Abstract
Background
A greater percent loss of concentric versus eccentric muscle torque (i.e., relative eccentric muscle torque preservation) has been reported in the paretic limb of individuals with stroke and has been attributed to hypertonia and/or co-contractions. Stroke provides a unique condition for examining mechanisms underlying eccentric muscle preservation because both limbs experience similar amounts of general physical activity, but the paretic side is impaired directly by the brain lesion.
Purpose
The purpose of this study was to determine 1) whether eccentric preservation also exists in the nonparetic limb and 2) the relationship of eccentric or concentric torque preservation with physical activity in stroke. We hypothesized that the nonparetic muscles would demonstrate eccentric muscle preservation, which would suggest that non-neural mechanisms may also contribute to its relative preservation.
Methods
Eighteen stroke and 18 healthy control subjects (age and sex matched) completed a physical activity questionnaire. Maximum voluntary concentric and eccentric joint torques of the ankle, knee and hip flexors and extensors were measured using an isokinetic dynamometer at 30°/s for the paretic and nonparetic muscles. Relative concentric and eccentric peak torque preservation were expressed as a percentage of control subject torque.
Results
Relative eccentric torque was higher (more preserved) than relative concentric torque for paretic, as well as nonparetic muscles. Physical activity correlated with paretic (r=0.640, p=0.001) and nonparetic concentric torque preservation (r=0.508, p=0.009), but not with eccentric torque preservation for either leg.
Conclusions
The relative preservation of eccentric torque in the nonparetic muscles suggest a role of non-neural mechanisms and could also explain the preservation observed in other chronic health conditions. Loss of concentric, but not eccentric muscle torque was related to physical inactivity in stroke.
Keywords: strength, rehabilitation, force, CVA
Introduction
Muscle weakness is a common and persistent consequence of stroke due to primary cerebral damage (21,29). Stroke significantly reduces isometric (static contraction) and concentric (shortening contraction) torque production in the paretic leg muscles of individuals with stroke, and minor reductions in the nonparetic muscles have also been reported (1,29). However, research examining the effect of stroke on eccentric (lengthening contraction) torque production is scant despite its importance for the performance of daily tasks. A greater percent loss of concentric versus eccentric muscle torque (i.e., relative eccentric muscle torque preservation) has been reported in several neurological conditions. Clark et al. (6) reported relative preservation of paretic knee eccentric extensor torque in 17 individuals with chronic stroke compared to controls, but they did not examine the nonparetic muscles. Preservation of eccentric torque compared to concentric torque has also been reported in amyotrophic and primary lateral sclerosis (17), multiple sclerosis (23,30), and cerebral palsy (9,23). Authors have suggested that during concentric contractions, abnormal antagonistic muscle activity impedes the concentric contraction, while hyperactive reflexes of the stretched agonist muscle facilitate the eccentric contraction (9,17). Understanding the mechanisms of muscle strength deficits can lead to the development of more effective exercise programs to regain motor function in chronic health conditions.
The contributing factors to eccentric preservation may be more complex and not only neurological in nature. There is evidence that older adults (31), as well as individuals with chronic obstructive pulmonary disease, also exhibit eccentric muscle preservation (26). Thus, perhaps the presence of the chronic condition, aging process and/or the ensuing sedentary lifestyle have some influence on this eccentric preservation. In fact, in a population-based survey of over 24,000 adults aged 65 years and older, the group with stroke had the highest proportion of individuals who did no leisure time physical activity (27%) compared to groups with other chronic conditions (3). Stroke is a unique condition in that impairment exists predominantly on the body side contralateral to the brain lesion with generally small changes on the ipsilesional nonparetic side. Examining the nonparetic side can provide important information as the paretic and nonparetic limb both experience similar amounts of general physical activity (e.g., walking, rising from a chair), yet the paretic side is impaired directly by the brain lesion.
We hypothesized that both the paretic and nonparetic leg muscles of individuals with stroke would exhibit relative eccentric torque preservation compared to torque values of healthy older adults. To test this hypothesis, we conducted a comprehensive evaluation of concentric and eccentric torque productions for the ankle, knee, and hip flexor and extensor muscles of individuals with stroke and healthy controls. We also used correlational analyses to determine the relationship of a sedentary lifestyle (i.e., amount of physical inactivity) to torque preservation.
Methods
Subjects
Eighteen subjects (6 women and 12 men) with residual hemiparesis following a single stroke were recruited voluntarily from the community. Subjects were at least 1 year post-stroke, 50 years of age or older, able to walk independently for 10 metres (with or without an assistive device), and able to follow two commands in a row. In addition, 18 gender-matched neurologically healthy subjects (6 women and 12 men) were recruited and were similar in age to the subjects with stroke. There was no attempt to match the stroke and control groups for physical activity as we expected even people with mild stroke to have less physical activity than controls. Approval was obtained from the local university and hospital ethics committees and all subjects provided an informed consent. For subjects with stroke, motor recovery of the paretic lower extremity was assessed with active movements using the leg and foot portion of the Chedoke-McMaster Stroke Assessment Score Form (16). Of the seven stages, stage 1 represents flaccid paralysis and stage 7 represents normal movement patterns. This measure has excellent test-retest reliability and construct validity (16). Disability was assessed using the Stroke Functional Classification levels from the American Heart Association Stroke Outcome Classification Score (20). Of the five levels, level I represents complete independence in basic and instrumental activities of daily living, and level V represents complete dependence and requires full-time care. Lower extremity muscle tone of the ankle, knee, and hip flexors and extensors was assessed using the Modified Ashworth Scale (MAS) (5) which describes the resistance of muscle to manual passive movement. The scale ranges from 0 = no increase in muscle tone to a maximum 4 = paretic part(s) rigid in flexion or extension. The MAS has been shown to be reliable in the lower extremity (2) and relates to alpha motor neuron excitability (4). The activity level of all subjects was assessed using the Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) (38). This self-report questionnaire provides an estimate of how many days per week, and hours per day are spent being active during leisure, household and work-related activities. Scores are calculated as the average hours of daily activity for each task, multiplied by a metabolic equivalent value, and summed over the different tasks. The PASIPD is a modification from the well-established Physical Activity Scale for the Elderly (PASE) (37). The main difference is that the PASIPD also includes examples of activities which accommodate older adults, as well as people using assistive devices such as wheelchairs or crutches. This physical activity measure has good test-retest reliability in people with chronic conditions (including stroke) (25) and acceptable construct validity (38). Group characteristics are summarized in Table 1. Approximately 5 weeks after the initial assessment, 9 subjects with stroke returned for repeat testing to establish test-retest intrarater reliability.
Table 1.
Characteristics of subjects with stroke (N = 18) and control subjects (N = 18)
| Stroke | Control | |||||
|---|---|---|---|---|---|---|
| Mean or n | SD | Range | Mean or n | SD | Range | |
| Age (years) | 64.9 | 7.8 | 53–77 | 63.3 | 8.0 | 52–83 |
| Gender (female/male) | 6/12 | 6/12 | ||||
| Mass (kg) | 76.7 | 13.0 | 53.0–101.0 | 80.9 | 12.1 | 63.2–103.0 |
| Height (m) | 1.71 | 0.10 | 1.54–1.90 | 1.77 | 0.10 | 1.59–1.97 |
| Body Mass Index (kg/m2) | 25.9 | 4.3 | 20.2–35.0 | 25.8 | 3.3 | 19.7–30.9 |
| Physical Activity* (MET b hours/day) | 13.8 | 9.7 | 2.2–40.3 | 23.8 | 12.2 | 5.2–53.2 |
| Time since stroke (years) | 7.7 | 7.4 | 1–29 | |||
| Stroke Type (ischemic/ hemorrhagic) | 10/8 | |||||
| Paretic side (right/left) | 6/12 | |||||
| Functional Classification†a (class I–V) | II | I–IV | ||||
| Impairment‡a (stages 1–7) | ||||||
| Leg | 6 | 4–7 | ||||
| Foot | 4 | 2–7 | ||||
| Modified Ashworth Scalea (0–4; flexors/extensors) | ||||||
| Ankle | 0/1 | 0–1/0–3 | ||||
| Knee | 1/0 | 0–3/0–2 | ||||
| Hip | 0/0 | 0–0/0–1+ | ||||
Physical Activity Scale for Individuals with Physical Disabilities
Stroke Functional Classification level (American Heart Association)
Chedoke-McMaster Stroke Assessment Score
median is reported for the ordinal MAS, Functional Classification, and Impairment scores
MET metabolic equivalent
Isokinetic joint torque assessment
A Kin-Com isokinetic dynamometer (Chattanooga Group, TN) was used to assess maximum voluntary concentric and eccentric joint torques of the ankle, knee, and hip flexors and extensors of the paretic and nonparetic sides of subjects with stroke and the nondominant leg of control subjects (determined by preference for kicking a ball). Unlike the upper extremity, it has been shown that there is no dominance for maximal muscle strength (18,35) or power (12) in the lower extremity. Thus the decision to test the nondominant leg should not bias the results in either direction.
Instrument calibration was tested prior to the study with known weights and was accurate to within +/− 1 N. All joint torques were assessed at an angular velocity of 30°/second as our lab has previously shown that subjects with stroke have difficulty achieving higher concentric speeds at the knee and ankle joints on the paretic side (13). Furthermore, the risk for muscle damage and impaired force production increases as the velocity of eccentric contractions increases (27).
The ankle and knee torques were tested at a 90° sitting angle and the hip torques were tested in a 40° semi-reclined position. An upright position was considered safer and more comfortable than a reclined position due to the incidence of hypertension in this population. Blood pressure was monitored throughout the test protocol and testing proceeded only when blood pressure was at or below 140/90. For stabilization, three straps crossed the trunk and pelvis (two over the chest and one just distal to the anterior-superior iliac spines), and for measurement of knee torques an additional rigid clamp was placed over the distal thigh musculature of the test leg. The dynamometer axis was aligned with the joint line of the ankle, knee, and hip (lateral malleolus, lateral femoral condyle, and greater trochanter respectively) by the same examiner throughout testing. A rigid metallic ankle attachment provided by the manufacturer was used for assessment of ankle torques. For knee and hip torques, the cuff of the dynamometer was positioned three finger breaths above the medial malleolus and three finger breaths proximal to the popliteal fossa respectively. The subject’s hands rested comfortably in their lap during testing.
Joint angle or muscle length affects torque production. Therefore to allow for an equivalent comparison, the paretic, nonparetic, and control joints were all tested through the same limited range of motion. Ankle torques were tested through a range of 10–35° of plantarflexion (0° = neutral; foot perpendicular to shank), knee torques through a range of 15–95° of flexion (0° = full knee extension), and hip torques through a range of 55–105° of flexion (0° = neutral; pelvis aligned with thigh). The selection of these joint angle values were based from mean values obtained from the paretic leg during pilot testing.
Gravity compensation was performed in accordance with the manufacturer’s instructions before each new muscle was tested. As warm-up, and to ensure the subject understood the requested action, two submaximal and one maximal practice contractions were performed before each muscle was tested. Preload is defined as the preset torque which must be overcome before movement of the dynamometer can occur. The preload was individualized for each subject, muscle, and contraction type. For concentric actions it was set at 75% of the peak torque value obtained from the maximal practice concentric contraction. For eccentric actions it was set at 75% of the final torque value obtained from the maximal practice concentric contraction (which was the start position for the eccentric contraction). This was done because muscle torque is length-dependent and during eccentric testing the subjects could not overcome the preload value used for concentric testing (i.e. the eccentric start position often placed the joint in a relatively weaker position).
Concentric and eccentric contractions were alternated and separated by 5 second rest intervals. Each concentric-eccentric cycle was separated by 30 seconds of rest, and each new muscle group tested was separated by 5 minutes of rest. Torque-angle curves were visually inspected by the same examiner and submaximal trials were rejected. Testing was completed when the peak torque and shape of the 3 curves closely overlaid one another, thus ensuring that only consistant maximum effort trials were recorded. The highest number of repetitions performed by any subject was 5 per contraction type. To reduce the chance of an order effect, testing began with extension for approximately half of the subjects and flexion for the remainder.
Data analysis
Preliminary analyses of 9 subjects demonstrated that the mean peak torque was slightly more reliable across two separate days than the mean average torque intraclass correlation coeffcient (ICC). For the paretic lower extremity muscles, the mean ICC(3,3) (SEM) for intrarater reliability were 0.98 (3.4N) for peak concentric torque and 0.96 (7.2N) for peak eccentric torque. For the nonparetic lower extremity muscles the mean ICCs (SEM) were 0.92 (6.0N) for peak concentric torque and 0.96 (8.4N) for peak eccentric torque. The average concentric and eccentric torque ICCs were slightly lower (range: 0.91–0.96) and therefore only peak torque was used for further analyses.
A single ensemble-averaged torque-angle curve was calculated from the three recorded curves for each muscle (ankle, knee, and hip flexors and extensors) and contraction type (concentric and eccentric). Each subject’s absolute torque values were normalized by body mass, but not segment length. To describe paretic and nonparetic relative concentric and eccentric torque preservation, the normalized torques for the paretic and nonparetic sides of subjects with stroke were divided by the normalized torques of their matched control subjects for each muscle contraction (paretic and nonparetic torque values were expressed as a percentage of control torque values).
A multivariate analysis of variance (MANOVA) blocked for subject was performed to determine whether concentric relative torque preservation was different than eccentric preservation (independent variables: concentric relative torque and eccentric relative torque) across all lower extremity muscles (6 dependent variables: ankle, knee, and hip flexors and extensors) for the paretic side of individuals with stroke. A second MANOVA blocked for subject used the same variables for the nonparetic side. Multivariate normality and lack of collinearity were confirmed with the models (collinearity statistics of tolerances > 0.3 and variance inflation factors < 5; Box’s M for equality of the group covariance matrices demonstrated multivariate normality). When the MANOVA was significant, pair-wise t-tests were performed to identify which specific muscles (i.e., of the ankle, knee and hip extensors and flexors) demonstrated differences between concentric relative torque and eccentric relative torque. As some of the individual pair comparisons were not normally distributed according to the Shapiro-Wilks Test, post-hoc paired T-tests (parametric) and Wilcoxon Signed Rank Test were performed depending on the distribution of the variables.
To determine the relationship of a sedentary lifestyle to the preservation of torque, we calculated four correlations between PASIPD scores and 1) relative paretic concentric preservation (i.e., paretic over control torque), 2) relative paretic eccentric preservation, 3) relative nonparetic concentric preservation and 4) relative nonparetic eccentric preservation. Torque preservation values were an average of the 6 muscles (ankle, knee, hip flexors and extensors). Pearson correlations were used as these dependent variables were normally distributed.
All statistical analyses were performed using SPSS 11.5 software with an alpha value of 0.05.
Results
The subjects with stroke had mild to moderate stroke impairment as demonstrated by their Functional Classification (II out of IV), Chedoke-McMaster Impairment Scores (6/7 on the leg and 4/7 on the foot score) and spasticity scores (median of 0 or 1 for the ankle, knee and hip). The control group had the identical ratio of males/females as the stroke group, and the mean age of the two groups were within one year. The control group was almost twice as physically active as the subjects with stroke as measured by the PASIPD.
As expected, for all concentric and eccentric muscles (flexors and extensors of the ankle, knee, and hip joints), the paretic torque was lower than the nonparetic torque (Table 2). However, the nonparetic torques approached control values for the concentric knee extensors, as well as all eccentric muscles except for the ankle plantarflexors. Ankle plantarflexor torque was the most impaired muscle. Paretic plantarflexor torque was only 31% and 48% of control values of concentric and eccentric torque, respectively. Nonparetic plantarflexor torque was 65% and 79% of control values of concentric and eccentric torque, respectively (Table 2). As expected, for all muscles in the subjects with stroke (paretic and non-paretic sides) and controls, the absolute eccentric torque was higher than the concentric torque (Table 2). Over the six muscles, eccentric torque was 1.6 times, 1.8 and 2.0 times the value of concentric torque for the control group, stroke nonparetic side and stroke paretic side, respectively. Thus, the eccentric to concentric torque ratio was higher for both the paretic and nonparetic sides of the stroke group compared to the control group due to the greater loss of concentric torque that accompanied the stroke group.
Table 2.
Absolute torque (Nm) in persons with stroke and controls
| Variable | Stroke (Paretic side) Mean (SD) | Stroke (Nonparetic side) Mean (SD) | Control Mean (SD) |
|---|---|---|---|
| Knee Extension concentric | 53.6 (23.7) | 90.7 (37.9) | 93.8 (25. 6) |
| Knee Extensors Eccentric | 99.8 (39.1) | 138. 7 (52.9) | 148.2 (38.1) |
| Knee Flexors Concentric | 26.7 (11.7) | 40.0 (15.7) | 54.8 (19.0) |
| Knee Flexors Eccentric | 45.4 (24.0) | 69.0 (36.8) | 75.1 (28.2) |
| Hip Extensors Concentric | 61.3 (30.3) | 82.7 (42.2) | 108.2 (39.3) |
| Hip Extensors Eccentric | 133.4 (55.0) | 172.8 (72.0) | 213.1 (72.4) |
| Hip Flexors Concentric | 46.3(16.0) | 63.9(20.7) | 86.5(29.8) |
| Hip Flexors Eccentric | 71.4 (25.2) | 97.6(38.3) | 110.2 (34.1) |
| Ankle Plantarflexors Concentric | 28.4 (20.2) | 59.6 (22.0) | 91.9 (29.7) |
| Ankle Plantarflexors Eccentric | 78.5 (38.5) | 129.6 (34.7) | 163.9 (40.8) |
| Ankle Dorsiflexors Concentric | 31.9 (16.8) | 44.6 (14.0) | 53.1 (12.9) |
| Ankle Dorsiflexors Eccentric | 61.3 (25.9) | 73.7 (21.9) | 79.3 (19.4) |
Paretic leg relative torque
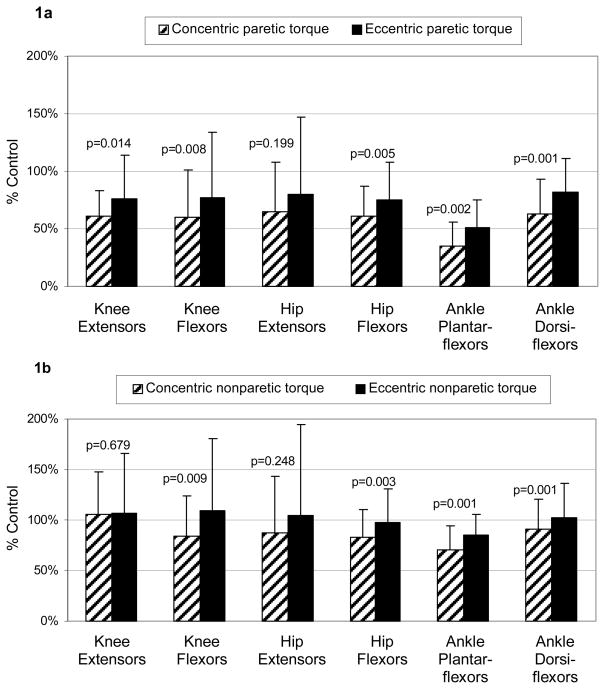
The MANOVA for the paretic lower extremity revealed a significant difference between concentric and eccentric relative torque preservation across ankle, knee, and hip flexors and extensors (Hotelling’s Trace = 1.726, P = 0.032). Concentric relative torque preservation of paretic muscles (mean = 58%) was less than eccentric preservation (mean = 74%) and post-hoc tests revealed statistical significance for five out of six muscles (Figure 1a).
Figure 1.
Concentric and eccentric torque preservation for (1a) paretic and (1b) nonparetic side of subjects with stroke (expressed as percentage of control torque) for 6 lower extremity muscle groups. The error bar represents 1 standard deviation.
Despite the varying levels of absolute torque across the six muscles, the differences between the concentric and eccentric relative torque preservation were remarkably consistent across the paretic muscles (mean of 16%, range from 14–18%).
Nonparetic leg relative torque
The MANOVA for the nonparetic lower extremity revealed a significant difference between concentric and eccentric relative torque preservation across ankle, knee, and hip flexors and extensors (Hotelling’s Trace = 2.271, P = 0.012). Concentric relative torque preservation of nonparetic muscles (mean = 87%) was less than eccentric preservation (mean = 101%) and post-hoc tests revealed statistical significance for four out of six muscles (Figure 1b). The differences between the concentric and eccentric relative torque preservation ranged from a minimum of 0 (knee extensors) to a maximum of 25% (knee flexors). However, on average across the six nonparetic muscles, the difference between the concentric and eccentric preservation was 14%.
Correlations between physical activity and torque preservation
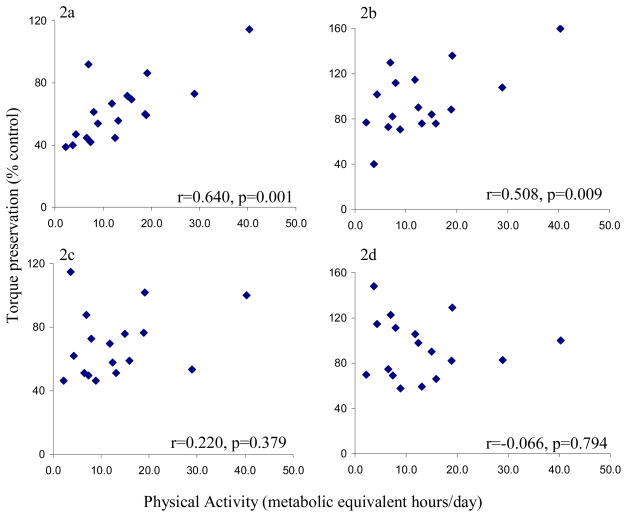
Physical activity (PASIPD) correlated with paretic (r=0.640, p<0.006) and nonparetic concentric torque preservation (r=0.508, p<0.038) (Figure 2). Physical activity did not correlate with eccentric torque preservation for the paretic or nonparetic muscles (p >0.5).
Figure 2.
Scatterplots of torque preservation versus physical activity using 2a) paretic concentric torque, 2b) nonparetic concentric torque, 2c) paretic eccentric torque and 2d) nonparetic eccentric torque. Torque preservation values were an average of the 6 muscle groups (ankle, knee, hip flexors and extensors) and expressed as a percent of control torque. R-values generated from Pearson correlations.
Discussion
As expected, absolute eccentric torque was greater than concentric torque for both the stroke and control subjects across all muscles. This observation is consistent with other studies in stroke (6) and healthy populations and is partly attributed to the additional force from the elastic components in series with the contractile elements (24,33).
The greater eccentric torque preservation (relative to healthy values) than concentric torque preservation demonstrated that eccentric torque production in the paretic leg was less affected by stroke than concentric torque production across all muscles assessed. This striking result supports the hypothesis that individuals with upper motor neuron lesions will have a larger eccentric to concentric torque ratio in their paretic muscles due to the greater loss in concentric torque. Clark et al. (7) suggested that the eccentric paretic knee preservation that they observed in their study reflected reduced regulation of high force levels post-stroke, resulting possibly from a reduction in autogenic inhibition or lower absolute levels of force. As eccentric torque preservation has also been reported in individuals with amyotrophic and primary lateral sclerosis (17), as well as children with cerebral palsy (9), it has been proposed that hyperactive stretch reflexes may contribute to this phenonemon in upper motorneuron lesions (17,22,23). Thus, stretch of the agonist during eccentric contractions would facilitate agonist activation and increase torque production, while stretch of the antagonist during concentric contractions may lead to abnormal co-activation with the agonist and reduce torque production. However, this explanation may be challenged, as others have reported no evidence of abnormal antagonist co-contraction during maximal voluntary concentric knee flexion or extension in the paretic or nonparetic legs of individuals with stroke, even at fast isokinetic velocities of 300°/s (11). Hence, other mechanisms besides hyperactive stretch reflexes and co-contraction may contribute to eccentric torque preservation.
There are potential cortical changes post-stroke which could also contribute to the eccentric preservation. Eccentric contractions have been shown to require greater brain activation (e.g., cortico evoked potentials) than concentric contractions in healthy individuals (14,15). The neural recovery following stroke involves increases in recruitment of secondary and ipsilateral cortical motor areas (8), and it is possible that such re-organization may alter the concentric/eccentric relationship.
However, our novel finding that the nonparetic muscles of individuals with chronic stroke also exhibit eccentric preservation provides evidence that mechanisms non-neural in nature must contribute to this altered concentric/eccentric relationship. The difference between the concentric and eccentric preservation was almost identical for the paretic (16%) and nonparetic muscles (14%), despite the finding that the absolute nonparetic torques were an average of 143% of the paretic torques across the six muscles and two contraction types (eccentric and concentric). The nonparetic muscles cannot be considered unimpaired by the stroke as it is known that a small percentage of descending corticospinal tracts that originate from the lesion site remain ipsilateral (10). However, the nonparetic muscles had no clinical evidence of spasticity and several of the nonparetic muscles had no loss of eccentric torque. Thus, hyperactive stretch reflexes do not appear to be the contributing factor for the nonparetic eccentric preservation.
Overall, concentric torque preservation was lower than eccentric preservation for both the paretic and nonparetic muscles as shown by the MANOVAs. Of the 12 pair-wise post-hoc comparisons, three cases were not significant. In two of these cases, the means for eccentric preservation were more than 15% higher than concentric preservation and we likely lacked power to detect significant differences. However, in the nonparetic knee extensors, the concentric and eccentric torque preservation values were similar. On closer investigation, we noted that the absolute concentric torque for the nonparetic knee extensor muscles was similar to the controls. In contrast, all other nonparetic muscles showed a loss of concentric torque. It is common for people with stroke to place their paretic foot further in front when they rise from a chair to compensate with the nonparetic knee extensors. Thus, frequent use of the nonparetic knee extensors during daily activities may have contributed to the concentric preservation in this muscle.
We suggest that the sedentary lifestyle of the individuals with stroke plays a role in determining the loss of concentric torque as more physically active participants had greater relative concentric torque values (i.e., closer to healthy values) for the paretic and nonparetic sides. Such a relationship is logical given that physical activity must be undertaken by contractile activity of the muscle. One might have expected that less physical activity would be associated with an equal loss of both eccentric and concentric torque. The striking observation of this study is that despite less physical activity than the control group, the individuals with stroke showed eccentric torque preservation. Furthermore, the lack of correlation between eccentric torque preservation and physical activity is complex and a number of explanations may account for this finding. It would be ideal to have a third stroke group who undertook the same amount of physical activity as the controls, but that would not be possible. There may be competing mechanisms involved with eccentric torque and physical activity. A lack of physical activity will lead to reduction in contractile activity which will negatively impact both concentric and eccentric ability. However, physical inactivity will also lead to a reduction in contractile content and infiltration of connective tissue (i.e., collagen) which may increase the mechanical stiffness of the muscle. During eccentric contractions, the passive contribution of the connective tissue (including stiffness of the lengthening muscle) would add to the tension development (24). Thus, losses from eccentric contractile inactivity may be partially or completely compensated by gains in mechanical stiffness and hence, a relationship between physical activity and eccentric torque is not found. We suggest that these bilateral changes arise in part from physical inactivity which may lead to structural changes in the muscle that increase passive stiffness.
It is possible that the presence of stroke induces an exaggerated aging effect as eccentric preservation is one characteristic found in muscles of older adults. Eccentric muscle preservation in older adults (19,31,32,36) has been attributed partly to structural changes (e.g., increase in non-contractile content). However, Ochala et al. (28) showed that the eccentric muscle preservation in single skeletal muscle fibers from older men were partly due to the contribution of the contractile elements of muscle cells. Hence, future research could determine whether the non-contractile and contractile changes found in older adults are similar to those with stroke.
Structural changes in the mechanical properties of the paretic muscles in individuals with stroke may be more severe than in older adults without a stroke. Sinkjaer and Magnussen (34) examined the ankle plantarflexors in individuals with stroke (mean time after onset = 31 months; range = 2 to 80 months). They found that intrinsic stiffness (contractile properties of the muscle fibers) did not differ for paretic, nonparetic and control ankle plantarflexors but passive stiffness (response from the passive tissues) was increased a remarkable 278% in the paretic leg, and 95% in the nonparetic leg when compared to controls. Such changes in the mechanical stiffness could reflect an accelerated aging process, and contribute to the eccentric preservation found in stroke.
Clinical Implications and Future Directions
Our study showed that eccentric torque was less impaired than concentric in individuals with stroke, thus clinicians should assess limb strength under both contraction conditions to better quantify muscle performance. In other words, due to the relative preservation of eccentric torque in individuals with stroke, care must be taken to avoid underloading of paretic and nonparetic muscles during the eccentric phase of exercises. Relative preservation of eccentric torque may allow individuals with stroke who have very weak muscles, such as those with acute hemiparesis, to perform eccentric actions before concentric actions to stimulate muscle activity. For example, a patient may not be able to complete a sit to stand exercise due to concentric quadriceps weakness. However, they might be able to practice a controlled stand to sit exercise to activate eccentric quadriceps, then use the arm rests to assist concentric activity to get to the standing position again. Methods to increase physical activity should be explored with individuals with stroke to maintain contractile ability of the muscles. Lastly, other health conditions should be examined as our study would suggest that eccentric preservation may exist in chronic health conditions where movement inefficiency or sedentary lifestyles exist.
Limitations
Several limitations should be noted about this study. The sample size was relatively small and the results should be interpreted with caution. The correlations between physical activity and muscle preservation are not causational but provide directions for future hypothesis testing. We acknowledge potential type I errors with the multiple post-hoc comparisons. However, the use of MANOVA assists in reducing type I error inflation.
Conclusions
In conclusion, our results demonstrate eccentric torque preservation was greater than concentric preservation with both the paretic and nonparetic muscles. Preservation of concentric torque, but not eccentric muscle torque was related to physical inactivity in stroke.
Acknowledgments
This work was supported by the Canadian Institute of Health Research, the Heart and Stroke Foundation of British Columbia and the Yukon, and the Michael Smith Foundation of Health Research (JJ E) and salary support (to JJE) from the Canadian Institutes of Health Research (MSH-63617). The authors wish to thank Sarah Einarson for her assistance with data collection. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Andrews AW, Bohannon RW. Distribution of muscle strength impairments following stroke. Clin Rehabil. 2000;14(1):79–87. doi: 10.1191/026921500673950113. [DOI] [PubMed] [Google Scholar]
- 2.Ansari NN, Naghdi S, Younesian P, Shayeghan M. Inter- and intrarater reliability of the Modified Modified Ashworth Scale in patients with knee extensor poststroke spasticity. Physiother Theory Pract. 2008;24(3):205–13. doi: 10.1080/09593980701523802. [DOI] [PubMed] [Google Scholar]
- 3.Ashe MC, Miller WC, Eng JJ, Noreau L. Older Adults, Chronic Disease and Leisure-Time Physical Activity. Gerontology. 2008 Jun 20; doi: 10.1159/000141518. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakheit AM, Maynard VA, Curnow J, Hudson N, Kodapala S. The relation between Ashworth scale scores and the excitability of the alpha motor neurones in patients with post-stroke muscle spasticity. J Neurol Neurosurg Psychiatry. 2003;74(5):646–8. doi: 10.1136/jnnp.74.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 6.Clark DJ, Condliffe EG, Patten C. Reliability of concentric and eccentric torque during isokinetic knee extension in post-stroke hemiparesis. Clin Biomech (Bristol, Avon) 2006;21(4):395–404. doi: 10.1016/j.clinbiomech.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Clark DJ, Condliffe EG, Patten C. Activation impairment alters muscle torque-velocity in the knee extensors of persons with post-stroke hemiparesis. Clin Neurophysiol. 2006;117(10):2328–37. doi: 10.1016/j.clinph.2006.07.131. [DOI] [PubMed] [Google Scholar]
- 8.Cramer SC. Functional magnetic resonance imaging in stroke recovery. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S47–55. doi: 10.1016/s1047-9651(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 9.Damiano DL, Martellotta TL, Quinlivan JM, Abel MF. Deficits in eccentric versus concentric torque in children with spastic cerebral palsy. Med Sci Sports Exerc. 2001;33(1):117–22. doi: 10.1097/00005768-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Davidoff RA. The pyramidal tract. Neurology. 1990;40(2):332–9. doi: 10.1212/wnl.40.2.332. [DOI] [PubMed] [Google Scholar]
- 11.Davies JM, Mayston MJ, Newham DJ. Electrical and mechanical output of the knee muscles during isometric and isokinetic activity in stroke and healthy adults. Disabil Rehabil. 1996;18(2):83–90. doi: 10.3109/09638289609166022. [DOI] [PubMed] [Google Scholar]
- 12.Demura S, Yamaji S, Goshi F, Nagasawa Y. Lateral dominance of legs in maximal muscle power, muscular endurance, and grading ability. Percept Mot Skills. 2001;93(1):11–23. doi: 10.2466/pms.2001.93.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Eng JJ, Kim CM, Macintyre DL. Reliability of lower extremity strength measures in persons with chronic stroke. Arch Phys Med Rehabil. 2002;83(3):322–8. doi: 10.1053/apmr.2002.29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Siemionow V, Sahgal V, Xiong F, Yue GH. Distinct brain activation patterns for human maximal voluntary eccentric and concentric muscle actions. Brain Research. 2004;1023(2):200–12. doi: 10.1016/j.brainres.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Fang Y, Siemionow V, Sahgal V, Xiong F, Yue GH. Greater movement-related cortical potential during human eccentric versus concentric muscle contractions. J Neurophysiol. 2001;86(4):1764–72. doi: 10.1152/jn.2001.86.4.1764. [DOI] [PubMed] [Google Scholar]
- 16.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 17.Griffin JW, Tooms RE, Vander Zwaag R, O’Toole ML, Bertorini TE. Eccentric and concentric muscle performance in patients with spastic paresis secondary to motor neuron disease. A preliminary report. Neuromuscul Disord. 1994;4(2):131–8. doi: 10.1016/0960-8966(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 18.Holder-Powell HM, Rutherford OM. Unilateral lower-limb musculoskeletal injury: its long-term effect on balance. Arch Phys Med Rehabil. 2000;81(3):265–8. doi: 10.1016/s0003-9993(00)90069-8. [DOI] [PubMed] [Google Scholar]
- 19.Hortobágyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50(6):B399–406. doi: 10.1093/gerona/50a.6.b399. [DOI] [PubMed] [Google Scholar]
- 20.Kelly-Hayes M, Robertson JT, Broderick JP, et al. The American Heart Association Stroke Outcome Classification: executive summary. Circulation. 1998;97(24):2474–8. doi: 10.1161/01.cir.97.24.2474. [DOI] [PubMed] [Google Scholar]
- 21.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83(1):49–57. [PubMed] [Google Scholar]
- 22.Knutsson E, Gransberg L, Martensson A. Facilitation and inhibition of maximal voluntary contractions by the activation of muscle stretch reflexes in patients with spastic paresis. Electromyogr Clin Neurophysiol. 1988;70:37–8. [Google Scholar]
- 23.Knutsson E, Mårtensson A, Gransberg L. Influences of muscle stretch reflexes on voluntary, velocity-controlled movements in spastic paraparesis. Brain. 1997;120 (Pt 9):1621–33. doi: 10.1093/brain/120.9.1621. [DOI] [PubMed] [Google Scholar]
- 24.Lieber RL, editor. Skeletal muscle structure, function and plasticity. 2. Philadelphia: Lippincott Williams and Wilkins; 2002. p. 63. [Google Scholar]
- 25.Linksvan der Ploeg HP, Streppel KR, van der Beek AJ, van der Woude LH, Vollenbroek-Hutten M, van Mechelen W. The Physical Activity Scale for Individuals with Physical Disabilities: test-retest reliability and comparison with an accelerometer. J Phys Act Health. 2007;4(1):96–100. doi: 10.1123/jpah.4.1.96. [DOI] [PubMed] [Google Scholar]
- 26.Mathur S, MacIntyre DL, Forster BB, Road JD, Levy RD, Reid WD. Preservation of eccentric torque of the knee extensors and flexors in patients with COPD. J Cardiopulm Rehabil Prev. 2007;27(6):411–6. doi: 10.1097/01.HCR.0000300271.45881.99. [DOI] [PubMed] [Google Scholar]
- 27.McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol. 1986;61(1):293–9. doi: 10.1152/jappl.1986.61.1.293. [DOI] [PubMed] [Google Scholar]
- 28.Ochala J, Dorer DJ, Frontera WR, Krivickas LS. Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age. Pflugers Arch. 2006;452(4):464–70. doi: 10.1007/s00424-006-0065-6. [DOI] [PubMed] [Google Scholar]
- 29.Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev. 2004;41(3A):293–312. doi: 10.1682/jrrd.2004.03.0293. [DOI] [PubMed] [Google Scholar]
- 30.Ponichtera JA, Rodgers MM, Glaser RM, Mathews TA, Camaione DN. Concentric and eccentric isokinetic lower extremity strength in persons with multiple sclerosis. J Orthop Sports Phys Ther. 1992;16(3):114–22. doi: 10.2519/jospt.1992.16.3.114. [DOI] [PubMed] [Google Scholar]
- 31.Porter MM, Vandervoort AA, Kramer JF. Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol A Biol Sci Med Sci. 1997;52(2):B125–31. doi: 10.1093/gerona/52a.2.b125. [DOI] [PubMed] [Google Scholar]
- 32.Pousson M, Lepers R, Van Hoecke J. Changes in isokinetic torque and muscular activity of elbow flexors muscles with age. Exp Gerontol. 2001;36(10):1687–98. doi: 10.1016/s0531-5565(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 33.Reeves ND, Narici MV. Behavior of human muscle fascicles during shortening and lengthening contractions in vivo. J Appl Physiol. 2003;95(3):1090–6. doi: 10.1152/japplphysiol.01046.2002. [DOI] [PubMed] [Google Scholar]
- 34.Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain. 1994;117 (Pt 2):355–63. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- 35.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31(2):119–25. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- 36.Vandervoort AA, Kramer JF, Wharram ER. Eccentric knee strength of elderly females. J Gerontol. 1990;45(4):B125–8. doi: 10.1093/geronj/45.4.b125. [DOI] [PubMed] [Google Scholar]
- 37.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 38.Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83(2):193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]