Abstract
Increasing evidence has supported the important role of mesenchymal stem cells (MSCs) in wound healing, however, the underlying mechanism remains unclear. Recently, we have isolated a unique population of MSCs from human gingiva (GMSCs) with similar stem cell-like properties, immunosuppressive, and anti-inflammatory functions as human bone marrow-derived MSCs (BMSCs). We describe here the interplay between GMSCs and macrophages and the potential relevance in skin wound healing. When cocultured with GMSCs, macrophages acquired an anti-inflammatory M2 phenotype characterized by an increased expression of mannose receptor (MR; CD206) and secretory cytokines interleukin (IL)-10 and IL-6, a suppressed production of tumor necrosis factor (TNF)-α, and decreased ability to induce Th-17 cell expansion. In vivo, we demonstrated that systemically infused GMSCs could home to the wound site in a tight spatial interaction with host macrophages, promoted them toward M2 polarization, and significantly enhanced wound repair. Mechanistically, GMSC treatment mitigated local inflammation mediated by a suppressed infiltration of inflammatory cells and production of IL-6 and TNF-α, and an increased expression of IL-10. The GMSC-induced suppression of TNF-α secretion by macrophages appears to correlate with impaired activation of NFκB p50. These findings provide first evidence that GMSCs are capable to elicit M2 polarization of macrophages, which might contribute to a marked acceleration of wound healing.
Keywords: Human gingival, Mesenchymal stem cells, M2 macrophages, Wound healing
Introduction
Mesenchymal stem cells (MSCs) represent a heterogeneous population of fibroblast-like stromal cells with self-renewal and multipotent differentiation capacities [1, 2]. MSCs display both tropic and trophic properties, with unique capabilities of homing to injury or inflammatory sites via a myriad of growth factors and cytokines that may facilitate the repair of damaged tissues [3, 4]. In recent years, one of the breakthroughs in MSC research is the discovery that MSCs possess immunosuppressive and anti-inflammatory functions both in vitro and in vivo [3, 4]. MSCs exert their profound immunomodulatory effects by suppressing the proliferation and function of both innate and adaptive immune cells and by activating regulatory T-cells (Tregs) through mechanisms involving direct cell-cell contact and/or various soluble factors [3–6]. These unique properties render MSCs a potential novel immunotherapeutic tool for a variety of autoimmune and inflammation-related diseases [3, 4].
To date, the interactions between MSCs and T lymphocytes [7], natural killer cells [7, 8], and dendritic cells [9] have been extensively studied. However, there remains scanty evidence of potential interplay between MSCs and macrophages, an essential component of the innate immune response [10–13]. Macrophages have been characterized as: (a) classically activated or M1 macrophages which are induced in response to stimulation by Th1 cytokines such as interferon (IFN)-γ or lipopolysaccharide (LPS); and (b) alternatively activated or M2 macrophages, also known as wound healing macrophages, which are induced by Th2 cytokines such as interleukin (IL)-4 or IL-13 [11, 14]. M1 macrophages, characterized by the release of nitric oxide (NO), reactive oxygen species (ROS), and tumor necrosis factor (TNF)-α, are generally considered as proinflammatory; while M2 macrophages, characterized by the production of IL-10, transforming growth factor (TGF)-β, and arginase-1 (Arg1), are considered as anti-inflammatory [15–19]. Most recently, studies have shown that MSCs are capable of reprogramming macrophages into M2 phenotype characterized by the increased phagocytic ability, upregulated expression of anti-inflammatory cytokine IL-10, and suppressed expression of proinflammatory cytokines such as IL-12 and TNF-α [20–22]. These findings suggest that macrophages can be skewed to an M2-like phenotype in the presence of MSCs under various pathological conditions. However, the underlying mechanisms of MSC-guided transition from conventional M1 to alternative M2 macrophages under normal physiological condition, specifically tissue regeneration or wound repair, remain largely unknown.
Cutaneous wound healing represents a highly coordinated process to achieve tissue homeostasis, which involves complex interactions of different types of resident cells and infiltrating immune cells as well as their secreted soluble mediators [23]. The repair process involves three distinct but overlapping phases: inflammation, tissue formation, and remodeling [23]. On tissue insult, the immediate inflammatory response is characterized by infiltration and activation of leukocytes, whereas a delayed or excessive inflammatory response may lead to abnormal wound healing in diabetic patients, scarring, and fibrotic diseases. Aside from leukocytes that act as the principal cellular component of the early inflammatory response, macrophages contribute to all stages of wound repair [23–25]. Particularly, several studies have shown that M2 macrophages can produce mediators essential in the resolution of inflammation and tissue modeling, thus, promoting wound repair [26, 27]. Recent studies have demonstrated that systemically injected MSCs can home to injury sites [28–30], differentiate into multiple types of skin cells [30, 31], and secrete various factors with proliferative, anti-inflammatory, angiogenic, or chemotactic effects [30, 31], thus, facilitating survival/proliferation of both resident and replacing cells, and consequently accelerating wound repair [31]. Although the role of macrophages [23–25] and MSCs [24, 28, 29] have been implicated in wound repair, little is known about their interactions, specifically whether MSCs can promote the transition of M1 to M2 macrophage in accelerating the healing of skin wounds.
Most recently, we have isolated a unique population of MSCs from the easily accessible human gingival tissues, designated as GMSCs [32]. Similar to human bone marrow-derived MSCs (BMSC), GMSCs not only possess multipotent differentiation capabilities but also display potent immunosuppressive and anti-inflammatory functions through inhibiting the proliferation of T lymphocytes and promoting the generation of Tregs [32]. Herein, we further explore whether GMSCs possess immunomodulatory effects on the innate immune cells, specifically macrophages. We show that macrophage cocultured with GMSCs acquired the phenotype of M2 macrophages characterized by increased expression of CD206, a high level of IL-10 and IL-6, and a low level of TNF-α as compared with control macrophages. Using an excisional wound model in mice, we demonstrated that systemic injection of GMSCs attenuated local inflammation, promoted angiogenesis, and significantly enhanced wound repair. Mechanistically, GMSCs were capable of polarizing M2 macrophages during wound repair. These findings provide first evidence that GMSCs can promote skin wound repair by eliciting the polarization of macrophages toward an anti-inflammatory M2 phenotype.
Materials and Methods
Animals
C57BL/6J mice (male, 8- to 10-week-old) were obtained from Jackson Laboratories (Bar Harbor, ME, http://www.jax.org) and group-housed at the Animal Facility of University of Southern California (USC). All animal care and experiments were performed under the institutional protocols approved by the Institutional Animal Care and Use Committee (IACUC) at USC.
Cytokines and Reagents
Recombinant human IL-4, CCL-2 (macrophage chemotactic protein-1 [MCP-1]), IL-6, and macrophage-colony stimulating factor (M-CSF) were purchased from PeproTech (Rocky Hill, NJ, http://www.peprotech.com). LPS from Escherichia coli 055:B5, phorbol 12-myristate 13-acetate (PMA), and Brefeldin A were obtained from Sigma-Aldrich (St. Louis, MO, http://www.sigmaaldrich.com). Antibodies include anti-CD14 allophycocyanin, anti-CD11a fluorescein isothiocyanate (FITC), anti-CD90 peridinin chlorophyll protein (PerCp)-Cy5.5, anti-IL-6-phycoerythrin (PE), anti-IL-10-PE, anti-TNFα-PE, and anti-IL-17-FITC (eBiosciences, San Diego, CA, http://www.ebioscience.com), anti-CD206 (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), anti-CD14-PE, anti-CD4-(PerCp)-Cy5.5, anti-CD86-PE, anti-CD209 (DC-SIGN)-PE (BioLegend, San Diego, CA, http://www.biolegend.com).
Cell Culture
The isolation and culture of human bone marrow and gingival tissue-derived MSCs, human peripheral blood-derived CD14+ monocytes, human acute monocytic leukemia cell line THP-1 [33], and human foreskin fibroblasts (Hs68) were described in details in Supporting Information. Both THP-1 and Hs68 cell lines were from ATCC (Manassas, VA, http://www.atcc.org). The gingival tissues were obtained as remnants of discarded tissues following routine dental procedures at USC School of Dentistry and the Outpatient Dental Clinic at Los Angeles County (LAC)-USC Medical Center under the approved Institutional Review Board (IRB) protocol at USC.
Coculture of Macrophage with GMSCs
For coculture studies, 2 × 105 GMSCs were seeded with human peripheral blood monocyte (PBMC)-derived macrophages on day 7 and cultured for another 3 days. For trans-well coculture, 0.4-μm-pore size Corning transwell inserts (VWR, West Chester, PA, https://www.vwrsp.com) were placed into the 6-well plate with macrophages initially seeded at the bottom well, while 2 × 105 GMSCs were seeded onto the inserts and continued to culture for another 3 days [20].
Flow Cytometry
Cells were processed for standard flow cytometric analysis of cell surface markers and analyzed using a FACS Calibur (BD Biosciences). To detect intracellular cytokine, macrophages were stimulated with 1 μg/ml LPS either for 24 hours (IL-10), or 5 hours (IL-6), or 1 μg/ml ionomycin with 50 nM PMA for 5 hours (TNF-α), in the presence of 10 μg/ml Brefeldin A (Sigma) to block the secretion of cytokines [20]. After stained for CD206, cells were processed with BD Cytofix/ Cytoper Fixation/Permeabilization kit (BD Biosciences), followed by incubation with specific antibodies for different cytokines, and analyzed by flow cytometry.
Phagocytic Assay
To determine the phagocytic activity of macrophages cocultured with GMSCs in transwells, FITC-coupled Zymosan particles (Sigma; 25 μg/ml) were added into the cultures and incubated at 37°C or 4°C for 1 hour. After washing, cells were fixed with 1% paraformaldehyde and the uptake of FITC-coupled zymosan particles was determined by flow cytometry.
Cytokine Antibody Array
Cytokine expression profiles in the supernatants of GMSCs, macrophage, and their cocultures were detected using RayBio Human Cytokine Antibody Array three (RayBiotech, Inc., Norcross, GA, http://www.raybiotech.com) and semiquantified following the manufacturer’s instructions. The medium alone was used as background control and arbitrarily set as 1.0.
Skin Wound Healing Model and GMSC Treatment
Mice were randomly divided into control and GMSC-treated groups, and the excisional full-thickness skin wound splinting model was generated as described previously [29, 31] and detailed in Supporting Information.
Myeloperoxidase Activity Assay
The infiltration of neutrophils in skin wound was assessed by measuring myeloperoxidase (MPO) activity as described previously [34, 35] and detailed in Supporting Information.
Histological and Immunohistochemical Studies
Standard H&E staining and dual-color immunofluorescence studies using specific primary antibodies for mice F4/80 and resistin-like molecule (RELM)-α were performed as previously described [32]. Isotype-matched control antibodies (eBiosciences) were used as negative controls. For semiquantification, positive signals in at least five random high-power fields were visualized, counted, and expressed as percentage of total DAPI-positive cells (mean ± SD).
Western Blot Analysis
Cell lysates or mice skin homogenates (50–100 μg of total protein) were separated on polyacrylamide-SDS gel and electroblotted onto nitrocellulose membrane (BioRad, Hercules, CA, http://www.bio-rad.com). After blocking with TBS/5% nonfat dry milk, the membrane was incubated with antibodies against mice arginase-1 (Santa Cruz Biotech, Inc., Santa Cruz, CA. http://www.scbt.com), RELM-α (PeproTech) or human nuclear factor kappa B (NFκB) p50 (BioLegend) or p65 (Millipore, Billerica, MA, http://www.millipore.com) followed by incubation with a horseradish peroxidase-conjugated secondary antibody, and the signals were visualized by enhanced chemiluminescence detection (PIERCE, Rockford, IL, http://www.piercenet.com). The blots were also reprobed with a specific antibody against β-actin (Sigma).
Enzyme-Linked Immunosorbent Assay
The concentration of IL-6, IL-10, and TNF-α in skin wound lysates of mice, and human IL-6, IL-10, and TNF-α levels in the supernatants of cultured cells were detected using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience).
Statistical Analysis
All data are expressed as mean ± SEM from at least three independent experiments. Differences between experimental and control groups were analyzed by two-tailed unpaired Student’s t-test using SPSS. p-values less than 0.05 were considered statistically significant.
Results
GMSCs Converted Macrophages into M2 Phenotype
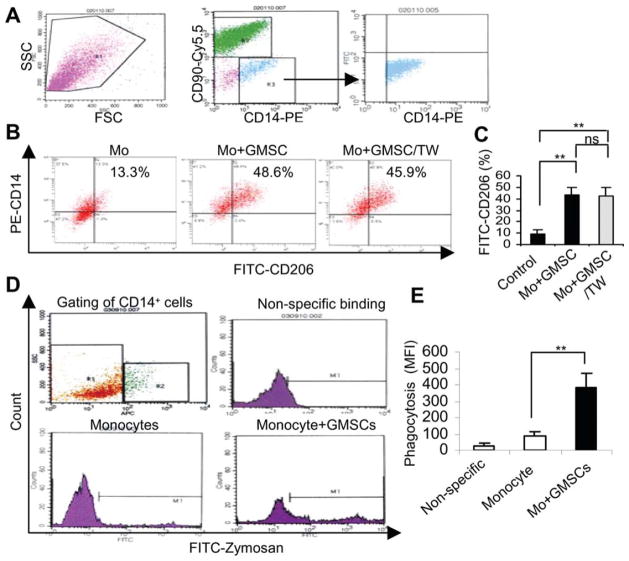
We have recently shown that GMSCs display similar immunomodulatory capacities to human BMSCs (hBMSCs) via interacting with T cells [32]. Herein, we further explore the potential interplay between GMSCs and macrophages, specifically, whether GMSCs can induce an anti-inflammatory M2 phenotype [15–19]. To this end, human PBMC-derived macrophages were cocultured with GMSCs at equal cell densities for 72 hours under direct cell-cell contact, and the expression of MR (CD206), one of the well-accepted markers for M2 macrophage [11, 20, 36], was determined by flow cytometry. Our results showed that coculture with GMSCs under direct cell-cell contact led to a significant increase in the expression of CD206 among CD14+ macrophages gated from the coculture (Fig. 1A) as compared with macrophage cultured alone (43.6% ± 6.68% vs. 9.33% ± 3.48%; p < .01; Fig. 1B, 1C). To determine whether upregulation of CD206 induced by GMSCs is dependent on direct cell-cell contact and/or soluble factors, macrophages and GMSCs were cocultured in the transwell system. Similarly, coculture with GMSCs in trans-wells increased the CD206+ macrophage population to the same extent as under condition of direct cell-cell contact (42.27% ± 7.84% vs. 43.6% ± 6.68%; p > .05; Fig. 1B, 1C), suggesting that soluble factors contributed an essential role in macrophage plasticity. To confirm that M2 macrophage population is specifically induced by soluble factors secreted by MSCs, we cultured macrophages with human BMSCs under the transwell condition. Similar with aforementioned findings, we observed a reproducible induction of CD206+ macrophage population in coculture with BMSCs (42.27% ± 7.84%), whereas no obvious changes were detected in cultures with normal skin fibroblasts or with macrophages alone (9.96% ± 1.5% vs. 9.33% ± 3.48%; p > .05; Supporting Information Fig. 1B, 1C). Meanwhile, we observed about a fourfold increase in the phagocytic activity in macrophages cocultured with GMSCs in the transwell as compared with macrophages cultured alone (p < .01; Fig. 1D, 1E). The nonspecific adhesion of zymosan particles to macrophages after incubation at 4°C was low to undetectable, suggesting that the increased zymosan uptake in macrophages cocultured with GMSCs was specifically caused by phagocytosis. These results confirmed that soluble factors released by cocultured MSCs contribute to the polarization of macrophages to the M2 phenotype.
Figure 1.
GMSCs promote the polarization of M2 macrophages. Monocytes isolated from PBMCs using human monocyte isolation kit were seeded in 6-well plates (2 × 105 per well) and cultured in macrophage growth medium for 7 days, followed by coculture with the same number of GMSCs for 3 days. Cocultured cells were collected and immunostained with PerCp/Cy5.5-CD90, PE-CD14, and FITC-CD206 and analyzed by flow cytometry. (A): Strategy of gating CD14-positive cells from the coculture with GMSCs (CD90-PerCp/Cy5.5). (B, C): Comparison of CD206 expression on macrophages cultured alone (Control) and cocultured in direct contact with GMSCs or in a transwell system (GMSCs/TW). (D): After coculture with GMSCs in the transwell for 72 hours, macrophages were incubated with 25 μg/ml FITC-Zymosan for 1 hour at 4°C (nonspecific binding) and 37°C, respectively, and analyzed by flow cytometry. Macrophages cultured alone were used as controls. (E): The average MFI of phagocytosed particles is shown for three different cultures. The results represent three independent experiments (mean ± SEM). **, p < .01. Abbreviations: FITC, fluorescein isothiocyanate; FSC, forward-scattered light; GMSC, mesenchymal stem cells from human gingival; MFI, mean fluorescence intensity; ns, no significance; PE, phycoerythrin; SSC, side-scattered light; TW, transwell.
GMSCs Induced an Anti-Inflammatory Immune Profile in Macrophages in Coculture
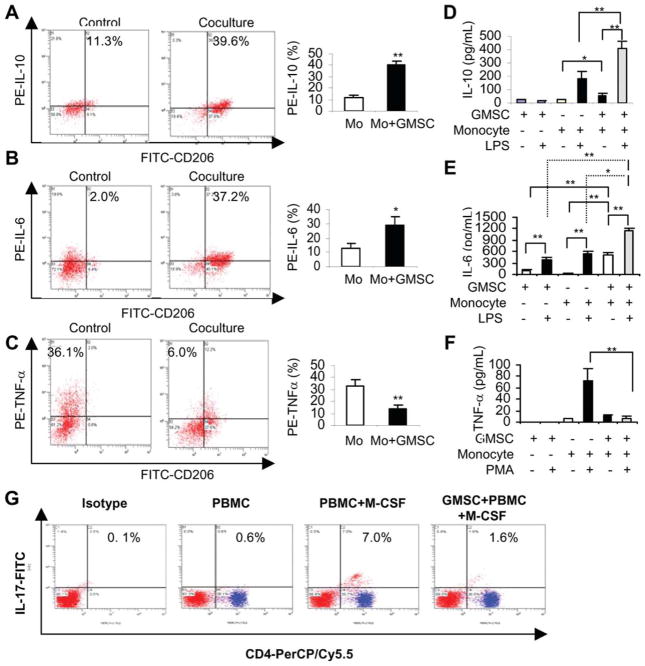
Next, we determined the cytokine expression profile by macrophages cocultured with GMSCs. Flow cytometric analysis showed that, in comparison with macrophages cultured alone, coculture with GMSCs significantly increased the percentage of macrophages expressing IL-10 (Fig. 2A) and IL-6 (Fig. 2B), while decreased TNF-α-positive macrophages (Fig. 2C) following stimulation using different protocols as previously described [20]. The differential cytokine profile of secretory IL-10, IL-6, and TNF-α in the supernatants of macrophages, GMSCs, and their cocultures was further confirmed by ELISA, respectively. Minimal release of cytokines was observed in macrophages or GMSCs alone in the absence of stimuli (Fig. 2D, 2F). Addition of stimulating agents triggered a burst of IL-10 and TNF-α secretion by macrophages, but had minimal effect on GMSCs cultured alone (Fig. 2D, 2F). The increased secretion of IL-10 triggered by LPS was significantly augmented in the coculture of GMSCs and macrophages (Fig. 2D); on the contrary, in the same coculture, PMA-triggered release of TNF-α was dramatically abolished (Fig. 2F). In addition, LPS also induced a marked increase in the production of IL-6 in the coculture of GMSCs and macrophages as compared with macrophages and GMSCs alone (Fig. 2E).
Figure 2.
Cytokine expression profiles in human macrophages cocultured with GMSCs. (A–C): After coculture with GMSCs in the transwell for 72 hours, macrophages were stained with FITC-CD206, followed by intracellular cytokine staining with PE-conjugated antibodies human IL-10 (A), IL-6 (B), and TNFα (C) and subjected to flow cytometry analysis, wherein cells stained with FITC- and PE-conjugated isotype control antibodies and macrophages cultured alone were used as controls. The graphs showed the average values from three independent experiments (mean ± SEM). (D–F): The secretion of IL-10 (D), IL-6 (E), and TNFα (F) in the supernatants of cocultured macrophages/GMSCs (2 × 105) was determined using enzyme-linked immunosorbent assay as compared with GMSCs and macrophages cultured alone. (G): Peripheral blood monocytes (PBMCs) (2 × 105) were cultured alone or cocultured with the same number of GMSCs in the presence of macrophage-colony stimulating factor (M-CSF; 30 ng/ml) for 72 hours. Then PBMCs were collected and immunostained with isotype-matched IgGs or CD4-PerCP/Cy5.5 and IL-17-FITC and analyzed by flow cytometry. PBMCs cultured alone in the absence of M-CSF were used as controls. The results represent three independent experiments (mean ± SEM). *, p < .05; **, p < .01. Abbreviations: FITC, fluorescein isothiocyanate; GMSC, mesenchymal stem cells from human gingival; IL, interleukin; PE, phycoerythrin; TNF, tumor necrosis factor.
Most recently, it has been shown that the presence of macrophage colony-stimulating factor (M-CSF) in a coculture system of peripheral blood monocytes and T lymphocytes could stimulate Th-17 cell expansion, possibly via M-CSF-induced macrophages [37]. Using this coculture system, we observed that GMSCs were capable of suppressing Th-17 cells expansion mediated by M-CSF-induced macrophages (Fig. 2G). Taken together, these results suggest that GMSCs are capable of switching macrophages from classical activation or proinflammatory M1 phenotype to an anti-inflammatory profile of M2 macrophages.
GMSCs Converted Inflammatory Human THP-1 Monocytes to M2 Macrophages
THP-1, an established human monocyte leukemic cell line, has been widely used as a cellular model to dissect the molecular mechanisms underlying monocyte-macrophage or dendritic cell differentiation [33]. Previous studies have shown that during PMA-induced THP-1 differentiation, IL-4 or IL-13, two well-known inducers for polarization of M2 macrophages [11, 14], could enhance the expression of CD86 and dendritic cell-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin (DC-SIGN, CD209), a marker for both immature dendritic cells and M2 macrophages [33]. To further explore the role of GMSC in the modulation of monocyte/macrophage differentiation, we cocultured differentiating THP-1 cells with GMSCs in transwells. In agreement with a previous study [33], we showed that addition of IL-4 suppressed CD14 and significantly increased CD86 and DC-SIGN expression (Fig. 3A–3D). Interestingly, coculture with GMSCs not only led to increased expression of CD86 and DC-SIGN but also markedly promoted CD14 expression during PMA-induced THP-1 differentiation (Fig. 3A–3D). In addition, our results indicated that LPS-stimulated increase in the secretion of TNF-α was almost abolished in THP-1 cells when cocultured with GMSC in both direct cell-cell contact and the transwell systems (Fig. 3E). GMSCs cultured alone neither express constitutive nor inducible TNF-α expression in response to LPS stimulation (data not shown). On the contrary, stimulation of coculture of THP-1 cells and GMSCs with LPS led to a concentration-dependent increase in secretory IL-10, whereas only a slight increase was observed in THP-1 or GMSCs cultured alone (Fig. 3F). At the mechanistic level, LPS stimulation failed to upregulate NFκB p50 in THP-1 cells cocultured with GMSCs as compared with THP-1 cells alone (Fig. 3G). The impaired LPS-induced activation of NFκB p50 appears to correlate with the suppression of proinflammatory cytokine TNF-α released by THP-1 cells in the presence of GMSCs. All together, these results suggest that GMSCs were capable to reprogram differentiation of monocytes/macrophages under different conditions to acquire phenotypes characteristic of M2 macrophages.
Figure 3.
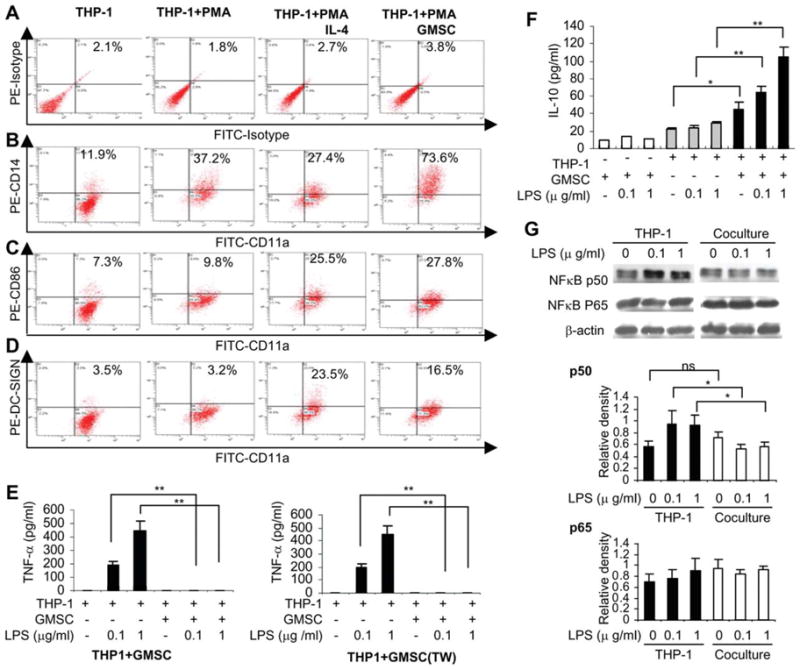
GMSCs regulate the immunophenotype and function of human monocyte leukemic cells (THP-1) during differentiation. (A–D): THP-1 cells (2 × 105) were differentiated on stimulation with PMA (10 ng/ml) in the absence or presence of IL-4 (100 ng/ml) or coculture with GMSCs in the transwell for 96 hours. Cells were stained with isotype-matched control IgGs (A), FITC-CD11a, and PE-conjugated CD14 (B), CD86 (C), or DC-SIGN (D) antibodies (A) and then analyzed by flow cytometry. THP-1 cells without any treatment were used as controls. (E–F): After coculture with GMSCs for 72 hours, THP-1 cells were stimulated with 0.1 and 1 μg/ml LPS for 4 hours (TNF-α) or 24 hours (IL-10), and the secretion of TNF-α (E) and IL-10 (F) in the supernatants was determined by enzyme-linked immunosorbent assay. (G): After coculture with GMSCs for 72 hours, THP-1 cells were stimulated with 0.1 and 1 μg/ml LPS for 1 hour, and the expression of NFκB p50 and p65 were determined by western blot, where the graphs represent the relative densities to the band of β-actin as the internal control. The results were representative of four independent experiments (mean ± SEM). *, p < .05; **, p < .01. Abbreviations: FITC, fluorescein isothiocyanate; GMSC, mesenchymal stem cells from human gingival; IL, interleukin; LPS, lipopolysaccharide; NFκB, nuclear factor kappa B; PE, phycoerythrin; PMA, phorbol 12-myristate 13-acetate; THP-1, human acute monocytic leukemia cell line; TNF, tumor necrosis factor.
Cytokines Involved in GMSC-Mediated Polarization of M2 Macrophages
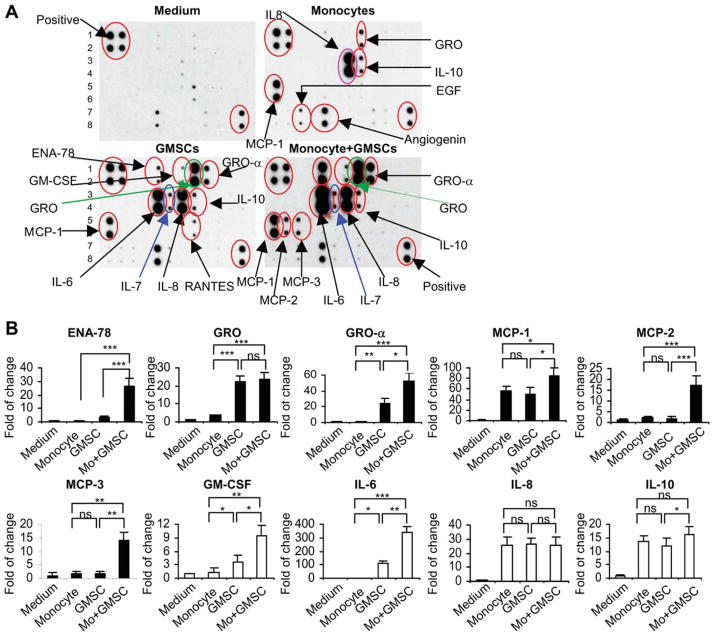
As described earlier, secretory factors may play an essential role in GMSC-induced polarization of M2 macrophages. We next screened for candidate soluble factors involved in GMSC-mediated M2 macrophage polarization using cytokine array analysis (Fig. 4A). Our results showed that PBMC-derived macrophages constitutively expressed high levels of IL-8 (26-fold), chemokine (C-C motif) ligand 2 (CCL-2) (MCP-1, 57-fold), and IL-10 (14-fold) and moderate levels of epidermal growth factor (4.8-fold), chemokine growth-related oncogene (GRO-α, 3.6-fold), and angiogenin (2.5-fold). Compared with macrophages, GMSCs also constitutively expressed similarly high levels of IL-8 (26-fold), MCP-1 (49-fold), and IL-10 (12-fold), but a much higher level of GRO-α (22-fold vs. 3.6-fold). Uniquely, MSCs constitutively expressed a relatively high level of IL-6 (107-fold) and GRO-α (24-fold) and a moderate level of IL-7 (7-fold), granulocyte macrophage colony-stimulating factor (GM-CSF) (3.6-fold), and ENA-78 (3-fold). However, when compared with GMSC cultured alone, a dramatic increase in the secretion of epithelial neutrophil-activating peptide 78 (ENA-78) (26-fold vs. 3-fold), GM-CSF (9.5-fold vs. 3.6-fold), IL-6 (340-fold vs. 107-fold), GRO-α (53-fold vs. 24-fold), MCP-1 (84-fold vs. 49-fold), MCP-2 (17-fold vs. 1.8-fold), and MCP-3 (14-fold vs. 1.8-fold) and a moderate increase in IL-10 (16-fold vs.12-fold) were observed in the supernatants obtained from the cocultured macrophages and GMSCs, whereas no additive increase in IL-8 and GRO-α was noticed (Fig. 4B).
Figure 4.
Cytokine expression profile determined by antibody array. The cytokine expression profile in the conditioned media collected from macrophage, GMSC, and their coculture were detected using the RayBio Human Cytokine Antibody Array 3 (RayBiotech, Inc., Norcross, GA), which allows the detection of 42 cytokines, chemokines, and growth factors in one experiment. The fresh medium without cell culture was used as a background control. (A): The representative image of cytokine antibody array. (B): The graphs show the relative intensity of spots of individual protein, whereby the intensity of the medium control was arbitrarily set as 1.0. The results were representative of three independent arrays. *, p < .05; **, p < .01; ***, p < .0001. Abbreviations: EGF, epithelial growth factor; ENA, epithelial neutrophil-activating peptide; GMSC, mesenchymal stem cells from human gingival; GRO, growth-related oncogene; IL, interleukin; MCP-1, macrophage chemotactic protein-1.
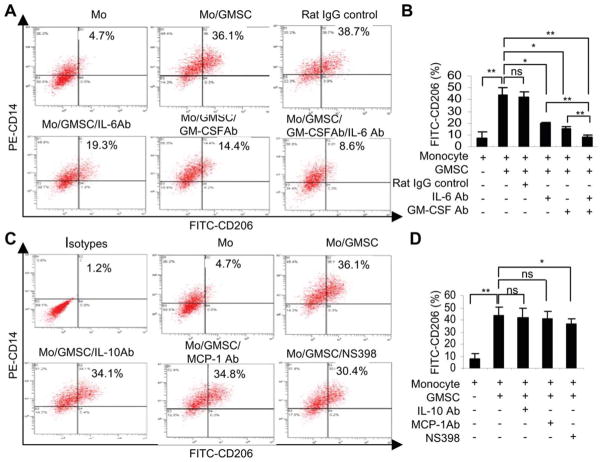
Based on previous findings that GMSCs constitutively expressed COX-2 [32] and that abundant levels of IL-6, CCL-2, IL-10, GM-CSF were detected in the supernatants of cocultured macrophages and GMSCs (Fig. 4), we explore whether these secretory factors contribute to the polarization of macrophages toward an M2 phenotype. To this end, PBMC-derived macrophages were cocultured with GMSCs in transwells in the presence or absence COX-2 inhibitor or various specific neutralizing antibodies for IL-6, CCL-2, IL-10, and GM-CSF for 72 hours, and the percentage of M2 macrophages characterized as CD14+/CD206+ double-positive cells was determined by flow cytometry. We showed that addition of neutralizing antibodies specific for IL-6 and GM-CSF significantly decreased the percentage of M2 macrophages (p < .05) as compared with the coculture control treated with nonspecific antibodies (Fig. 5A, 5B). A synergistic inhibitory effect on M2 macrophage generation was observed when both IL-6 and GM-CSF were neutralized (p < .01; Fig. 5A, 5B). Inhibition of COX-2 activity led to a moderate decrease of M2 macrophages, whereas neutralizing IL-10 and CCL-2 showed no obvious effects (Fig. 5C, 5D). These results suggest that both IL-6 and GM-CSF synergistically contribute to the induction of M2 macrophages mediated by coculture with GMSCs.
Figure 5.
Blocking IL-6 and GM-CSF synergistically inhibit GMSC-mediated induction of M2 macrophages. Macrophages were cocultured with GMSCs in transwells for 72 hours in the presence or absence of COX-2 inhibitor NS398 (10 μM) or specific neutralizing antibodies for IL-6, GM-CSF, CCL-2/MCP-1, or IL-10 (10 μg/ml). An isotype-matched rat IgG was used as negative controls. (A, C): Cells were immuno-stained with PE-CD14 and FITC-CD206 antibodies and subjected to flow cytometry analysis. (B, D): The graphs show the average values from three independent experiments (mean ± SEM). *, p < .05; **, p < .01. Abbreviations: FITC, fluorescein isothiocyanate; GM-CSFAb, granulocyte macrophage-colony stimulating factor neutralizing antibody; GMSC, mesenchymal stem cells from human gingival; IL, interleukin; MCP, macrophage chemotactic protein-1; ns, no significance; PE, phycoerythrin.
GMSCs-Based Therapy Enhanced Skin Wound Healing in Mice
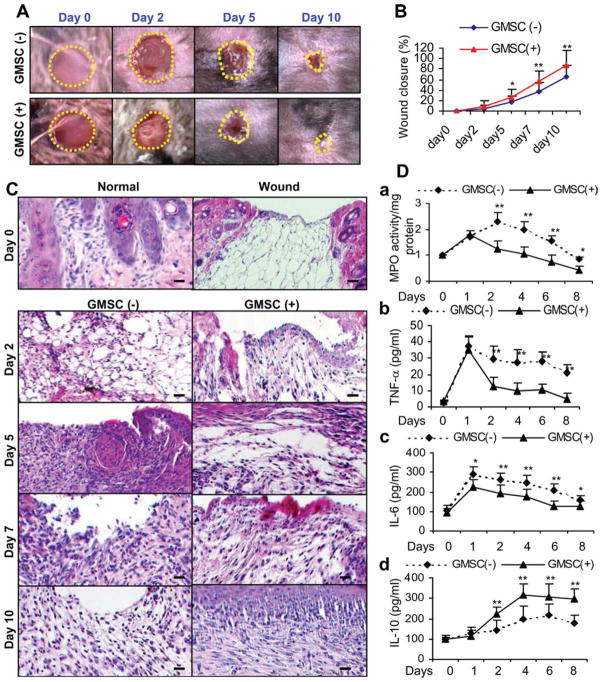
Given the essential roles of both MSCs [24, 30, 31] and M2 macrophages [10, 12, 27, 38] in wound healing, we explore the in vivo relevance of GMSC-induced M2 switch in wound repair using an excisional skin healing model in mice. First, we investigated whether GMSCs were capable of enhancing cutaneous excisional wound repair. To this end, GMSCs (2 × 106 per mice) were systemically injected into mice 1 day post full-thickness skin excision and wound closure was carefully measured daily (n = 4). Our results showed that mice receiving systemic infusion of GMSCs displayed accelerated skin wound closure compared with the control mice without treatment, wherein the enhancement in wound closure appeared as early as day 3, and the wound became completely closed on day 10 (p < .01; Fig. 6A, 6B).
Figure 6.
Systemic administration of GMSCs accelerates wound closure and suppress local inflammatory responses in C57BL/6 mice. One day after excisional skin wound, GMSCs (2 × 106 per mice) were systemically infused by tail vein (i.v.) into mice and wound closure was daily observed. (A): Representative photographs of wounds at different time postwounding with or without GMSC treatment. (B): Measurement of wound closure at different time points (n = 4). The percentage of wound closure was calculated as: (area of original wound – area of measured wound)/area of original wound × 100. (C): Representative H&E-stained paraffin-embedded sections of full-thickness incisional skin wounds from mice with or without receiving systemic administration of GMSCs (n = 4). Mice were sacrificed at different days post-wounding. Scale bar = 100 μm. (D): At different time points after wounding, skin samples were collected and tissue lysates were prepared for further analysis: (A): MPO activity assay. (B–D): Enzyme-linked immunosorbent assay on inflammatory cytokines, including TNF-α (B), IL-6 (C) and anti-inflammatory cytokine IL-10 (D). The results were representative of three independent experiments (mean ± SEM). *, p < .05; **, p < .01. Abbreviations: GMSC, mesenchymal stem cells from human gingival; IL, interleukin; MPO, myeloperoxidase; TNF, tumor necrosis factor.
Histological analysis of wounds on day 3, 5, and 7 showed a more organized granulation tissue proper at the excisional wound site in GMSC-treated mice as compared with the untreated group (Supporting Information Fig. 2A). Masson trichrome staining of GMSC-treated skin wounds on day 7 showed thick and densely packed collagen fibers, whereas thin and loosely packed basket-weaved collagen bundles were more apparent in untreated skin wounds (Supporting Information Fig. 2B). In addition, abundant presence of microvascular structures and CD31-positive endothelial cells were observed in GMSC-treated wounds as compared with controls (Supporting Information Fig. 2C). Interestingly, the CD31+ endothelial cells were localized in close spatial relationship to GMSCs prelabeled with CM-DiI dye (Supporting Information Fig. 3). Overall, we observed rapid re-epithelialization in GMSC-treated wounds (complete epithelialization in all six of eight wounds examined; n = 4) compared with untreated wounds (complete epithelialization in two of eight wounds examined; n = 4) on day 10 (Fig. 6C). These results suggest that enhancement of wound healing by systemic infusion of GMSCs involves enhanced re-epithelialization, collagen deposition and angiogenesis. In agreement with previous studies [28, 29], we also observed no apparent benefit in wound healing when treated with normal skin fibroblasts (data not shown).
Interplay Between GMSCs and Macrophages Regulated the Local Inflammatory Response During Skin Wound Healing
We next investigated the in vivo effects of GMSCs on inflammatory cell response and production of local inflammatory cytokines in skin wounds. Analysis of skin wounds on day 3, 5, and 7 following GMSC injection indicated that infiltration of inflammatory cells was significantly decreased in GMSC-treated wounds as compared with controls (Fig. 6C). GMSC treatment decreased neutrophil infiltration as represented by a time-dependent decrease in MPO activity in wounded skin at several time points postwounding (Fig. 6Da). In addition, ELISA analysis showed that GMSC treatment significantly decreased the local levels of both proinflammatory cytokines, TNF-α and IL-6, and increased the anti-inflammatory cytokine IL-10 (Fig. 6Db–6Dd). These findings suggest that GMSC treatment promotes skin wound healing, at least in part, by suppressing inflammatory cell infiltration and proinflammatory cytokine secretion as well as by increasing the production of IL-10 at the local wound sites.
To explore the interactions of homed GMSCs and host macrophages at the wounds, GMSCs prelabeled with CM-DiI were systemically injected into mice. As shown in Figure 7A, the number of GMSCs homing to injured sites significantly increased as compared with that in normal skin, and the homed GMSCs were in close proximity with F4/80-positive macrophages (Fig. 7B). Then, we further investigated the in vivo effects of GMSCs on the phenotype of macrophages in skin wounds. Dual-color immunofluorescence studies were performed using specific antibodies for F4/80 and RELM-α, also known as Fizz1 (found in inflammatory zone 1), a well-known marker for M2 macrophages [11, 38]. As shown, GMSC treatment led to a time-dependent increase in the number of F4/80 and RELM-α-positive macrophages (Fig. 7C, 7D; Supporting Information Fig. 4). The increased expression of RELM-α in skin wounds on day 7 after GMSC treatment was further confirmed by western blot (Fig. 7E). Meanwhile, we also detected the expression of arginase-1 in skin wounds, another well-known marker for M2 macrophage [11, 39]. We observed a similar increase in the expression of arginase-1 protein in skin wounds on day 7 after GMSC injection as compared with controls (Fig. 7E). Furthermore, a time-dependent increase in arginase-1 protein expression was also demonstrated in skin wounds after GMSC treatment on different days postwounding (Fig. 7F). These compelling findings suggest that GMSCs were capable to promote alternative activation of host macrophages infiltrated at the wounded skin sites, potentially contributing to the regulation of the inflammatory response and enhancing the healing of excisional skin in mice.
Figure 7.
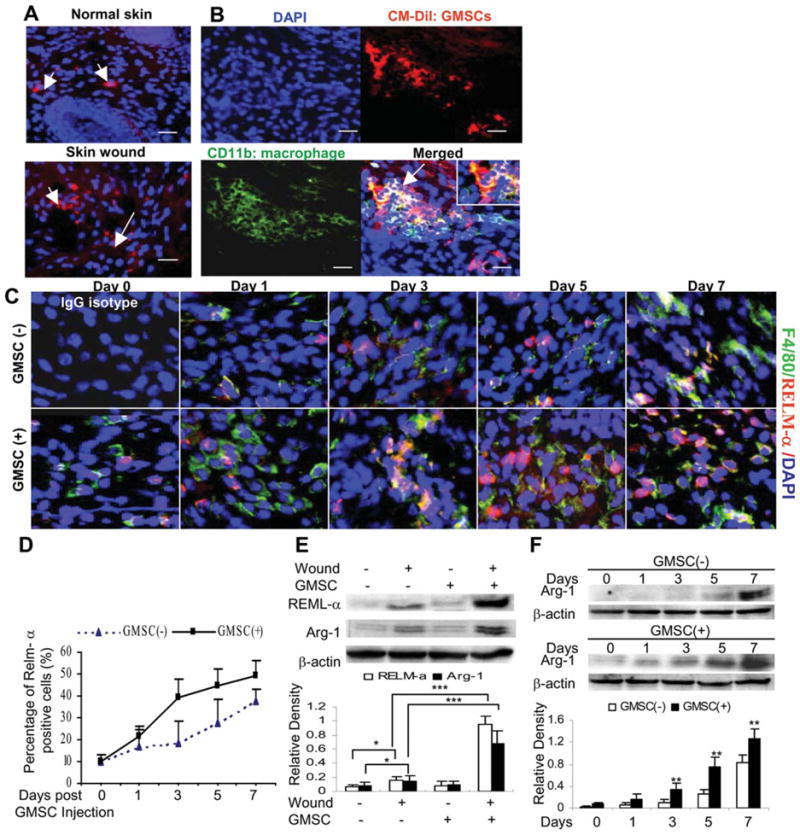
Interactions of homed GMSCs with macrophages during wound healing. (A): GMSCs prelabeled with CM-DiI were systemically infused by tail vein (i.v.) into mice one day after skin wounding. Seven days after cell injection, skin tissues were frozen sectioned and observed under a fluorescence microscope, whereby normal skin on the other side of the same mice were used as controls. (B): Frozen sections of wounded skins from mice after injection with CM-DiI pre-labeled GMSCs were immunostained with fluorescein isothiocyanate-conjugated antibody for mice CD11b. Scale bar = 50 μm. The results were representative of at least three independent experiments. (C): Frozen sections of full-thickness incisional skin wounds from mice after treatment with GMSCs for different days were dual-color immunostained with specific antibodies for F4/80 (Green) and RELM-α (Red). Scale bar = 50 μm. (D): Quantification of M2 macrophages positive for RELM-α. (E): Western blot analysis of arginase-1 (Arg-1) and RELM-α expression in skin wounds 7 days post GMSC treatment. (F): Time-dependent increases in Arg-1 and RELM-α expression induced by GMSC treatment. The results are representative of three independent experiments. *, p < .05; **, p < .01; ***, p < .001. Abbreviations: DAPI, 4′, 6-diamidino-2-phenylindole; GMSC, mesenchymal stem cells from human gingival; RELM, resistin-like molecule.
Discussion
Macrophages, one of the major types of innate immune cells, can produce a plethora of mediators that play various functions in inflammation, immunity, and wound healing [11, 14]. On recruitment to the injury sites, macrophages encounter various signals that drive their differentiation toward distinct phenotypes [11, 14]. M1 macrophages are generally induced by Th1 cytokines such as IFN-γ or Toll-like receptor agonists like LPS and play important roles in inflammation and pathogen clearance via secreting proinflammatory mediators, including nitric oxide (NO), TNF-α, and IL-12 [11, 14]. On the contrary, M2 macrophages can be induced in response to diverse noninflammatory cues, including Th2-related cytokines such as IL-4 and IL-13, IL-10, TGF-β1, glucocorticoids, and apoptotic cells [11, 14]. M2 macrophages are characterized by secretion of high levels of anti-inflammatory cytokines, IL-10 and TGF-β1, as well as by the expression of specific markers such as MR (CD206), chitinase-like secretory lectins (Ym1), and Fizz1 (found in inflammatory zone 1, also known as RELM-α) [11, 14]. Accumulating evidence has shown that M2 macrophages are not only implicated in Th2-driven pathologies such as helminth infection and asthma [19] but also can coordinate adaptive immune responses by interacting with Tregs [36, 40], ameliorate the outcome of several inflammatory diseases by counteracting Th1-initiated inflammatory responses [17–19, 41], and contribute to tissue homeostasis by promoting inflammatory resolution [13].
In addition to IL-4 and IL-13, several other soluble factors, including IL-10 [39], GM-CSF [42–44], prostaglandin E (PGE2) [15, 21], CCL-2 (MCP-1), and IL-6 [45], are also capable to induce polarization of M2 macrophages under different experimental settings. For instance, CCL-2 and IL-6 can promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2 macrophage polarization [45]. GM-CSF can skew mice macrophage progenitors toward an M2 phenotype, especially in the absence of SHIP [44]. Recent studies have shown that PGE2 constitutively produced by MSCs might be responsible for MSC-induced M2 phenotype of macrophages [15, 21]. In this study, we observed a relatively abundant level of IL-6, CCL-2, IL-10, and GM-CSF in cocultured human macrophages and GMSCs (Fig. 4), wherein GMSCs promoted the switch of macrophages to an M2-like profile in a soluble factor-dependent manner (Fig. 1B, 1C). However, only specific blocking of IL-6 and GM-CSF inhibited the induction of M2-like macrophages (Fig. 5A, 5B), suggesting that both IL-6 and GM-CSF could contribute to GMSC-induced polarization of M2 macrophages.
Cutaneous wound healing is a complex process of well-defined overlapping events [23], wherein macrophages play an essential role in the removal of infiltrated leukocytes and cellular debris at the wound sites [13, 25, 46]. The depletion or selective ablation of macrophages could be detrimental to wound healing because of the failure of clearance of dead and damaged cells [25, 46]. It is recognized that both M1 and M2 macrophages play a pivotal role in different stages of physiological wound repair [12, 13, 47]. M1 macrophages are abundant during the initial inflammatory response and produce a high amount of proinflammatory cytokines and ROS, whereas M2 macrophages are predominated in the resolution phase and secrete mainly anti-inflammatory cytokines and exert a higher phagocytic activity. In a recent study, looking at the gene expression profile at the early stage of wound repair whereas the inflammatory response is relatively dominant, a mixture of M1 activation gene transcripts such as IL-6 and Toll-like receptors and M2 activation gene transcripts such as IL-13, and arginase were detected at the wound sites. On the contrary, as wound repair proceeds without infection, and tissue remodeling gradually takes place, the profile of macrophage-related transcripts was predominantly M2 activation genes such as TGF-β1 and IL-1 receptor agonist [47]. Recently, a population of resolution-phase macrophages was described during the resolving phase of acute peritonitis, which possessed a unique hybrid phenotype of both M2 and M1 as they not only express CD206 and synthesize IL-10 and arginase 1 but also express other markers typical of M1 (i.e., inducible nitric oxide synthase (iNOS)) [13]. Similarly, a recent study has shown that wound macrophages on day 1 express more TNF-α and IL-6, but less TGF-β1 than those on day 7, supporting the notion that wound macrophages exhibit a complex phenotype, which not only require IL-4 or IL-13 but also include traits associated with both M1 and M2 activation and phenotypic changes as the wound matures [27]. Routley et al. have reported that estrogen or progesterone can contribute toward M2 activation of macrophages to drive wound repair, angiogenesis, and remodeling [38]. Consistently, our current study also showed a dynamic increase in the number of M2 macrophages and the level of anti-inflammatory cytokine IL-10 and a decrease in the expression of M1-cytokines (TNF-α and IL-6) during the wound healing process. All together, these findings support the notion that both the presence and the activation/phenotype of macrophages within the wound are fundamental elements in guiding normal wound repair [38] and manipulating the differentiation of plastic macrophages toward an M2 macrophage phenotype would provide novel strategies to promote normal wound healing or aid in the resolution of impaired wound healing.
Recent studies have implicated the application of MSCs from bone marrow [24, 28–31] and adipose tissues [48] in skin wound healing. The enhancement of wound repair by MSCs may be attributed to their unique properties of multipotent differentiation and homing to injury sites [29–31], and their abilities to secrete various soluble factors with trophic, proangiogenic, and anti-inflammatory functions [24, 28, 31]. A recent study has shown that treatment with BMSC enhances wound healing possibly by increasing the recruitment of macrophages to the wound sites [28]. However, the interaction between MSCs and macrophages and specifically whether MSCs have any effects on the phenotype or activation of macrophages during wound healing remains unknown. Most recently, several studies have implicated the effects of MSC on regulating the phenotype of macrophages in different settings. In an in vitro study, human macrophages after cocultured with human BMSCs acquired an M2-like phenotype characterized by a high expression of IL-10 and IL-6, but a low expression of IL-12 and TNF-α as compared with control macrophages [20]. In a mouse model of global ischemia, the beneficial neuroprotective effects of hBMSCs were largely explained by their modulation of inflammatory and immune responses, apparently by alternative activation of microglia and/or macrophages in the central nervous system [22]. Meanwhile, it has been shown that macrophages from septic lungs secreted more IL-10 when prepared from mice treated with BMSCs versus untreated mice, suggesting that BMSCs (activated by LPS or TNF-α) reprogrammed macrophages by releasing prostaglandin E2 [15]. In addition, both in vitro and in vivo studies have shown that mouse BMSCs are capable to convert activated peritoneal macrophages into a regulatory-like profile similar to M2 macrophage, characterized by a low ability to produce inflammatory cytokines and a high ability to produce IL-10 and phagocyte apoptotic cells [21].
In the present study, we have demonstrated, for the first time to our knowledge, that human-derived GMSCs [32] can switch the differentiation and activation of in vitro-cultured human macrophages into an M2-like phenotype characterized by an increased expression of MR/CD206 and phagocytic activity, a high ability to express IL-10 and IL-6, and a low ability to express TNF-α, a phenotype similar to that of human macrophages after cocultured with hBMSCs [20]. More importantly, using an established mouse model of skin wound healing, we showed that systemic administration of GMSCs attenuated local inflammatory responses, increased angiogenesis and extracellular matrix (ECM) deposition, and consequently enhanced skin wound healing. The GMSC-mediated rapid cutaneous wound repair is associated with a dynamic increase in the number of M2 macrophages characterized by an increased expression of arginase-1 and RELM-α. These findings suggest that GMSCs are effective in enhancing skin wound repair possibly through switching the activation of host macrophages to an anti-inflammatory M2 phenotype.
Conclusion
In conclusion, we have demonstrated that GMSCs can reprogram macrophages toward an anti-inflammatory M2 phenotype, which may contribute to the rapid re-epithelialization, improved angiogenesis and tissue remodeling of skin wound healing. These findings further support the notion that GMSCs, a unique population of MSCs with functional similarities to BMSCs, and specifically, their ease of isolation, accessible tissue source, and rapid ex vivo expansion, are a promising cell source for stem cell-based therapies of inflammatory diseases and skin wound.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Research Grant R01DE 019,932 and USC Institutional fundings, CTSI, and Zumberge award.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author contributions: Q.-Z.Z.: conception and design, collection and assembly of data, manuscript writing, final approval of the manuscript; W.-R.S.: collection and assembly of data, final approval of the manuscript; S.-H.S.: collection and assembly of data; P.W.S.: manuscript writing, final approval of the manuscript; A.P.X.: manuscript writing, final approval of the manuscript; A.W.: manuscript writing, final approval of the manuscript; A.L.N: collection and assembly of data; B.K.: collection and assembly of data; A.D.L.: conception and design, manuscript writing, final approval of the manuscript, financial support.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilieage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 4.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 6.Ryan JM, Barry F, Murphy JM, et al. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFoxP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 8.Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 9.Spaggiari GM, Abdelrazik H, Becchetti F, et al. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien J, Lyons T, Monks J, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176:1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009;33:222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troidl C, Möllmann H, Nef H, et al. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med. 2009;13:3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bystrom J, Evans I, Newson J, et al. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 15.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E (2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aki K, Shimizu A, Masuda Y, et al. ANG II receptor blockade enhances anti-inflammatory macrophages in anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F870–F882. doi: 10.1152/ajprenal.00374.2009. [DOI] [PubMed] [Google Scholar]
- 17.Hunter MM, Wang A, Parhar KS, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wang YP, Zheng G, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 19.Smith P, Mangan NE, Walsh CM, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. Plos One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtaki H, Ylostalo JH, Foraker JE, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 24.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 25.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies FM, Henriquez FL, Alexander J, et al. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol. 2010;160:369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daley JM, Brancato SK, Thomay AA, et al. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. Plos One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Tredget EE, Liu C, et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. Plos One. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by trans-differentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puig-Kröger A, Serrano-Gómez D, Caparrós E, et al. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem. 2004;279:25680–25688. doi: 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Rey E, Anderson P, González MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 35.Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiemessen MM, Jagger AL, Evans HG, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/ macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Kim HJ, Chang EJ, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: Implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 38.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17:42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 39.Park-Min KH, Antoniv TT, Ivashkiv LB. Regulation of macrophage phenotype by long-term exposure to IL-10. Immunobiology. 2005;210:77–86. doi: 10.1016/j.imbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Savage ND, de Boer T, Walburg KV, et al. Human anti-inflammatory macrophages induce Foxp3+GITR+CD25+regulatory T cells, which suppress via membrane-bound TGF-1. J Immunol. 2008;181:2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 41.Weber MS, Prod’homme T, Youssef S, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 42.Grant V, King AE, Faccenda E, et al. PGE/cAMP and GM-CSF synergise to induce a pro-tolerance cytokine profile in monocytic cell lines. Biochem Biophys Res Commun. 2005;331:187–193. doi: 10.1016/j.bbrc.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 43.Chen GH, Olszewski MA, McDonald RA, et al. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. Am J Pathol. 2007;170:1028–1040. doi: 10.2353/ajpath.2007.060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroda E, Ho V, Ruschmann J, et al. SHIP represses the generation of IL-3-induced M2 macrophages by inhibiting IL-4 production from basophils. J Immunol. 2009;183:3652–3660. doi: 10.4049/jimmunol.0900864. [DOI] [PubMed] [Google Scholar]
- 45.Roca H, Varsos ZS, Sud S, et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 47.Deonarine K, Panelli MC, Stashower ME, et al. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:1–11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim WS, Park BS, Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9:879–887. doi: 10.1517/14712590903039684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.