Abstract
Study Objectives:
The hypocretin system enhances signaling in the mesolimbic pathways regulating reward processing and addiction. Because individuals with narcolepsy with cataplexy have low hypocretin levels, we hypothesized that they may be less prone to risk- and reward-seeking behaviors, including substance abuse.
Design:
Endpoints were performance on an array of psychometric tests (including the Eysenck Impulsiveness Scale, the Zuckerman Sensation Seeking Scale, the Gormally Binge Eating Scale, and the Beck Depression and Anxiety Inventory) and on the Balloon Analogue Risk Task (BART).
Setting:
Tertiary narcolepsy referral centers in Leiden (The Netherlands) and Boston (USA).
Patients:
Subjects with narcolepsy with cataplexy (n = 30), narcolepsy without cataplexy (n = 15), and controls (n = 32) matched for age, sex, and smoking behavior.
Interventions:
None.
Measurements and Results:
There was no difference in risk-taking behavior between narcolepsy with or without cataplexy and the control group, as measured using the BART and the array of questionnaires. However, subjects in the narcolepsy with cataplexy group had significantly higher scores on the Eysenck Impulsiveness Scale (p < 0.05), with 10.0% categorized as impulsive, compared to 6.7% of the narcolepsy without cataplexy group and none of the controls. Narcoleptics with cataplexy also scored significantly higher than controls on the Binge Eating Scale (p < 0.05), with moderate or severe binge eating in 23%. On the depression and anxiety scales, all narcolepsy patients, especially those with cataplexy, scored significantly higher than controls.
Conclusions:
We found that narcoleptics with or without cataplexy generally have normal risk-taking behavior, but narcoleptics with cataplexy were more impulsive and more prone to binge eating than patients without cataplexy and controls. Our findings shed new light on the relation between sleepiness and impulsiveness. Furthermore, rates of depression and anxiety were higher in all narcoleptic subjects. However, using the current methods, no evidence could be found to support the hypothesis that hypocretin deficiency would affect reward-processing in humans.
Citation:
Dimitrova A; Fronczek R; Van der Ploeg J; Scammell T; Gautam S; Pascual-Leone A; Lammers GJ. Reward-seeking behavior in human narcolepsy. J Clin Sleep Med 2011;7(3):293-300.
Keywords: Narcolepsy, cataplexy, hypocretin, orexin, risk-taking, addiction, reward-seeking, substance abuse, sleep, sleepiness
Narcolepsy with cataplexy is caused by an extensive loss of the neurons producing the hypocretin (orexin) neuropeptides.1,2 Loss of these essential signaling molecules results in chronic daytime sleepiness, cataplexy, and other REM sleep associated phenomena.3 In addition, considerable research indicates that the hypocretin system enhances signaling in the mesolimbic pathways that regulate reward processing and addiction, and hypocretin is now considered a key factor in of the neural mechanisms of drug addiction.4,5 In response to drugs of abuse and other rewarding stimuli, neurons of the ventral tegmental area (VTA) release dopamine in the nucleus accumbens (NAc). The hypocretin neurons heavily innervate and directly excite neurons in both the VTA and NAc, and hypocretin increases the excitability of VTA neurons by increasing their expression of glutamate receptors.6–8 Most likely, hypocretin amplifies signaling in the mesolimbic pathway. The importance of hypocretin in reward processing is very clear from the observations that mice lacking hypocretin show little or no addiction to morphine, and hypocretin can reinstate drug-seeking behavior in rodents in which this behavior was previously extinguished.4,5,8
BRIEF SUMMARY
Current Knowledge/Study Rationale: Because individuals with narcolepsy with cataplexy are generally hypocretin deficient, and rodent studies show that the hypocretin system plays a vital role in reward mechanisms, we assessed reward-seeking behaviour in narcolepsy with and without cataplexy, and healthy controls.
Study Impact: Using the current methods, no evidence could be found to support the hypothesis that hypocretin-deficiency would affect reward-processing in humans. However, narcoleptics with cataplexy were more prone to binge eating and more impulsive than patients without cataplexy and controls. Rates of depression and anxiety were higher in all narcoleptic subjects.
These studies suggest that hypocretin may be necessary in the development, maintenance and re-acquisition of addiction and reward-seeking behaviors in animals. The effects of hypocretin on these behaviors in humans have not been studied. Because of their low hypocretin levels, people with narcolepsy may have reduced signaling in the mesolimbic pathways that would make them less prone to risk- and reward-seeking in their decision-making behavior. Support for this hypothesis comes from the clinical observation that while narcoleptic patients are often treated with highly addictive drugs including amphetamines, they rarely become addicted.9,10
When considering hypocretin signaling in people with narcolepsy, it is important to distinguish between narcolepsy with cataplexy (N+C) and narcolepsy without cataplexy (N-C). Cataplexy is sudden muscle weakness triggered by strong emotional stimuli such as joking, laughter, or anger. Among people with N+C, 90% have no detectable hypocretin-1 in their cerebrospinal fluid (CSF). In contrast, nearly all individuals with N-C have normal CSF concentrations of hypocretin.1,11,12 Considering the role of hypocretin in reward-seeking behavior and addiction, we hypothesized that individuals with N+C (low or absent CSF hypocretin) would show less risk-taking behavior than individuals with N-C (normal hypocretin levels).
MATERIALS AND METHODS
We examined decision-making and reward-seeking in N+C and N-C in comparison to age- and gender-matched healthy controls. Endpoints were performance on an array of psychometric tests and the Balloon Analogue Risk Task (BART).
Participating Sites
This research protocol was carried out simultaneously at the Beth Israel Deaconess Medical Center in Boston, MA and also at the Leiden University Medical Centre in Leiden, Netherlands. The BIDMC and Leiden Committees on Clinical Investigations approved the research protocols.
Recruitment
In Boston, subjects with N+C and N-C were recruited from sleep clinics in the Boston area and through the Narcolepsy Network. Healthy controls were recruited through the internet. In Leiden, subjects with narcolepsy were recruited from the sleep clinic at Leiden University Medical Center, and healthy controls were recruited through newspaper advertisements.
Subject Characteristics
We tested a total of 77 subjects: 47 women and 30 men (N+C, N = 30, 10 male; N-C, N = 16, 9 male; and healthy controls, N = 32, 11 male). These were matched across groups for sex, age, and smoking behavior (Table 1). All subjects took their regular medications during this study, including medications for narcolepsy. Dosages used at the time of participation were not included in the analyses.
Table 1.
Subject characteristics
| N+C | N-C | Control | |
|---|---|---|---|
| Basic Characteristics | |||
| Number | 30 (10 Male) | 15 (9 Male) | 32 (11 Male) |
| Mean age ± SD (y) | 36.4 ± 13.6 | 39.37 ± 13.1 | 35.5 ± 13.5 |
| Smoking | 16.7% | 13.3% | 15.6% |
| Medication use | |||
| Modafinil | 33.3% | 53.3% | 0.0% |
| Amphetamines | 33.3% | 46.7% | 0.0% |
| Sodium oxybate | 27.0% | 13.3% | 0.0% |
| Antidepressants | 16.7% | 6.7% | 6.3% |
| Benzodiazepines | 6.7% | 6.7% | 0.0% |
| Past drug use | |||
| Marijuana | 26.7% | 40.0% | 40.6% |
| LSD/psilocybin/PCP/ketamine | 10.0% | 5.0% | 3.1% |
| Cocaine/metamph/MDMA | 6.7% | 20.0% | 6.3% |
| Benzo/barb | 0.0% | 0.0% | 3.1% |
| History – Past Diagnoses | |||
| Substance/alcohol addiction | 0.0% | 13.3% | 3.1% |
| Problematic gambling | 0.0% | 6.7% | 3.1% |
| Binge eating‡ | 6.7% | 0.0% | 3.1% |
| Depression | 23.3% | 13.3% | 12.5% |
| Anxiety | 10.0% | 6.7% | 3.1% |
LSD, lysergic acid diethylamide; PCP, phencyclidine; metamph, methamphetamine; MDMA, 4-methylenedioxy-N-methylamphetamine; Benzo, benzodiazepines; Barb, barbiturates;
2 N+C and 1 C subject reported past overeating problems, which are included in these tallies, but had not been formally diagnosed or treated.
Inclusion/Exclusion Criteria
Participants 18-64 years of age were included. The diagnoses of narcolepsy with and without cataplexy were confirmed using ICSD-2 criteria,13 including review of each individual’s overnight PSG and MSLT by a board-certified sleep neurologist. To assure that patients were clinically stable, narcolepsy patients were selected for the study only if they had a definite diagnosis for > 6 months and if they were free of medication changes for over a month. Informed consent was obtained prior to medical record review.
Subjects were excluded if they met current DSM-IV14 diagnostic criteria for substance or alcohol abuse or dependence,15 gambling addiction, binge-eating disorder, generalized anxiety disorder, depression, or other psychiatric illness, as these conditions have characteristic neurobehavioral profiles that would have skewed the results.16–18 While the protocol allowed for recreational drug use in the past (defined as drug use without diagnosis of abuse, dependence, or participation in a detoxification program), individuals were excluded for recreational drug use within the past week. Smoking was not considered an exclusion criterion.
Procedures
Part I: Questionnaires
After giving informed consent, the subjects took the following pen-and paper tests in random order:
The Eysenk Impulsiveness Scale (EIS) is a 19-question Yes/No measure of propensity to engage in impulsive behavior.15 Typical questions include Do you usually make up your mind quickly? and Do you often get involved in things you later wish you could get out of? Scores range from 0 to 19, with higher scores indicating higher impulsivity.
The Zuckerman Sensation Seeking Scale (SSS) is widely used in psychological studies for evaluation of sensation seeking, thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility.19 Subjects make a forced choice between a total of 72 pairs of contrasting statements, such as I would like to try parachute jumping, versus I would never want to try jumping out of a plane with or without a parachute.
The Binge Eating Scale (BES) is a 16-question scale used in the diagnosis of binge eating disorder.20 Typically, subjects scoring ≤ 17 on the BES are classified as non-binge eaters, those with scores from 18 to 26 as moderate binge eaters, and those scoring ≥ 27 as severe binge eaters.
The World Health Organization-developed AUDIT (Alcohol Use Disorders Identification Test) screen is a 10-question screening tool for detecting excessive drinking.21,22
The CAGE questionnaire for substance abuse is a 4-question tool widely used in primary care as an initial screen for alcohol and substance abuse.23,24 CAGE is a mnemonic for a questionnaire that asks about attempts to Cut down on drinking, Annoyance with criticisms about drinking, Guilt about drinking, and using alcohol/substances as an Eye opener. When the answers to ≥ 2 questions are positive, the test is considered to be positive.25
Gamblers Anonymous 20 Questions (GA20) is a highly sensitive and specific screening tool for the detection of problematic gamblers.26 It included questions such as: Have you ever felt remorse after gambling? Did you ever gamble to get money with which to pay debts or otherwise solve financial difficulties? and Did you ever borrow to finance your gambling?
The Beck Depression Inventory-II (BDI-II) is a 21-question, multiple choice, self-report inventory widely used for measuring the severity of depression.27,28 It measures feelings of hopelessness and irritability, guilt, feelings of being punished, as well as physical expressions of depression such as fatigue, weight loss, and lack of interest in sex. These symptoms may be difficult to interpret in the narcoleptic population due to excessive daytime sleepiness and the corresponding fatigue and decreased energy levels.
The Hospital Anxiety and Depression Scale (HADS) is an accurate measure of depressive moods and anxiety in both inpatient and outpatient populations.29,30 The HADS was selected for use in conjunction with the BDI-II because the HADS lacks questions on altered sleep patterns, fatigue, and energy levels, which makes it more suitable for use in the narcoleptic population.
The Beck Anxiety Inventory (BAI) consists of 21 items, each describing a common symptom of anxiety. The respondent is asked to rate how much he or she has been bothered by each symptom over the past week.31,32
The Epworth Sleepiness Scale (ESS) is an 8-item widely recognized method for measuring subjective daytime sleepiness.33
Participants were given ample time to complete the questionnaires, followed by a 25-min break for scoring. Subjects who scored positive on the BES, AUDIT, CAGE, or GA20, were encouraged to follow up with their primary care physician and were given a 24-h substance abuse hotline number. Psychiatrists were available on call in case participants gave a positive response to the BDI-II suicidality question. However, this was never necessary.
Part II: Computer Tests
The Balloon Analogue Risk Task (BART) is a computer-administered behavioral measure of risk taking that has convergent validity with real-world risk related situations.16,34 Specifically, risk-taking on the BART is highly correlated with measures of sensation seeking, impulsivity, and deficiencies in behavioral constraint, as well as with self-reported occurrence of addiction and other risky behaviors.16–18,34
The BART simulates a balloon being inflated by the participant with each click of a mouse. Each pump is worth 5 cents. The number of pumps used on the current balloon and the amount of money earned on the current balloon is shown on the computer screen. At any point, the participant can bank the amount of money accumulated from the current balloon and add it to their total earnings. However, if the balloon explodes before the participant banks the money, the money is lost. Each balloon has a different explosion point. Therefore, the probability of losing the money, as well as the potential loss, increases with each pump.
Subjects performed the BART twice. Each time they were presented with 30 balloons. Prior to the first trial they were not given any instructions regarding compensation. Before the second BART trial, subjects were told that they would be paid what they earned during the second BART test.
After a 5-min break, subjects then performed the Psychomotor Vigilance Task (PVT). The PVT is a reliable test of behavioral alertness, which consists of a series of reaction time (RT) measurements.35 Subjects were asked to press the space bar once as soon as they saw a counter running on the screen. The total test lasted for 10 minutes. To minimize distraction, the experimenter left the lab for the duration of the PVT testing.
Statistics
Groups were compared using the independent samples t-test. Dichotomous values were analyzed using the χ2 test. Possible medication effects on BART and questionnaire scores were investigated in a multivariate analysis. P-values lower than 0.05 were considered to be significant. For the categorical analysis of the results on the EIS, BES, BDI-II, and the BAI, validated cut-off points were used.15,20,27,31
RESULTS
Sleepiness and Vigilance
Even though subjects with N+C and N-C were taking their regular medications, they had higher ESS scores than the control group (p < 0.001) (Table 2). Similarly, subjects with N+C or N-C had longer reaction times on the PVT than controls (p < 0.001).
Table 2.
Test results
| Narcolepsy With Cataplexy Mean (± SD) | Narcolepsy Without Cataplexy Mean (± SD) | Controls Mean (± SD) | |
|---|---|---|---|
| Epworth Sleepiness Scale | 14.6 (± 4.0)*** | 13.0 (± 5.0)*** | 5.4 (± 3.0) |
| Psychomotor Vigilance Task | |||
| Average reaction time (msec) | 357 (± 68)*** | 339 (± 54)* | 308 (± 34) |
| Zuckerman SSS | |||
| General Sensation Seeking | 10.3 (± 4.5) | 11.6 (± 4.4) | 10.6 (± 4.4) |
| Thrill – Adventure Seeking | 7.2 (± 3.6) | 8.3 (± 3.3) | 7.4 (± 3.4) |
| Experience Seeking | 7.8 (± 3.0) | 7.5 (± 3.0) | 8.5 (± 3.9) |
| Disinhibition | 5.1 (± 3.1) | 5.3 (± 2.8) | 5.6 (± 3.1) |
| Boredom Susceptibility | 6.8 (± 3.5) | 6.8 (± 4.7) | 7.3 (± 3.5) |
| Eysenck Impulsiveness Scale | 6.6 (± 3.9)* | 6.5 (± 4.7)* | 4.2 (± 3.6)* |
| Depression – Anxiety | |||
| Beck Depression Inventory (BDI-II) | 13.3 (± 9.2)*** | 13.0 (± 5.5)** | 5.2 (± 7.3) |
| HADS Depression | 5.2 (± 3.3)*** | 5.2 (± 4.1)** | 2.0 (± 2.6) |
| Beck Anxiety Inventory | 12.0 (± 9.0)*** | 9.9 (± 5.3)** | 4.7 (± 5.1) |
| HADS Anxiety | 6.8 (± 3.8)** | 5.8 (± 3.2)** | 3.9 (± 3.5) |
| Addiction | |||
| Gormally BES | 9.7 (± 8.3)* | 7.5 (± 5.8) | 5.6 (± 5.0) |
| Substance Abuse (CAGE) | 0.3 (± 0.8) | 0.3 (± 0.6) | 0.4 (± 1.0) |
| Alcohol Use (AUDIT) | 3.8 (± 3.5) | 4.1 (± 2.5) | 4.6 (± 3.9) |
| Gambler’s Anonymous 20 (GA20) | 1.1 (± 2.0) | 0.7 (± 1.6) | 0.8 (± 1.8) |
| Balloon Analogue Risk Task | |||
| Total # of pumps BART#1 | 1091 (± 264) | 1086 (± 284) | 1102 (± 337) |
| Avg # of pumps/balloon BART#1 | 36.5 (± 8.8) | 36.2 (± 9.5) | 36.8 (± 11.2) |
| Exploded Balloons (out of 30) B1 | 10.3 (± 4.1) | 9.7 (± 5.0) | 10.1 (± 4.6) |
| Total # of pumps BART#2 | 1163 (± 237) | 1135 (± 216) | 1199 (± 207) |
| Avg # of pumps/balloon BART#2 | 38.7 (± 7.9) | 37.8 (± 7.2) | 40.0 (± 6.9) |
| Exploded Balloons (out of 30) B2 | 10.0 (± 3.8) | 9.0 (± 3.6) | 10.1 (± 3.7) |
| Delta Total pumps (B1-B2) | +70.9 | +49.1 | +96.1 |
| Delta Avg # pumps/balloon (B2-B1) | +2.2 | +1.6 | +3.2 |
| Delta exploded Balloons (B2-B1) | -0.4 | -0.7 | 0.0 |
RT, reaction time; msec, milliseconds; SSS, Sensation Seeking Scale; BES, Binge Eating Scale; AUDIT, Alcohol Use Disorders Identification Test; GA20 - Gamblers Anonymous 20 questions; Avg, average; B1, BART#1; B2, BART#2; HADS, Hospital Anxiety and Depression Scale. Independent samples t-test - all comparing either the N+C or the N-C group with controls ( 0.05 > p < 0.10), (*p < 0.05), (**p < 0.01), (***p < 0.001).
Personality
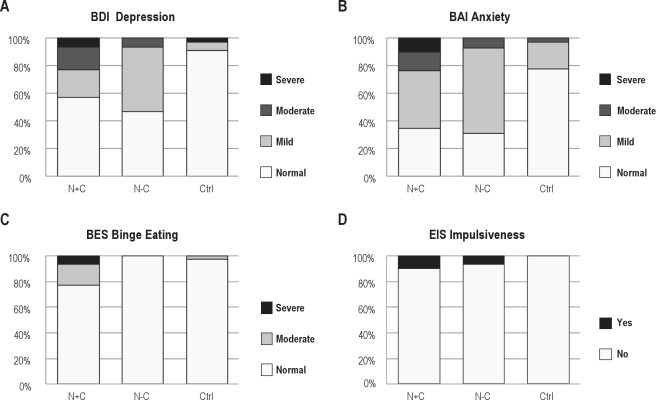
The responses on the Zuckerman Sensation Scale were comparable among the 3 groups in all 5 categories—general sensation seeking, thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility (Table 2). On the Eysenck Impulsiveness Scale (EIS), subjects in the N+C group had significantly higher scores than controls (p < 0.05), while there was a trend for the N-C group to score higher than controls (p = 0.07). After applying the age and sex-specific cutoffs for the EIS, we found that 10% of the N+C subjects were categorized as impulsive, compared to 6.7% of the N-C and none of the controls (Figure 1D). Upon closer analysis of the EIS responses, we found that the N+C and N-C groups differed greatly from controls in the way in which they answered 7 of the 19 EIS Questions (Table 3).
Figure 1.
Categorical analysis of the results on the Beck Depression Inventory II (BDI-II), the Beck Anxiety Inventory (BAI), the Gormally Binge Eating Scale (BES), and the Eysenck Impulsiveness Scale (EIS) for the groups Narcolepsy with Cataplexy (N+C), Narcolepsy without Cataplexy (N-C), and Controls (Ctrl). Validated cut-off points were used.15,20,27,31
Table 3.
Significant differences
| Eysenck Impulsiveness Scale Question | N+C | N-C | C |
|---|---|---|---|
| 2. Do you generally do and say things without stopping to think? (Y/N) | 60.0%* | 33.3% | 21.9% |
| 3. Do you often get into a jam because you do things without thinking? (Y/N) | 36.7%*** | 20.0%** | 6.3% |
| 4. Are you an impulsive person? (Y/N) | 43.4%* | 26.7% | 21.9% |
| 7. Do you mostly speak without thinking things out? (Y/N) | 46.7%* | 33.3% | 21.9% |
| 8. Do you often get involved in things you later wish you could get out of? (Y/N) | 43.4%** | 20.0% | 12.5% |
| 10. Do you need to use a lot of self-control to keep out of trouble? (Y/N) | 10.0% | 26.7%* | 12.5% |
| 13. Do you think an evening out is more successful if it is unplanned or arranged at the last moment? (Y/N) | 36.7% | 66.7%** | 21.9% |
Ratio of positive responses narcolepsy with/without cataplexy versus controls: * > 2; ** > 3; *** > 5.
Depression and Anxiety
On both depression scales—the BDI-II and HADS Depression Scales—the N+C and the N-C groups scored significantly higher than the control group, with responses in the N+C group indicating higher levels of depression (Table 2, Figure 1A).
On the Beck Anxiety Scale (BAI), the N+C and the N-C group scored more anxious than the control group. On the HADS anxiety scale, the N+C group again scored significantly higher than controls or the N-C group (Table 2, Figure 1B).
Addiction and Substance Abuse
The surveys of substance and alcohol abuse and compulsive gambling showed no significant differences between groups. However, on the Binge Eating Scale (BES) the N+C subjects scored significantly higher than controls (p < 0.05), with 16.7% Of the N+C subjects scored as moderate binge eaters and 6.7% as severe binge eaters (Figure 1C). All of the N-C subjects scored in the non-binge-eating range. Among the control subjects, only 3.1% were found to be moderate binge eaters and none were severe binge eaters.
Balloon Analogue Risk Task
There were no statistically significant differences among the groups on either BART trial, including the total number of pumps, the average number of pumps per balloon, and the number of exploded balloons (Table 2). All 3 groups scored higher (more risk-taking) on the second BART trial when the stakes were higher, with similar increases in all groups.
Effects of Medications
As the N+C and N-C subjects were maintained on their regular medications, we examined the effects on medication using a multivariate analysis. There were no significant effects of age, sex, modafinil, amphetamines, GHB, antidepressants, or benzodiazepines/opioids/muscle relaxants on BART, PVT, or questionnaire scores.
Differences Between Sites
Comparing the Boston and Leiden populations, no variables differed significantly across all 3 groups. However, the following differences were highly significant between 2 groups (all p < 0.001): Narcolepsy with cataplexy subjects in Boston were more likely to use modafinil (53% vs. 12%); narcolepsy without cataplexy subjects in Boston were more likely to have used LSD/PCP/ketamine (50% vs. 0%); and controls in Leiden had higher scores on the AUDIT (7.1 ± 0.5 vs. 2.3 ± 1.0). Supplemental Table S1 presents a complete overview of differences between the 2 research sites.
DISCUSSION
Hypocretin is hypothesized to help mediate addiction and reward-seeking behaviors5; we predicted that narcoleptic subjects with cataplexy (N+C) would exhibit less reward seeking and risk taking and have lower levels of substance abuse than normal. Surprisingly, we found no significant differences among the three groups on a variety of tests measuring sensation seeking, substance and alcohol abuse, gambling, and risky behaviors. However, narcoleptics with cataplexy had significantly higher levels of impulsiveness and binge eating than controls. In the N-C group, there was a trend towards higher levels of impulsiveness, but binge-eating scores were similar to those of controls.
We also found higher rates of anxiety and depression in subjects with N+C and N-C, which is consistent with prior studies of psychosocial impairment in narcolepsy.36,37 Interestingly, the rates of depression and anxiety were higher in subjects with N+C than those with N-C.38
Hypocretin Deficiency and Addiction
While narcoleptic patients are often treated with highly addictive drugs (including amphetamines), the clinical picture is that they rarely become addicted.9,10 However, our results do not support the idea that hypocretin-deficient individuals are less prone to addiction. It is possible that hypocretin-deficient patients may process reward via a different mechanism, not solely involving the mesolimbic pathway. Recent fMRI findings by Ponz et al.39 suggest that in contrast to controls, in non-medicated narcoleptics with cataplexy, activity in the ventral tegmental area is not modulated by high reward expectancy, and activity in the ventral striatum is reduced during winning. Interestingly, in the 12 hypocretin-deficient patients studied, there was reward-associated increased activity in the amygdala and dorsal striatum.
Risk-taking, Impulsiveness, and Sleepiness
In the current study, all narcolepsy patients reported excessive daytime sleepiness, yet they had normal levels of risk taking. In contrast, healthy sleep deprived subjects have shown increased risk-taking on various gambling tasks and questionnaires, such as the Brief Sensation Seeking Scale (BSSS), the Evaluation of Risk (EVAR) scale, and the BART.38,40,41 Possibly, sleepiness also increases risk-taking behavior in narcolepsy with cataplexy, but this effect is masked by their hypocretin deficiency. However, this would not explain the findings in the non-hypocretin deficient narcolepsy without cataplexy group. More likely, the sleepiness experienced by narcoleptic subjects is of a different nature than the sleepiness caused by sleep deprivation in otherwise healthy persons.
Though risk taking appeared normal, N+C subjects had significantly higher Eysenck Impulsiveness Scale (EIS) scores than controls, and the N-C subjects had a trend towards higher EIS scores. Specifically, N+C subjects differed from controls on questions such as Do you generally do and say things without stopping to think? and Do you mostly speak without thinking things out? Differently, N-C subjects more often answered in a positive way to questions such as Do you need to use a lot of self-control to keep out of trouble? and Do you think an evening out is more successful if it is unplanned or arranged at the last moment? These observations could shed light on the nature of narcolepsy with and without cataplexy. Perhaps those with cataplexy speak or act impulsively because they appreciate that their sleepiness can impair their ability to sustain attention. In their fight with chronic sleepiness, it may work best for them to grab the moment. The different responses on the EIS between narcoleptics with and without cataplexy suggest that some quality of sleepiness may differ between the two groups.
Binge Eating
Narcoleptics with cataplexy were more likely to report moderate and severe binge eating. This may reflect more impulsive behavior or a strategy for staving off sleepiness, but based on work in rodents, it seems unlikely that people with narcolepsy would find food more rewarding.6 The higher prevalence of eating disorders and possibly increased food intake in narcolepsy is clinically important, as people with narcolepsy are more obese than healthy controls.42 Prior studies of obesity in narcolepsy have yielded mixed results regarding eating habits, total food intake, food choice, basal metabolic rate, and physical activity. In a study of narcoleptics with and without cataplexy, both groups had increased scores on a questionnaire developed for detecting eating disorders (EAT-40).43 Similarly, narcoleptics with cataplexy scored significantly higher than age-and sex-matched controls on measures of eating disorders contained in the Schedules for Clinical Assessment in Neuropsychiatry (SCAN 2.1) and on DSM-IV criteria for bulimia and Eating Disorder NOS.44 Conversely, in a study of 116 patients with narcolepsy with and without cataplexy, there was no increased incidence of eating disorders (anorexia nervosa, bulimia, and binge eating disorder), as defined by the DSM-IV criteria.45 Furthermore, self-reported total food intake and physical activity as measured by actigraphy appeared normal in narcoleptic subjects.46,47 Many questions remain about the causes of obesity in narcolepsy, but our results indicate that binge eating is common in narcolepsy with cataplexy.
We chose to include subjects with self-reported past overeating who had never been formally diagnosed or treated for a binge-eating disorder. On close review of the results, only one of the four N+C subjects who reported past overeating scored high on the BES. The remaining moderate and severe BES scores came from subjects, who did not report a problem on initial screening. This may indicate a larger scale binge-eating problem associated with orexin deficiency than captured by our testing and necessitates further investigation.
Anxiety and Depression
As in previous studies, we found higher levels of depression and anxiety in patients with narcolepsy using the BDI-II, the BAI, and the HADS questionnaires.48–50 One limitation is that the BDI-II includes measures of physical aspects of depression that overlap with the symptoms of narcolepsy, such as fatigue, excessive daytime sleepiness, and decreased energy levels. However, the HADS scale does not contain these questions, and it also revealed higher levels of depression and anxiety in both N+C and N-C. This suggests that hypocretin deficiency or the life impact of narcolepsy affects mood independent of daytime sleepiness.
A closer look reveals that subjects in the N+C group were more often diagnosed with depression than those in the N-C and control groups. This finding and the higher depression scores in the N+C group raise the possibility that severe hypocretin deficiency may cause or aggravate depression. In fact, prior studies showed that low hypocretin-1 levels correlated with the severity of depressive symptoms and the prevalence of attempted suicide.51,52 Perhaps decreased hypocretin signaling reduces signaling in reward and motivation pathways that then contributes to depression.
Limitations
Our study provides new perspectives on reward- and risk-seeking behaviors in narcolepsy, but a few limitations should be mentioned. One is that the scales we used for the assessment of depression, anxiety, risk taking, impulsiveness, and binge eating are not validated for use in these populations. Another is that all participants continued their regular medications, which could have influenced mood or have affected impulsiveness. For example, amphetamines or antidepressants could have improved mood in the narcolepsy subjects, masking the true prevalence of depression and influencing impulsivity. However, it is unlikely that the final results were influenced by this, as a multivariate analysis did not show any effects of medications.
Hypocretin-1 concentrations in the cerebrospinal fluid were not known for the subjects included in this study. As such, it is not certain that all N+C subjects were really hypocretin deficient. However, 90% of N+C patients have no detectable hypocretin-1 in their cerebrospinal fluid (CSF). In contrast, nearly all individuals with N-C have normal CSF concentrations of hypocretin.1,11,12
We excluded subjects with a prior history of addiction, substance abuse, or psychiatric illness. This may seem counterintuitive, as these exclusion criteria are directly related to our main hypothesis. However, to truly study the prevalence of addiction and substance abuse, one would need a substantially larger study population and a more epidemiological approach, which is very difficult in a relatively rare disease such as narcolepsy. Inclusion of subjects with ongoing substance abuse or psychiatric problems would have skewed the results.
Smoking was not considered an exclusion criterion. To rule out any influence of smoking, subjects were matched for smoking behavior. In future studies, it would be of interest to consider smoking as addictive behavior. However, in our clinical experience, patients often smoke because nicotine has an alerting effect. This would make it difficult to distinguish between addictive behavior and behavior to counterbalance sleepiness.
The study included populations from Boston and Leiden, and unique cultural characteristics could have influenced risk-taking behavior. The main differences between sites were that in Boston, more N+C subjects used modafinil, more N-C subjects used LSD/PCP/ketamine, and controls had higher alcohol consumption (Supplemental Table S1) However, post hoc analysis showed that these factors did not significantly influence the final results, and the main findings of our study were present in both populations.
CONCLUSION
Although animal studies have shown that hypocretin plays an essential role in reward-seeking and addiction, we found that subjects with narcolepsy had normal risk-taking and sensation-seeking behaviors. Unexpectedly, we found higher levels of impulsiveness and a higher prevalence of binge-eating in narcolepsy with cataplexy. These results highlight some of the everyday behavioral consequences of narcolepsy, and future work may be able to establish whether some traits such as impulsiveness are a consequence of sleepiness or altered reward mechanisms. Hypocretin deficiency clearly reduces the propensity of animals to seek alcohol or abusable drugs, but the question whether people with narcolepsy are less prone towards substance abuse remains open. Answering this question could require a large cohort study directly focusing on substance abuse. Our results do not support the idea that hypocretin-deficient individuals are less prone to addiction.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Scammell consulted with Merck, GSK, and Valeant Pharmaceuticals. He has also received honorarium from Jazz Pharmaceuticals. Dr. Pascual-Leone is an advisory board member and has received an equipment grant from Nexstim. He is also an advisory board member for Neuronix Ltd. and NeoSync Inc. and has received grants from the NIH, IBRF, and NLMFF. The other authors have indicated no financial conflicts of interest.
Supplemental Material
Table S1.
Location differences
| Boston | Leiden | ||
|---|---|---|---|
| Narcolepsy With Cataplexy | N = 15 | N = 15 | |
| Use of Modafinil | 53.3% | 13.3% | *** |
| Psychomotor Vigilance (ms) | 318 ± 11.4 | 381.3 ± 20.8 | * |
| General Sensation Seeking Score | 8.3 ± 1.0 | 12.3 ± 1.1 | * |
| Narcolepsy Without Cataplexy | N = 6 | N = 9 | |
| Epworth Sleepiness Scale | 16.8 ± 2.0 | 10.4 ± 1.1 | ** |
| Use of LSD/PCP/Ketamine | 50.0% | 0.0% | *** |
| Use of Cocaine/Metamph/MDMA | 50.0% | 0.0% | ** |
| Eysenck Impulsiveness Scale (EIS) | 9.8 ± 2.3 | 4.3 ± 0.8 | * |
| Controls | N = 17 | N = 15 | |
| History of Depression | 23.5% | 0.0% | * |
| Psychomotor Vigilance (ms) | 286.7 ± 7.2 | 323.4 ± 7.2 | ** |
| Binge Eating Scale | 7.8 ± 1.3 | 3.1 ± 0.8 | ** |
| Alcohol Use (AUDIT) | 2.3 ± 0.5 | 7.1 ± 1.0 | *** |
*p < 0.05;
**p < 0.01;
***p < 0.001
REFERENCES
- 1.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 2.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 3.Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–66. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- 4.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 6.Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–90. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narita M, Nagumo Y, Hashimoto S, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 10.Akimoto H, Honda Y, Takahashi Y. Pharmacotherapy in narcolepsy. Dis Nerv Syst. 1960;21:704–6. [PubMed] [Google Scholar]
- 11.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 12.Kanbayashi T, Inoue Y, Chiba S, et al. CSF hypocretin-1 (orexin-A) concentrations in narcolepsy with and without cataplexy and idiopathic hypersomnia. J Sleep Res. 2002;11:91–3. doi: 10.1046/j.1365-2869.2002.00284.x. [DOI] [PubMed] [Google Scholar]
- 13..American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed: Diagnostic and coding manual. Westchester, IL: 2005. [Google Scholar]
- 14.American Psychiatric Association. (DSM-IV-TR) Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington, DC: American Psychiatric Press, Inc.; 2000. [Google Scholar]
- 15.Eysenck SB, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness, and empathy in adults. Pers Indiv Differ. 1985:613–9. [Google Scholar]
- 16.Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 17.Lejuez CW, Aklin WM, Jones HA, et al. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Exp Clin Psychopharmacol. 2003;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- 18.Lejuez CW, Aklin W, Bornovalova M, Moolchan ET. Differences in risk-taking propensity across inner-city adolescent ever- and never-smokers. Nicotine Tob Res. 2005;7:71–9. doi: 10.1080/14622200412331328484. [DOI] [PubMed] [Google Scholar]
- 19.Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46:139–49. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- 20.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 21.Babor TF, Higgins-Biddle J, Monteiro M. 2001. AUDIT - Alcohol Use Disorders Identification Test. Guidelines for use in primary care. 2nd edition. WHO DoMHaSDDnWMMa, ed. [Google Scholar]
- 22.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 23.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–3. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 24.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–7. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 25.Brown BR., Jr. Office management of common musculoskeletal pain syndromes. Am Fam Physician. 1972;6:92–8. [PubMed] [Google Scholar]
- 26.Prieto Ursua L, Llavona-Uribelarrea L. 20 Questions of Gamblers Anonymous: A psychometric study with population of Spain. J Gabl Stud. 1998;14:3–15. doi: 10.1023/a:1023033924960. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 28.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Olsson I, Mykletun A, Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 2005;5:46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 32.Steer RA, Ranieri W, Beck AT, Clark DA. Further evidence for the validity of the BAI with psychiatric outpatients. J Anxiety Disord. 1993;7:195–205. [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 2003;26:475–9. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 35.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 36.Broughton RJ, Guberman A, Roberts J. Comparison of the psychosocial effects of epilepsy and narcolepsy/cataplexy: a controlled study. Epilepsia. 1984;25:423–33. doi: 10.1111/j.1528-1157.1984.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 37.Broughton WA, Broughton RJ. Psychosocial impact of narcolepsy. Sleep. 1994;17:S45–9. doi: 10.1093/sleep/17.suppl_8.s45. [DOI] [PubMed] [Google Scholar]
- 38.McKenna BS, Dicjinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16:245–52. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 39.Ponz A, Khatami R, Poryazova R, et al. Abnormal activity in reward brain circuits in human narcolepsy with cataplexy. Ann Neurol. 2010;67:190–200. doi: 10.1002/ana.21825. [DOI] [PubMed] [Google Scholar]
- 40.Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 41.Killgore WD, Grugle NL, Killgore DB, et al. Restoration of risk-propensity during sleep deprivation: caffeine, dextroamphetamine, and modafinil. Aviat Space Environ Med. 2008;79:867–74. doi: 10.3357/asem.2259.2008. [DOI] [PubMed] [Google Scholar]
- 42.Kok SW, Overeem S, Visscher TL, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 43.Chabas D, Foulon C, Gonzalez J, et al. Eating disorder and metabolism in narcoleptic patients. Sleep. 2007;30:1267–73. doi: 10.1093/sleep/30.10.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortuyn HA, Swinkels S, Buitelaar J, et al. High prevalence of eating disorders in narcolepsy with cataplexy: a case-control study. Sleep. 2008;31:335–41. doi: 10.1093/sleep/31.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahmen N, Becht J, Engel A, Thommes M, Tonn P. Prevalence of eating disorders and eating attacks in narcolepsy. Neuropsychiatr Dis Treat. 2008;4:257–61. [PMC free article] [PubMed] [Google Scholar]
- 46.Lammers GJ, Pijl H, Iestra J, Langius JA, Buunk G, Meinders AE. Spontaneous food choice in narcolepsy. Sleep. 1996;19:75–6. doi: 10.1093/sleep/19.1.75. [DOI] [PubMed] [Google Scholar]
- 47.Middelkoop HA, Lammers GJ, Van Hilten BJ, Ruwhof C, Pijl H, Kamphuisen HA. Circadian distribution of motor activity and immobility in narcolepsy: assessment with continuous motor activity monitoring. Psychophysiology. 1995;32:286–91. doi: 10.1111/j.1469-8986.1995.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 48.Dodel R, Peter H, Spottke A, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007;8:733–41. doi: 10.1016/j.sleep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Dodel R, Peter H, Walbert T, et al. The socioeconomic impact of narcolepsy. Sleep. 2004;27:1123–8. doi: 10.1093/sleep/27.6.1123. [DOI] [PubMed] [Google Scholar]
- 50.Krishnan RR, Volow MR, Miller PP, Carwile ST. Narcolepsy: preliminary retrospective study of psychiatric and psychosocial aspects. Am J Psychiatry. 1984;141:428–31. doi: 10.1176/ajp.141.3.428. [DOI] [PubMed] [Google Scholar]
- 51.Brundin L, Petersen A, Bjorkqvist M, Traskman-Bendz L. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord. 2007;100:259–63. doi: 10.1016/j.jad.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Salomon RM, Ripley B, Kennedy JS, et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]