Abstract
Objectives:
To study the acute effect of the new SensAwake CPAP modality (reducing pressure on awakenings) on wake after sleep onset (WASO) and other polysomnographic measures in patients with obstructive sleep apnea (OSA).
Study Design:
Randomized crossover trial comparing an automatic continuous positive airway pressure device (AutoCPAP) with and without SensAwake on sleep architecture. CPAP naive patients received each therapy for a single night in the laboratory with at least 1-week washout. Both patients' and technicians' subjective satisfaction was assessed. Pressure data measured and stored by the AutoCPAP device were also analyzed.
Results:
OSA was controlled adequately by both modes (SensAwake ON apnea hypopnea index ± SD, AHI = 5.3 ± 5.6/h vs. SensAwake OFF = 5.4 ± 5.8, p = 0.9) in the 42 patients who completed the protocol. Mean and 90% pressures were significantly lower with SensAwake (mean ON = 6.9 ± 1.9 vs. OFF = 7.7 ± 2.5 cm H2O, p < 0.05; 90% pressure ON = 9.6 ± 2.7 vs. OFF = 10.6 ± 2.7 cm H2O, p < 0.02). SensAwake did not improve WASO (ON = 74 ± 54 min vs. OFF = 78 ± 51 min, p = 0.6). There were no differences in other sleep architecture measures or patient satisfaction between the 2 modalities. AutoCPAP-measured AHI closely approximated PSG-derived (ROC AUC = 0.81 [95% CI 0.71-0.92], p = 0.0001).
Conclusions:
SensAwake provides similar control of the AHI to the standard AutoCPAP mode but does so at lower mean and 90% pressures. However, no measure of sleep architecture was significantly improved by the SensAwake mode during this initial acute exposure. The internal AutoCPAP AHI detection and calculation was similar to PSG-derived AHI measures. Longer term studies are needed to evaluate any long-term influence of SensAwake on WASO.
Citation:
Dungan GC; Marshall NS; Hoyos CM; Yee BJ; Grunstein RR. A randomized crossover trial of the effect of a novel method of pressure control (SensAwake) in automatic continuous positive airway pressure therapy to treat sleep disordered breathing. J Clin Sleep Med 2011;7(3):261-267.
Keywords: CPAP, AutoCPAP, wake after sleep onset, polysomnography
Obstructive sleep apnea (OSA) is a common disorder associated with serious sequelae. 1–3 Continuous positive airway pressure (CPAP) therapy has been shown to be efficacious in the management of neurocognitive and cardiovascular risks associated with obstructive sleep apnea4–6 and remains the first-line therapy for OSA. Effectiveness of CPAP is limited by poor acceptance and adherence, with long-term compliance estimated between 29% and 83%.7 The causes of reduced compliance with CPAP are multifactorial including CPAP interface, blower mechanics and control, patient perception, personality, training, and other factors.7,8 Modalities intended to improve the delivery of the pressurized air necessary for CPAP have been developed specifically to address patient compliance problems. Heated humidification, ramping, auto-titrating pressure control, expiratory pressure relief, and other delivery (hardware) and control (software) approaches have been marketed with claims of improved compliance.9
One possible source of impaired compliance may be the perception by the patient of excessive pressure, particularly during exhalation, noted during wakefulness.10 This has been a design consideration since early AutoCPAP devices.11 Therapy devices often include a “ramp” feature, which reduces the system pressure to some pre-set low value, and progressively increases the pressure over a specific period of time. This ramp is designed to allow the patient to drop the pressure during wakefulness, reducing the waking perception of excessive pressure on the face. This is believed to be useful in allowing patients to fall asleep, especially when on high therapeutic CPAP pressures. Activation of the ramp typically requires a conscious effort of the patient, often requiring activation of a switch or other mechanism on the CPAP blower itself.
BRIEF SUMMARY
Current Knowledge/Study Rationale: SensAwake is a new technological innovation in positive airway pressure treatment for sleep apnea. It detects when patients are awake and then lowers pressure in an attempt to improve patients comfort and hopefully compliance- but no clinical trial has tested this.
Study Impact: SensAwake seems to control sleep apnea just as well as normal automatically titrating CPAP does. However, it did not improve the patients' sleep architecture or sleep consolidation. Whether it improves medium or long-term compliance with PAP therapy remains to be tested in a clinical trial.
A novel method of pressure modification has been developed (SensAwake; Fisher and Paykel Healthcare, Ltd., Auckland NZ), which evaluates breathing patterns during CPAP therapy.12 When breathing patterns suggest a patient may be awake, the CPAP pressure is reduced in much the same way as would happen after activating a pressure ramp feature. The possible advantage may be that the reduction in pressure is automatic, not requiring a full awakening or overt action on the part of the patient; thus rendering a reduced pressure without the stimulation attendant to activating a ramp. Such a feature may result in a quicker return to sleep after arousal/awakening; potentially improving both sleep quality and the amount of CPAP- protected sleep. This may ultimately have an impact on acceptance or adherence to CPAP therapy and subsequent outcomes.
We sought to evaluate the effects of the SensAwake (SAw) modality in addition to AutoCPAP therapy (SleepStyle 200 Series AutoCPAP, Fisher – Paykel Healthcare, Ltd., Auckland NZ) on polysomnographic wake after sleep onset and sleep architecture compared with standard AutoCPAP therapy in a randomized crossover trial. This new modality has never been tested in a randomized trial for any outcome. We also evaluated whether the in-device recordings were able to determine whether a patient was being adequately treated whilst in auto-titration mode compared to gold standard in-laboratory polysomnography, specifically by evaluating the ability of the embedded algorithm within the autoCPAP device to detect apnea/hypopnea events as compared to PSG.
MATERIALS AND METHODS
The study was approved by the Human Research Ethics Committee of the Sydney Southwest Area Health Service and registered at www.clinicaltrials.gov (NCT00811213). Patients provided informed written consent.
Patient Sample
Recently diagnosed patients with moderate-to-severe obstructive sleep apnea were invited into the study if they were between 18 and 65 years of age; had a baseline diagnostic apnea/hypopnea index (AHI) ≥ 15 events/h of sleep (determined using attended in-laboratory polysomnography); fluent in spoken English; had an Epworth Sleepiness Scale score between 0 and 15 (normal to moderate daytime sleepiness; due to safety considerations related to driving among more severely sleepy patients); and did not have psychiatric or other significant sleep disorders. Participants were excluded from the study if they had been prescribed and fitted with any PAP device in the past 2 years; if they had unstable cardiovascular disease including heart failure (untreated or resistant hypertension acceptable); inability to tolerate CPAP due to nasal obstruction or claustrophobia as determined by the study investigator; any known factor or disease that might interfere with treatment compliance, study conduct, or interpretation of the results such as psychiatric disease; a history of non-compliance to medical regimens; unwillingness to comply with study requirements; or participation in another clinical trial in the previous month.
Assessments
Baseline Assessment
Patients were examined at baseline for basic anthropometrics and sleepiness (Epworth Sleepiness Scale). As the patients had all recently been diagnosed with sleep apnea, their polysomnogram (PSG) report was used to derive a baseline AHI value. There was no CPAP pressure titration performed prior to exposure to the new device.
Randomization, Allocation Concealment, and Blinding
The order of treatment (i.e., SAw ON or OFF first) was randomized in a 1:1 ratio using a computer algorithm prepared by an investigator who never met any patients nor had any role in recruitment or determination of eligibility. This investigator placed the treatment allocations into sequentially numbered opaque envelopes. A sleep technologist opened the randomization envelope after the patient was determined to be eligible and provided informed consent. This technologist would then prepare the therapy room with the appropriate SAw modality. The technologist would ensure the alternative modality was administered during the subsequent study night. Patients were told that the study evaluated two different methods of providing PAP therapy. Technicians performing the studies were aware of which modality was being tested in each patient. The technologists who scored the study were blinded to the treatment allocation.
Data Acquisition
Patients attended the diagnostic services facility for a standard comprehensive CPAP polysomnographic study. Auto-titration pressure was set to range between 4 and 20 cm H2O. Heated humidification was used for all patients. Masks were fitted by a CPAP therapist prior to the study, and patients wore the same mask for both in-laboratory PSG evaluations.
PSG data were collected using a digital polysomnographic recording system (Sandman SD32+/Sandman Elite, Covidien, Boulder, CO, USA). These data included EEG (central and occipital), bilateral EOG, chin and leg EMG, thoracic and abdominal respiratory effort measured with inductive plethysmography, Lead 2 ECG, finger pulse oxygen saturation (SpO2), body position, and continuous video monitoring. In addition, CPAP mask pressure, airflow, and leak were monitored using an external apparatus integrating analogue signals into the PSG (PTAF 2, Pro-Tech, Mukilteo, WA, USA). PSG studies were scored using the current AASM standard scoring methodology, specifically using the defined “alternative” hypopnea scoring method.13 While more than one technologist performed PSG scoring in this study, every single patient had all their studies scored by a single technologist.
We used two 10-cm visual-analogue scales to assess technician and patient satisfaction. Technicians were asked “Overall, how satisfied were you with the machine they tried last night”? Patients were asked “Overall, how satisfied are you with the machine you tried last night”? The 10-cm scale spanned between “Not at all satisfied” (0 cm) and “Extremely satisfied”.
The patients had no PAP therapy during the one-week washout period. The patients were advised of the possibility of experiencing ongoing symptoms of OSA during the washout period and the need to refrain from driving motor vehicles or operating heavy machinery if daytime sleepiness was experienced.
Data from the CPAP device was archived onto proprietary software (Performance Maximizer, Fisher and Paykel Healthcare, Ltd, Auckland, NZ). This included all pressure variables, machine derived AHI, and verification of the delivered modality. The AutoCPAP device includes a detection and recording algorithm for apnea and hypopnea events. This recording was available upon post-study download to the proprietary software. The AHI was compared to the AHI derived from the manually scored polysomnographic data.
Statistical Analyses
We calculated that we needed to randomize 45 patients to detect a 0.45 standard deviation difference in a within-patients comparison with 80% power coupled with an 11% dropout rate (i.e., 40 patients). This size of difference in the primary outcome (wake after sleep onset; WASO) was selected as clinically significant given the relatively small decreases in WASO that are reported in association with pharmaceutical treatments for insomnia. The 2 secondary outcomes were PSG-derived sleep efficiency and the percentage of slow wave sleep. All other outcomes are classed as tertiary in importance. Data analyses were conducted by GCD using a statistical software package (SPSS v 17; SPSS; Chicago, IL). Data are presented as mean ± standard deviation unless otherwise indicated. Comparisons were made using paired t-tests or Wilcoxon test as appropriate. Agreement between in-laboratory PSG-derived AHI and the machine-derived AHI was ascertained using the Bland-Altman test14 and a receiver-operating characteristic curve15 set to discriminate positive events if the PSG-scored AHI ≥ 5. Statistical significance on all tests was reached with p < 0.05.
RESULTS
Patients
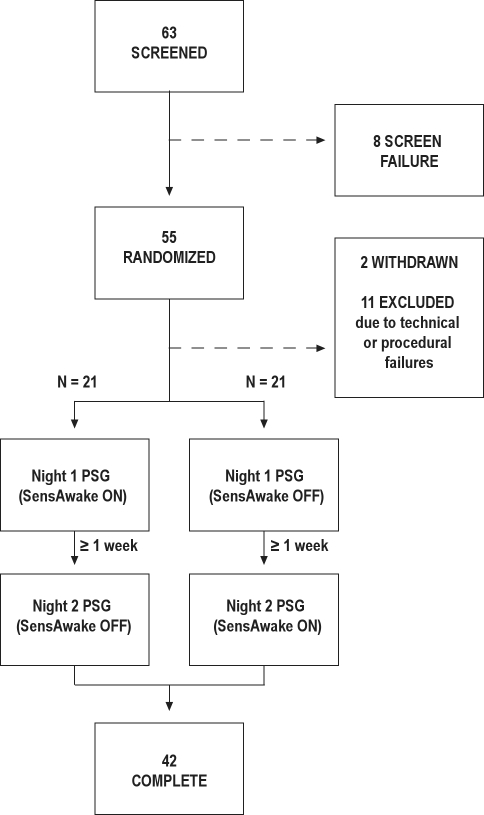
Sixty-three candidates were screened for inclusion in the trial and the patient flow through the trial is summarized in Figure 1. Eight patients failed screening. Of the 55 patients randomized, 2 withdrew after the first night of the trial (none due to treatment-specific adverse events). Eleven patients were subsequently excluded from analysis due to improper SAw mode selection. This mode selection error occurred due to a combination of technician error compounded by a heretofore unknown characteristic of the software used in this laboratory based study. As such, the analyses presented are based on per-protocol data only. Forty-two patients completed both arms of the trial and were included in the analysis, with half each being randomized to the differing treatment orders. The patients were typical for obstructive sleep apnea patients in our clinic (Table 1).
Figure 1.
Patient flow diagram
Table 1.
Baseline participant characteristics
| Characteristic | Mean ± SD unless otherwise stated |
|---|---|
| Gender | |
| Male (number) | 32 (76%) |
| Female (number) | 10 (24%) |
| Age (y) | 50.3 ± 9.4 |
| BMI (kg/m2) | 32.1 ± 5.9 |
| BMI ≥ 30 kg/m2 (%) | 27 (65%) |
| ESS Score (/24) | 10.8 ± 4.9 |
| ESS Score ≥ 10 (number, %) | 24 (57%) |
| Baseline AHI (1/h) | 32.8 ± 19.6 (14.1 min, 85.1 max) |
| Baseline SpO2 Mean (% saturation) | 93.9 ± 3.2 |
| Baseline SpO2 Minimum (% saturation) | 77.6 ± 11.1 |
Outcomes
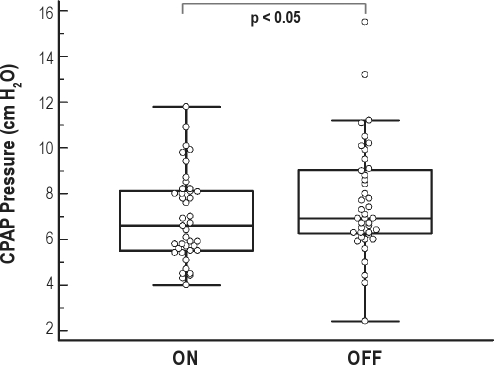
Table 2 presents salient clinical data which was expected to be similar between the modes of therapy. These data represent measures describing the overall “success” of the therapy to treat the underlying sleep disordered breathing. AHI was not different between the two SAw conditions. AHI was significantly reduced under both SAw conditions (AHI baseline v AHI SAw ON 32.8 ± 19.6/h v. 5.3 ± 5.6/h, p < 0.001; AHI baseline v. AHI SAw OFF 32.8 ± 19.6/h v. 5.4 ± 5.8/h, p < 0.001). Blood oxygen desaturation was also significantly improved on both SAw modalities (ODI3 baseline v ODI3 SAw ON 33.4 ± 24.3/h v. 5.8 ± 5.6/h, p < 0.001; ODI3 baseline v. ODI3 SAw OFF 33.4 ± 24.3/h v. 5.4 ± 5.7/h, p < 0.001; SpO2mean baseline v. SpO2mean SAw ON 93.9% ± 3.2% v. 96.1% ± 1.5%, p = 0.001; SpO2mean baseline v. SpO2mean SAw OFF 93.9% ± 3.2% v. 95.9% ± 1.4%, p = 0.001). The mean pressure (Figure 2) and 90th percent pressure delivered during AutoCPAP therapy was significantly lower while SAw was ON versus OFF.
Table 2.
Non-outcome and manipulation data
| Parameter | SAw ON | SAw OFF | Mean Difference (95% CI) | p |
|---|---|---|---|---|
| Time in bed (min) | 451.2 ± 30.6 | 449.2 ± 32.9 | −2.0 (−13.1 to 9.0) | 0.712 |
| Arousal index (1/h) | 12.7 ± 9.9 | 11.9 ± 7.7 | −0.8 (−3.3 to 1.7) | 0.525 |
| Apnea hypopnea index (1/h) | 5.3 ± 5.6 | 5.4 ± 5.8 | 0.09 (−1.47 to 1.65) | 0.905 |
| Desaturation index (3%) (1/h) | 6.6 ± 8.1 | 5.8 ± 6.4 | −0.8 (−2.6 to 1.0) | 0.372 |
| Mean TST - SpO2 (%) | 96.0 ± 1.4 | 95.9 ± 1.3 | −0.13 (−0.5 to 0.2) | 0.479 |
| PLM index (1/h) | 0.35 ± 2.0 | 0.21 ± 1.3 | −0.15 (−0.39 to 0.09) | 0.207 |
| Mean pressure (cm H2O) | 6.9 ± 1.9 | 7.7 ± 2.5 | 0.7 (0.1 to 1.4) | 0.035 |
| 90% pressure (cm H2O) | 9.5 ± 2.7 | 10.6 ± 2.7 | 1.1 (0.3 to 1.9) | 0.011 |
| Leak (L/min) | 46.2 ± 9.5 | 47.2 ± 11.3 | 1.1 (−1.2 to 3.3) | 0.353 |
| Percent of study with excessive leak (%) | 13.5 ± 21.7 | 10.6 ± 16.2 | −2.9 (−9.1 to 3.4) | 0.359 |
| Machine recorded AHI (1/h) | 3.9 ± 4.2 | 3.7 ± 3.5 | 0.23 (−0.96 to 1.42) | 0.756 |
Figure 2.
Box and whisker plot indicating the median and interquartile ranges in mean pressure delivered delivered by AutoCPAP with SAw condition mode ON and OFF
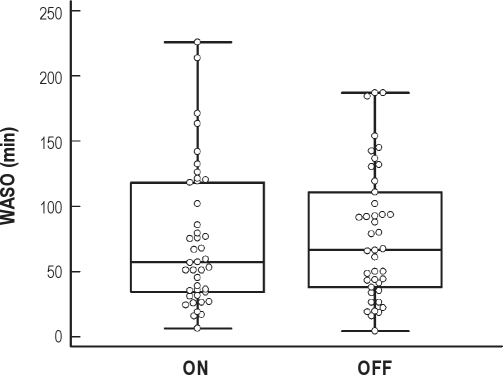
Table 3 presents the effects of SAw on all outcome measures. Wake after sleep onset was comparable under both conditions (Figure 3). No outcome measure was significantly affected by SAw except technician satisfaction with the therapy which was significantly lower under the SAw condition.
Table 3.
Outcome variables
| Parameter | SAw ON | SAw OFF | Mean Difference (95% CI) | p |
|---|---|---|---|---|
| Wake after sleep onset (min) | 74.1 ± 53.7 | 78.0 ± 50.6 | 3.9 (−11.3 to 19.2) | 0.605 |
| Sleep efficiency index (% TIB) | 81.6 ± 13.0 | 79.3 ± 14.8 | −2.2 (−6.1. to −1.6) | 0.25 |
| Slow wave sleep (% TST) | 29.5 ± 9.6 | 30.7 ± 11.1 | 1.2 (−2.4 to 4.7) | 0.511 |
| Stage 1 (% TST) | 6.3 ± 9.9 | 4.1 ± 3.7 | 2.2 (−1.0 to 5.4) | 0.12 |
| Stage 2 (% TST) | 43.9 ± 9.6 | 43.3 ± 11.9 | −0.6 (−3.9 to 2.7) | 0.73 |
| Stage REM (% TST) | 20.3 ± 7.8 | 21.9 ± 9.6 | 1.6 (−1.2 to 4.3) | 0.252 |
| Sleep latency | 8.3 ± 7.3 | 13.8 ± 25.4 | −5.6 (−12.9 to 1.8) | 0.288 |
| REM latency | 106.7 ± 70.9 | 106.5 ± 59.6 | −0.22 (−22.4 to 22.0) | 0.984 |
| Arousal index (1/h) | 12.7 ± 9.9 | 11.9 ± 7.7 | −0.8 (−3.3 to 1.7) | 0.525 |
| Total sleep time (min) | 368.8 ± 67.6 | 357.1 ± 74.5 | −11.6 (−31.1 to 7.9) | 0.235 |
| Technician VAS (1/10 cm) | 5.4 ± 2.2 | 6.3 ± 1.6 | 0.96 (0.17 to 1.7) | 0.019 |
| Patient VAS (1/10 cm) | 6.0 ± 2.0 | 6.2 ± 2.1 | 0.18 (−0.62 to 0.97) | 0.654 |
Figure 3.
Box and whisker plot indicating the median and interquartile ranges in wake after sleep onset with the SAw mode ON and OFF (p = N.S.)
Additional Analyses
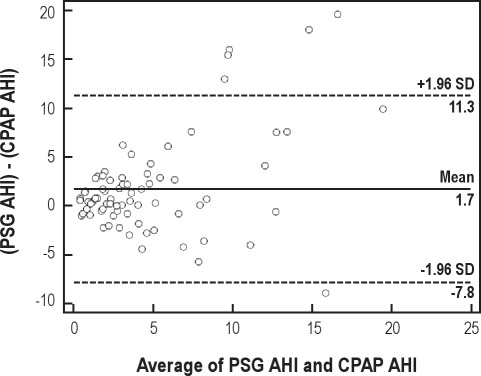
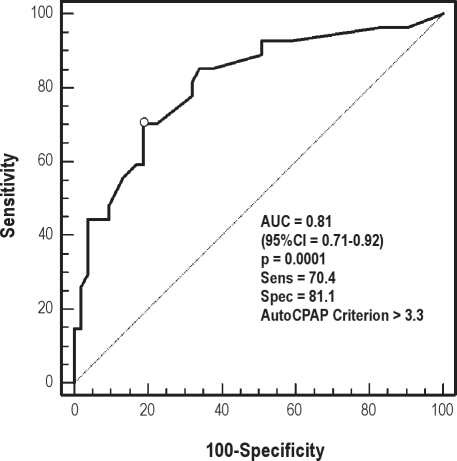
AHI as measured on the AutoCPAP device was compared to the AHI determined during the simultaneous in-laboratory polysomnographic recording. The Bland-Altman plot of AHI derived from PSG vs. AutoCPAP-derived AHI suggests that the data were largely comparable, with a modest underestimation of the AHI by the AutoCPAP device (Figure 4). Area under the receiver operator characteristic (ROC) curve (Figure 5) with a PSG-scored diagnostic threshold of 5 events/hr as the diagnostic cut-off AHI diagnostic criterion is 0.81 (95% CI = 0.708 to 0.915, p < 0.0001), suggesting reasonable agreement between the AutoCPAP AHI and the “gold-standard” PSG AHI. Optimum sensitivity and specificity for an AHI 5/h cutoff is 70.4 and 81.1, respectively, corresponding to an AutoCPAP-derived threshold of 3.3 events/hour.
Figure 4.
Bland-Altman plot of AHI derived from PSG-scored events (PSG AHI) v. AutoCPAP-derived events (CPAP AHI); comparing the difference between the 2 methods to the average of the corresponding values from the 2 methods
Figure 5.
Receiver operator characteristic curve comparing AutoCPAP-derived AHI to a “gold-standard” PSG-scored AHI, using a PSG AHI cutoff of 5/h for conditional classification
DISCUSSION
Used on a single night, the SAw modality does not result in improvements in wake after sleep onset, other sleep architectural measures, or subjective patient perception of therapy compared with this modality switched off. AutoCPAP with SAw was as effective as the AutoCPAP alone in managing sleep disordered breathing events, with both modalities reducing overall exposure to events to within generally acceptable therapeutic target ranges for AHI (0 to 5 events/h). However a null-effect on outcomes measured during a single-night in-laboratory study cannot be assumed to have any bearing on long term adherence and thus effectiveness of therapy. Factors not assessed in the single night laboratory setting may affect the course of therapy in a longer term study. Such a study is necessary to evaluate the potential impact of the SAw modality on PAP compliance and patient-centered outcomes.
SAw did deliver lower pressure exposure than AutoCPAP alone. This is likely due to the designed function of the SAw algorithm, namely to lower pressure upon detecting wakefulness, with pressure returning to therapeutic levels only after the patient has returned to sleep. Thus, with detection of such “wake” events, the pressure delivered to the patient would be lower than that pressure likely delivered without such depressurizing algorithms. One theoretical advantage of AutoCPAP over fixed-pressure CPAP is exposure to lower pressure (peak and mean) with equivalent management of sleep disordered breathing.16,17 Such reduced pressure has been suggested as a means of improving comfort on CPAP therapy which may improve compliance although such an effect on compliance has not been clearly demonstrated in clinical trials.7,9,18,19 The effect of SAw on compliance remains to be quantified. It should not be automatically assumed that lowering pressure will cause improved compliance. Compliance with full pressure and pressure < 1 cm H2O are highly correlated.20
The ability to monitor the efficacy of therapy longitudinally remains an important aspect of long-term care for sleep apnea patients receiving CPAP therapy. Many CPAP devices can report on usage statistics. Detection of apnea and hypopnea events during therapy can help clinicians evaluate unresolved symptoms as well as make long-term therapy decisions. The ability to embed analysis has been described for other CPAP devices.21 The AutoCPAP platform used in this study also used an embedded detection algorithm to report apnea/hypopnea events as an index (of recording time). The embedded detection algorithm and reporting software does allow an adequate estimate of AHI, as demonstrated in this simultaneous study. The area under the ROC curve is 0.81 (95% CI 0.708 to 0.915, p = 0.0001). This suggests that the ability to accurately detect residual sleep disordered breathing at a PSG-determined detection level of 5/h is adequate in this device. In this experiment the best case sensitivity and specificity was 70% and 81%, respectively, at a CPAP derived AHI cut-off of 3.3/hr, when compared to a 5/hr PSG cut-off.
The small systematic underestimation of the AHI by the device (as shown in the Bland-Altman plot) is not unexpected as it does not make the index calculation with respect to polysomnographically validated sleep time. Rather, the index of respiratory event occurrence uses the larger “recording” time as the denominator of the index calculation. In addition, some patients showed clinically significant disagreement (both overestimate and underestimate) between the machine event index and the PSG-derived gold-standard AHI. These findings are important because the ability to base therapeutic decisions on data derived from embedded event detection and calculation is often not validated, but may provide clinically useful information.
The reduced satisfaction with the overall therapy as experienced by the technician may be explained by the single-blinded character of the study. Owing to technical constraints in the laboratory the SAw condition was un-blinded to the night technician. This blinding limitation may have provided a basis for other experimental bias. This was mitigated by both using blinded scorers for the evaluation of outcome data, and by maintaining blinding of the patients.
A limitation of this experiment's design is the single-night character of the experiment. These findings may not relate to the actual ability of the SAw modality to affect either acceptance or adherence to AutoCPAP therapy over the medium to long term.
AutoCPAP with and without SAw was equally effective in managing sleep disordered breathing events in our two-night in-laboratory only randomized trial. However, SAw did not acutely cause beneficial effects in any sleep quality measures that we employed in this study. SAw may act on sleep quality in less obvious ways, and consideration should be made to an acute study of SAw versus AutoCPAP evaluating spectral analysis of the EEG. Further, a long-term home study of SAw should be performed to evaluate both the longitudinal effect of the modality on acceptance and adherence to therapy, as well as determine any beneficial effect of the therapy on either neurocognitive functioning or known cardiovascular sequelae (e.g., hypertension) associated with sleep apnea.
DISCLOSURE STATEMENT
This was an investigator initiated trial that was funded by Fisher and Paykel Healthcare Ltd., Auckland, New Zealand. Mr. Dungan has worked as a research consultant to Respironics, Inc. The other authors have indicated no financial conflicts of interest.
SensAwake, SleepStyle, and PerformanceMaximizer are trademarks of Fisher and Paykel Healthcare, Ltd.
ABBREVIATIONS
- AHI
Apnea/hypopnea index
- AUC
Area under the curve
- AutoCPAP
Automatically adjusting CPAP
- BMI
Body mass index
- BP
Blood pressure
- CPAP
Continuous positive airway pressure
- LPM
Liters per minute
- ODI3
Oxygen desaturation index (3%)
- OSA
Obstructive sleep apnea
- PAP
Positive Airway Pressure
- ROC
Receiver operator characteristic curve
- SAw
SensAwake
- WASO
Wake after sleep onset
REFERENCES
- 1.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George CF. Reduction in motor vehicle collisions following treatmetn of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Augusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6.Peker Y, Hedner J, Norum J, Kralczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 7.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparrow D, Aloia M, DeMolles DA, Gottlieb DJ. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65:1061–6. doi: 10.1136/thx.2009.133215. [DOI] [PubMed] [Google Scholar]
- 9.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnea. Cochrane Database Syst Rev. 2009;(4):CD003531. doi: 10.1002/14651858.CD003531.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Freedman N. Treatment of obstructive sleep apnea syndrome. Clin Chest Med. 2010;32:187–201. doi: 10.1016/j.ccm.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Meurice J-C, Cornette A, Philip-Joet F, et al. Evaluation of autoCPAP devices in home treatment of sleep apnea/hypopnea syndrome. Sleep Med. 2007;8:695–703. doi: 10.1016/j.sleep.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Ayappa I, Norman RG, Whiting D, et al. Irregular respiration as a marker of wakefulness during titration of CPAP. Sleep. 2009;32:99–104. [PMC free article] [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st. ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10. [PubMed] [Google Scholar]
- 15.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 16.Senn O, Brack T, Matthews F, Russi EW, Bloch KE. Randomized short-term trial of two AutoCPAP devices versus fixed continuous positive airway pressure for the treatment of sleep apnea. Am J Respir Crit Care Med. 2003;168:1506–11. doi: 10.1164/rccm.200304-542OC. [DOI] [PubMed] [Google Scholar]
- 17.Hukins C. Comparative study of autotitrating and fixed-pressure CPAP in the home: a randomized, single-blind crossover trial. Sleep. 2004;27:1512–7. doi: 10.1093/sleep/27.8.1512. [DOI] [PubMed] [Google Scholar]
- 18.Vennelle M, White S, Riha RL, Mckay T, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS) Sleep. 2010;33:267–71. doi: 10.1093/sleep/33.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker JP, Marshall NS. Flexible pressure delivery modification of continuous positive airway pressure for obstructive sleep apnea does not improve compliance with therapy: systematic review and meta-analysis. Chest. 2010 Dec 30; doi: 10.1378/chest.10-2379. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax. 2005;60:427–32. doi: 10.1136/thx.2004.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer P, Meurice J-C, Philip-Joet F, et al. Simultaneous laboratory-based comparison of ResMed Autoset™ with polysomnography in the diagnosis of sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12:770–5. doi: 10.1183/09031936.98.12040770. [DOI] [PubMed] [Google Scholar]