessary to further explain or identify risk factors for the development of antimicrobial resistance in horses.
Acknowledgements
The authors wish to thank Yvonne Abbott and Bernadette Legget for technical assistance with this project.
References
- Anzai T, Kamada M, Ike K. et al. Drug susceptibility of Escherichia coli isolated from foals with diarrhea and mares with metritis. Bulletin of Equine Research Institute. 1987;24:42–50. [Google Scholar]
- Blake DP, Hillman K, Fenlon DR. et al. Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ileal conditions. J Appl Microbiol. 2003;95:428–436. doi: 10.1046/j.1365-2672.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- Boerlin P, Eugster S, Gaschen F. et al. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet Microbiol. 2001;82:347–359. doi: 10.1016/S0378-1135(01)00396-0. [DOI] [PubMed] [Google Scholar]
- Bucknell DG, Gasser RB, Irving A. et al. Antimicrobial resistance in Salmonella and Escherichia coli isolated from horses. Aust Vet J. 1997;75:355–356. doi: 10.1111/j.1751-0813.1997.tb15713.x. [DOI] [PubMed] [Google Scholar]
- CLSI. Performance standards for antimicrobial disk diffusion susceptibility tests; Approved Standard. Clinical and Laboratory Standards Institute, Pennsylvania. 2005.
- Collignon P, Aarestrup FM. Extended-spectrum β-lactamases, food, and cephalosporin use in food animals. Clin Infect Dis. 2007;44:1391–1392. doi: 10.1086/516612. [DOI] [PubMed] [Google Scholar]
- Dargatz DA, Traub-Dargatz JL, Sangster NC. Antimicrobic and anthelmintic resistance. Vet Clin North Am Equine Pract. 2000;16:515–536. doi: 10.1016/s0749-0739(17)30093-7. [DOI] [PubMed] [Google Scholar]
- Dunowska M, Morley PS, Traub-Dargatz JL. et al. Impact of hospitalization and antimicrobial drug administration on antimicrobial susceptibility patterns of commensal Escherichia coli isolated from the feces of horses. J Am Vet Med Assoc. 2006;228:1909–1917. doi: 10.2460/javma.228.12.1909. [DOI] [PubMed] [Google Scholar]
- Guérin-Faublée V, Carret G, Houffschmitt P. In vitro activity of 10 antimicrobial agents against bacteria isolated from cows with clinical mastitis. Vet Rec. 2003;152:466–471. doi: 10.1136/vr.152.15.466. [DOI] [PubMed] [Google Scholar]
- Gustafsson I, Sjölund M, Torell E. et al. Bacteria with increased mutation frequency and antibiotic resistance are enriched in the commensal flora of patients with high antibiotic usage. J Antimicrob Chemother. 2003;52:645–650. doi: 10.1093/jac/dkg427. [DOI] [PubMed] [Google Scholar]
- Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med Supplement. 2004;10:S122–129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- Li XZ, Mehrotra M, Ghimire S. et al. β-Lactam resistance and β-lactamases in bacteria of animal origin. Vet Microbiol. 2007;121:197–214. doi: 10.1016/j.vetmic.2007.01.015. [DOI] [PubMed] [Google Scholar]
- McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34(Suppl 3):S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- Morley PS, Apley MD, Besser TE. et al. Antimicrobial drug use in veterinary medicine. J Vet Int Med. 2005;19:617–629. doi: 10.1111/j.1939-1676.2005.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Mulvey MR, Simor AE. Antimicrobial resistance in hospitals: How concerned should we be? CMAJ. 2009;180:408–415. doi: 10.1503/cmaj.080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Infect Control. 2006;34:S20–28. doi: 10.1016/j.ajic.2006.05.238. [DOI] [PubMed] [Google Scholar]
- Prescott JF. In: Antimicrobial Therapy in Veterinary Medicine. Prescott JF, Baggot JD, Walker RD, editor. Ames, Iowa State University Press; 2000. Antimicrobial drug resistance and its epidemiology; pp. 35–36. [Google Scholar]
- Seguin JC, Walker RD, Caron JP. et al. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J Clin Microbiol. 1999;37:1459–1463. doi: 10.1128/jcm.37.5.1459-1463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood LL. Principles of antimicrobial therapy: what should we be using? Vet Clin North Am Equine Pract. 2006;22:279–296. doi: 10.1016/j.cveq.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119:S3–S10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- van de Sande-Bruinsma N, Grundmann H, Verloo D. et al. European antimicrobial resistance surveillance system and European surveillance of antimicrobial consumption project groups. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14:1722–1730. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard AE, Stobberingh EE. Antibiotic usage in animals: impact on bacterial resistance and public health. Drugs. 1999;58:589–607. doi: 10.2165/00003495-199958040-00002. [DOI] [PubMed] [Google Scholar]
- Vo ATT, van Duijkeren E, Fluit AC. et al. Characteristics of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates from horses. Vet Microbiol. 2007;124:248–255. doi: 10.1016/j.vetmic.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Ward MP, Brady TH, Couëtil LL. et al. Investigation and control of an outbreak of salmonellosis caused by multidrug-resistant Salmonella Typhimurium in a population of hospitalized horses. Vet Microbiol. 2005;107:233–240. doi: 10.1016/j.vetmic.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Weese JS, Lefebvre SL. Risk factors for methicillin-resistant Staphylococcus aureus colonization in horses admitted to a veterinary teaching hospital. Can Vet J. 2007;48:921–926. [PMC free article] [PubMed] [Google Scholar]