Abstract
Rationale: Exposure to ozone causes a decrease in spirometric lung function and an increase in airway inflammation in healthy young adults at concentrations as low as 0.08 ppm, close to the National Ambient Air Quality Standard for ground level ozone.
Objectives: To test whether airway effects occur below the current ozone standard and if they are more pronounced in potentially susceptible individuals, such as those deficient in the antioxidant gene glutathione S-transferase mu 1 (GSTM1).
Methods: Pulmonary function and subjective symptoms were measured in 59 healthy young adults (19–35 yr) immediately before and after exposure to 0.0 (clean air, CA) and 0.06 ppm ozone for 6.6 hours in a chamber while undergoing intermittent moderate exercise. The polymorphonuclear neutrophil (PMN) influx was measured in 24 subjects 16 to 18 hours postexposure.
Measurements and Main Results: Subjects experienced a significantly greater (P = 0.008) change in FEV1 (± SE) immediately after exposure to 0.06 ppm ozone compared with CA (−1.71 ± 0.50% vs. −0.002 ± 0.46%). The decrement in FVC was also greater (P = 0.02) after ozone versus CA (−2.32 ± 0.41% vs. −1.13 ± 0.34%). Similarly, changes in %PMN were greater after ozone (54.0 ± 4.6%) than CA (38.3 ± 3.7%) exposure (P < 0.001). Symptom scores were not different between ozone versus CA. There were no significant differences in changes in FEV1, FVC, and %PMN between subjects with GSTM1-positive and GSTM1-null genotypes.
Conclusions: Exposure of healthy young adults to 0.06 ppm ozone for 6.6 hours causes a significant decrement of FEV1 and an increase in neutrophilic inflammation in the airways. GSTM1 genotype alone appears to have no significant role in modifying the effects.
Keywords: pulmonary function, airway inflammation, polymorphism, ozone exposure, exercise
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Inhalation of ozone causes decrements in lung function and an increase in airway inflammation at concentrations near the current Ambient Ozone Standard. It is not known what the effects are at concentrations lower than this.
What This Study Adds to the Field
This study reports that acute exposure to ozone for 6.6 hours at a level of 0.06 ppm (a level below the current Ambient Ozone Standard) causes significant effects on pulmonary function and airway inflammation in healthy young adults.
Ozone is a major component of photochemical smog. Controlled human exposure studies have been critical in demonstrating that it can cause decrements in lung function (1–7) and lung inflammation (8–11). Although the majority of these studies involved exposures to relatively high (0.1–0.4 ppm) concentrations for short periods of time (typically 2 h), prolonged exposure studies at lower levels were largely responsible for the U.S. Environmental Protection Agency (EPA) lowering the National Ambient Air Quality Standard for ground level ozone in 2008. Several studies have now confirmed the initial observation of Horstman and colleagues (4) that exposure of healthy young adults to 0.08 ppm ozone for 6.6 hours while undergoing intermittent moderate exercise will result in a significant drop (5–8%) in FEV1 (5, 6). Similarly, studies using this exposure regime demonstrated that 0.08 ppm ozone can initiate inflammatory responses in the lungs of healthy young adults (9), characterized by increases in polymorphonuclear neutrophils (PMN) in bronchoalveolar lavage fluid collected 24 hours postexposure. Recently, we have confirmed and extended these findings in 15 healthy young adults by showing a significant increase in sputum neutrophilic inflammation 18 hours postexposure to 0.08 ppm ozone (12).
Recent community-based air pollution studies using emergency department records have reported associations for increased risk of pediatric asthma at very low ozone concentrations (13). This has led some to question whether adverse responses could occur in healthy adults at concentrations below the current standard. However, only two controlled human exposures have investigated this and none to our knowledge have addressed inflammatory effects. Adams reported that healthy young adults exposed to multiple ozone concentrations experienced a significant decrease in lung function at 0.08 ppm but not at 0.06 ppm or 0.04 ppm (7). However, a secondary analysis by others of the same data concluded that 0.06 ppm may have induced a change in FEV1 (14). In 2009, Schelegle and colleagues (15) reported that healthy young volunteers exposed to step-wise ozone concentrations experienced a significant decrement of FEV1 at concentrations as low as 0.07 ppm but not at 0.06 ppm.
Large heterogeneity in responses to ozone between individuals has been reported (16, 17). Ozone exerts oxidant stress and results in airway inflammation, and therefore genes that modulate inflammation and antioxidant defense mechanisms have been proposed as potential effect modifiers. In particular, glutathione S-transferase mu 1 (GSTM1) has attracted attention as it is present in the airways, and gene deletions (GSTM1-null genotype) can be present in up to 50% of the population, resulting in complete absence of the enzyme. This polymorphism has been associated with reduced lung function (18, 19), and numerous epidemiology and controlled exposure studies have suggested that it may confer susceptibility to increased airway inflammation to ozone as well as other oxidant air pollutants (20, 21).
More than 100 million people in the United States now live in the counties that do not meet the current ozone standard, and public health consequences are enormous. In the present study, we performed a randomized controlled human exposure crossover study and investigated specifically if exposure of healthy young adults to 0.06 ppm ozone for 6.6 hours with exercise would cause measurable airway effects assessed by pulmonary function (chosen a priori to be FEV1 and FVC) and inflammatory markers (sputum PMN). We also examined if responses were more pronounced in individuals with the GSTM1-null genotype. Some of the preliminary results of these studies have been previously reported in the form of abstracts (22).
METHODS
Subjects
Fifty-nine healthy young adult subjects aged 19 to 35 years, with no history of smoking in the past 2 years, completed the study. All subjects underwent a screening procedure that included a complete medical history and physical examination and a pregnancy test for females. Exclusion criteria included respiratory illness or symptoms within 4 weeks or a positive pregnancy test. All subjects were genotyped for GSTM1. The study protocol was approved by the Institutional Review Board at the University of North Carolina Medical School in Chapel Hill and the EPA, and informed consent was obtained from all subjects before their participation in the study. Subject characteristics and baseline lung function test values are shown in Table 1.
TABLE 1.
SUBJECT CHARACTERISTICS AND BASELINE LUNG FUNCTION MEASURES
| All Subjects | Males | Females | GSTM1-p | GSTM1-n | |
|---|---|---|---|---|---|
| No. | 59 | 27 | 32 | 30 | 29 |
| Age, yr | 25.0 (0.5) | 26.1 (0.9) | 24.0 (0.5) | 24.9 (0.8) | 25.1 (0.7) |
| Height, cm | 171.2 (1.2) | 178.9 (1.2) | 164.7 (1.0) | 172.2 (1.5) | 170.2 (1.9) |
| Weight, kg | 70.5 (1.8) | 78.8 (2.3) | 63.4 (2.1) | 71.5 (2.5) | 69.4 (2.6) |
| BSA, m2 | 1.8 (0.03) | 2.0 (0.03) | 1.7 (0.03) | 1.8 (0.04) | 1.8 (0.04) |
| BMI, kg/m2 | 23.8 (0.48) | 24.5 (0.72) | 23.3 (0.64) | 23.9 (0.68) | 23.8 (0.69) |
| FEV1, L | 4.02 (0.10) | 4.64 (0.12) | 3.49 (0.07) | 4.14 (0.12) | 3.89 (0.16) |
| FVC, L | 4.83 (0.13) | 5.69 (0.14) | 4.10 (0.11) | 4.94 (0.17) | 4.72 (0.21) |
| FEV1/FVC, % | 84.1 (0.8) | 82.0 (1.1) | 85.8 (1.2) | 84.6 (1.2) | 83.5 (1.2) |
| FEF25-75%, L/s | 4.22 (0.14) | 4.64 (0.23) | 3.87 (0.14 | 4.39 (0.21) | 4.05 (0.18) |
| FEFmax, L/s | 9.03 (0.25) | 10.61 (0.28) | 7.69 (0.17) | 9.49 (0.35) | 8.54 (0.34) |
Definition of abbreviations: BMI = body mass index; BSA = body surface area; FEF25-75% = forced expiratory flow between 25 and 75% of FVC; FEFmax = maximum forced expiratory flow; GSTM1-n = glutathione S-transferase mu 1 null; GSTM1-p = glutathione S-transferase mu 1 positive.
Values are mean (± SEM)
Study Design
The study design was similar to those used in previous studies (4, 7) to facilitate comparison. Each subject was exposed to 0.00 ppm (clean air, CA) and 0.06 ppm ozone for 6.6 hours with moderate exercise in a stainless steel chamber (4 × 6 × 3.2 m). Exposures were randomized, double-blinded, and separated by at least 1 week. Minute ventilation (Ve) was measured hourly and exercise levels adjusted to Ve = 20 L/min/m2 body surface area to ensure that subjects breathed consistently throughout exposure. Spirometric lung function and symptom scores were assessed immediately before and after the 6.6-hour exposure period. Sputum was collected the next morning approximately 16 to 18 hours postexposure. Exposures were conducted only during the cool weather season in Chapel Hill (November–March) to minimize exposure to elevated ambient ozone.
Study Protocol
Training day.
On a training day, all subjects were trained for lung function measurements. Settings for the treadmill and bicycle ergometer required to produce a desired value of Ve was determined for each subject.
Exposure day.
On the day of each exposure, subjects were assessed for vital signs, completed a symptom questionnaire, and performed preexposure lung function spirometry and body plethysmography maneuvers. They then entered the chamber set for the appropriate condition (CA or 0.06 ppm ozone) and began exercising for a 50-minute period at 20 L/min/m2 body surface area in Ve followed by a 10-minute rest period. The exercise session was repeated six times alternating between the treadmill and bicycle with Ve maintained within ± 2 L/min of the subject's target value. Subjects were also given 35 minutes to eat lunch. Thus, they remained in the chamber for a total of 6.6 hours, during which ventilatory parameters (Ve, Vt, breathing frequency) were measured and electrocardiac signals, heart rate, and blood oxygen saturation monitored continuously to ensure subject safety. Subjects were exposed in pairs (of the same sex). At the end of exposure, spirometric and plethysmographic lung function measurements were performed and a symptom questionnaire was obtained.
Follow-up day.
On the next morning, subjects returned to the laboratory and sputum samples were collected.
Measurements
Spirometry was performed on a 10.2-L dry seal digital spirometer interfaced to a computer (SensorMedics Model 1022; SensorMedics; Palm Springs, CA). At least three sets of qualified data were obtained and the largest value selected for FEV1 and FVC as per American Thoracic Society guidelines (23). Pulmonary function on all subjects was measured using one dedicated spirometer and by one certified pulmonary function technician to minimize variability. Specific airway resistance (sRaw) was assessed by body plethysmography (SensorMedics Model V6200) and the average of two highest values out of three to five measurements obtained. Measurements were performed before and immediately after exposure for use in endpoint analysis. Additional spirometry measurements were performed in the chamber during 10-minute rest periods primarily for monitoring subject safety and were not necessarily performed by the same technician.
Symptoms were assessed before and after 6.6-hour exposure. The subjects were asked to rate the severity of cough, pain on deep inspiration (PDI), shortness of breath (SOB), and throat irritation on a five-point scale ranging from 0 (none) to 4 (most severe). Subjects recorded the severity score directly on the computerized questionnaire. Total symptom severity was obtained by adding scores of all four symptoms.
GSTM1 genotypes were determined using real-time polymerase chain reaction as previously described by Gilliland and colleagues (24) from DNA isolated from white blood cells using the QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA).
Sputum Collection and Analysis
Sputum samples were obtained and processed by the method described by Alexis and colleagues (25). Briefly, subjects underwent sputum induction with hypertonic saline. Acquired samples were subjected to plug selection and subsequent treatment with dithiothreitol. Cell viability and total cell counts were evaluated and differential cell counts examined (Hema-Stain3; Fisher Scientific). Acquired sputum samples considered acceptable for processing had a minimum of 75 mg of selected plug material, cell viability greater than 50%, and squamous epithelial cells less than 40%. All sample processing and slide preparations were performed on the same day of collection.
Ozone Generation and Monitoring
Ozone was generated by a silent electric discharge method (Model 502; Meckenheim, Bonn, Germany) and introduced into the chamber that was maintained at 22 ± 1.0°C and 40 ± 5% relative humidity. The concentration of ozone was continuously monitored using ultraviolet photometric analyzers (TECO Model 49; Thermo Scientific, Franklin, MA) that were periodically calibrated for ± 5% accuracy by NIST traceable ozone calibrator (TECO Model 49PS).
Data and Statistical Analyses
The lung function endpoints were expressed as percent changes from the preexposure (or baseline) values for each subject. Neutrophil content in the sputum samples was expressed as percent of total cell count (%PMN) and the measurements after each exposure were compared. Linear mixed-effects models with a subject-specific random intercept was used to test changes in response endpoints between clean air and ozone exposures at the group level to account for subject-level variability and repeated measures. The effect of GSTM1 and separately sex was examined using a two-factor mixed-effects model with repeated measures on a single factor, exposure and subject-level random effects. We report the magnitude and direction of the expected change along with its associated 95% equal two-tail confidence intervals. R statistical software (Version 2.10.1) was used for the analyses. α of 0.05 was used to determine statistical significance.
RESULTS
Exercise and Minute Ventilation
Means of six hourly measurements of Ve, Vt, breathing frequency, and heart rate during 6.6-hour exposure to CA and ozone are summarized in Table 2. Overall, there was no difference in both ventilation parameters and heart rates between CA and 0.06 ppm ozone exposure.
TABLE 2.
BREATHING PARAMETERS AND HEART RATE OF SUBJECTS DURING 6.6-HOUR EXPOSURE
| Ve (L/min) | Vt (L) | F (min-1) | Heart Rate (min-1) | |
|---|---|---|---|---|
| Clean air | 37.1 (0.2) | 1.38 (0.05) | 29.1 (0.9) | 128.4 (1.1) |
| Ozone | 36.5 (0.2) | 1.37 (0.05) | 29.0 (0.9) | 127.0 (1.1) |
Definition of abbreviations: F = breathing frequency.
Values are mean (± SEM). Note that the target value of Ve = 20 L/min/m2. BSA corresponds to unadjusted mean value of 36.5 L/min.
Exposure to 0.06 ppm Ozone Causes Decrements in Lung Function
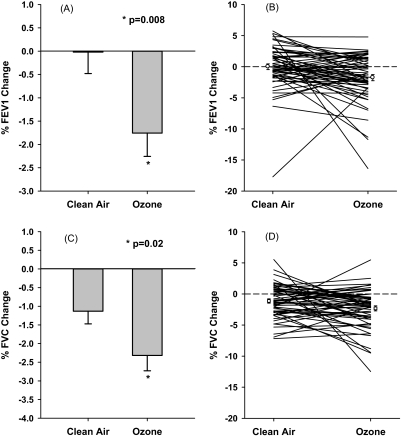
The primary hypothesis tested in this study was that exposure to 0.06 ppm ozone would decrease FEV1 and FVC after 6.6 hours. The results are summarized in Table 3. Exposure to ozone resulted in a 1.71 ± 0.50% (mean ± SEM) decrease in FEV1 compared with virtually no change (0.002 ± 0.46%) after exposure to CA (Figure 1A). Thus, relative to CA, exposure to 0.06 ppm ozone for 6.6 hours resulted in a 1.71 ± 0.64% decrement in FEV1 (P = 0.008). These decrements did not appear to be driven by a small subset of subjects (Figure 1B). Of the 59 individuals studied, only three subjects showed greater than 10% drop after ozone exposure. Similarly, FVC decreased by 2.32 ± 0.41% after ozone exposure versus 1.13 ± 0.34% after CA (Figure 1C). Ozone exposure thus caused a relative decrement of 1.19 ± 0.51% (P = 0.02). Again, individual response to ozone exposure was mostly within ± 5% change, as shown in Figure 1D. Changes in other lung function parameters (forced expiratory flow between 25% and 75% of FVC, maximum forced expiratory flow, and sRaw) were not significant.
TABLE 3.
LUNG FUNCTION RESPONSES TO 6.6-HOUR EXPOSURE TO CLEAN AIR AND 0.06 PPM OZONE IN HEALTHY YOUNG ADULTS
| All Subjects | Males | Females | GSTM1-p | GSTM1-n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 59) |
(n = 27) |
(n = 32) |
(n = 30) |
(n = 29) |
||||||
| %Δ | 95% CI | %Δ | 95% CI | %Δ | 95% CI | %Δ | 95% CI | %Δ | 95% CI | |
| FEV1 | ||||||||||
| CA | −0.002 (0.46) | −0.9, 0.9 | 0.59 (0.53) | −0.5, 1.6 | −0.51 (0.72) | −1.9, 0.9 | −0.08 (0.46) | −1.0, 0.8 | 0.078 (0.82) | −1.5, 1.7 |
| Ozone | −1.71 (0.50) | −2.7, −0.8 | −0.86 (0.62) | −2.1, 0.4 | −2.43 (0.74) | −4.0, −1.1 | −2.07 (0.71) | −3.5, −0.7 | −1.34 (0.69) | −2.8, −0.1 |
| Ozone-CA | −1.71 (0.64)* | −3.0, −0.5 | −1.45 (0.95) | −3.4, 0.5 | −1.93 (0.88)* | −3.8, −0.3 | −1.99 (0.90)* | −4.0, −0.2 | −1.42 (0.92) | −3.4, 0.3 |
| FVC | ||||||||||
| CA | −1.13 (0.34) | −1.8, −0.5 | −0.77 (0.33) | −1.4, −0.1 | −1.44 (0.56) | −2.5, −0.3 | −1.11 (0.49) | −2.1, −0.2 | −1.15 (0.48) | −2.1, −0.2 |
| Ozone | −2.32 (0.41) | −3.1, −1.5 | −1.26 (0.52) | −2.3, −0.2 | −3.22 (0.57) | −4.3, −2.1 | −2.41 (0.52) | −3.4, −1.4 | −2.23 (0.65) | −3.5, −1.0 |
| Ozone-CA | 1.19 (0.51)* | −2.2, −0.2 | −0.49 (0.74) | −2.0, 1.0 | −1.78 (0.68)* | −3.1, −0.4 | −1.30 (0.73) | −2.7, 0.1 | −1.07 (0.71) | −2.5, 0.4 |
Definition of abbreviations: CA = clean air; CI = confidence interval; GSTM1-n = glutathione S-transferase mu 1 null; GSTM1-p = glutathione S-transferase mu 1 positive.
%Δ indicates change relative to preexposure, mean (± SEM).
P < 0.05.
Figure 1.
Percent changes in FEV1 and FVC after 6.6-hour exposure to clean air and 0.06 ppm ozone. (A, B) Group means (± SEM) and individual changes, respectively, for FEV1. (C, D) Group means (± SEM) and individual changes, respectively, for FVC.
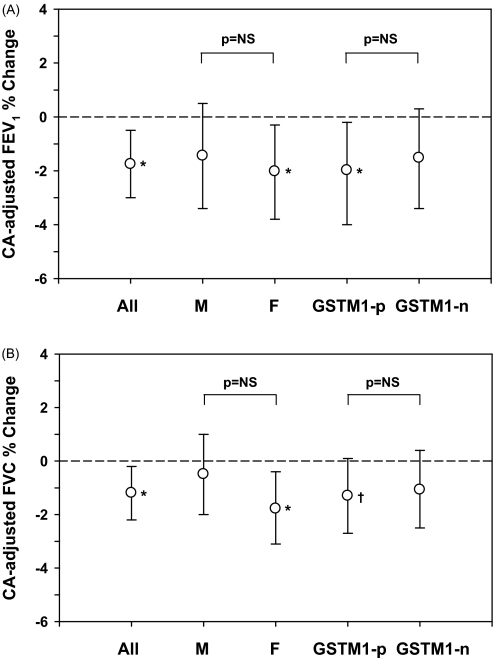
A second aim of the study was to determine the role of GSTM1 in determining responses to ozone. Although both genotypes had decrements in FEV1 after ozone exposure relative to air, changes were only statistically significant for GSTM1-positive subjects (Figure 2A). However, the difference in FEV1 response between GSTM1-null and -positive subjects was not statistically significant (P = 0.72). Similarly, females had a significant decrement in CA-adjusted FEV1 (1.93 ± 0.88%, P = 0.02), whereas males did not (1.45 ± 0.95%, P = 0.14), but the difference between sexes was not significant (P = 0.66). No differences between GSTM1-null versus -positive and males versus females were seen for FVC (Figure 2B).
Figure 2.
Clean air (CA) adjusted % changes (mean and 95% confidence interval) in (A) FEV1 and (B) FVC after ozone exposure for all subjects, and by sex and GSTM1 genotype. *P < 0.05; †0.05 < P < 0.1. F = females; GSTM1-n = glutathione S-transferase mu 1 null; GSTM1-p = glutathione S-transferase mu 1 positive; M = males; NS = not significant.
Exposure to 0.06 ppm Ozone Causes Pulmonary Inflammation
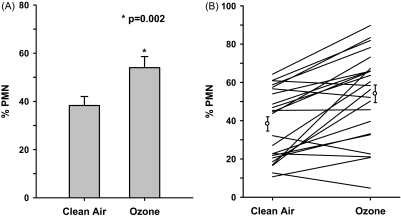
This study is the first to examine ozone concentrations below the current standard to cause pulmonary inflammation. The results are summarized in Table 4. Graphic illustration in Figure 3A shows that ozone exposure alters the airway milieu as evidenced by increases in %PMN in induced sputum samples. After air exposure, %PMN averaged 38.3 ± 3.7%. In contrast, ozone-exposed samples averaged 54.0 ± 4.6%. Thus, relative to clean air, ozone exposure resulted in a 15.7 ± 3.1% increase in %PMN for the whole group (P < 0.002). Of the 24 subjects studied, all but 5 subjects showed an ozone-induced increase in %PMNs and 10 showed greater than or equal to 20% increase (Figure 3B).
TABLE 4.
POLYMORPHONUCLEAR NEUTROPHIL RESPONSES TO 6.6-HOUR EXPOSURE TO CLEAN AIR AND 0.06 PPM OZONE IN HEALTHY YOUNG ADULTS
| All Subjects | Males | Females | GSTM1-p | GSTM1-n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 24) |
(n = 11) |
(n = 13) |
(n = 12) |
(n = 12) |
||||||
| Mean (± SEM) | 95% CI | Mean (± SEM) | 95% CI | Mean (± SEM) | 95% CI | Mean (± SEM) | 95% CI | Mean (± SEM) | 95% CI | |
| % Polymorphonuclear neutrophils | ||||||||||
| CA | 38.3 (3.7) | 31.1, 45.6 | 33.4 (5.3) | 23.0, 43.8 | 42.4 (5.1) | 32.4, 52.4 | 34.7 (5.6) | 23.7, 45.7 | 41.9 (4.8) | 32.5, 51.3 |
| Ozone | 54.0 (4.6) | 45.0, 63.0 | 57.6 (6.4) | 45.1, 70.1 | 50.9 (6.6) | 38.0, 63.8 | 46.1 (6.7) | 33.0, 59.2 | 61.9 (5.5) | 51.1, 72.7 |
| Ozone-CA | 15.7 (3.1)* | 9.6, 21.8 | 24.2 (4.3)* | 15.8, 32.6 | 8.5 (3.7)* | 0.79, 16.2 | 11.3 (4.5)* | 2.3, 20.3 | 20.0 (4.6)* | 11.0, 29.0 |
| Total cell count | ||||||||||
| CA | 5.05 × 106 (0.82) | 4.08 × 106 (0.98) | 5.86 × 106 (1.26) | 3.97 × 106 (0.90) | 6.13 × 106 (1.34) | |||||
| Ozone | 6.93 × 106 (1.52) | 9.42 × 106 (3.03) | 4.83 × 106 (0.96) | 5.58 × 106 (2.23) | 8.28 × 106 (2.10) | |||||
Definition of abbreviations: CA = clean air; CI = confidence interval; GSTM1-n = glutathione S-transferase mu 1 null; GSTM1-p = glutathione S-transferase mu 1 positive; PMN = polymorphonuclear neutrophils.
%PMN indicates polymorphonuclear neutrophils as % of total cell counts. SEM of total cell count is for the base number of 106.
P < 0.05.
Figure 3.
% Polymorphonuclear neutrophils (PMN) changes in sputum samples after 6.6-hour exposure to clean air (CA) and 0.06 ppm ozone for (A) the group mean, and (B) each individual. Error bars represent standard error. %PMN is defined by neutrophil number as % of total cell counts in the sample. F = females; GSTM1-n = glutathione S-transferase mu 1 null; GSTM1-p = glutathione S-transferase mu 1 positive; M = males; NS = not significant.
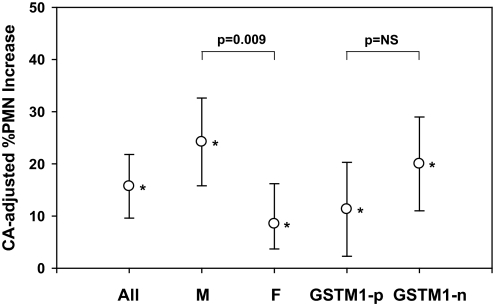
Figure 4 shows a significant increase in ozone-induced %PMN for both GSTM1-null (20.0%; 95% CI, 11.0–29.0; P = 0.001) and GSTM1-positive subjects (11.3%; 95% CI, 2.3–20.3; P = 0.02). Those carrying the null allele had a stronger response (P = 0.001) than those carrying the positive allele (P = 0.02); however, the estimate of the modifying effect of GSTM1 did not reach significance (P = 0.17; also see Table 4). Both males (24.2%; 95% CI, 15.8–32.6; P = 0.001) and females (8.5%; 95% CI, 0.79–16.20; P = 0.03) had statistically significant increases in ozone-induced %PMNs. The modifying effect of sex was significant (P = 0.009). The changes in %PMN were not accompanied by changes in total cell numbers for the whole group or any subgroup after ozone versus air. Total cell counts in sputum samples were 5.05 (± 0.82) × 106 after CA and 6.93 (± 1.52) × 106 after ozone (P = not significant [NS] vs. CA) for the whole group.
Figure 4.
Clean air (CA) adjusted % changes in polymorphonuclear neutrophils (PMN) (mean and 95% confidence interval) after ozone exposure for all subjects, and by sex and GSTM1 genotype. *P < 0.05. F = females; GSTM1-n = glutathione S-transferase mu 1 null; GSTM1-p = glutathione S-transferase mu 1 positive; M = males; NS = not significant.
Symptom Questionnaire
Of 56 subjects who had no symptoms at baseline, 20 subjects reported symptoms after either CA or 0.06 ppm ozone exposure. The most commonly reported symptom was throat irritation followed by shortness of breath, pain on deep inspiration, and cough. The mean (± SEM) total symptom score was 0.43 ± 0.11 for CA and 0.41 ± 0.11 for ozone (P = NS versus CA). For genotype subgroups, total symptom score was 0.40 ± 0.16 for CA and 0.47 ± 0.17 for ozone in GSTM1-positive subjects and 0.46 ± 0.16 for CA and 0.35 ± 0.13 for ozone in GSTM1-null subjects (P = NS versus CA for both groups). The score and nature of the symptoms were similar between CA and ozone exposures.
DISCUSSION
In 2008 the EPA revised National Ambient Air Quality Standard for ground level ozone down to 0.075 ppm (3-yr average of the fourth highest daily maximum 8-h average) (26). This was based primarily on controlled human chamber studies of FEV1 changes postexposure after 6.6 hours. Inflammation of the airway, as well as toxicology and epidemiology studies, were used as supportive evidence. In this study, we demonstrate, using the same exposure approach, decrements in FEV1 and FVC at a concentration (0.06 ppm) below the current standard. Furthermore, we demonstrate increased neutrophil airways inflammation at this low ozone concentration.
To date, two previous studies have investigated lung function at 0.06 ppm ozone using a 6.6-hour protocol; neither found statistically significant effects (7, 15) even though their observed effect size was greater than in our study. For example, they reported clean air–adjusted FEV1 after 0.06 ppm ozone decrements of 3.52 and 2.86%, whereas we observed a smaller drop of 1.71%, yet our results were highly statistically significance (P = 0.008). Similarly, our reported change in FVC of 1.18% was statistically significant (P = 0.02), although in the range of the 3.16 and 0.45% reported previously (7, 15). The key difference between the previous and the current study is that although their studies were designed to compare multiple concentrations at multiple time points, ours was specifically designed to limit the need for multiple comparisons.
To accomplish this, we used several strategies to increase the statistical power and to minimize experimental variability. First, our analysis was focused on only one ozone concentration and a comparison to clean air. Second, we enrolled nearly twice as many subjects (n = 59) as previous studies, as the study was powered to examine the differences between two GSTM1 polymorphisms. Third, our analysis was determined a priori to focus only on changes observed immediately postexposure for lung function. Similarly, analysis of airway inflammation was limited to %PMN 16 to 18 hours after exposure. Fourth, we used only one dedicated pulmonary technician and spirometer for primary lung function measurements. Last, our studies were performed exclusively in the winter season, during which ambient ozone level was lower than 0.06 ppm (see Figure E1 in the online supplement), and thus potential influence of prior exposure to ambient ozone was minimized.
Our results may have significant public health implications. Although most subjects (> 60%) showed less than 10% decrease in lung function after 0.06 ppm ozone, 3 of 59 subjects in the present and 2 of 30 subjects in the Adams study (7) showed a lung function decrement greater than 10%. This suggests that 0.06 ppm ozone may cause lung function decrements in the majority of young individuals with some 6% (e.g., 5 of 89 subjects) of them having a greater response. In addition, this is the first study to examine and observe airway inflammatory effects for ozone at concentration levels below 0.08 ppm. Chronic airway inflammation may cause airway damage and thereby bronchoconstriction and bronchial hyperresponsiveness. An increase in neutrophilic inflammation has been shown to be strongly associated with exacerbation of airway disease in people with asthma. Thus, ozone is uniquely associated with worsening of asthma and increased hospitalization in children with asthma (13). Although the current study is limited to healthy subjects, the health consequences may be more severe in individuals with preexisting diseases, particularly in asthma.
Effects of sex on response to ozone exposure have been reported variably. Some studies found a greater response in FEV1 in female than in male subjects, whereas others found a comparable response between them, mostly after a short-term exposure to high-concentration ozone (27, 28). For a prolonged exposure to low-concentration ozone, most studies report combined results of both males and females with no specific analysis for sex effects (6, 7, 15). The studies, however, appear to have assumed or found no sex effects in pulmonary function response. In our study, FEV1 and FVC decrement was not significant in males but was significant in females; however, we found no significant difference between males and females. In contrast, %PMN was increased significantly in both sexes, with males showing a greater response than females (P = 0.009). Thus, it appears that ozone sensitivity may differ between sexes depending on endpoints.
A two-factor mixed-effects model with repeated measures was used to test if there was a difference in FEV1 responses between GSTM1-positive and GSTM1-null subjects. We did not find such an association in either FEV1 or FVC. This was consistent with earlier studies (20, 29) that have shown no independent role of GSTM1-null genotype on lung function decrement. The role of GSTM1-null, however, may become evident when it presents with other genotypes (NQO1 and GSTP1) (19, 29). Regarding airway neutrophilia, although the difference was not significant, GSTM1-null subjects appeared to have a greater neutrophilic inflammatory response than GSTM1-positive subjects. A sample size estimate, however, revealed that the nonsignificant increase would likely become significant with a larger cohort of approximately 47 subjects, which would be consistent with our earlier report on response of GSTM1-null genotype to 0.4 ppm ozone (20). This seemingly contradicting trend versus lung function response may be due to differences in the putative modes of action of ozone. Although changes in lung function are believed to occur via activation of a subset of airway C-fibers (30), inflammation is believed to originate from activation of nuclear factor-κB induced by reactive oxygen species generation (31). Thus, corticosteroids can blunt ozone-induced neutrophilia but not lung function responses (32). GSTM1 is believed to act by reducing oxidative stress, including detoxification of byproducts generated by inflammation. In line with previous studies (33), we found no relationship between lung function and inflammation.
In summary, our study shows ozone effects on two independent markers of airway health at a level as low as 0.06 ppm in healthy young adults. We did not find a significant role of GSTM1 genotype alone on the ozone-induced airway effects, but there may be individuals or subpopulations with enhanced sensitivity (such as in asthma) at these levels. We therefore conclude that exposure to ozone levels below the current standard can cause changes in lung function and initiate an airway inflammatory response in a young adult population and pose a health risk, particularly to susceptible populations.
Supplementary Material
Acknowledgments
The authors thank the nurses and the technical staff of the U.S. Environmental Protection agency, the University of North Carolina–Chapel Hill, and TRC Environmental Corp for their invaluable assistance, and Brent Coull of Harvard University for reviewing statistical analyses.
Supported by U.S. Environmental Protection Agency Internal Fund, U.S. Environmental Protection Agency cooperative agreement CR83346301, and National Institutes of Health grants RC1ES018417 and R01ES012706 (D.B.P.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201011-1813OC on January 7, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Disclaimer: Although the research described in this article has been supported by the U.S. Environmental Protection Agency, it has not been subjected to Agency review and therefore does not necessarily reflect the views of the Agency, and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- 1.Silverman F, Folinsbee LJ, Barnard JW, Shephard RJ. Pulmonary function changes in ozone: interaction of concentration and ventilation. J Appl Physiol 1976;41:859–864. [DOI] [PubMed] [Google Scholar]
- 2.Hazucha MJ. Relationship between ozone exposure and pulmonary function changes. J Appl Physiol 1987;62:1671–1680. [DOI] [PubMed] [Google Scholar]
- 3.Folinsbee LJ, McDonnell WF, Horstman DH. Pulmonary function and symptom responses after 6.6-hour exposure to 0.12 ppm ozone with moderate exercise. J Air Pollut Control Assoc 1988;38:28–35. [DOI] [PubMed] [Google Scholar]
- 4.Horstman DH, Folinsbee LJ, Ives PJ, Abdul-Salaam S, McDonnell WF. Ozone concentration and pulmonary response relationships for 6.6-hour exposure with five hours of moderate exercise to 0.08, 0.10, and 0.12 ppm. Am Rev Respir Dis 1990;142:1158–1163. [DOI] [PubMed] [Google Scholar]
- 5.McDonnell WF, Kehrl HR, Abdul-Salaam S, Ives PJ, Folinsbee LJ, Devlin RB, O'Neil JJ, Horstman DH. Respiratory response of humans exposed to low levels of ozone for 6.6 hours. Arch Environ Health 1991;46:145–150. [DOI] [PubMed] [Google Scholar]
- 6.Adams WC. Comparison of chamber and face mask 6.6-hour exposure to 0.08 ppm ozone via square-wave and triangular profiles on pulmonary responses. Inhal Toxicol 2003;15:265–281. [DOI] [PubMed] [Google Scholar]
- 7.Adams WC. Comparison of chamber 6.6-h exposure to 0.04–0.08 ppm ozone via square-wave and triangular profiles on pulmonary responses. Inhal Toxicol 2006;18:127–136. [DOI] [PubMed] [Google Scholar]
- 8.Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo WJ, Becker S, House DE, McDonnell WF, et al. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis 1989;139:407–415. [DOI] [PubMed] [Google Scholar]
- 9.Devlin RB, McDonnell WF, Mann R, Becker S, House DE, Koren HS. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am Rev Respir Dis 1991;4:72–81. [DOI] [PubMed] [Google Scholar]
- 10.Schelegle ES, Siefkin AD, McDonald RJ. Time course of ozone-induced neutrophilia in normal humans. Am Rev Respir Dis 1991;142:1353–1358. [DOI] [PubMed] [Google Scholar]
- 11.Devlin RB, McDonnell WF, Becker S, Madden MC, McGee MP, Perez R, Hatch G, House DE, Koren HS. Time-dependent changes of inflammatory mediators in the lungs of humans exposed to 0.4 ppm ozone for 2 hr: a comparison of mediators found in bronchoalveolar lavage fluid 1 and 18 hr after exposure. Toxicol Appl Pharmacol 1996;138:176–185. [DOI] [PubMed] [Google Scholar]
- 12.Alexis NE, Lay JC, Hazucha M, Harris B, Hernandez ML, Bromberg PA, Kehrl H, Diaz- Sanchez D, Kim CS, Devlin RB, et al. Low-level ozone exposure induces airways inflammation and modifies cell surface phenotypes in healthy humans. Inhal Toxicol 2010;22:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnet JA, Walter LA, Sarnet SE, Mulholland JA, Tolbert PE. Short-term association between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med 2010;182:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JB, Bateson TF, McDonnell WF. Effects of exposure to 0.06 ppm ozone on FEV1 in humans: a secondary analysis of existing data. Environ Health Perspect 2008;16:1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schelegle ES, Morales CA, Walby WF, Marion S, Allen RP. 6.6-hour inhalation of ozone concentration from 60 to 87 parts per million in healthy humans. Am J Respir Crit Care Med 2009;180:265–272. [DOI] [PubMed] [Google Scholar]
- 16.McDonnel WF, Horstman DH, Abdul-Salaam S, House DE. Reproducibility of individual responses to ozone exposure. Am Rev Respir Dis 1985;131:36–40. [DOI] [PubMed] [Google Scholar]
- 17.McDonnel WF, Muller KE, Bromberg PA, Shy CM. Predictors of individual differences in acute response to ozone exposure. Am Rev Respir Dis 1993;147:818–825. [DOI] [PubMed] [Google Scholar]
- 18.Romieu I, Sienra-Monge JJ, Ramierez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, Hernandez-Avila M, London SJ. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Gilliland FD, Gauderman WJ, Vora H, Rappaport E, Dubeau L. Effects of glutathione-S- transferase M1, T1, and P1 on childhood lung function growth. Am J Respir Crit Care Med 2002;166:710–716. [DOI] [PubMed] [Google Scholar]
- 20.Alexis NE, Zhou H, Lay JC, Harris B, Hernandez ML, Lu TS, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, et al. The glutathione-S-transferase Mu 1 null genotype modulates ozone-induced airway inflammation in human subjects. J Allergy Clin Immunol 2009;124:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang IA, Fong KM, Zimmerman PV, Holgate ST, Holloway JW. Genetic susceptibility to the respiratory effects of air pollution. Thorax 2008;63:555–563. [DOI] [PubMed] [Google Scholar]
- 22.Kim CS, Kehrl H, Hazucha MJ, Rappold AG, Brown JB, Devlin RB, Diaz-Sanchez D. Pulmonary response in healthy young adults exposed to low concentration of ozone for 6.6 hours with mild exercise. Am J Respir Crit Care Med 2010;181:A1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco HV, Burgos F, Casaburi R, Coates A, Carpo R, Enright P, van der Grinten CPM, Gustafsson P, et al. Standardization of spirometry. In: Brusco V, Carpo R, Viegi G, editors. ATS/ERS task force: standardization of lung function testing. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, Gauderman WJ, Peters JM. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2002;166:457–463. [DOI] [PubMed] [Google Scholar]
- 25.Alexis NE, Hu SH, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using radiolabelled aerosol bolus delivery technique. Am J Respir Crit Care Med 2001;164:1964–1970. [DOI] [PubMed] [Google Scholar]
- 26.US Environmental Protection Agency. National Ambient Air Quality Standard for ozone: Final Rule 40 CFR Parts 50 and 58. Fed Regist 2008;73:16436–16514. [Google Scholar]
- 27.Hazucha MJ, Folinsbee LJ, Bromberg PA. Distribution and reproducibility of spirometric response to ozone by gender and age. J Appl Physiol 2003;95:1917–1925. [DOI] [PubMed] [Google Scholar]
- 28.Lauritzen SK, Adams WC. Ozone inhalation effects consequent to continuous exercise in females: comparison with males. J Appl Physiol 1985;59:1601–1606. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Arjomandi N, Tager IB, Holland N, Balmes JR. Effects of antioxidant enzyme polymorphisms on ozone-induced lung function changes. Eur Respir J 2007;30:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol 2010;588:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes PJ, Karin M. Nuclear factor-κB – a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–1071. [DOI] [PubMed] [Google Scholar]
- 32.Vagaggini B, Taccola M, Conti I, Carnevali S, Cianchetti S, Batoli ML, Bacci E, Dente FL, Franco A, Giannini D, et al. Budesonide reduces neutrophilic but not functional airway response to ozone in mild asthmatics. Am J Respir Crit Care Med 2001;164:2172–2176. [DOI] [PubMed] [Google Scholar]
- 33.Blomberg A, Mudway IS, Nordenhail C, Hedenström H, Kelly FJ, Frew AJ, Holgate ST, Sandstörm T. Ozone-induced lung function decrements do not correlate with early airway inflammation or antioxidant responses. Eur Respir J 1999;13:1418–1428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.