Abstract
Rationale: Vascular endothelial growth factor (VEGF) regulates vascular, inflammatory, remodeling, and cell death responses. It plays a critical role in normal pulmonary physiology, and VEGF excess and deficiency have been implicated in the pathogenesis of asthma and chronic obstructive pulmonary disease, respectively. Although viruses are an important cause of chronic obstructive pulmonary disease exacerbations and innate responses play an important role in these exacerbations, the effects of antiviral responses on VEGF homeostasis have not been evaluated.
Objectives: We hypothesized that antiviral innate immunity regulates VEGF tissue responses.
Methods: We compared the effects of transgenic VEGF165 in mice treated with viral pathogen–associated molecular pattern polyinosinic:polycytidylic acid [poly(I:C)], mice treated with live virus, and control mice.
Measurements and Main Results: Transgenic VEGF stimulated angiogenesis, edema, inflammation, and mucin accumulation. Each of these was abrogated by poly(I:C). These inhibitory effects were dose dependent, noted when poly(I:C) was administered before and after transgene activation, and mediated by a Toll-like receptor-3–independent and RIG-like helicase (RLH)– and type I IFN receptor–dependent pathway. VEGF stimulated the expression of VEGF receptor-1 and poly(I:C) inhibited this stimulation. Poly(I:C) also inhibited the ability of VEGF to activate extracellular signal–regulated kinase-1, Akt, focal adhesion kinase, and endothelial nitric oxide synthase, and aeroallergen-induced adaptive helper T-cell type 2 inflammation. Influenza and respiratory syncytial virus also inhibited VEGF-induced angiogenesis.
Conclusions: These studies demonstrate that poly(I:C) and respiratory viruses inhibit VEGF-induced tissue responses and adaptive helper T-cell type 2 inflammation and highlight the importance of a RLH- and type I IFN receptor–dependent pathway(s) in these regulatory events. They define a novel link between VEGF and antiviral and RLH innate immune responses and a novel pathway that regulates pulmonary VEGF activity.
Keywords: RIG-like helicase, mitochondrial antiviral signaling molecule, influenza virus, chronic obstructive pulmonary disease
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Vascular endothelial growth factor (VEGF) plays a critical role in the pathogenesis of asthma and chronic obstructive pulmonary disease (COPD). Although viruses are important causes of COPD exacerbations and innate immune responses, the effects of antiviral responses on VEGF homeostasis have not been evaluated.
What This Study Adds to the Field
Our studies demonstrate that the retinoic acid–inducible gene (RIG)–like helicase innate immune pathway inhibits the effector of VEGF and defines the mechanism of this regulation.
Vascular endothelial growth factor (VEGF) is a critical regulator of angiogenesis, vascular permeability, and vascular remodeling (1–6). It also induces inflammation and tissue remodeling (5), contributes to the pathogenesis of wound healing (7, 8), and has a remarkable ability to confer endothelial cell and epithelial cell cytoprotection (9–11). This may be clearly seen in the lung, where studies from our laboratory and others have demonstrated that VEGF overexpression induces tracheal angiogenesis, tissue edema, parenchymal inflammation, dendritic cell activation, airway fibrosis, mucus metaplasia, enhanced helper T-cell type 2 (Th2)- and IL-13–induced tissue responses and cytoprotection in the setting of oxidant injury (5, 12). In accordance with these varied responses, normal lung development, baseline lung function, and the ability of the lung to respond to injury appear to be critically dependent on the presence of local functional VEGF (12–16). VEGF excess has been implicated in the pathogenesis of a variety of disorders including pulmonary edema and asthma (5, 16, 17). In keeping with the ability of VEGF to serve as an endothelial cell survival factor that plays a critical role in the maintenance of lung structure, VEGF deficiency augments oxidant injury and tissue destruction and has been implicated in the pathogenesis of pulmonary emphysema and other disorders (reviewed in Reference 16). Surprisingly, the processes that regulate VEGF effector responses in normal and diseased lungs are not well understood.
Chronic obstructive pulmonary disease (COPD) is a composite term that is used for patients with emphysema and chronic bronchitis. It is the fourth leading cause of death in the world and much of its morbidity and mortality can be attributed to acute disease exacerbations (18–21). Studies have demonstrated that a variety of viruses including influenza virus and respiratory syncytial virus (RSV) are important causes of COPD exacerbations (20, 22–26) and that virus-induced COPD exacerbations are more severe, last longer, and are associated with heightened airway and systemic inflammatory responses compared with exacerbations due to other causes (21, 27–29). It has been demonstrated that innate immune responses play an important role in the pathogenesis of these exacerbations (30, 31). However, the mechanisms that mediate these responses have not been adequately defined. In addition, although interventions that decrease lung VEGF levels and abrogate VEGF signaling have been shown to induce emphysema in animal modeling systems (16, 32–34), and cohorts of patients with COPD with decreased levels of VEGF (16, 33, 35–37) have been described, the ability of antiviral responses to regulate VEGF-induced tissue responses has not been defined.
We hypothesized that antiviral innate immunity regulates VEGF-induced tissue responses. To test this hypothesis, we compared the effects of the viral pathogen-associated molecular pattern (PAMP) stimulator polyinosinic:polycytidylic acid [poly(I:C)] and respiratory viruses on mice transgenic (Tg+) for VEGF, with the transgene under the control of the Clara cell 10-kD protein (CC10) promoter. These studies demonstrate that poly(I:C) is a potent inhibitor of VEGF-induced inflammation, permeability, angiogenesis, mucus metaplasia, and adoptive Th2 inflammation. RSV and influenza virus inhibited VEGF-induced angiogenesis in a similar manner. These studies also demonstrate that the inhibitory effects of poly(I:C) are mediated by a Toll-like receptor-3 (TLR3)–independent and retinoic acid–inducible gene (RIG)–like helicase (RLH) and type I IFN receptor–dependent pathway(s) that regulates VEGF receptor type 1 (VEGFR1) expression and VEGF activation of focal adhesion kinase (FAK), mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3 kinase), and endothelial nitric oxide synthase (eNOS).
METHODS
Wild-type and Genetically Modified Mice
Wild-type and CC10-rtTA-VEGF mice were used in these studies. The latter use the CC10 promoter and two transgenic constructs to target VEGF to the lung in an externally regulatable fashion. The human (h) VEGF165 transgene in these mice was activated via the administration of doxycycline (dox) as previously described by our laboratories (5). In wild-type mice receiving normal or dox water and Tg+ mice receiving normal water, hVEGF levels in bronchoalveolar lavage (BAL) fluids were less than or equal to 10 pg/ml. Increased levels of BAL VEGF were noted within 24 hours and steady state levels between 0.5 and 1.2 ng/ml were seen after 1 week of dox administration (see Figure E1 in the online supplement). TLR3−/− mice were a gift from R. A. Flavell (Yale University, New Haven, CT), mitochondrial antiviral signaling protein (MAVS) null mice were a gift from Z. J. Chen (University of Texas Southwestern Medical Center, Dallas, TX) (38), double-stranded RNA–dependent protein kinase null mutant mice (PKR−/−) were a gift from B. R. G. Williams (Cleveland Clinic Foundation, Cleveland, OH) (39), and IFN α receptor 1 (IFNAR1) null mice were obtained from R. Enelow (Dartmouth Medical School, Hanover, NH). These mice were bred with the VEGF Tg+ mice to generate transgenic mice with wild-type (+/+) or null (−/−) TLR3, MAVS, PKR, and IFNAR1 loci. Thus, in each given experiment we compared the effects of our intervention in transgene negative (Tg−) and transgenic (Tg+) mice with wild-type and null TLR3, MAVS, PKR, or IFNAR1 loci.
Administration of Poly(I:C)
To define the effects of poly(I:C) a number of protocols were employed. In most experiments a pretreatment−2-week protocol was employed. In these experiments, wild-type and Tg+ mice were treated with poly(I:C) (30 μg in 50 μl unless otherwise indicated; Amersham Biosciences, Piscataway, NJ) or vehicle control via nasal aspiration and transgene activation was accomplished via the administration of dox (0.5 mg/ml) in the animal's drinking water 24 hours later. Poly(I:C) was administered every other day and dox was administered continuously for up to 2 weeks. The doses of poly(I:C) (≤30 μg) that were employed were chosen after preliminary experiments demonstrating that they did not alter CC10 promoter–driven production of transgenic hVEGF (Figure E1). In selected experiments a pretreatment protocol and shorter treatment interval were employed. In these experiments, wild-type and Tg+ mice were treated with poly(I:C) (dose per mouse, 30 μg in 50 μl) or vehicle control via nasal aspiration and transgene activation was accomplished with dox 24 hours later. The animals were then randomized not to receive or to receive one additional dose of poly(I:C) 24 hours later and dox was administered continuously during this 2-week interval. Last, in selected experiments post–transgene activation protocols were employed. In these experiments dox was administered for 2 days or 2 weeks. The poly(I:C) was then administered via intranasal aspiration every other day for 2 weeks as noted previously. In selected pretreatment and post–transgene activation experiments unmethylated CpG (CpG-B) (Keck Center, Yale University) was also employed at similar doses. At the end of these intervals, assessments of angiogenesis (anti-CD31 immunohistochemistry), vascular permeability (Evans blue dye extravasation, wet-to-dry lung weight measurements, BAL protein concentration), and BAL and tissue inflammatory cell infiltration and mucus metaplasia (periodic acid–Schiff staining, mucin 5AC [MUC5AC] immunoblotting) were undertaken (see below). In all cases we compared Tg− and Tg+ mice receiving normal water and Tg− and Tg+ mice receiving dox water.
Characterization of the Effects of Influenza Virus and RSV
Tg− and Tg+ mice were lightly anesthetized and 5.0 × 103.375 TCID50 (median tissue culture infective dose) of A/PR/8/34 influenza virus (equivalent to 0.05 LD50 [median lethal dose] in C57BL/6 mice) or 106 plaque-forming units of RSV (A2 strain) was administered via nasal aspiration in 50 μl of serum-free medium to each mouse, using techniques that have been previously described (40). The mice were killed 2 weeks postinfection and assessments of angiogenesis (anti-CD31 immunohistochemistry) were undertaken as described below.
Anti-CD31 Immunohistochemistry and Morphometric Analysis
Tracheal whole mounts were stained, using immunohistochemical procedures. Endothelial cells were identified with rat monoclonal antibodies to CD31 (BD Pharmingen, San Diego, CA). After several rinses with phosphate-buffered saline, specimens were incubated for 6 hours at room temperature with fluorescent (Cy3) donkey anti-rat secondary antibodies (Jackson ImmunoResearch, West Grove, PA). The percent area covered by vessels (vessel area density) was analyzed with ImageJ software (http://rsb.info.nih.gov/ij). At least four tracheal whole mounts were stained for each antibody and treatment group. Ten regions of 1.4 μm2 each (total area, 14 μm2) were measured in each trachea at a final magnification of ×184, and the average percentage area of mucosa occupied by vessels was calculated.
Evans Blue Dye Extravasation
Evans blue dye (Sigma-Aldrich Inc., St. Louis, MO) in 0.9% saline at a concentration of 5 mg/ml was used to assess plasma leakage as previously described (5). The results are expressed as the ratio of Evans blue dye absorbance at 620 nm of paired lung homogenates and serum.
Calculation of Whole Lung Wet-to-Dry Ratios
Lungs were excised en bloc and extrapulmonary tissues were removed. They were then weighed, placed in a desiccating oven at 65°C for 48 hours, and reweighed.
Bronchoalveolar Lavage
Mice were killed by intraperitoneal ketamine–xylazine injection and each trachea was cannulated and perfused with two 0.9-ml aliquots of cold saline. Cellular contents and BAL fluid were separated by centrifugation, BAL cell number was quantitated, and the cell differential was assessed. BAL fluid was stored in aliquots at −80°C.
Measurements of BAL Protein
BAL was undertaken and protein levels were assessed with a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. The optical density of each sample was read at a wavelength of 750 nm with a SmartSpec 3000 spectrophotometer (Bio-Rad Laboratories).
Histologic Analysis
Lungs were removed en bloc as described previously, inflated at 25 cm Hg pressure with phosphate-buffered saline containing 0.5% low melting point agarose gel, fixed in Streck solution (Streck Laboratories, La Vista, NE), embedded in paraffin, sectioned, and stained. Hematoxylin–eosin and periodic acid–Schiff stains were performed in the Research Histology Laboratory of the Department of Pathology at Yale University School of Medicine.
Analysis of Mucin Gene Expression
Levels of MUC5AC, the major airway mucin in BAL fluids, were assessed as previously described (41). In these evaluations, 0.1 ml of 1:100 diluted BAL fluid was slot-blotted onto nitrocellulose membranes, using a Minifold II slot-blot apparatus (Schleicher and Schuell, Keene, NH). After air drying, each membrane was blocked with 5% skim milk, washed three times, and incubated overnight at 4°C with a monoclonal antibody against MUC5AC (45M1; NeoMarkers, Union City, CA). After washing, the membranes were incubated for 1 hour at room temperature with horseradish peroxidase–conjugated anti-mouse IgG (Pierce, Rockford, IL). Immunoreactive mucins were detected by a chemiluminescence procedure (ECL Plus Western blotting detection system; Amersham Biosciences) according to the instructions of the manufacturer.
ELISA Evaluations
ELISA and activity assay kits for IFN-α and IFN-β analysis were purchased from PBL Biomedical Laboratories (Piscataway, NJ). ELISAs for eotaxin/CCL11, monocyte chemotactic protein (MCP)-1/CCL2, keratinocyte chemoattractant (KC)/CXCL1, and macrophage inflammatory protein (MIP)-2/CXCL14 were obtained from R&D Systems (Minneapolis, MN). Each was performed according to the manufacturer's instructions.
Assessments of VEGF Signaling
Lung lysates were subjected to immunoblot analysis using antibodies against phosphorylated (p)- extracellular signal–regulated kinase-1 (ERK), total ERK, p-FAK, total FAK, p-Akt, total Akt, p-eNOS, and total eNOS (Cell Signaling, Beverly, MA).
Assessment of VEGF Receptor Expression
Cell preparations were obtained from Tg− and Tg+ mice that had been randomized to receive poly(I:C) or its vehicle control according to the pretreatment−2-week protocol described previously. They were then incubated with antibodies against CD45, CD11c, MHCII, CD31, VEGFR1 (R&D Systems), VEGFR2 (BioLegend, San Diego, CA), or VEGFR3 (R&D Systems). Fluorescence-activated cell-sorting (FACS) analysis was used to define the levels of receptor expression on dendritic cells (CD45+, CD11c+, MHCII+) and endothelial cells (CD45−, CD31+).
Ovalbumin Sensitization and Challenge
Ovalbumin (OVA) sensitization (with alum) and aerosol OVA challenge were undertaken as previously described by our laboratories (41, 42). Responses of mice that had been treated with poly(I:C) or vehicle 24 hours before aerosol challenge were compared. BAL and tissue responses were evaluated 24 hours after antigen challenge.
Statistics
Data were assessed by Student t test, analysis of variance, or Wilcoxon rank sum test as appropriate. Data are expressed as means ± SEM and are representative of evaluations done in a minimum of four animals.
RESULTS
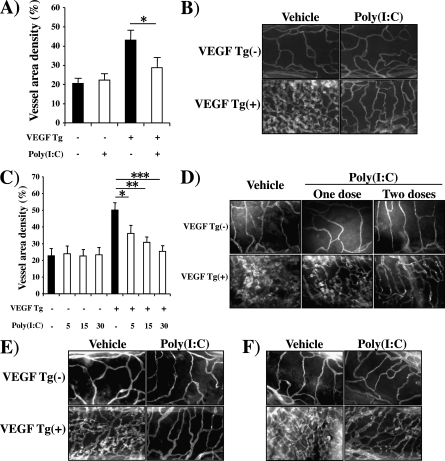
Effect of Poly(I:C) on VEGF-induced Angiogenesis
To begin to define the relationship between antiviral innate immune responses and VEGF, we compared the VEGF-induced vascular responses in Tg− and Tg+ mice treated with poly(I:C) or vehicle control. In these experiments comparisons of vehicle-treated Tg− and Tg+ mice revealed an angiogenic response in the Tg+ animals. In these animals vascular density was markedly increased (Figure 1A). These vessels were patulous and manifested an increase in density of vascular sprouting (Figure 1B). These responses were seen after as little as 48 hours of transgene activation and were greater after 14 days of dox administration (Figure 1 and data not shown). In contrast, VEGF-induced alterations in vascular density, appearance, and sprouting were all inhibited by poly(I:C) pretreatment (Figure 1). These effects were dose dependent, with maximal inhibition being noted with 30-μg doses of poly(I:C) (the highest dose tested) and significant inhibition being seen with doses as low as 5 μg (Figure 1C). They were also seen with shorter treatment intervals, with as little as one dose of poly(I:C) causing a significant decrease in vascular density (Figure 1D). Importantly, this effect did not require a pretreatment protocol because significant inhibition of VEGF-induced vascular alterations was also seen in experiments in which poly(I:C) was administered after 48 hours or 2 weeks of transgene activation (Figures 1E and 1F). It was also at least partially poly(I:C) specific because CpG (a TLR9 agonist) did not have similar effects (data not shown).
Figure 1.
Effect of polyinosinic:polycytidylic acid [poly(I:C)] on vascular endothelial growth factor (VEGF)–induced angiogenesis. Wild-type (VEGF transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were treated with poly(I:C) (30 μg unless otherwise noted) or vehicle, transgene activation was accomplished with doxycycline (dox), and tracheal angiogenesis was assessed on the basis of (A and C) morphometric assessments of the percentage of tracheal surface area that was covered by bronchial blood vessels (vessel area density %) or (B, D–F) anti-CD31 immunohistochemistry. In (A–C), the 2-week pretreatment protocol was employed. Poly(I:C) or vehicle [poly(I:C)−] treatment was initiated 1 day before dox was administered and continued every other day for 2 weeks. In (D), poly(I:C) or vehicle was administered 1 day before dox was administered. Some mice received only one dose of poly(I:C) or vehicle (one dose) and others received a second dose 1 week later (two doses). In all cases dox was continued for 2 weeks and analysis was then undertaken. In (E), dox was started, poly(I:C) or vehicle was administered 2 days later, and dox and poly(I:C)/vehicle were continued for 14 days. In (F), dox was started, treatment with poly(I:C) or vehicle was initiated 2 weeks later, and dox and poly(I:C)/vehicle were continued for an additional 2 weeks. In (A) and (C), each value represents the mean ± SEM of a minimum of four experiments. (B) and (D–F) are representative of a minimum of four similar experiments (*P < 0.05; **P < 0.01; ***P < 0.005).
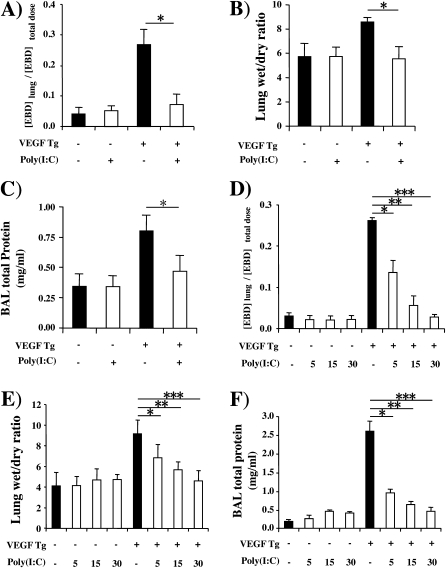
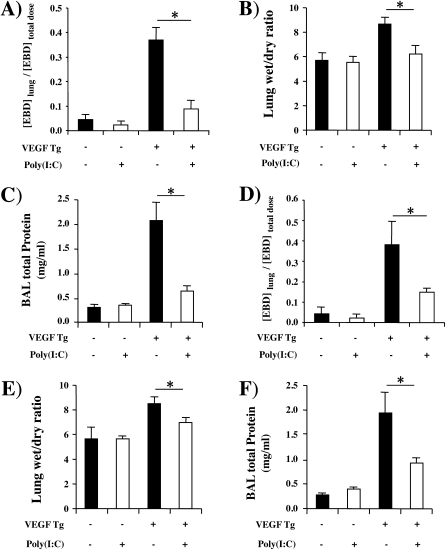
Effect of Poly(I:C) on VEGF-induced Permeability Alterations
Because VEGF is also well known to increase vascular permeability, we compared Evans blue dye extravasation, lung wet-to-dry weight ratios, and levels of BAL protein in wild-type (Tg−) and Tg+ mice treated with poly(I:C) or vehicle control. All three were significantly increased in vehicle-treated Tg+ mice (Figures 2A−2C). These changes were seen after as little as 48 hours of dox-induced transgene activation and were more impressive after 14 days of dox administration (Figures 2A−2C and data not shown). In contrast, all three VEGF-induced alterations were inhibited by poly(I:C) pretreatment (Figures 2A−2C). These effects were dose dependent, with maximal inhibition being noted with 30-μg doses of poly(I:C) (the highest dose tested) and significant inhibition being seen with doses as low as 5 μg (Figures 2D−2F and data not shown). Importantly, this effect did not require a pretreatment protocol because significant inhibition of VEGF-induced vascular permeability alterations was also seen when poly(I:C) was administered 48 hours (Figures 3A−3C) or 14 days (Figures 3D−3F) after dox- induced transgene activation. It was also at least partially poly(I:C) specific because CpG did not have similar effects (data not shown).
Figure 2.
Effect of polyinosinic:polycytidylic acid [poly(I:C)] on vascular endothelial growth factor (VEGF)–induced vascular permeability. Wild-type (VEGF transgene negative [Tg−]) and transgenic (VEGF Tg+) mice were treated with poly(I:C) (30 μg unless otherwise noted) or vehicle [poly(I:C)−], transgene activation was accomplished with doxycycline (dox) 1 day later, and pulmonary permeability was evaluated by (A and D) quantitating Evans blue dye (EBD) extravasation, (B and E) assessing lung wet-to-dry weight ratios, and (C and F) measuring bronchoalveolar lavage (BAL) protein. Each value represents the mean ± SEM of a minimum of four experiments (*P < 0.05; **P < 0.01; ***P < 0.005).
Figure 3.
Effect of delayed addition of polyinosinic:polycytidylic acid [poly(I:C)] on vascular endothelial growth factor (VEGF)–induced vascular permeability. Wild-type (VEGF transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were treated with poly(I:C) (30 μg) or vehicle [poly(I:C)−] at intervals after transgene activation was accomplished with doxycycline (dox) and vascular permeability was assessed by (A and D) measurements of Evans blue dye (EBD) extravasation, (B and E) assessments of lung wet-to-dry weight ratios, and (C and F) measurements of bronchoalveolar lavage (BAL) protein. In (A–C), treatment with poly(I:C) or vehicle was initiated 2 days after the dox and poly(I:C)/vehicle and dox were continued for 14 days. In (D–F) dox was started, treatment with poly(I:C) or vehicle was started 2 weeks later, and dox and poly(I:C) were continued for an additional 2 weeks. Each value represents the mean ± SEM of a minimum of four evaluations (*P < 0.05).
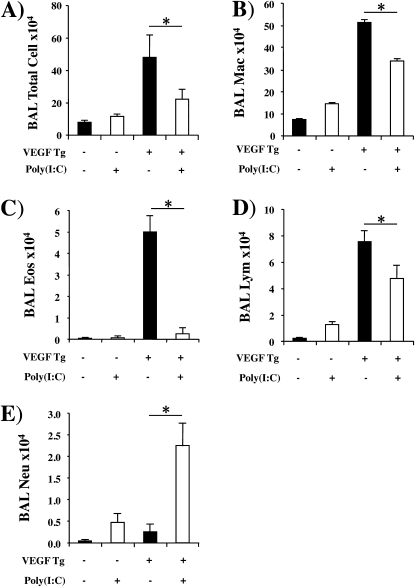
Effect of Poly(I:C) on VEGF-induced Inflammation
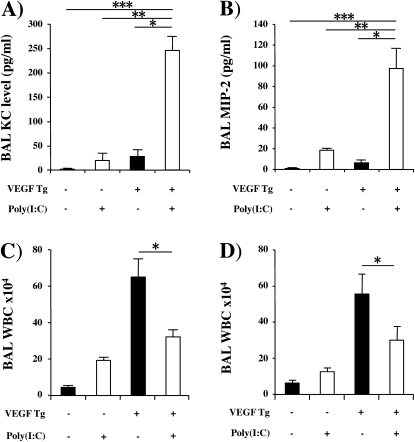
We next compared the levels of BAL and tissue inflammation in wild-type (Tg−) and Tg+ mice treated with poly(I:C) or vehicle control. As previously reported (5), transgenic VEGF caused BAL and tissue inflammation with elevated levels of macrophage, eosinophil, and lymphocyte recovery (Figures 4A−4D). These changes were seen after as little as 48 hours of dox-induced transgene activation and were more impressive after 14 days of dox administration (Figure 4 and data not shown). In keeping with the inflammatory nature of these responses, the levels of eotaxin/CCL11, MCP-1/CCL2, KC/CXCL1, and MIP-2/CXCL14 were increased in the BAL from Tg+ versus Tg− mice (Figure E2). These VEGF-induced inflammatory alterations were inhibited by poly(I:C) pretreatment (Figures 4A−4D). In contrast, BAL neutrophil accumulation was not increased in VEGF Tg+ mice treated with vehicle but was increased in Tg+ mice treated with poly(I:C) (Figure 4E). This neutrophilia was associated with significant increases in the levels of neutrophil chemotactic chemokines KC/CXCL1 and MIP-2/CXCL14 (Figures 5A and 5B) in BAL. Overall these regulatory effects were dose dependent, with maximal regulation being noted with 30-μg doses of poly(I:C) (the highest dose tested) and significant alterations being seen with doses as low as 5 μg (data not shown). Importantly, significant regulation of VEGF-induced inflammatory alterations was also seen when poly(I:C) was administered 48 hours or 14 days after dox-induced transgene activation (Figures 5C and 5D).
Figure 4.
Effect of polyinosinic:polycytidylic acid [poly(I:C)] on vascular endothelial growth factor (VEGF)–induced inflammation. Wild-type (VEGF transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were treated with poly(I:C) (30 μg) or vehicle [poly(I:C)−], transgene activation was accomplished with doxycycline (dox), and bronchoalveolar lavage (BAL) was undertaken. (A) Total cell, (B) macrophage (Mac), (C) eosinophil (Eos), (D) lymphocyte (Lym), and (E) neutrophil (Neu) recovery were assessed. In (A–E), poly(I:C) treatment was initiated 1 day before dox and both were then continued for 14 days (*P < 0.05).
Figure 5.
Neutrophilic chemokines and delayed addition of polyinosinic:polycytidylic acid [poly(I:C)]. In (A) and (B), wild-type (vascular endothelial growth factor [VEGF] transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were treated with poly(I:C) (30 μg) or vehicle [poly(I:C)−], transgene activation was accomplished with doxycycline (dox), bronchoalveolar lavage (BAL) was undertaken 2 weeks later, and keratinocyte chemoattractant (KC)/CXCL1 and macrophage inflammatory protein (MIP)-2/CXCL14 were assessed by ELISA. In (C), poly(I:C) was administered 2 days after dox, poly(I:C) and dox were continued for 14 days, and total BAL cell recovery was assessed. In (D) dox was started, poly(I:C) treatment was initiated 2 weeks later, dox and poly(I:C) were continued for an additional 2 weeks, and total BAL cell recovery was assessed. Each value represents the mean ± SEM of a minimum of four experiments (*P < 0.05; **P < 0.01; ***P < 0.005). WBC = white blood cells.
Effects of Poly(I:C) on VEGF-induced Mucus Metaplasia
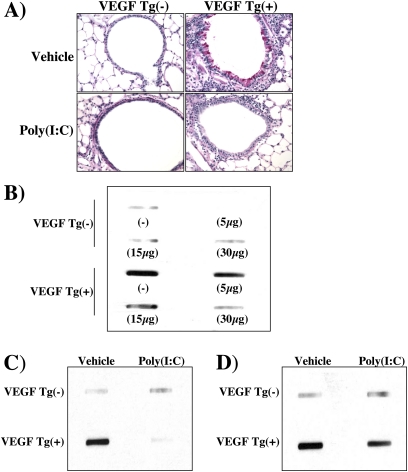
We next compared the mucus responses in wild-type (Tg−) and Tg+ mice treated with poly(I:C) or vehicle control. As we previously reported (5), transgenic VEGF caused mucus cell metaplasia and BAL MUC5AC accumulation (Figures 6A and 6B). These changes were seen after as little as 48 hours of dox-induced transgene activation and were more impressive after 14 days of dox administration (Figures 6A and 6B and data not shown). Each of these VEGF-induced alterations was inhibited by poly(I:C) pretreatment (Figures 6A and 6B). These effects were dose dependent, with maximal inhibition being noted with 30 μg doses of poly(I:C) (the highest dose tested) and significant inhibition being seen with doses as low as 5 μg (Figure 6B and data not shown). Importantly, significant inhibition of VEGF-induced mucus metaplasia was also seen when the poly(I:C) was administered 48 hours or 14 days after dox-induced transgene activation (Figures 6C and 6D).
Figure 6.
Effect of polyinosinic:polycytidylic acid [poly(I:C)] on vascular endothelial growth factor (VEGF)–induced mucus metaplasia. Wild-type (VEGF transgene negative [Tg−]) and transgenic (VEGF Tg+) mice were treated with poly(I:C) (30 μg unless otherwise indicated) or vehicle [poly(I:C)−], transgene activation was accomplished with doxycycline (dox), and bronchoalveolar lavage (BAL) was undertaken. In (A), periodic acid–Schiff staining was performed. In (B–D), BAL mucin 5AC (MUC5AC) accumulation was assessed by slot and immunoblot analysis. In (A) and (B), poly(I:C) treatment was initiated 1 day before dox and both were then continued for 14 days. In (C), poly(I:C) was administered 2 days after dox, and poly(I:C) and dox were continued for 14 days. In (D) dox was started, poly(I:C) treatment was initiated 2 weeks later, and dox and poly(I:C) were continued for an additional 2 weeks. The noted data are representative of a minimum of four similar evaluations.
Effects of Poly(I:C) on VEGF Production
The studies noted previously demonstrate that poly(I:C) inhibits VEGF-induced angiogenesis, edema, inflammation, and mucus metaplasia. These effects could be due to alterations in the production of transgenic VEGF or alterations in its ability to induce these tissue responses. To differentiate among these possibilities, we compared the levels of VEGF in BAL fluids from Tg+ mice treated with vehicle or poly(I:C). These studies demonstrated that doses of poly(I:C) less than or equal to 30 μg did not alter the levels of production of transgenic VEGF (Figure E1). Thus, poly(I:C) inhibits VEGF-induced tissue responses without altering VEGF production.
Role(s) of TLR3
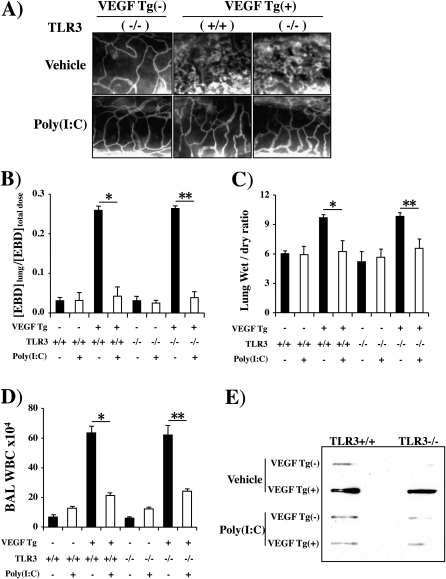
To define the innate immune pathway(s) that poly(I:C) uses to inhibit VEGF-induced tissue responses, we evaluated the effects of poly(I:C) in VEGF Tg− and Tg+ mice with wild-type and null TLR3 loci. As noted previously, poly(I:C) was an effective inhibitor of VEGF-induced angiogenesis, edema, mucus metaplasia, and inflammation (Figure 7). Interestingly, poly(I:C) had a similar ability to inhibit these VEGF responses in TLR3-sufficient and TLR3-deficient mice (Figure 7). Thus, TLR3 did not play a significant role in the pathogenesis of the regulatory effects of poly(I:C) in VEGF Tg+ animals.
Figure 7.
Role of Toll-like receptor-3 (TLR3) in polyinosinic:polycytidylic acid [poly(I:C)] regulation of vascular endothelial growth factor (VEGF) responses. Wild-type (VEGF transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were bred with mice with a wild-type (+/+) or null mutant TLR3 locus. This generated transgene negative mice with a wild-type TLR3 locus (VEGF Tg−/TLR3+/+), transgene negative mice with a null TLR3 locus (VEGF Tg−/TLR3−/−), transgenic mice with a wild-type TLR3 locus (VEGF Tg+/TLR3+/+), and transgenic mice with a null TLR3 locus (VEGF Tg+/TLR3−/−). These mice were treated with poly(I:C) (30 μg) or vehicle [poly(I:C)−], transgene activation was accomplished with doxycycline (dox) 24 hours later, and treatment with poly(I:C) or vehicle and dox was continued for 2 weeks. (A) Angiogenesis, (B) Evans blue dye (EBD) extravasation, (C) lung wet-to-dry weight ratios, (D) bronchoalveolar lavage (BAL) total cell recovery, and (E) BAL mucin 5AC (MUC5AC) levels were assessed. (A) and (E) are representative of a minimum of four similar experiments. In (B–D) each value represents the mean ± SEM of a minimum of four evaluations (*P < 0.05; **P < 0.01). WBC = white blood cells.
Role of the RLH Pathway
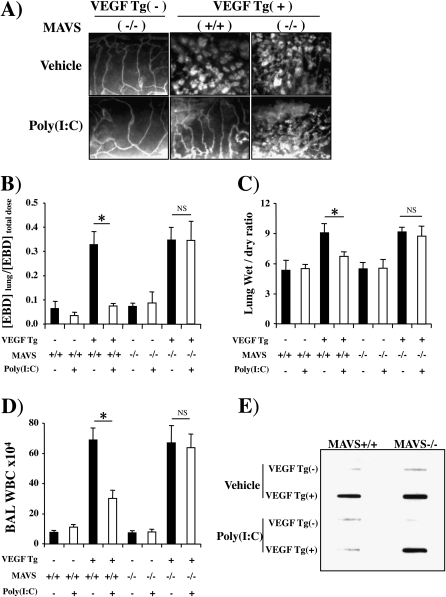
To further define the innate pathway(s) that mediates the interactions of VEGF and poly(I:C), we compared the angiogenic, edema, inflammatory, and mucus responses in wild-type mice and mice with null mutations of the mitochondrial antiviral signaling protein (MAVS). MAVS was chosen because it is the central integrator of the RLH pathway that links the viral nucleic acid–sensing RNA helicases such as RIG-I and melanoma differentiation antigen-5 (Mda5) to antiviral effector responses (38, 43). These studies demonstrated that MAVS plays a critical role in these responses because the ability of poly(I:C) to inhibit the VEGF-induced angiogenic, edema, inflammatory, and mucus responses was abrogated in MAVS-deficient animals (Figure 8). This demonstrates that poly(I:C) inhibits the tissue effects of VEGF via a mechanism that is critically dependent on the RLH pathway.
Figure 8.
Role of mitochondrial antiviral signaling protein (MAVS) in polyinosinic:polycytidylic acid [poly(I:C)] regulation of vascular endothelial growth factor (VEGF) responses. Wild-type (VEGF transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were bred with mice with a wild-type (+/+) or null mutant MAVS locus. This generated transgene negative mice with a wild-type MAVS locus (VEGF Tg−/MAVS+/+), transgene negative mice with a null MAVS locus (VEGF Tg−/MAVS−/−), transgenic mice with a wild-type MAVS locus (VEGF Tg+/MAVS+/+), and transgenic mice with a null MAVS locus (VEGF Tg+/MAVS−/−). These mice were treated with poly(I:C) (30 μg) or vehicle [poly(I:C)−], transgene activation was accomplished with doxycycline (dox) 24 hours later, poly(I:C) and dox were continued for 2 weeks, and (A) angiogenesis, (B) Evans blue dye (EBD) extravasation, (C) lung wet-to-dry weight ratios, (D) bronchoalveolar lavage (BAL) total cell recovery, and (E) BAL mucin 5AC (MUC5AC) levels were assessed. (A) and (E) are representative of a minimum of four similar experiments. In (B–D) each value represents the mean ± SEM of a minimum of four evaluations (*P < 0.05; **P < 0.01). NS = not significant; WBC = white blood cells.
Role of Type I IFNs and PKR
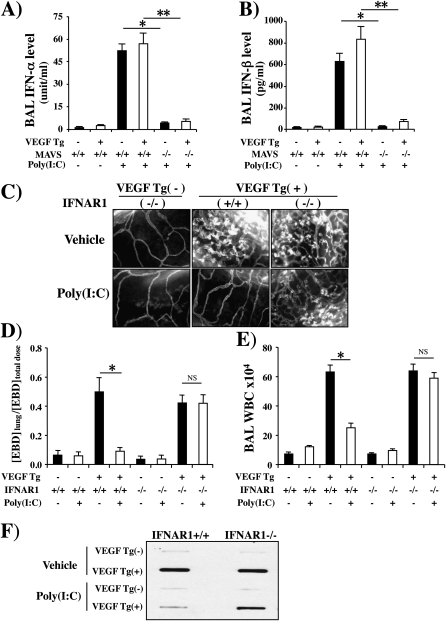
Because RLH pathway activation has been linked to the production of type I IFNs and the activation of PKR, we compared the production of IFN-α and IFN-β in Tg− and Tg+ mice treated with vehicle or poly(I:C) and the VEGF-inhibitory effects of poly(I:C) in Tg+ mice with wild-type and null type I IFN receptors (IFNAR1) and PKR. Poly(I:C) was a potent stimulator of both type I IFNs in wild-type (Tg−) and VEGF Tg+ animals (Figures 9A and 9B). These inductive events were MAVS dependent because they were significantly ameliorated in mice with a null MAVS locus (Figures 9A and 9B). In the latter experiments, the ability of poly(I:C) to inhibit VEGF-induced angiogenesis, edema, inflammation, and mucin production was significantly decreased in Tg+ mice with null mutations of IFNAR1 (Figures 9B−9E). In contrast, the effects of poly(I:C) were not significantly altered in PKR null mice (Figure E3). When viewed in combination these studies demonstrate that type I IFNs are induced during and play a critical role in the pathogenesis of the VEGF-inhibitory effects of poly(I:C). These studies also demonstrate that the regulatory effects of the type I IFNs are mediated via a PKR-independent mechanism(s).
Figure 9.
Induction and role of type I IFNs in polyinosinic:polycytidylic acid [poly(I:C)] regulation of vascular endothelial growth factor (VEGF) responses. Wild-type (VEGF transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were bred with mice with wild-type (+/+) or null mutant (−/−) mitochondrial antiviral signaling protein (MAVS) or IFN α receptor 1 (IFNAR1) loci. This generated transgene negative mice with a wild-type MAVS locus (VEGF Tg−/MAVS+/+), transgene negative mice with a null MAVS locus (VEGF Tg−/MAVS−/−), transgenic mice with a wild-type MAVS locus (VEGF Tg+/MAVS+/+), and transgenic mice with a null MAVS locus (VEGF Tg+/MAVS−/−). It also generated transgene negative mice with a wild-type IFNAR1 locus (VEGF Tg−/IFNAR1+/+), transgene negative mice with a null IFNAR1 locus (VEGF Tg−/IFNAR1−/−), transgenic mice with a wild-type IFNAR1 locus (VEGF Tg+/IFNAR1+/+), and transgenic mice with a null IFNAR1 locus (VEGF Tg+/IFNAR1−/−). These mice were treated with poly(I:C) (30 μg) or vehicle [poly(I:C)−], transgene activation was accomplished with doxycycline (dox) 24 hours later, and poly(I:C) or vehicle and dox were continued for 2 weeks. The levels of (A and B) bronchoalveolar lavage (BAL) type I IFNs, (C) angiogenesis, (D) Evans blue dye (EBD) extravasation, (E) BAL total cell recovery, and (F) BAL mucin 5AC (MUC5AC) were assessed. Wild-type and MAVS−/− mice are compared in (A) and (B). Wild-type and IFNAR1−/− mice are compared in (C)–(F). (A), (B), (D), and (E) are representative of a minimum of four similar experiments. In (A), (B), (D), and (E) each value represents the mean ± SEM of a minimum of four evaluations. (C) and (F) are representative of four similar experiments (*P < 0.05; **P < 0.01). NS = not significant; WBC = white blood cells.
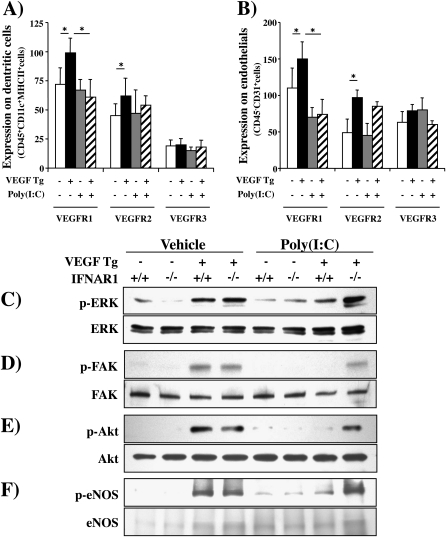
Poly(I:C) Regulation of VEGF Receptor Expression and Signaling
The studies noted previously demonstrate that poly(I:C) inhibits VEGF effector responses without altering VEGF production in our Tg+ mice. This suggests that this inhibition is mediated via a postligand event such as VEGF receptor expression and/or signaling. To address these possibilities we compared the expression of VEGFR1, VEGFR2, and VEGFR3 and known VEGF signaling pathways in Tg− and Tg+ mice treated with poly(I:C) or vehicle control. As seen in Figure 10, VEGF stimulated VEGFR1 and VEGFR2 expression on dendritic cells and endothelial cells (Figures 10A and 10B). These effects were at least partially receptor specific because VEGFR3 was not similarly regulated (Figures 10A and 10B). Interestingly, poly(I:C) inhibited the expression of VEGFR1 but not VEGFR2 or VEGFR3 in this experimental system (Figures 10A and 10B). Importantly, VEGF also activated/phosphorylated ERK, FAK, Akt, and eNOS. Each of these activation events was also significantly decreased by treatment with poly(I:C) (Figure 10). Interestingly, the ability of poly(I:C) to inhibit these signaling pathways was significantly decreased in mice lacking the IFNAR1 receptor (Figure 10). These studies demonstrate that the ability of poly(I:C) to inhibit VEGF tissue responses is associated with a decrease in VEGF stimulation of VEGFR1 and a decrease in the ability of VEGF to activate the ERK, FAK, Akt, and eNOS signaling pathways. They also demonstrate that these inhibitory effects are mediated, at least in part, by a type I IFN receptor–dependent mechanism.
Figure 10.
Polyinosinic:polycytidylic acid [poly(I:C)] regulation of vascular endothelial growth factor (VEGF) receptor expression and signaling. In (A) and (B) cell preparations were obtained from transgene-negative (Tg−) and transgene-positive (Tg+) mice that had been randomized to receive poly(I:C) or vehicle [poly(I:C)−] according to the pretreatment−2-week protocol. Fluorescence-activated cell-sorting (FACS) analysis was used to define the levels of VEGF receptor type 1 (VEGFR1), VEGFR2, and VEGFR3 expression on (A) dendritic cells and (B) endothelial cells. In (C–F), wild-type (VEGF Tg−) and transgenic (VEGF Tg+) mice were treated with poly(I:C) (30 μg) or vehicle, transgene activation was accomplished with doxycycline (dox) 24 hours later, and poly(I:C) or vehicle and dox were continued for 2 weeks. The lungs were then harvested and immunoblotting against total and phosphorylated (p) signaling moieties was undertaken as noted. The effects of poly(I:C) and on vehicle on (C) ERK, (D) focal adhesion kinase (FAK), (E) Akt, and (F) endothelial nitric oxide synthase (eNOS) are noted. Comparisons are made of mice that are sufficient (+/+) and deficient (−/−) in IFN α/β receptor chain 1 (IFNAR1). Each blot is representative of a minimum of three similar experiments.
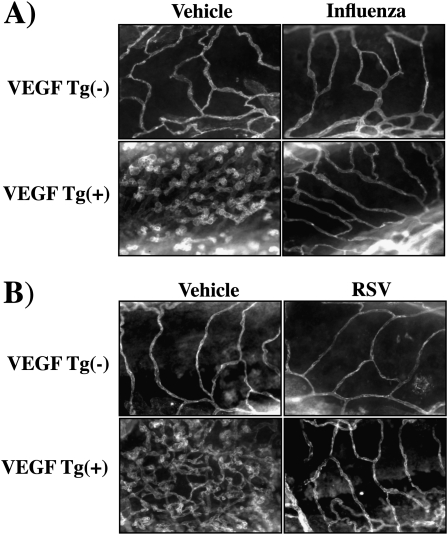
Effect of Influenza and RSV on VEGF-induced Angiogenesis
The studies noted previously used poly(I:C) as a surrogate for double-stranded RNA viruses and single-stranded RNA viruses that go through a double-stranded stage during viral replication. To begin to evaluate the validity of this assumption, we compared the effects of influenza virus and RSV on VEGF-induced angiogenesis in the murine lung. In keeping with our findings with poly(I:C), both viruses were potent inhibitors of VEGF-induced angiogenesis (Figures 11A and 11B).
Figure 11.
Effect of respiratory viruses on vascular endothelial growth factor (VEGF)–induced vascular angiogenesis. Wild-type (VEGF transgene negative [Tg−]) and VEGF transgenic (VEGF Tg+) mice were treated with (A) influenza virus or (B) respiratory syncytial virus (RSV), transgene activation was accomplished with doxycycline (dox), and angiogenesis was assessed by anti-CD31 immunohistochemistry. The figures are representative of at least three similar experiments.
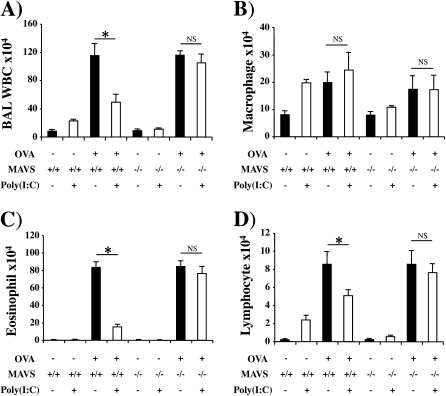
Poly(I:C) Regulation of Th2 Inflammation
Previous studies from our laboratory demonstrated that VEGF plays a critical role in the generation of type 2 adaptive immune responses such as are seen in asthma (5). This led us to determine whether poly(I:C) also inhibited aeroallergen-induced Th2 inflammation. As previously described by our laboratory and others (41, 42), ovalbumin (OVA) sensitization and challenge increased BAL and tissue total cell, macrophage, eosinophil, and lymphocyte recovery (Figures 12A−12D and data not shown). In all cases these inductive effects were abrogated by treatment with poly(I:C) (Figures 12A−12D and data not shown). These inhibitory effects were mediated by an MAVS-dependent pathway because they were abrogated in MAVS null animals (Figures 12A−12D). Thus, in accordance with the critical role that VEGF plays in Th2 inflammation and the ability of RLH activation to abrogate the tissue effects of VEGF, poly(I:C) is a potent inhibitor of adaptive Th2 inflammation.
Figure 12.
Polyinosinic:polycytidylic acid [poly(I:C)] inhibition of helper T-cell type 2 (Th2) inflammation. Mice were sensitized with ovalbumin (OVA), treated with poly(I:C) (30 μg) or vehicle [poly(I:C)−], and challenged 24 hours later with an OVA aerosol. Bronchoalveolar lavage (BAL) (A) total cell, (B) macrophage, (C) eosinophil, and (D) lymphocyte recovery was assessed. These studies compare the effects of poly(I:C) in mice sufficient (+/+) and deficient (−/−) for mitochondrial antiviral signaling protein (MAVS). Each value represents the mean ± SEM of evaluations in a minimum of four mice (*P < 0.05; **P < 0.01). NS = not significant; WBC = white blood cells.
DISCUSSION
In keeping with the pleiotropic effects of VEGF in injury and repair and its roles in normal homeostasis and disease, studies were undertaken to define the mechanisms that control its tissue effector responses. Specifically, we hypothesized that antiviral innate immune responses are powerful regulators of VEGF effector function. The present studies confirm this hypothesis by demonstrating that the viral PAMP, poly(I:C), inhibits VEGF-induced angiogenic responses and tissue edema. The viral relevance of these findings was confirmed in studies that demonstrated that influenza virus and RSV also inhibit VEGF angiogenesis. Innate immune regulation of VEGF responses was not restricted to vascular targets because VEGF-induced inflammation, mucous metaplasia, and Th2 inflammation were also abrogated by poly(I:C). Importantly, these studies also demonstrated that these effects of poly(I:C) are mediated by a TLR3-independent, RLH-dependent pathway that selectively regulates VEGF receptor expression and inhibits VEGF activation of ERK, FAK, Akt, and eNOS. When viewed in combination, these studies define a novel link between VEGF and antiviral and RLH-mediated innate immune responses. In so doing, they provide essential insights into the consequences of viral recognition and innate immune activation. These insights define mechanisms that can mediate the adverse consequences of viral infections and RLH-mediated immune responses. They also define an agonist and pathway that can be used to regulate VEGF-induced tissue responses in health and disease.
Host antiviral responses are initiated via the detection of viral PAMPs by host pattern recognition receptors (PRRs). On recognition, PRR signaling results in the expression of type I IFNs that suppress viral replication and facilitate adaptive immune responses (31, 38, 43–45). Double-stranded (ds) RNA, which is produced during the replication of many viruses, is recognized by several innate pathways including TLR3 and the RNA helicases RIG-I and Mda5 (31, 38, 43–45). In addition, many singled-stranded RNA viruses including influenza virus have been shown to activate the RIG-I pathway via the generation of 5′-triphosphorylated single-stranded RNA. TLR3 resides in endosomal membranes, where it recognizes dsRNA and poly(I:C). RIG-I and Mda5 detect dsRNA and poly(I:C) in the cytoplasm, where they are linked to downstream signaling molecules via MAVS (38, 43). When our studies demonstrated that poly(I:C) inhibited VEGF-induced tissue responses we expected that this inhibition would be mediated, to a great extent, by TLR3. Our studies, however, proved that this is not correct because similar poly(I:C)-induced VEGF-inhibitory effects were seen in mice that were sufficient and deficient in this receptor. In contrast, poly(I:C) regulation of VEGF-induced tissue responses appeared to be RLH mediated because it was entirely abrogated by the elimination of MAVS. In accordance with the prominent role that the RLH pathway plays in the induction of type I IFNs, our studies also demonstrated that poly(I:C) stimulated the production of IFN-α and IFN-β and that the inhibitory effects of poly(I:C) are significantly diminished in the absence of a common type I IFN receptor. In contrast, null mutations of PKR did not alter the VEGF-regulatory effects of poly(I:C). These are the first studies to define a relationship between VEGF and the RLH innate immune signaling pathway. They are also the first to demonstrate that type I IFNs inhibit VEGF-induced tissue responses via a PKR-independent mechanism. Additional investigation will be required to define the pathways that type I IFNs use to inhibit VEGF effector responses.
VEGF mediates its tissue effects by recruiting and activating multiple signaling networks that regulate cell and organ function. These ligands bind and signal through VEGF receptors, which are receptor tyrosine kinases, and neuropilin-1 and -2, which are coreceptors. Studies of VEGF signaling in a variety of tissues and modeling systems have demonstrated that phospholipase C-γ, PI3 kinase, Akt, Ras pathways, and MAP kinases play important roles in many VEGF-induced cellular and tissue responses (4, 6, 9, 46–48). To further understand the mechanisms that RLH pathway activation might use to control VEGF-induced tissue responses, we compared the expression of VEGF receptors and the activation of these pathways in mice treated with poly(I:C) or vehicle control. Our studies demonstrate that the ability of VEGF to stimulate the expression of VEGFR1 on dendritic cells and endothelial cells and to activate pulmonary FAK, ERK, Akt, and eNOS was significantly diminished after treatment with poly(I:C). These studies also demonstrated that the inhibition of VEGF signaling is mediated via a type I IFN receptor–dependent mechanism. In accordance with the widespread inhibition that was noted, these studies, in composite, demonstrate that poly(I:C) selectively regulates VEGF receptor expression and inhibits a variety of VEGF-induced signaling events.
The lung is among the organs with the highest levels of basal VEGF expression and activity. In this setting VEGF plays a critical role in early lung development. More recently it has been shown to confer protection against injury and oxidative stress. This protection may be particularly important in the respiratory alveolus because pharmacological inhibition of VEGF activity and lung-targeted inactivation of the VEGF gene result in emphysematous phenotypes whereas VEGF expression diminishes emphysema in murine modeling systems (11, 14, 16, 32–34, 49). In accordance with this finding, a number of investigators have reported decreased levels of VEGF expression/accumulation in biological samples from patients with emphysema (16, 33, 35–37). Our demonstration that VEGF effector function is abrogated by antiviral innate immune responses has important implications regarding the pathogenesis of COPD. First, these studies highlight a mechanism by which the protective effects of VEGF can be abrogated in the setting of viral infection. One can easily see how this inhibition of VEGF effector responses could contribute to the RLH-mediated, exaggerated inflammatory, apoptotic, and emphysematous responses that are seen in mice simultaneously exposed to cigarette smoke and poly(I:C) or influenza virus (31) and to the pathological effects of virus-induced COPD exacerbations. These studies also suggest that one cannot develop a clear understanding of the integrity of the effects of VEGF in the lung simply by measuring BAL VEGF levels. Because the tissue effects of VEGF can be abrogated without corresponding alterations in the levels of VEGF in a biological fluid the levels of BAL VEGF are a “best case” estimate of the tissue effects of this important cytokine.
A number of lines of evidence have emerged that suggest that VEGF plays an important role in asthma pathogenesis. This includes studies from our laboratory that demonstrated that lung-targeted VEGF transgenic mice have an asthma-like phenotype with eosinophilic inflammation, airway remodeling, and airway hyperresponsiveness and that VEGFR blockade diminishes aeroallergen-induced Th2 inflammation and Th2 cytokine production in vivo (5). It also includes human investigations that demonstrated that the levels of expression of VEGF and the VEGFRs are increased in asthma (50–52) and studies that noted associations between VEGF polymorphisms and the disease (53). To add to this literature we characterized the effects of poly(I:C) on OVA-induced adaptive Th2 immune responses. In accordance with reports from others (54), our studies demonstrated that poly(I:C) inhibits tissue inflammation in this setting. In contrast, others have reported that poly(I:C) can exacerbate pulmonary allergic reactions (55). These divergent results may be due to differences in the protocols that were employed or the appreciation that different doses of poly(I:C) can activate different immune pathways (56). However, it is tempting to hypothesize that both reports are correct and that poly(I:C) inhibits when it predominantly activates the RLH pathway (as described in this article) and stimulates when it predominantly activates TLR3 as described by Torres and colleagues (55). It will be interesting to see whether subsequent studies confirm this speculation.
In summary, our studies demonstrate that RLH activation blocks VEGF tissue responses and ameliorates aeroallergen-induced adaptive Th2 inflammation. These studies suggest that innate immunity agonists such as poly(I:C) can be used to activate the RLH pathway and control VEGF tissue responses in diseases such as asthma. Elevated levels of VEGF are also believed to be involved in the pathogenesis of a variety of other diseases including tumor angiogenesis, rheumatoid arthritis, inflammatory bowel disease, psoriasis, allergic rhinitis, and wet macular degeneration (3, 57–61). Thus, it is tempting to speculate that poly(I:C) and/or other RLH agonists can be used to treat these disorders as well. Additional investigations into the agonists that can be employed, the mechanisms of their effect, and the utility of RLH agonists in the regulation of VEGF-induced tissues responses are warranted.
Supplementary Material
Acknowledgments
The authors thank Kathleen Bertier and Karen DeAngelo for excellent secretarial assistance.
Supported by NIH grants HL-078744 and HL-079328 and by a grant from the Flight Attendants Medical Research Institute (FAMRI) (J. A. Elias).
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201008-1276OC on January 28, 2011
Author Disclosure: J.A.E. received consultancy fees from Promedior Inc., served on the advisory board of Intermune Inc., and owns stock in Intermune Inc. and Merck, Inc. J.A.E. also received patents from Yale University for chitinases in lung inflammation, mir-1 in VEGF tissue responses, IL-18 in COPD, and VEGF in asthma. B.M., C.S.D.C., D.H., M.-J.K., S.T., R.J.H., and C.G.L. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005;438:932–936. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 2005;94:209–231. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005;438:967–974. [DOI] [PubMed] [Google Scholar]
- 4.Lal BK, Varma S, Pappas PJ, Hobson RW II, Duran WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res 2001;62:252–262. [DOI] [PubMed] [Google Scholar]
- 5.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances Th2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel C, Bauer A, Wiesnet M, Preissner KT, Schaper W, Marti HH, Fischer S. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol 2007;212:236–243. [DOI] [PubMed] [Google Scholar]
- 7.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 9.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor–mediated cytoprotection in endothelial cells. J Biol Chem 2001;276:30359–30365. [DOI] [PubMed] [Google Scholar]
- 10.Lunn JS, Sakowski SA, Kim B, Rosenberg AA, Feldman EL. Vascular endothelial growth factor prevents G93A-SOD1–induced motor neuron degeneration. Dev Neurobiol 2009;69:871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y, Zhang Z. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 12:605–614. [DOI] [PubMed]
- 12.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 2009;119:1298–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtagic E, Jedrychowski MP, Nugent MA. Neutrophil elastase cleaves VEGF to generate a VEGF fragment with altered activity. Am J Physiol Lung Cell Mol Physiol 2009;296:L534–L546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medford AR, Ibrahim NB, Millar AB. Vascular endothelial cell growth factor receptor and coreceptor expression in human acute respiratory distress syndrome. J Crit Care 2009;24:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuder RM, Yun JH. Vascular endothelial growth factor of the lung: friend or foe. Curr Opin Pharmacol 2008;8:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 2000;22:657–664. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. The relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2000;57:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the Lung Health Study. Am J Respir Crit Care Med 2001;164:358–364. [DOI] [PubMed] [Google Scholar]
- 20.Sapey E, Stockley RA. COPD exacerbations. 2. Aetiology. Thorax 2006;61:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004;1:115–120. [DOI] [PubMed] [Google Scholar]
- 22.Ko FW, Ip M, Chan PK, Fok JP, Chan MC, Ngai JC, Chan DP, Hui DS. A 1-year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest 2007;131:44–52. [DOI] [PubMed] [Google Scholar]
- 23.Mallia P, Contoli M, Caraqmori G, Pandit A, Johnston SL, Papi A. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr Pharm Des 2007;13:73–97. [DOI] [PubMed] [Google Scholar]
- 24.Mallia P, Johnston SL. How viral infections cause exacerbation of airway diseases. Chest 2006;130:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Gorman C, McHenry E, Coyle PV. Human metapneumovirus in adults: a short case series. Eur J Clin Microbiol Infect Dis 2006;25:190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sajjan US, Jia Y, Newcomb DC, Bentley JK, Lukacs NW, LiPuma JJ, Hershenson MB. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J 2006;20:2121–2123. [DOI] [PubMed] [Google Scholar]
- 27.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000;55:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. [DOI] [PubMed] [Google Scholar]
- 29.Tan WC, Xiang X, Qiu D, Ng TP, Lam SF, Hegele RG. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med 2003;115:272–277. [DOI] [PubMed] [Google Scholar]
- 30.D'Hulst AI, Maes T, Bracke KR, Demedts IK, Tournoy KG, Joos GF, Brusselle GG. Cigarette smoke–induced pulmonary emphysema in SCID-mice: is the acquired immune system required? Respir Res 2005;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP– and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 2008;118:2771–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem 2008;283:29447–29460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 34.Tuder RM, Yoshida T, Fijalkowka I, Biswal S, Petrache I. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc 2006;3:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bae YJ, Kim TB, Moon KA, Lee KY, Park CS, Seo JB, Chae EJ, Moon HB, Cho YS. Vascular endothelial growth factor levels in induced sputum and emphysematous changes in smoking asthmatic patients. Ann Allergy Asthma Immunol 2009;103:51–56. [DOI] [PubMed] [Google Scholar]
- 36.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med 2003;114:354–358. [DOI] [PubMed] [Google Scholar]
- 37.Marwick JA, Stevenson CS, Giddings J, MacNee W, Butler K, Rahman I, Kirkham PA. Cigarette smoke disrupts VEGF165–VEGFr-2 receptor signaling complex in rat lungs and patients with COPD: morphological impact of VEGFr-2 inhibition. Am J Physiol Lung Cell Mol Physiol 2006;290:L897–L908. [DOI] [PubMed] [Google Scholar]
- 38.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 2006;24:633–642. [DOI] [PubMed] [Google Scholar]
- 39.Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams BR, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA–dependent protein kinase. EMBO J 1995;14:6095–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Zhao MQ, Xu L, Ramana CV, Declercq W, Vandenabeele P, Enelow RI. Requirement for tumor necrosis factor-receptor 2 in alveolar chemokine expression depends upon the form of the ligand. Am J Respir Cell Mol Biol 2005;33:463–469. [DOI] [PubMed] [Google Scholar]
- 41.Lee CG, Hartl D, Matsuura H, Dunlop FM, Scotney PD, Fabri LJ, Nash AD, Chen NY, Tang CY, Chen Q, et al. Endogenous IL-11 signaling is essential in Th2- and IL-13–induced inflammation and mucus production. Am J Respir Cell Mol Biol 2008;39:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13–induced tissue responses and apoptosis. J Exp Med 2009;206:1149–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, Yamamoto M, Uematsu S, Ishii KJ, Takeuchi O, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med 2006;203:1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, et al. 5′-Triphosphate-siRNA: turning gene silencing and RIG-I activation against melanoma. Nat Med 2008;14:1256–1263. [DOI] [PubMed] [Google Scholar]
- 45.Vitour D, Meurs EF. Regulation of interferon production by RIG-I and LGP2: a lesson in self-control. Sci STKE 2007;2007:pe20. [DOI] [PubMed] [Google Scholar]
- 46.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, et al. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci USA 2006;103:11021–11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derakhshan B, Harrison KD, Miao QR, Sessa WC. Approaches for studying angiogenesis-related signal transduction. Methods Enzymol 2008;443:1–23. [DOI] [PubMed] [Google Scholar]
- 48.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 1999;274:16349–16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 50.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol 2001;107:1034–1038. [DOI] [PubMed] [Google Scholar]
- 51.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol 2001;107:295–301. [DOI] [PubMed] [Google Scholar]
- 52.Lee YC, Lee HK. Vascular endothelial growth factor in patients with acute asthma. J Allergy Clin Immunol 2001;107:1106. [DOI] [PubMed] [Google Scholar]
- 53.Sharma S, Murphy AJ, Soto-Quiros ME, Avila L, Klanderman BJ, Sylvia JS, Celedon JC, Raby BA, Weiss ST. Association of vascular endothelial growth factor polymorphisms with childhood asthma, lung function, and airways responsiveness. Eur Respir J 2009;33:1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aumeunier A, Grela F, Ramadan A, Pham Van L, Bardel E, Gomez Alcala A, Jeannin P, Akira S, Bach JF, Thieblemont N. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in nod mice. PLoS ONE 2010;5:e11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres D, Dieudonne A, Ryffel B, Vilain E, Si-Tahar M, Pichavant M, Lassalle P, Trottein F, Gosset P. Double-stranded RNA exacerbates pulmonary allergic reaction through TLR3: implication of airway epithelium and dendritic cells. J Immunol 2010;185:451–459. [DOI] [PubMed] [Google Scholar]
- 56.Jeon SG, Oh SY, Park HK, Kim YS, Shim EJ, Lee HS, Oh MH, Bang B, Chun EY, Kim SH, et al. Th2 and Th1 lung inflammation induced by airway allergen sensitization with low and high doses of double-stranded RNA. J Allergy Clin Immunol 2007;120:803–812. [DOI] [PubMed] [Google Scholar]
- 57.Choi GS, Park HJ, Hur GY, Choi SJ, Shin SY, Ye YM, Park HS. Vascular endothelial growth factor in allergen-induced nasal inflammation. Clin Exp Allergy 2009;39:655–661. [DOI] [PubMed] [Google Scholar]
- 58.Choi ST, Kim JH, Seok JY, Park YB, Lee SK. Therapeutic effect of anti-vascular endothelial growth factor receptor I antibody in the established collagen-induced arthritis mouse model. Clin Rheumatol 2009;28:333–337. [DOI] [PubMed] [Google Scholar]
- 59.Pourgholami MH, Morris DL. Inhibitors of vascular endothelial growth factor in cancer. Cardiovasc Hematol Agents Med Chem 2008;6:343–347. [DOI] [PubMed] [Google Scholar]
- 60.Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 2009;136:585–595. [DOI] [PubMed] [Google Scholar]
- 61.Yoo SA, Kwok SK, Kim WU. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediators Inflamm 2008;2008:129873. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.