In this issue of the Journal, Grasemann and coworkers (pp. 1363–1368) report that levels of asymmetric dimethyl arginine (ADMA) are increased in cystic fibrosis (CF) airways (1). ADMA inhibits cellular arginine uptake and nitric oxide (NO) synthase (NOS) activity. Levels of ADMA decrease during antibiotic therapy in association with improved lung function. This observation may prove to have therapeutic relevance. However, it is important to note that increased ADMA in CF airways may be both beneficial (through inhibition of NO production) and harmful (through inhibition of S-nitrosothiol production).
Nitric oxide in the concentrations measured in exhaled air (parts per billion) is generally irrelevant to normal lung physiology (2). However, NO can be relevant to lung pathology. It interacts with oxygen, superoxide, and other molecules to injure airway epithelium. Products of these reactions, such as nitrous acid and peroxynitrous acid, are potent cytotoxins downstream of inducible NOS activity (3).
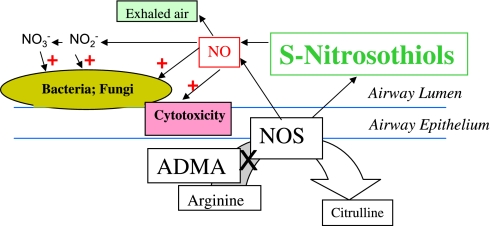
Nitric oxide can also affect bacteria in the CF airway (4, 5). Indeed, the CF airway is a complex ecosystem in which nitrogen oxides, oxygen, protons, and more complex chemical species are exchanged between prokaryotic and eukaryotic cells (Figure 1; 4–7). For example, levels of NO are lower than normal in the CF airway, in part because NO is consumed by Pseudomonas, Aspergillus, and other denitrifying organisms (4). NO can serve both as an electron acceptor (dissimilatory denitrification, ultimately forming ammonia) and as a precursor for amino acid formation (assimilatory denitrification). Airway NO levels rise and NH3 levels fall with antibiotic therapy in CF (4). Levels of oxidized NO in the form of nitrate are high in the CF airway (6); and nitrate, like NO, can feed denitrifying organisms. Together, the effects of NOS products to cause cytotoxicity and promote prokaryotic growth suggest that the high levels of ADMA should be advantageous for patients with CF, and that a decrease in ADMA levels with antibiotic therapy might be disadvantageous.
Figure 1.
Cystic fibrosis airway nitrogen redox ecology. Nitric oxide (NO) synthase (NOS) isoforms in the cystic fibrosis (CF) lung produce both NO and S-nitrosothiols. NO is oxidized to nitrite (NO2−) and nitrate (NO3−). In this issue of the Journal, Grasemann and coworkers show that asymmetric dimethylarginine (ADMA), which blocks arginine uptake and NOS activity, is increased in CF airways (1). High levels of ADMA could benefit the CF airways by inhibiting adverse effects of NOS (red), including its cytotoxicity and its promotion of the growth of denitrifying organisms. However, high levels of ADMA could also adversely affect the CF airways by inhibiting the beneficial formation of S-nitrosothiols (green). S-nitrosothiols are endogenous bronchodilators that inhibit microbial growth, augment ciliary beat frequency, inhibit amiloride-sensitive sodium transport, and increase expression and maturation of the delF 508 CF transmembrane regulatory protein (2, 8–13).
However, NOS also produces S-nitrosothiols in concentrations two log orders higher than NO (8–10). S-nitrosothiols are antimicrobial. They augment ciliary beat frequency (2). They relax human airway smooth muscle and prevent tachyphylaxis to β2-adrenergic agonists (2, 10, 11). They inhibit amiloride-sensitive sodium transport (12). They augment expression, maturation and function of delF508 CF transmembrane conductance regulator through inhibition of the expression of Hsp70/Hsp90-organizing protein (13). S-nitrosothiol levels are decreased in the CF airway (14), consistent with high ADMA levels (1); indeed, S-nitrosothiol replacement therapy improves oxygenation in CF (9). Prevention of S-nitrosothiol formation is therefore likely to be an important disadvantage of having high levels of ADMA in the CF airway (1).
We do not know why ADMA levels are high in CF. One possible mechanism is based on what we know about the CF airway ecosystem. Anti-pseudomonal therapy decreases ADMA levels in patients with CF (1). Biochemically, ADMA levels are decreased by dimethylarginine dimethylaminohydrolases (DDAHs). DDAHs are inhibited by S-nitrosylation, or physiological protein modification by NO (15). S-nitrosylation is driven both by NOS activation and—in relatively acidic conditions in the CF airway ecosystem (5)—by nitrite protonation; at baseline, this should increase ADMA levels. Antimicrobial therapy can decrease bacterial conversion of abundant airway nitrate to nitrite. Nitrite depletion would decrease DDAH S-nitrosylation, thereby increasing ADMA breakdown during the course of therapy. However, this proposed mechanism is speculative. Much work remains to be done.
The CF airway is dark, damp, and largely anaerobic (7). It is also surprisingly well-suited to denitrifying prokaryotic species, given that NOS expression is decreased and ADMA levels are increased. To understand why—and to learn how to use nitrogen oxide redox ecology to therapeutic advantage—we need to get beyond simply modeling NO radical diffusion (2, 3). The Cystic Fibrosis Foundation has shown the benefit of promoting interactions among scientists across disciplines. The work of Grasemann and coworkers suggests that there might be much to gain by organizing a small working group to bring together nitrogen balance ecologists, biochemists, CF airway epithelial biologists, and mathematicians experienced in biochemical modeling. This group could model prokaryotic and eukaryotic outcomes of specific CF interventions to identify strategies to optimize therapeutic development in CF. The elegant insights of Grasemann and coworkers serve to alert us that both the benefits and toxicities of airway nitrogen oxides need to be better understood to improve clinical outcomes in CF.
Contributions: Drafting of the manuscript and intellectual design: N.V.M., B.G.
Supported by National Institutes of Health grant RO1 HL59337.
Author Disclosure: N.V.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.G. has received consultancy fees and expert testimony fees from Aperion, and holds a patent with N30 Pharma.
References
- 1.Grasemann H, Al-Saleh S, Scott JA, Shehnaz D, Mehl A, Amin R, Rafii M, Pencharz P, Belik J, Ratjen F. Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with cystic fibrosis. Am J Respir Crit Care Med 2011;183:1363–1368. [DOI] [PubMed] [Google Scholar]
- 2.Gaston B, Singel D, Doctor A, Stamler JS. S-Nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 2006;173:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricciardolo F, Sterk P, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev 2004;84:731–765. [DOI] [PubMed] [Google Scholar]
- 4.Gaston B, Ratjen F, Vaughan JW, Malhotra NR, Canady RG, Snyder AH, Hunt JF, Gaertig S, Goldberg JB. Nitrogen redox balance in the cystic fibrosis airway: effects of anti-pseudomonal therapy. Am J Respir Crit Care Med 2002;165:387–390. [DOI] [PubMed] [Google Scholar]
- 5.Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, Hennigan RF, Hwang SH, Buettner G, Schurr MJ, et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite under conditions characteristic of the cystic fibrosis airway. J Clin Invest 2006;116:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasemann H, Ioannidis I, Tomkiewicz RP, de Groot H, Rubin BK, Ratjen F. Nitric oxide metabolites in cystic fibrosis lung disease. Arch Dis Child 1998;78:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 2002;109:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lui L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001;410:490–494. [DOI] [PubMed] [Google Scholar]
- 9.Snyder A, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med 2002;165:922–926. [DOI] [PubMed] [Google Scholar]
- 10.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Heys JR, Rockman HA, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 2007;129:511–522. [DOI] [PubMed] [Google Scholar]
- 11.Gaston B, Drazen JM, Jansen A, Sugarbaker DA, Loscalzo J, Stamler JS. Relaxation of human bronchial smooth muscle by S-nitrosothiols in vitro. J Pharmacol Exp Ther 1994;268:978–984. [PubMed] [Google Scholar]
- 12.Jain L, Chen XJ, Brown LA, Eaton DC. Nitric oxide inhibits lung sodium transport through a cGMP-mediated inhibition of epithelial cation channels. Am J Physiol 1998;274:475–484. [DOI] [PubMed] [Google Scholar]
- 13.Marozkina NV, Borowitz M, Yemen S, Liu L, Sun F, Islam R, Erdmann-Gilmore P, Townsend RR, Lichti CF, Mantri S, et al. S-Nitrosoglutathione targets Hsp 70/Hsp 90 organizing protein as a corrector therapy for cystic fibrosis. Proc Natl Acad Sci USA 2010;107:11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J Pediatr 1999;135:770–772. [DOI] [PubMed] [Google Scholar]
- 15.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci USA 2002;99:13527–13532. [DOI] [PMC free article] [PubMed] [Google Scholar]