Abstract
Study Objective:
To evaluate the efficacy and tolerability of gabapentin enacarbil (GEn) 1200 mg or 600 mg compared with placebo in subjects with moderate-to-severe primary restless legs syndrome (RLS).
Methods:
This 12-week, multicenter, double-blind, placebo-controlled study randomized subjects (1:1:1) to GEn 1200 mg, 600 mg, or placebo. Co-primary endpoints: mean change from baseline in International Restless Legs Scale (IRLS) total score and proportion of responders (rated as “very much” or “much” improved) on the investigator-rated Clinical Global Impression–Improvement scale (CGI-I) at Week 12 LOCF for GEn 1200 mg compared with placebo. Secondary endpoints included GEn 600 mg compared with placebo on the IRLS and CGI-I at Week 12 LOCF and subjective measures for sleep. Safety and tolerability assessments included adverse events.
Results:
325 subjects were randomized (GEn 1200 mg = 113; 600 mg = 115; placebo = 97). GEn 1200 mg significantly improved mean [SD] IRLS total score at Week 12 LOCF (baseline: 23.2 [5.32]; Week 12: 10.2 [8.03]) compared with placebo (baseline: 23.8 [4.58]; Week 12: 14.0 [7.87]; adjusted mean treatment difference [AMTD]: –3.5; p = 0.0015), and significantly more GEn 1200 mg-treated (77.5%) than placebo-treated (44.8%) subjects were CGI-I responders (p < 0.0001). Similar significant results were observed with GEn 600 mg for IRLS (AMTD: –4.3; p < 0.0001) and CGI-I (72.8% compared with 44.8%; p < 0.0001). GEn also significantly improved sleep outcomes (Post-Sleep Questionnaire, Pittsburgh Sleep Diary and Medical Outcomes Sleep Scale) compared with placebo. The most commonly reported adverse events were somnolence (GEn 1200 mg = 18.0%; 600 mg = 21.7%; placebo = 2.1%) and dizziness (GEn 1200 mg = 24.3%; 600 mg = 10.4%; placebo = 5.2%). Dizziness increased with increased dose and led to discontinuation in 2 subjects (GEn 1200 mg, n = 1; GEn 600 mg, n = 1). Somnolence led to discontinuation in 3 subjects (GEn 600 mg).
Conclusions:
GEn 1200 mg and 600 mg significantly improve RLS symptoms and sleep disturbance compared with placebo and are generally well tolerated.
Citation:
Lee DO; Ziman RB; Perkins AT; Poceta JS; Walters AS; Barrett RW. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med 2011;7(3):282-292.
Keywords: Gabapentin enacarbil, PIVOT RLS II, restless legs syndrome, GSK1838262, XP13512
Restless legs syndrome (RLS) is a neurologic disorder characterized by an urge to move the legs, usually accompanied or caused by unpleasant sensations in the legs.1 Symptoms generally begin or worsen during periods of rest or inactivity, are relieved by movement, and are worse in the evening or at night than during the day. Thus, RLS symptoms may delay sleep onset, reduce sleep efficiency, and cause multiple awakenings,2 resulting in significant sleep disturbance for patients.3,4
Two dopamine agonists, ropinirole and pramipexole, are currently approved by the US Food and Drug Administration for the treatment of moderate-to-severe primary RLS. However, these agents are not effective in all patients and there are limited data on the potential benefit of dopamine agonists on the common RLS-associated problems of sleep fragmentation and pain. In addition, some patients report worsening of symptoms over time, or experience intolerable side effects and longer-term complications such as augmentation, tolerance and impulse control disorders.5,6 A non-dopaminergic alternative may benefit such patients.7
BRIEF SUMMARY
Current Knowledge/Study Rationale: The study purpose was to explore the efficacy and safety of 600 mg GEn dosed once a day (QD) and further confirm the safety and effectiveness of 1200 mg QD in a 12 week trial for the treatment of primary RLS.
Study Impact: Gabapentin enacarbil is the first clinically effective, non-dopaminergic FDA approved treatment for primary RLS. Both 600 mg and 1200 mg doses were found to be well tolerated and effective.
Early reports indicate that gabapentin may provide effective treatment for RLS.8–10 However, gabapentin has pharmacokinetic deficiencies that may limit its clinical effectiveness. Gabapentin absorption occurs through active transport by a low-capacity nutrient transporter expressed in a narrow region of the upper small intestine.11,12 As a result, gabapentin bioavailability decreases with increasing dose12 and plasma exposure to gabapentin is variable between patients.13,14 Additionally, the short half-life of gabapentin requires frequent dosing.13,15
Gabapentin enacarbil (GEn) is an actively transported prodrug of gabapentin that overcomes the pharmacokinetic limitations of gabapentin through absorption by high-capacity nutrient transporters located throughout the large and small intestine.16,17 After absorption, GEn is rapidly converted to gabapentin by non-specific carboxylesterases, primarily in intestinal epithelial cells.17 GEn delivers predictable and sustained gabapentin exposure (bioavailability ≥ 68% based on urinary recovery of gabapentin in healthy adults) compared with mg-equivalent doses of gabapentin.16,18,19
In a 12-week, placebo-controlled study, subjects with moderate-to-severe primary RLS reported significantly improved RLS symptoms with GEn 1200 mg compared with placebo.20 A shorter 2-week study demonstrated significant treatment benefit in subjects with primary RLS with GEn 1200 mg compared with placebo, but not with GEn 600 mg.21 The present study evaluated the efficacy and tolerability of GEn 1200 mg and 600 mg compared with placebo in subjects with moderate-to-severe primary RLS, to confirm the previous findings for GEn 1200 mg and to further evaluate whether 12-week exposure to GEn 600 mg provided significant treatment benefits.
SUBJECTS AND METHODS
Study Design
This study (XenoPort, Inc. protocol XP053, clinicaltrials.gov identifier: NCT00365352, Patient Improvements in Vital Outcomes following Treatment in Restless Legs Syndrome II [PIVOT RLS II]) was a Phase III, 12-week, randomized, double-blind, placebo-controlled, parallel-group comparison of GEn 1200 mg and 600 mg compared with placebo. The study was conducted between August 2006 and December 2007 at 28 research centers in the USA (see appendix at www.aasmnet.org/jcsm).
Subjects
Adults with a diagnosis of primary RLS, based on the International Restless Legs Syndrome Study Group diagnostic criteria,1 were recruited. Eligible subjects had RLS symptoms for ≥ 15 nights in the month prior to screening (or, if on treatment, the same frequency of symptoms before treatment was started), documented RLS symptoms for ≥ 4 of the 7 consecutive evenings/nights during the baseline period, an International Restless Legs Scale (IRLS)22 total score ≥ 15 at the beginning and end of the baseline period, and had discontinued dopamine agonists, gabapentin and any other RLS treatments for ≥ 2 weeks prior to baseline. No information was collected regarding patients' previous response to treatments.
The rationale for a homogeneous patient population resulted in the exclusion of subjects if they had a history of RLS symptom augmentation or end-of-dose rebound with previous dopamine agonist treatment. Subjects were also excluded if they had a body mass index of > 34 kg/m2, an estimated creatinine clearance of < 60 mL/min or serum ferritin level of < 20 ng/mL, were currently suffering from moderate or severe depression, a neurologic disease, a sleep disorder, or a movement disorder other than RLS. Other exclusion criteria were clinically significant or unstable medical conditions, or other medical conditions or drug therapy which could have affected RLS treatment efficacy. Subjects were also excluded if they were pregnant or lactating.
All subjects provided written informed consent prior to study participation. The study was conducted in accordance with good clinical practice guidelines and the Declaration of Helsinki (2004 revision).23 The protocol was reviewed and approved by a local or regional institutional review board, depending upon center requirements.
Randomization and Treatment Allocation
Subjects completed a 7-day screening and baseline study period prior to randomization. Subjects were randomized 1:1:1 to receive GEn 1200 mg (two 600-mg extended release tablets), GEn 600 mg (one 600-mg tablet and one placebo tablet), or placebo (2 placebo tablets), once daily at 5 pm with food, using a blocked randomization schedule stratified by study site. Blinding was ensured with matching placebo and GEn tablets. Subjects took one tablet of study drug (GEn 600 mg or placebo) on Days 1–3 and 2 tablets of study drug (GEn 600 mg or placebo) from Day 4 onwards, according to their randomization group. At study completion or following early withdrawal, subjects took one tablet of study drug during a 7-day downward taper (GEn 600 mg or placebo) or entered an open-label extension study (study completers only). Subjects attended clinic on Days –7 and 1 (baseline), and at Weeks 1, 2, 3, 4, 6, 8, 10, 12 (or early termination [ET]), and follow-up.
Efficacy Assessments
The co-primary efficacy endpoints were the mean change from baseline in IRLS total score at Week 12 last observation carried forward (LOCF) and the proportion of responders (rated “very much” or “much” improved) on the investigator-rated Clinical Global Impression-Improvement scale (CGI-I)24 at Week 12 LOCF, for GEn 1200 mg compared with placebo.
Secondary efficacy endpoints included the mean change from baseline in IRLS total score at Week 1 LOCF for GEn 1200 mg and 600 mg, and at Week 12 LOCF for GEn 600 mg, compared with placebo. Also assessed were the proportion of responders on the investigator-rated CGI-I at Week 1 observed case (OC) for GEn 1200 mg and 600 mg, and at Week 12 LOCF for GEn 600 mg, compared with placebo. The proportion of responders on the subject-rated CGI-I was also assessed for GEn 1200 mg and 600 mg at Week 1 OC and Week 12 LOCF, compared with placebo.
Maximum RLS symptom severity and the time to onset of RLS symptoms (length of time from the start of the 24-h assessment period [08:00] to the time when 50% of subjects experienced their first symptom) were assessed for GEn 1200 mg and 600 mg compared with placebo, using a 24-h RLS symptom diary (Appendix, Figure S1) beginning at 08:00. Subjects recorded the times they slept and their symptoms when awake, if present, as mild, moderate or severe. Symptom severity ratings were summarized in 6 non-overlapping 4-h periods beginning at 08:00.
Other secondary efficacy endpoints included mean change from baseline to Week 12 LOCF on the following subject-rated scales for GEn 1200 mg and 600 mg compared with placebo; the Pittsburgh Sleep Diary (PghSD)25,26 items average daily wake time after sleep onset (WASO) and average daily total sleep time (TST); and the validated Medical Outcomes Study (MOS)27,28 Sleep Scale domains sleep disturbance, sleep quantity, sleep adequacy (getting enough sleep to feel rested upon awakening and getting the amount of sleep needed), and daytime somnolence. A post-sleep questionnaire (PSQ) assessed sleep quality, next-day functioning, number of nights with RLS symptoms, number of nighttime awakenings, and number of hours awake due to RLS symptoms during the previous week at Week 12 LOCF.
The IRLS was completed at baseline and at the end of Weeks 1, 2, 3, 4, 6, 8, 10, and 12/ET; the investigator- and subject-rated CGI-I were completed at the end of Weeks 1, 2, 4, 8, and 12/ET; the 24-h RLS symptom diary was completed at baseline and at the end of Weeks 2 and 12/ET; the PghSD was completed at baseline and the end of Weeks 2, 4, 8, and 12/ET; and the MOS sleep scale and PSQ were completed at baseline and the end of Weeks 4, 8, and 12/ET. Following discontinuation of treatment, no efficacy measurements were performed.
Safety and Tolerability Assessments
The incidence and severity of treatment-emergent adverse events (AEs), serious AEs (SAEs), and events leading to study withdrawal were recorded. Clinical laboratory parameters (hematology, serum chemistry, and urinalysis) were assessed at baseline and Weeks 1, 2, 4, 8, and 12/ET. Vital signs were measured at every visit, and electrocardiograms (ECGs) were performed at baseline and at the end of Weeks 1, 4, 8, and 12/ET.
Daytime sleepiness was assessed at baseline and at the end of Weeks 4, 8, and 12/ET using the Epworth Sleepiness Scale (ESS).29 An ESS score > 10 was pre-specified as abnormal. A sponsor-developed sudden onset of sleep (SOS) questionnaire (Appendix, Figure S2) was completed by subjects at baseline and the end of Weeks 4, 8, and 12/ET to record possible sleep attacks (defined as “sudden onset of sleep that is irresistible and overwhelming and comes without warning”) and the activities during which these events occurred. Median time to onset of RLS symptoms and the distribution of symptom severity were calculated from the 24-h RLS symptom diaries at baseline and at the end of Week 12.
Statistical Analyses
Sample size was determined for each of the co-primary endpoints using the results of 2 previous GEn studies21,30; 105 subjects per treatment group were considered sufficient to detect with 90% power, a mean treatment difference (GEn 1200 mg compared with placebo) of −4.0 in mean change from baseline in IRLS total score at the 0.05 significance level using a 2-sided t-test, assuming a standard deviation (SD) of 8.8, and a difference in response rate of 23% on the investigator-rated CGI-I (GEn 68%, placebo 45%; odds ratio [OR] 2.6). Positive evidence of efficacy required both co-primary tests to be significant (p < 0.05). The study was not powered to compare GEn 1200 mg with GEn 600 mg.
The safety population comprised all subjects who had received at least one dose or portion of a dose of study medication. All efficacy analyses were performed on the modified intent-to-treat (mITT) population, comprising all subjects in the safety population who had completed the IRLS at baseline and at least once during the treatment period, using LOCF as an imputation method unless otherwise specified. No adjustment was made for multiple comparisons for secondary endpoints, as these were considered exploratory and supportive to the co-primary endpoints.
Study sites were pooled regionally, as pre-specified in the protocol, into 6 consolidated sites before performing analyses that included adjustment for site.
Changes from baseline in continuous efficacy endpoints were analyzed using an analysis of covariance (ANCOVA) model, adjusted for baseline value, pooled site, treatment, and treatment by pooled-site interaction (if the interaction term was significant at the 10% level); change from baseline in the ESS endpoint was analyzed using an ANCOVA adjusted for treatment group. Dichotomous efficacy endpoints were analyzed using logistic regression adjusted for pooled site and treatment. Multi-categorical efficacy endpoints were analyzed using the Cochran-Mantel-Haenszel mean score test (using equally spaced scores stratified by pooled site). The time to onset of first RLS symptoms in each treatment group was summarized descriptively using the Kaplan-Meier method.
RESULTS
Subjects
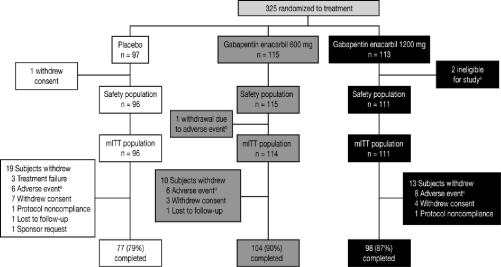
A total of 325 subjects were enrolled and randomized to receive GEn 1200 mg (n = 113), 600 mg (n = 115), or placebo (n = 97) (Figure 1). Three subjects were excluded from the safety population; 2 failed entry criteria; and one withdrew consent, all before taking study drug. One subject (GEn 600 mg) was excluded from the mITT population after an AE of somnolence; the subject withdrew from the study after 2 days of dosing and before an efficacy assessment. Subject demographics and baseline characteristics were similar across treatment groups (Table 1) and representative of a population with moderate-to-severe RLS. Treatment compliance rates were 95.5% (GEn 1200 mg), 93.9% (GEn 600 mg), and 91.7% (placebo).
Figure 1.
Subject disposition
a2 subjects were ineligible for study entry due to failed entry criteria at baseline. b1 subject withdrawn due to an adverse event of somnolence. c8 subjects withdrawn due to adverse events of depression (n = 2), hypotension (n = 1), vertigo (n = 1), decreased libido (n = 1), joint sprain (n = 1), sedation (n = 1), and nausea and dizziness (n = 1). d6 subjects withdrawn due to adverse events of somnolence (n = 1), fatigue and somnolence (n = 1), increased platelet count (n = 1), dizziness (n = 1), sedation (n = 1), and hypertension (n = 1). e6 subjects withdrawn due to adverse events of palpitations and chest discomfort (n = 1), mood swings (n = 1), headache (n = 1), pruritis (n = 1), joint swelling (n = 1), and sleep apnea syndrome (n = 1). mITT, modified intent-to-treat.
Table 1.
Subject demographic and clinical characteristics at baseline (safety population)
| Characteristic | Placebo (n = 96) | GEn 600 mg (n = 115) | GEn 1200 mg (n = 111) |
|---|---|---|---|
| Age, years | 49.1 (12.19) | 48.3 (12.83) | 49.5 (12.67) |
| Gender, % female | 59 | 58 | 59 |
| Race, White/Caucasian, % | 95 | 92 | 96 |
| Duration of RLS symptoms, years | 14.4 (12.85) | 13.5 (13.07) | 14.1 (12.36) |
| No previous RLS treatment, % | 61 | 67 | 65 |
| IRLS total scorea | 23.8 (4.58) | 23.1 (4.93)b | 23.2 (5.32) |
| Average daily wake time after sleep onset, minutesa | 38.7 (42.57) | 31.8 (26.71)c | 31.4 (28.48) |
| Average daily total sleep time, hoursa | 6.7 (1.20) | 6.7 (1.10)c | 6.5 (1.37) |
| MOS Sleep Scalea | |||
| Sleep quantity, hours | 6.0 (1.31) | 6.1 (1.14)b | 6.1 (1.42) |
| Sleep adequacy score | 34.8 (24.62) | 30.5 (24.08)b | 34.7 (24.86) |
| Sleep disturbance score | 51.9 (23.16) | 53.1 (20.90)b | 52.1 (22.85) |
| Daytime somnolence score | 34.8 (19.45) | 34.0 (19.44)b | 36.7 (21.97) |
| Post-Sleep Questionnairea | |||
| Overall quality of sleep | |||
| Excellent | 0 | 0e | 0d |
| Reasonable | 41 (42.7) | 40 (35.7)e | 43 (39.1)d |
| Poor | 55 (57.3) | 72 (64.3)e | 67 (60.9)d |
| Ability to function | |||
| Excellent | 8 (8.3) | 9 (8.0)e | 5 (4.5)d |
| Good | 40 (41.7) | 50 (44.6)e | 55 (50.0)d |
| Moderate | 46 (47.9) | 47 (42.0)e | 39 (35.5)d |
| Poor | 2 (2.1) | 6 (5.4)e | 11 (10.0)d |
| Number of nights with RLS symptoms | |||
| 0 nights | 1 (1.0) | 0e | 1 (0.9)d |
| 1–2 nights | 2 (2.1) | 2 (1.8)e | 1 (0.9)d |
| 3–4 nights | 10 (10.4) | 11 (9.8)e | 7 (6.4)d |
| 5–6 nights | 30 (31.3) | 34 (30.4)e | 31 (28.2)d |
| 7 nights | 53 (55.2) | 65 (58.0)e | 70 (63.6)d |
| Number of awakenings during night due to RLS symptoms | |||
| 0 times | 8 (8.3) | 19 (17.0)e | 12 (10.9)d |
| 1–2 times | 47 (49.0) | 48 (42.9)e | 55 (50.0)d |
| 3–4 times | 29 (30.2) | 34 (30.4)e | 32 (29.1)d |
| ≥ 5 times | 12 (12.5) | 11 (9.8)e | 11 (10.0)d |
| Number of hours awake per night due to RLS symptoms | |||
| 0 hours | 8 (8.3) | 19 (17.0)e | 12 (10.9)d |
| < 1 hours | 40 (41.7) | 40 (35.7)e | 52 (47.3)d |
| 1 to < 2 hours | 24 (25.0) | 35 (31.3)e | 27 (24.5)d |
| 2 to < 3 hours | 16 (16.7) | 13 (11.6)e | 12 (10.9)d |
| ≥ 3 hours | 8 (8.3) | 5 (4.5)e | 7 (6.4)d |
| ESS total score | 9.6 (4.98) | 9.7 (5.22)c | 9.0 (4.76)d |
All values are mean (SD) unless otherwise stated.
mITT population;
n = 114;
n = 113;
n = 110;
n = 112.
ESS, Epworth Sleepiness Scale; GEn, gabapentin enacarbil; IRLS, International Restless Legs Scale; mITT, modified intent-to-treat; MOS, Medical Outcomes Study; SD, standard deviation; RLS, Restless Legs Syndrome.
Co-primary Endpoints
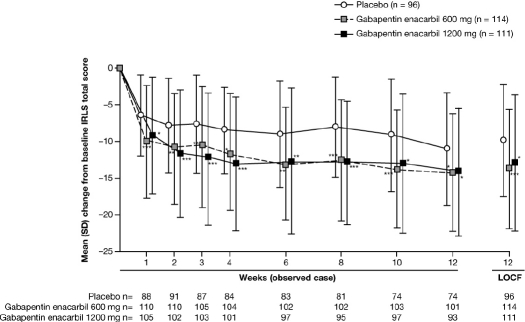
GEn 1200 mg significantly improved mean [SD] IRLS total score at Week 12 LOCF (baseline: 23.2 [5.32]; Week 12: 10.2 [8.03]; change from baseline to Week 12 LOCF: –13.0 [9.12]) compared with placebo (baseline: 23.8 [4.58]; Week 12: 14.0 [7.87]; change from baseline to Week 12 LOCF: –9.8 [7.69]; adjusted mean treatment difference [AMTD] for change from baseline: –3.5; 95% confidence intervals [CI]: –5.6, –1.3; p = 0.0015) (Figure 2). There was a statistically significant interaction for treatment by pooled site (p = 0.0062) that was driven by one site at which a larger response was observed with placebo than with GEn 1200 mg. When data from this site (n = 27) were excluded from a sensitivity analysis, it did not alter the significance of the efficacy findings, and the interaction for treatment by pooled site was no longer significant. At Week 12 LOCF, the majority of subjects in the GEn-treated groups had an IRLS total score ≤ 10 (64.9% and 55.0% of subjects in the GEn 600 mg and GEn 1200 mg groups, respectively), in contrast with the placebo group (32.3% of subjects). Of these, the proportion of subjects with an IRLS total score of 0 (absolute remitters) was 22.5%, 26.3%, and 11.5% in the GEn 1200 mg, GEn 600 mg, and placebo groups, respectively. The proportion of responders (subjects with ≥ 50% improvement in IRLS total score versus baseline at Week 12 LOCF) was 57.7% for GEn 1200 mg, 64.0% for GEn 600 mg, and 39.6% for placebo.
Figure 2.
Mean (SD) change from baseline in IRLS total score by visit (mITT population)
Adjusted mean treatment difference GEn compared with placebo at Week 12 LOCF: Co-primary endpoint: GEn 1200 mg, −3.5 (95% CI: −5.6, −1.3; p = 0.0015); Secondary endpoint: GEn 600 mg, −4.3 (95% CI: −6.4, −2.3; p < 0.0001). ***p < 0.0001; **p < 0.001; *p < 0.01. CI, confidence interval; GEn, gabapentin enacarbil; IRLS, International Restless Legs Scale; LOCF, last observation carried forward; mITT, modified intent-to-treat; PBO, placebo; SD, standard deviation. Mean (SD) change from baseline in IRLS total score over time using last observation carried forward was similar to the observed case data.
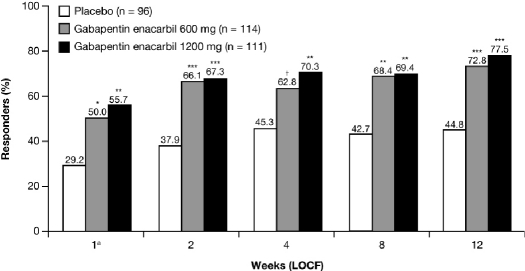
A significantly greater proportion of GEn 1200 mg-treated subjects (86/111; 77.5%) were responders on the investigator-rated CGI-I at Week 12 LOCF, compared with placebo (43/96; 44.8%; adjusted OR: 4.3; 95% CI: 2.34, 7.86; p < 0.0001) (Figure 3).
Figure 3.
Proportion of responders (much or very much improved) on the investigator-rated CGI-I by visit (mITT population)
Adjusted odds ratios for GEn compared with placebo at Week 12 LOCF: Co-primary endpoint: GEn 1200 mg, 4.3 (95% CI: 2.34, 7.86; p < 0.0001); Secondary endpoint: GEn 600 mg, 3.3 (95% CI: 1.84, 5.99; p < 0.0001). aObserved case. ***p < 0.0001; **p < 0.001; *p < 0.01; †p < 0.05 compared with placebo. CI, confidence interval; CGI-I, Clinical Global Impression–Improvement scale; GEn, gabapentin enacarbil; LOCF, last observation carried forward; mITT, modified intent-to-treat.
Secondary Endpoints
RLS Symptoms
GEn 600 mg significantly improved mean (SD) IRLS total score at Week 12 LOCF (baseline: 23.1 [4.93]; Week 12: 9.3 [7.77]; change from baseline to Week 12 LOCF: –13.8 [8.09]) compared with placebo (baseline: 23.8 [4.58]; Week 12: 14.0 [7.87]; change from baseline to Week 12 LOCF: –9.8 [7.69]; AMTD for change from baseline: –4.3; 95% CI: –6.4, –2.3; p < 0.0001) (Figure 2). Significant improvements in IRLS total score were seen as early as Week 1 LOCF for both GEn 1200 mg (–8.7 [8.09]; AMTD: –3.0; 95% CI: –4.8, –1.1; p = 0.0017) and GEn 600 mg (–9.8 [7.76]; AMTD: –4.1; 95% CI: –5.9, –2.3; p < 0.0001), compared with placebo (–6.0 [5.59]).
A significantly greater proportion of GEn 600 mg-treated subjects (83/114; 72.8%) were responders on the investigator-rated CGI-I at Week 12 LOCF, compared with placebo (43/96; 44.8%; adjusted OR: 3.3; 95% CI: 1.84, 5.99; p < 0.0001). The proportion of responders at Week 1 OC and at each subsequent assessment was also significantly greater for both GEn 1200 mg and GEn 600 mg compared with placebo (Figure 3).
A significantly greater proportion of GEn-treated subjects (GEn 1200 mg, 83/111 [74.8%], p < 0.0001; GEn 600 mg, 90/114 [78.9%], p < 0.0001) were responders on the subject-rated CGI-I at Week 12 LOCF, compared with placebo (46/96 [47.9%]), and significant improvements were seen as early as Week 1 OC with GEn 1200 mg (52/105 [49.5%]; p = 0.0001) and 600 mg (55/110 [50.0%]; p < 0.0001), compared with placebo (20/88 [22.7%]).
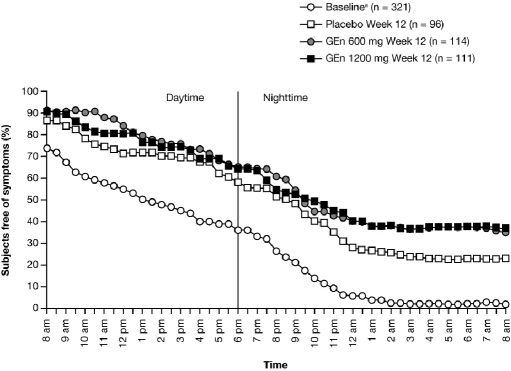
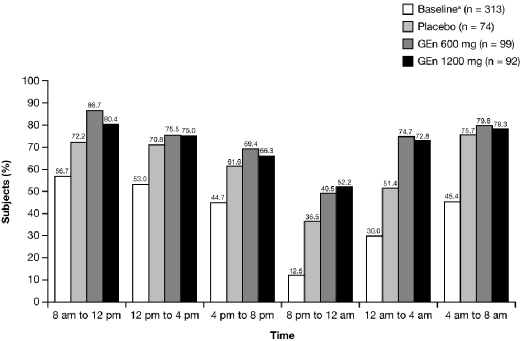
There was an increase in the estimated median time to onset of RLS symptoms from baseline to Week 12 in all treatment groups (24-h RLS symptom diary). At baseline, 98.0% of subjects experienced RLS symptoms by the end of the 24-h period. However, at Week 12, 37.0% (GEn 1200 mg), 35.3% (GEn 600 mg), and 23.0% (placebo) of subjects were free from symptoms at 24 hours (Figure 4).
Figure 4.
Proportion of subjects free of RLS symptoms over 24 hours, beginning at 8 am (mITT Population)
Median time to onset of RLS symptoms over 24 hours Baselinea: 5.5 hours (95% CI: 4.0, 7.5) Week 12: GEn 1200 mg, 13.8 h (95% CI: 11.5, 17.0), GEn 600 mg, 13.5 h (95% CI: 12.5, 16.5), Placebo, 12.8 h (95% CI: 9.5, 15.0) aCombined treatment groups. CI, confidence interval; GEn, gabapentin enacarbil; mITT, modified intent-to-treat.
During the 20:00 to 00:00 period at Week 12, 52.2% of GEn 1200 mg-treated subjects and 49.5% of GEn 600 mg-treated subjects reported no symptoms, compared with 36.5% of placebo-treated subjects (Figure 5).
Figure 5.
Proportion of subjects reporting no RLS symptoms at Baseline and Week 12 (mITT Population)
Assessed during the 24 hours prior to baseline and Week 12 visits. aCombined treatment groups. GEn, gabapentin enacarbil; mITT, modified intent-to-treat.
Sleep
Average daily WASO (PghSD) decreased significantly from baseline with GEn 1200 mg at Week 12 LOCF, compared with placebo (mean [SD] change from baseline: –18.5 [28.67] min, –12.5 [31.01] min; AMTD: –9.8; 95% CI: –15.5, –4.2; p = 0.0007) and at all other timepoints assessed (Figure 6a). Significant decreases were also seen with GEn 600 mg compared with placebo at each timepoint (Figure 6a).
Figure 6.
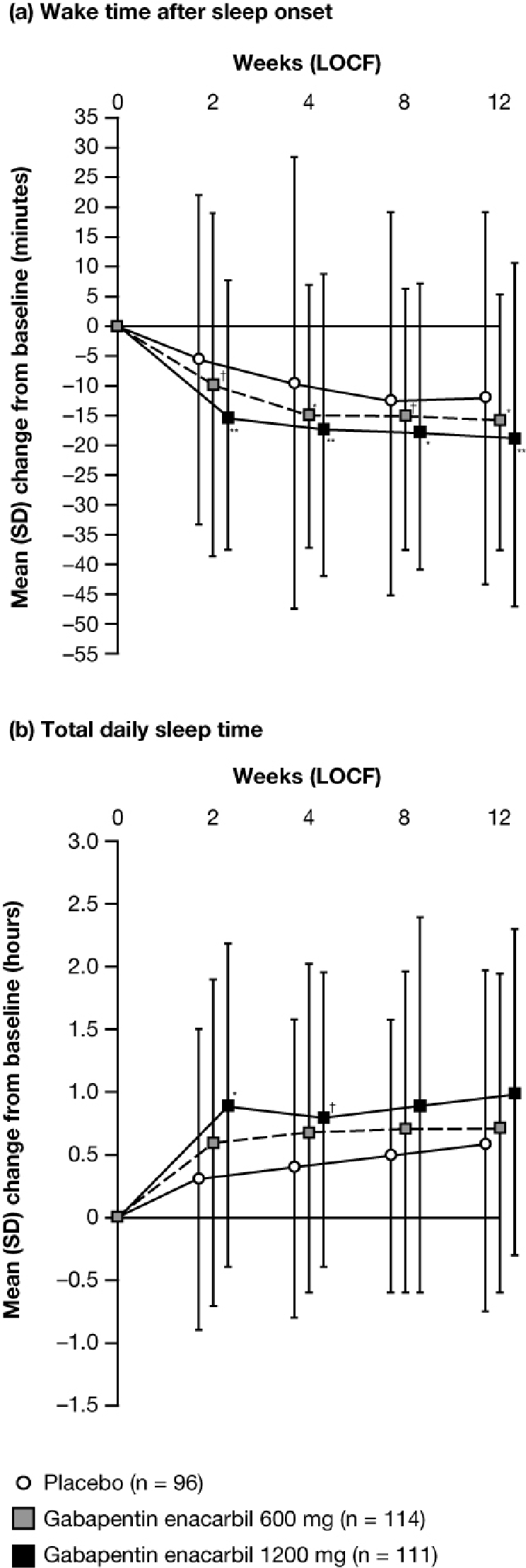
Mean (SD) change from baseline in daily (a) wake time after sleep onset and (b) total sleep time on the PghSD by visit (mITT Population)
(a) Adjusted mean treatment difference, GEn compared with placebo at Week 12 LOCF: GEn 1200 mg, –9.8 (95% CI: –15.5, –4.2; p = 0.0007); GEn 600 mg, –7.6 (95% CI: –13.3, –2.0; p = 0.0081). **p < 0.001; *p < 0.01; †p < 0.05. (b) Adjusted mean treatment difference, GEn compared with placebo at Week 12 LOCF: GEn 1200 mg, 0.2 (95% CI: −0.1, 0.6; p = 0.1161); GEn 600 mg, 0.1 (95% CI: −0.2, 0.4; p = 0.6778). *p < 0.01; †p < 0.05. CI, confidence interval; GEn, gabapentin enacarbil; LOCF, last observation carried forward; mITT, modified intent-to-treat; PghSD, Pittsburgh Sleep Diary; SD, standard deviation.
GEn 1200 mg did not significantly increase the daily TST (PghSD) from baseline compared with placebo at Week 12 LOCF (mean [SD] change from baseline: GEn 1200 mg, 1.0 [1.32] h; placebo, 0.6 [1.36] h; AMTD: 0.2; 95% CI: –0.1, 0.6; p = 0.1161), although significant improvement was seen at Weeks 2 and 4 LOCF (Figure 6b). No significant difference in daily TST was observed between GEn 600 mg and placebo at any visit.
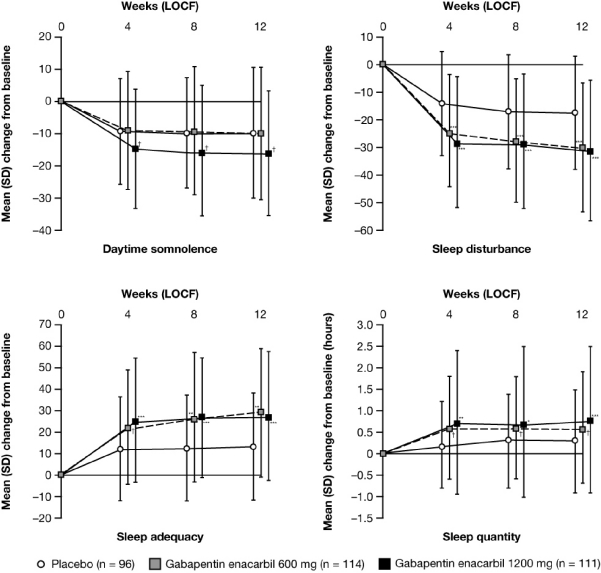
GEn 1200 mg significantly improved all MOS Sleep Scale domains compared with placebo at Week 12 LOCF, with greater improvements in mean [SD] change from baseline for sleep disturbance (–30.7 [25.45], –17.0 [20.40]; p < 0.0001), sleep quantity (0.8 [1.66] h, 0.3 [1.19] h; p = 0.0001), sleep adequacy (27.7 [29.91], 13.6 [24.59]; p < 0.0001), and daytime somnolence (–16.1 [19.58], –9.7 [20.29]; p = 0.0309) (Figure 7). Significant improvements in all domains were also seen at Week 4 LOCF (the earliest assessment) and Week 8 LOCF. GEn 600 mg-treated subjects had significantly greater improvements in mean [SD] change from baseline to Week 12 LOCF, compared with placebo, for sleep disturbance (–29.5 [23.27], –17.0 [20.40]; p < 0.0001), sleep quantity (0.6 [1.25] h, 0.3 [1.19] h; p = 0.0209), and sleep adequacy (29.1 [29.91], 13.6 [24.59]; p = 0.0003), but not for daytime somnolence (–9.8 [20.35], –9.7 [20.29]; p = 0.8926) (Figure 5). Significant improvements were also seen in sleep disturbance, quantity, and adequacy at Week 4 LOCF and Week 8 LOCF.
Figure 7.
Mean (SD) change from baseline in domains of the MOS Sleep Scalea by visit (mITT Population)
Adjusted mean treatment difference GEn compared with placebo at Week 12 LOCF: GEn 1200 mg: Sleep disturbance, –13.4 (95% CI: –18.5, –8.2; p < 0.0001); Sleep quantity, 0.6 (95% CI: 0.3, 0.9; p = 0.0001); Sleep adequacy, 13.9 (95% CI: 7.0, 20.8; p < 0.0001); Daytime somnolence, –5.4 (95% CI: –10.3, –0.5; p = 0.0309). GEn 600 mg: Sleep disturbance, –11.6 (95% CI: –16.8, –6.4; p < 0.0001); Sleep quantity, 0.3 (95% CI: 0.1, 0.6; p = 0.0209); Sleep adequacy, 13.0 (95% CI: 6.0, 19.9; p = 0.0003); Daytime somnolence, –0.3 (95% CI: –5.2, 4.6; p = 0.8926). ***p ≤ 0.0001; **p ≤ 0.001; *p ≤ 0.01; †p < 0.05. aScale 0–100, except for sleep quantity; decreases in daytime somnolence and sleep disturbance, and increases in sleep adequacy and quantity represent improvements. CI, confidence interval; GEn, gabapentin enacarbil; LOCF, last observation carried forward; mITT, modified intent-to-treat; MOS, Medical Outcomes Study; SD, standard deviation.
All items of the PSQ significantly improved with GEn 1200 mg and 600 mg compared with placebo at Week 4 LOCF, Week 8 LOCF, and Week 12 LOCF (Table 2).
Table 2.
Distribution of post-sleep questionnaire responses at Baseline and Week 12 last observation carried forward (LOCF) (modified intent-to-treat population)
| Week 4 LOCF |
Week 8 LOCF |
Week 12 LOCF |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 96) | GEn 600 mg (n = 114) | GEn 1200 mg (n = 111) | Placebo (n = 96) | GEn 600 mg (n = 114) | GEn 1200 mg (n = 111) | Placebo (n = 96) | GEn 600 mg (n = 114) | GEn 1200 mg (n = 111) | |
| Overall quality of sleep | |||||||||
| Excellent | 2 (2.1) | 24 (21.1) | 30 (27.0) | 5 (5.2) | 24 (21.1) | 25 (22.5) | 14 (14.6) | 24 (21.1) | 30 (27.0) |
| Reasonable | 64 (66.7) | 64 (56.1) | 66 (59.5) | 57 (59.4) | 64 (56.1) | 67 (60.4) | 53 (55.2) | 72 (63.2) | 65 (58.6) |
| Poor | 30 (31.3) | 26 (22.8) | 15 (13.5) | 34 (35.4) | 26 (22.8) | 19 (17.1) | 29 (30.2) | 18 (15.8) | 16 (14.4) |
| p-valuea | 0.0011 | < 0.0001 | 0.0014 | < 0.0001 | 0.0230 | 0.0023 | |||
| Ability to function | |||||||||
| Excellent | 18 (18.8) | 35 (30.7) | 37 (33.3) | 19 (19.8) | 35 (30.7) | 44 (39.6) | 23 (24.0) | 39 (34.2) | 45 (40.5) |
| Good | 50 (52.1) | 56 (49.1) | 54 (48.6) | 49 (51.0) | 55 (48.2) | 49 (44.1) | 49 (51.0) | 57 (50.0) | 49 (44.1) |
| Moderate | 24 (25.0) | 20 (17.5) | 17 (15.3) | 25 (26.0) | 23 (20.2) | 14 (12.6) | 19 (19.8) | 16 (14.0) | 13 (11.7) |
| Poor | 4 (4.2) | 3 (2.6) | 3 (2.7) | 3 (3.1) | 1 (0.9) | 4 (3.6) | 5 (5.2) | 2 (1.8) | 4 (3.6) |
| p-valuea | 0.0334 | 0.0120 | 0.0433 | 0.0034 | 0.0366 | 0.0152 | |||
| Number of nights with RLS symptoms | |||||||||
| 0 nights | 1 (1.0) | 19 (16.7) | 28 (25.2) | 7 (7.3) | 30 (26.3) | 31 (27.9) | 13 (13.5) | 35 (30.7) | 32 (28.8) |
| 1–2 nights | 21 (21.9) | 36 (31.6) | 28 (25.2) | 27 (28.1) | 33 (28.9) | 26 (23.4) | 17 (17.7) | 33 (28.9) | 29 (26.1) |
| 3–4 nights | 24 (25.0) | 23 (20.2) | 22 (19.8) | 17 (17.7) | 21 (18.4) | 20 (18.0) | 18 (18.8) | 15 (13.2) | 20 (18.0) |
| 5–6 nights | 20 (20.8) | 12 (10.5) | 15 (13.5) | 19 (19.8) | 10 (8.8) | 13 (11.7) | 25 (26.0) | 15 (13.2) | 12 (10.8) |
| 7 nights | 30 (31.3) | 24 (21.1) | 18 (16.2) | 26 (27.1) | 20 (17.5) | 21 (18.9) | 23 (24.0) | 16 (14.0) | 18 (16.2) |
| p-valuea | 0.0002 | < 0.0001 | 0.0006 | 0.0025 | 0.0001 | 0.0006 | |||
| Number of awakenings during night due to RLS symptoms | |||||||||
| 0 times | 24 (25.0) | 55 (48.2) | 54 (48.6) | 26 (27.1) | 58 (50.9) | 55 (49.5) | 35 (36.5) | 63 (55.3) | 62 (55.9) |
| 1–2 times | 48 (50.0) | 47 (41.2) | 42 (37.8) | 51 (53.1) | 42 (36.8) | 41 (36.9) | 41 (42.7) | 42 (36.8) | 41 (36.9) |
| 3–4 times | 17 (17.7) | 8 (7.0) | 9 (8.1) | 14 (14.6) | 11 (9.6) | 13 (11.7) | 13 (13.5) | 7 (6.1) | 7 (6.3) |
| 5 or more times | 7 (7.3) | 4 (3.5) | 6 (5.4) | 5 (5.2) | 3 (2.6) | 2 (1.8) | 7 (7.3) | 2 (1.8) | 1 (0.9) |
| p-valuea | 0.0003 | 0.0019 | 0.0025 | 0.0028 | 0.0009 | 0.0004 | |||
| Number of hours awake per night due to RLS symptoms | |||||||||
| 0 hours | 24 (25.0) | 55 (48.2) | 54 (48.6) | 26 (27.1) | 58 (50.9) | 55 (49.5) | 35 (36.5) | 63 (55.3) | 62 (55.9) |
| < 1 hours | 33 (34.4) | 45 (39.5) | 39 (35.1) | 39 (40.6) | 43 (37.7) | 37 (33.3) | 42 (43.8) | 37 (32.5) | 33 (29.7) |
| 1 to < 2 hours | 29 (30.2) | 12 (10.5) | 10 (9.0) | 19 (19.8) | 8 (7.0) | 12 (10.8) | 8 (8.3) | 12 (10.5) | 8 (7.2) |
| 2 to < 3 hours | 4 (4.2) | 0 | 4 (3.6) | 6 (6.3) | 3 (2.6) | 4 (3.6) | 2 (2.1) | 0 | 5 (4.5) |
| ≥ 3 hours | 6 (6.3) | 2 (1.8) | 4 (3.6) | 6 (6.3) | 2 (1.8) | 3 (2.7) | 9 (9.4) | 2 (1.8) | 3 (2.7) |
| p-valuea | < 0.0001 | 0.0003 | < 0.0001 | 0.0011 | 0.0019 | 0.0187 | |||
Number of subjects, n (%). Questions refer to symptoms experienced over the past week.
p-values derived from Cochran–Mantel–Haenszel testing using equally spaced scoring and stratification by pooled site. GEn, gabapentin enacarbil.
Tolerability
The most commonly reported treatment-emergent AEs overall with GEn 1200 mg and 600 mg were dizziness and somnolence (Table 3). The median (range) duration of dizziness was 4 (1–92) days (GEn 1200 mg, n = 27), 5 (1–84) days (GEn 600 mg, n = 12), and 3 (1–16) days (placebo, n = 5); the median (range) duration of somnolence was 16 (2–84) days (GEn 1200 mg, n = 20), 35 (1–85) days (GEn 600 mg, n = 25), and 39 (9–68) days (placebo, n = 2).
Table 3.
Treatment-emergent adverse events occurring in ≥ 5% of subjects in any treatment group (safety population)
| Subjects, n (%) |
|||
|---|---|---|---|
| Placebo (n = 96) | GEn 600 mg (n = 115) | GEn 1200 mg (n = 111) | |
| Adverse event, any | 76 (79.2) | 100 (87.0) | 94 (84.7) |
| Dizziness | 5 (5.2) | 12 (10.4) | 27 (24.3) |
| Somnolence | 2 (2.1) | 25 (21.7) | 20 (18.0) |
| Headache | 8 (8.3) | 17 (14.8) | 15 (13.5) |
| Nasopharyngitis | 7 (7.3) | 13 (11.3) | 11 (9.9) |
| Dry mouth | 2 (2.1) | 5 (4.3) | 9 (8.1) |
| Nausea | 4 (4.2) | 6 (5.2) | 6 (5.4) |
| Sedation | 2 (2.1) | 1 (0.9) | 6 (5.4) |
| Back pain | 3 (3.1) | 6 (5.2) | 3 (2.7) |
| Fatigue | 5 (5.2) | 6 (5.2) | 3 (2.7) |
| Upper respiratory tract infection | 2 (2.1) | 9 (7.8) | 2 (1.8) |
| Sinus congestion | 5 (5.2) | 1 (0.9) | 2 (1.8) |
GEn, gabapentin enacarbil.
Three subjects experienced SAEs: cholelithiasis (placebo), cellulitis (GEn 600 mg), and intervertebral disc protrusion (GEn 600 mg); all events resolved, were not considered treatment related, and the subjects continued in the study. All AEs leading to study withdrawal (Figure 1), except sleep apnea syndrome, were considered by investigators to be treatment related; all but 3 (sleep apnea syndrome, increased platelet count, and hypertension) resolved upon discontinuation of study drug.
No clinically relevant changes in vital signs, ECGs, or laboratory parameters were observed.
The mean (SD) ESS scores at baseline were < 10 for each treatment group (Table 1). Reductions in ESS scores (mean [SD] change from baseline) were similar between GEn 1200 mg (–2.8 [4.52]), GEn 600 mg (–2.9 [5.22]), and placebo (–2.4 [4.02]) at Week 12; treatment differences were not significant for GEn 1200 mg (AMTD: –0.5; 95% CI: –1.8, 0.8; p = 0.4790) or GEn 600 mg (AMTD: –0.5; 95% CI: –1.8, 0.8; p = 0.4704), compared with placebo. At Week 12, the proportion of subjects with an increase in ESS total score from baseline was 13.6% (n = 15) for GEn 1200 mg, 18.8% (n = 21) for GEn 600 mg and 21.1% (n = 19) for placebo.
One subject (GEn 1200 mg) reported 8 events of SOS, 3 prior to the 7-day baseline period and 5 during the 12-week treatment period, considered possibly related to study drug. The subject continued in the study and the events resolved without intervention. No subjects in the GEn 600 mg or placebo groups reported potential sleep attacks.
DISCUSSION
The results of this 12-week study of GEn in the treatment of moderate-to-severe primary RLS confirm the efficacy and tolerability reported by Kushida et al. in a similarly designed placebo-controlled study of GEn 1200 mg.20 At Week 12 LOCF, mean reductions in IRLS total score from baseline with GEn 1200 mg versus placebo (–13.0 and –9.8) were comparable with those reported by Kushida et al. (–13.2 and –8.8), and similar proportions of subjects were rated as responders on the investigator-rated CGI-I (77.5% and 44.8% compared with 76.1% and 38.9%, GEn 1200 mg and placebo, respectively).20 The reductions in IRLS total score observed in the present study are also comparable with those reported for dopamine agonists that are currently approved for treatment of RLS.31–35
A significant mean reduction in IRLS total score from baseline was also reported with GEn 600 mg (–13.8) compared with placebo (–9.8), and a greater proportion of GEn 600 mg-treated subjects were rated by investigators as CGI-I responders (72.8% compared with 44.8%, respectively). These results contrast with findings from a 2-week study of GEn 600 mg reported by Walters et al. that demonstrated a nonsignificant mean reduction from baseline in IRLS total score at Week 2 LOCF (–9.1) compared with placebo (–8.9), and no significant difference in the proportion of CGI-I responders (GEn 600 mg, 58.6%; placebo, 48.5%).21 At Week 2 LOCF in the present study, GEn 600 mg significantly improved mean IRLS total score compared with placebo (–11.0 versus –7.4; p < 0.0001) and significantly more subjects were investigator-rated CGI-I responders compared with placebo (66.1% and 37.9%). Therefore, it is unlikely that the duration of treatment accounted for the differences in outcomes between these studies. Similarly, there were no notable differences in study population or design that might explain the observed differences between these two studies.
Although not part of the RLS diagnostic criteria, patients often report sleep disturbance due to RLS symptoms.3,4 Subjects in the present study reported significant sleep disturbance at baseline on the MOS Sleep Scale domains compared with a healthy cohort sample of the US population.27 Both GEn doses demonstrated significant improvements on daily WASO as early as 2 weeks after treatment initiation, and on the MOS Sleep Scale domains of sleep disturbance, sleep quantity, and sleep adequacy, and all PSQ items at Week 4 LOCF (the first timepoint at which these domains and items were assessed). Improvements in these subject-reported sleep outcomes remained significant through Week 12 LOCF with both GEn doses. Significant reduction in daytime somnolence was demonstrated on the MOS Sleep Scale with GEn 1200 mg, but not with GEn 600 mg, compared with placebo. These improvements in sleep outcomes were consistent with those reported for GEn 1200 mg in a placebo-controlled study.17 Furthermore, after treatment with GEn, subject-reported MOS Sleep Scale domain scores were almost identical to those of the US healthy cohort sample, demonstrating the positive impact of RLS symptom reduction on sleep.
The robust efficacy observed with GEn in the present study may be the result of sustained gabapentin exposure throughout the evening and night,18 producing a prolonged and predictable clinical response throughout treatment. This durable response was seen in multiple independent subject-rated scales, suggesting that the clinical benefits of GEn treatment are perceived by subjects.
Both GEn doses were generally well tolerated across 12 weeks of treatment. The two most common AEs, dizziness and somnolence, occurred at rates similar to those reported in previous studies of GEn in RLS.20,21,30 Importantly, ESS findings indicated that increased daytime sleepiness did not occur with either GEn dose, despite reports of somnolence as an AE. The ESS questionnaire is designed to evaluate daytime sleepiness; questions on the ESS focus on the likelihood of dozing in any given situation and the impact this has on daytime activities. At Week 12, a reduction from baseline in ESS total score was seen in all three treatment groups, suggesting subjects had a reduced likelihood of dozing in the day. Conversely, when subjects were asked about AEs as part of the tolerability assessment, approximately one-fifth of subjects receiving GEn reported somnolence (GEn 1200 mg, 18%; GEn 600 mg, 22%; placebo, 2%). Reports of somnolence events in this context may have included those experienced at nighttime or those experienced after taking the medication just before bedtime, rather than daytime sleepiness per se, and may explain the apparent difference between the two measures. Moreover, the majority of reported somnolence events were mild (GEn 1200 mg, 15%; GEn 600 mg, 14%; placebo, 2%) or moderate (GEn 1200 mg, 2%; GEn 600 mg, 5%; placebo, 0%) in intensity.
Examination of the 24-hour RLS symptom diary showed no earlier onset of symptoms and a delay in median onset of RLS symptoms was observed with both GEn doses at Week 12 compared with placebo.
These results should be interpreted within the context of the study design, which was not powered to compare GEn 1200 mg with GEn 600 mg. The 12-week treatment duration may not have been long enough to fully assess RLS symptom augmentation or EMR; longer-term studies are needed to examine these clinical manifestations, which have been associated with dopaminergic therapy.
In summary, GEn 1200 mg and 600 mg significantly improved RLS symptoms and sleep disturbance compared with placebo after 12 weeks of treatment in subjects with moderate-to-severe primary RLS, and both were generally well tolerated.
DISCLOSURE STATEMENT
Research funding for design and conduct of this study, and collection, management, analysis, and interpretation of the data were sponsored by XenoPort, Inc., Santa Clara, CA, USA. Preparation, review, and approval of the manuscript were sponsored by GlaxoSmithKline, Research Triangle Park, NC, USA and XenoPort, Inc. Dr. Lee and Dr. Ziman have received research support from GlaxoSmithKline and XenoPort, Inc. Dr. Perkins has received research support from XenoPort, Inc. Dr. Poceta has received research support from GlaxoSmithKline and XenoPort, Inc. Dr. Walters has received compensation for consultancy and speaking engagements from GlaxoSmithKline, and has received research support from GlaxoSmithKline and XenoPort, Inc. He has also received compensation for consultancy and speaking engagements from UCB Pharma. Dr. Barrett is an employee of XenoPort, Inc. Gabapentin enacarbil is under investigation for the treatment of Restless Legs Syndrome, neuropathic pain, and prophylaxis of migraine.
ACKNOWLEDGMENTS
The authors acknowledge Barbara Wilson, M.Ed. (GlaxoSmithKline, Research Triangle Park, NC) for manuscript coordination and editorial assistance, Nicola Williams, M.Sc. (GlaxoSmithKline, Harlow, Essex, UK), Daniel Bonzo, Ph.D. (XenoPort, Inc., Santa Clara, CA), Ben Stein, Ph.D. (Premier Research International, Limited, San Diego, CA), and Mark J. Jaros, Ph.D. (Premier Research Group, Limited., Estes Park, CO) for statistical support and interpretation of the data, and Sarah Brown B.Sc (Hons), Phillippa Curran, Ph.D., and Sarah White, M.Sc. (Caudex Medical Ltd., Oxford, UK) for writing and editorial assistance.
APPENDIX.
Figure S1.
24-hour RLS symptom diary
Figure S2.
Sudden Onset of Sleep questionnaire
XP053 Study Investigators
Fares J. Arguello, M.D., Radiant Research, SLC, Salt Lake City, UT
Eric M. Ball, M.D., Walla Walla Clinic Research Department, Walla Walla, WA
Lisa Cohen, D.O., Suncoast Clinical Research, Inc., New Port Richet, FL
Michael J. Drass, M.D., Allegheny Pain Management, Altoona, PA
Stephen Duntley, M.D., Washington University, St. Louis, MO
Mitchell D. Feller, M.D., Coastal Carolina Research Center, Mt. Pleasant, SC
Gerald J. Ferencz, M.D., Shore Neurology, PA, Toms River, NJ
Mark A. Fisher, M.D., Lynn Health Science Center, Oklahoma City, OK
James E. Garrison III, M.D., Innovative Clinical Trials, San Antonio, TX
John R. Huddlestone, M.D., Neurology & Neurosurgery Associates of Tacoma, Tacoma, WA
Mark S. LeDoux, MD, Ph.D, University Of Tennessee Health Science Center, Memphis, TN
Daniel O. Lee, M.D., East Carolina Neurology, Greenville, NC
Kurt W. Lesh, M.D., Lynn Institute of the Rockies, Colorado Springs, CO
Daniel G. Lorch Jr., M.D., PAB Clinical Research, Brandon, FL
Stuart J. Menn, M.D., Pacific Sleep Medicine Services, Redlands, CA
Leslie Moldauer, M.B.A., M.D., Radiant Research, Denver, CO
Charulatha P. Nagar, M.D., Consultants in Neurology, Ltd., Northbrook, IL
Thomas Perkins, M.D., Ph.D., Raleigh Neurology Associates, Raleigh, NC
J. Steven Poceta, M.D., Scripps Clinic, La Jolla, CA
Michael Rokeach, M.D., Pacific Sleep Medicine, San Francisco, CA
Richard Shubin, M.D., Neuro-Therapeutics, Pasadena, CA
Daniel Vine, M.D., Pivotal Research Center, Salt Lake City, UT
Arthur S. Walters, M.D., NJ Neuroscience JFK Medical Center, Edison, NJ
J. Catesby Ware, Ph.D., Sleep Disorders Center-5th Floor, Norfolk, VA
Charles Wells Jr., M.D., Sleepmed, Inc., Macon, GA
Paul Wylie, M.D., Arkansas Center For Sleep Medicine, Little Rock, KS
Ronald B. Ziman, M.D., F.A.C.P., F.A.A.N., Northridge Neurological Center, Northridge, CA
REFERENCES
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 3.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–46. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Kushida CA, Allen RP, Atkinson MJ. Modeling the causal relationships between symptoms associated with restless legs syndrome and the patient-reported impact of RLS. Sleep Med. 2004;5:485–8. doi: 10.1016/j.sleep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Ondo W, Romanyshyn J, Vuong KD, Lai D. Long-term treatment of restless legs syndrome with dopamine agonists. Arch Neurol. 2004;61:1393–7. doi: 10.1001/archneur.61.9.1393. [DOI] [PubMed] [Google Scholar]
- 6.Winkelman JW, Johnston L. Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS) Sleep Med. 2004;5:9–14. doi: 10.1016/j.sleep.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Winkelman JW. A better future for patients with restless legs syndrome. Am J Med. 2007;120:S28–9. doi: 10.1016/j.amjmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59:1573–9. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 9.Happe S, Sauter C, Klosch G, Saletu B, Zeitlhofer J. Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology. 2003;48:82–6. doi: 10.1159/000072882. [DOI] [PubMed] [Google Scholar]
- 10.Happe S, Klosch G, Saletu B, Zeitlhofer J. Treatment of idiopathic restless legs syndrome (RLS) with gabapentin. Neurology. 2001;57:1717–9. doi: 10.1212/wnl.57.9.1717. [DOI] [PubMed] [Google Scholar]
- 11.Kriel RL, Birnbaum AK, Cloyd JC, Ricker BJ, Jones Saete C, Caruso KJ. Failure of absorption of gabapentin after rectal administration. Epilepsia. 1997;38:1242–4. doi: 10.1111/j.1528-1157.1997.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 12.Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993;10:276–81. doi: 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- 13.Gidal BE, DeCerce J, Bockbrader HN, et al. Gabapentin bioavailability: effect of dose and frequency of administration in adult patients with epilepsy. Epilepsy Res. 1998;31:91–9. doi: 10.1016/s0920-1211(98)00020-5. [DOI] [PubMed] [Google Scholar]
- 14.Gidal BE, Radulovic LL, Kruger S, Rutecki P, Pitterle M, Bockbrader HN. Inter- and intra-subject variability in gabapentin absorption and absolute bioavailability. Epilepsy Res. 2000;40:123–7. doi: 10.1016/s0920-1211(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 15.Vollmer KO, von Hodenberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung. 1986;36:830–9. [PubMed] [Google Scholar]
- 16.Cundy KC, Annamalai T, Bu L, et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311:324–33. doi: 10.1124/jpet.104.067959. [DOI] [PubMed] [Google Scholar]
- 17.Cundy KC, Branch R, Chernov-Rogan T, et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311:315–23. doi: 10.1124/jpet.104.067934. [DOI] [PubMed] [Google Scholar]
- 18.Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48:1378–88. doi: 10.1177/0091270008322909. [DOI] [PubMed] [Google Scholar]
- 19.Lal R, Sukbuntherng J, Luo W, et al. Pharmacokinetics of single escalating doses of gabapentin enacarbil in healthy adult volunteers: a randomized sequence, double-blind, placebo-controlled, crossover study. Clin Ther. 2009;31:1776–86. doi: 10.1016/j.clinthera.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Kushida CA, Becker PM, Ellenbogen AL, Canafax DM, Barrett RW, the XP052 Study Group Randomized, double-blind, placebo-controlled trial of XP13512/GSK1838262 in patients with RLS. Neurology. 2009;72:439–46. doi: 10.1212/01.wnl.0000341770.91926.cc. [DOI] [PubMed] [Google Scholar]
- 21.Walters AS, Ondo WG, Kushida CA, et al. Gabapentin enacarbil in restless legs syndrome: a phase 2b, two-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2009;32:311–20. doi: 10.1097/WNF.0b013e3181b3ab16. [DOI] [PubMed] [Google Scholar]
- 22.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects: 2004 Revision.; 2004. [PubMed] [Google Scholar]
- 24.National Institute of Mental Health (NIMH) Early Clinical Drug Evaluation Unit (ECDEU). Clinical Global Impressions. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: NIMH, USA; 1976. pp. 218–22. [Google Scholar]
- 25.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–40. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 26.Monk TH, Reynolds CF, III, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 27.Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JEJ, editors. Measuring functioning and well-being. The Medical Outcomes Study approach. Durham and London, UK: Duke University Press; 1992. pp. 235–59. [Google Scholar]
- 28.Allen RP, Kosinski M, Hill-Zabala CE, Calloway MO. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep) Sleep Med. 2008;10:531–9. doi: 10.1016/j.sleep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Kushida CA, Walters AS, Becker P, et al. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep. 2009;32:159–68. doi: 10.1093/sleep/32.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006;81:17–27. doi: 10.4065/81.1.17. [DOI] [PubMed] [Google Scholar]
- 32.Ferini-Strambi L, Aarskog D, Partinen M, et al. Effect of pramipexole on RLS symptoms and sleep: A randomized, double-blind, placebo-controlled trial. Sleep Med. 2008;9:874–81. doi: 10.1016/j.sleep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19:1414–23. doi: 10.1002/mds.20257. [DOI] [PubMed] [Google Scholar]
- 35.Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006;67:1034–39. doi: 10.1212/01.wnl.0000231513.23919.a1. [DOI] [PubMed] [Google Scholar]