Abstract
Aims
Although patients with syncope and bundle branch block (BBB) are at high risk of developing atrio-ventricular block, syncope may be due to other aetiologies. We performed a prospective, observational study of the clinical outcomes of patients with syncope and BBB following a systematic diagnostic approach.
Methods and results
Patients with ≥1 syncope in the last 6 months, with QRS duration ≥120 ms, were prospectively studied following a three-phase diagnostic strategy: Phase I, initial evaluation; Phase II, electrophysiological study (EPS); and Phase III, insertion of an implantable loop recorder (ILR). Overall, 323 patients (left ventricular ejection fraction 56 ± 12%) were studied. The aetiological diagnosis was established in 267 (82.7%) patients (102 at initial evaluation, 113 upon EPS, and 52 upon ILR) with the following aetiologies: bradyarrhythmia (202), carotid sinus syndrome (20), ventricular tachycardia (18), neurally mediated (9), orthostatic hypotension (4), drug-induced (3), secondary to cardiopulmonary disease (2), supraventricular tachycardia (1), bradycardia–tachycardia (1), and non-arrhythmic (7). A pacemaker was implanted in 220 (68.1%), an implantable cardioverter defibrillator in 19 (5.8%), and radiofrequency catheter ablation was performed in 3 patients. Twenty patients (6%) had died at an average follow-up of 19.2 ± 8.2 months.
Conclusion
In patients with syncope, BBB, and mean left ventricular ejection fraction of 56 ± 12%, a systematic diagnostic approach achieves a high rate of aetiological diagnosis and allows to select specific treatment.
Keywords: Syncope, Bundle branch block, Electrocardiography, Pacemakers
Introduction
The most common aetiology of syncope in patients with bundle branch block (BBB) is paroxysmal atrio-ventricular (A-V) block.1,2 However, other mechanisms such as ventricular tachycardia (VT), supraventricular tachycardia (SVT), carotid sinus syndrome (CSS), neurally mediated, or orthostatic hypotension can also cause syncope in this population.2 In addition, some of these patients are at high risk of sudden death, primarily related to the presence and severity of structural heart disease.3–6
The first step in the diagnostic strategy is to identify patients who are at high risk of sudden death.7 In these patients, an implantable cardioverter defibrillator (ICD)6,7 is indicated. The diagnostic and therapeutic strategy in the remaining patients is controversial. Some authors suggest that because the most common cause of syncope in these patients is paroxysmal A-V block, a pacemaker should be indicated,8,9 whereas others suggest following a comprehensive diagnostic approach that aims to document the cause of syncope before indicating any treatment.2,7
The Bradyarrhythmia detection in BBB (B4) Study is a multicentre, international, prospective, observational study that aims to analyse the clinical outcomes of patients with syncope and BBB following a systematic diagnostic approach, as recommended in guidelines for the diagnosis and management of syncope of European Society of Cardiology (ESC).7
Methods
Patients
Patients were included if they had at least one syncope in the last 6 months and BBB on EGG with a QRS duration of ≥120 ms. Patients with an indication for prophylactic ICD implantation due to low left ventricular ejection fraction (LVEF) were excluded from the study. Other exclusion criteria were pre-excitation, long QT syndrome, Brugada's syndrome, acute myocardial infarction, pregnancy, or life expectancy <1 year due to non-cardiac cause; patients who were geographically or otherwise inaccessible for follow-up or who were unwilling or unable to give informed consent were also excluded. The study was approved by the institutional review boards and signed informed consent was obtained from each patient at the time of enrolment.
Study protocol
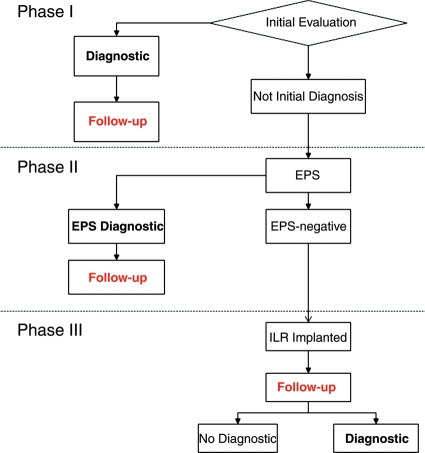
A systematic diagnostic strategy was designed with three consecutive phases (Figure 1). Phase I consisted of initial evaluation including clinical history, physical examination, 12-lead electrocardiogram (ECG), measurement of blood pressure in supine and orthostatic positions, and an echocardiogram. Electrocardiographic monitoring (in hospital or Holter) was also recommended. Phase II consisted of an electrophysiological study (EPS). Carotid sinus massage was performed on all patients, but could be performed either at Phase I or at Phase II, during EPS. Phase III consisted of implantable loop recorder (ILR) implantation (Reveal Plus, Medtronic Inc.)
Figure 1.
Proposed diagnostic strategy. Initial evaluation was performed in all patients. Those in whom initial evaluation achieved the diagnosed were treated accordingly. If initial evaluation was not diagnostic, an electrophysiological study (EPS) was performed: If it was diagnostic, patients were treated according to the findings, and if it was negative, an implantable loop recorder (ILR) was implanted.
When the diagnosis was achieved at a given phase, specific measures or treatment were instituted and patients were followed. When the diagnosis was not achieved at a given phase, patients entered the following phase.
Diagnostic criteria
According to the ESC guidelines,7 the following aetiological diagnoses were established: neurally mediated, when syncope was precipitated by emotional triggers and was preceded by typical prodromal symptoms; orthostatic hypotension, when syncope occurred in relation to orthostatism and orthostatic hypotension was elicited10; drug induced, when there was a clear relationship between syncope and the introduction of a new hypotensive drug or a change in dosage; secondary to cardiopulmonary disease, when syncope was clearly related to an acute cardiopulmonary disorder; CSS, when syncope or near-syncope was reproduced during or immediately after carotid sinus massage in the presence of asystole >3 s and/or a fall in systolic blood pressure of >50 mmHg; and arrhythmic syncope, when complete or advanced A-V block, asystole >3 s, or the presence of sustained VT or rapid SVT was documented,11 with or without syncope.12–14 Non-arrhythmic syncope was diagnosed when sinus rhythm was documented during a syncopal episode.
The EPS was considered diagnostic with the following findings7: sinus node recovery time >1500 ms; corrected sinus node recovery time >525 ms; baseline HV interval ≥70 ms; second- or third-degree His–Purkinje block during incremental atrial pacing or after intravenous class IC antiarrhythmic drugs or induction of sustained monomorphic VT or rapid SVT that provoked hypotension or reproduced spontaneous symptoms.
Study endpoints
Clinical endpoints were recurrent syncope, documented spontaneous arrhythmias, or death due to any cause.
All patients were followed quarterly during the first 12 months. Those with longer follow-up had additional visits at 18 and 24 months or at study closure. Whenever there was a syncopal recurrence, an unscheduled control visit was performed.
Statistical analysis
Data were sent by investigators via a dedicated Internet website that maintained the database and issued data-clarification forms.
The occurrence of clinical endpoints was compared between patients in whom diagnosis was achieved at Phase I or II, and who consequently were treated according to the diagnosis, and patients in whom an ILR was implanted after negative diagnostic work-up at Phases I and II. Comparison between groups was performed with Student's t-test or the Mann–Whitney non-parametric ‘U’ test, as appropriate, for continuous variables, and with Fisher's exact test or the χ2 test for proportions. Time to the onset of events was analysed by means of the Kaplan–Meier survival curves, which were compared using the log-rank test. Statistical significance was set at the standard value of P < 0.05. All reported P-values are two-tailed.
SPSS (SPSS Inc., Chicago, IL, USA) software version 12.0 statistical package was used for the statistical analyses.
Results
Patients
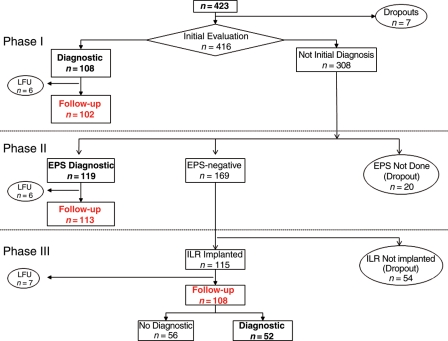
Between January 2003 and January 2006, 423 patients were eligible for the study (Figure 2). Overall, 100 patients were excluded from the analysis due to the following reasons: 7 patients had incomplete data at baseline, 19 had incomplete follow-up (6 after initial diagnosis, 6 after EPS, and 7 after ILR implantation), and 74 did not follow the proposed algorithm: EPS was not performed in 20 and ILR was not implanted in 54. There were not statistical differences in demographic data, the presence of structural heart disease, the type of BBB or previous history of syncope, between the 323 included and 100 excluded patients.
Figure 2.
Flowchart of included patients. From 423 patients initially eligible, only 323 (represented in red) entered in the study. The reasons for exclusion were incomplete data at baseline (7), lost of follow-up (LFU): 6 after initial diagnosis, 6 after electrophysiological study (EPS), and 7 after implantable loop recorder (ILR) or lack of adherence to protocol (20 without diagnosis at initial evaluation in whom an EPS was not performed and with negative EPS in whom ILR was not implanted).
The study population consisted of 323 patients. The demographic and clinical characteristics are listed in Table 1. It can be observed that patients who were diagnosed at Phase II had their first syncope episode at older age, had higher incidence of ischaemic or dilated cardiomyopathy, and had lower LVEF, but keeping in normal values.
Table 1.
Patient characteristics
| Total | Phase I | Phase II | Phase III | P-value | |
|---|---|---|---|---|---|
| Patients, n | 323 | 102 | 113 | 108 | |
| Age in years, mean ± SD | 73 ± 10 | 74 ± 11 | 75 ± 8 | 73 ± 10 | 0.396 |
| Male gender, n (%) | 206 (63.8) | 60 (58.8) | 82 (72.6) | 64 (59.3) | 0.055 |
| Total syncope episodes during lifetime, median (25°–75° percentile) | 2.0 (1.0–4.0) | 2.0 (1.0–3.0) | 2.0 (1.0–4.0) | 3.0 (1.0–5.0) | 0.076 |
| Syncope episodes lasting 6 months, median (25°–75° percentile) | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.114 |
| Age at first syncope, mean ± SD | 72 ± 11 | 72 ± 13 | 74 ± 9 | 70 ± 12 | 0.034 |
| History of pre-syncope, n (%) | 125 (38.7) | 46 (45.1) | 34 (30.1) | 45 (41.7) | 0.058 |
| Hospitalization for syncope, n (%) | 230 (71.2) | 74 (72.5) | 86 (76.1) | 70 (64.8) | 0.168 |
| Syncope without prodromes, n (%) | 183 (56.7) | 49 (48.0) | 65 (57.5) | 69 (63.9) | 0.067 |
| Syncope preceded by palpitations, n (%) | 18 (5.6) | 9 (8.8) | 6 (5.3) | 3 (2.8) | 0.160 |
| Trauma related to syncope (total), n (%) | 96 (29.7) | 24 (23.5) | 34 (30.1) | 38 (35.2) | 0.181 |
| Severe trauma related to syncope, n (%) | 44 (13.6) | 15 (14.7) | 15 (13.3) | 14 (13.0) | 0.926 |
| Baseline ECG, n (%) | |||||
| LBBB | 131 (40.6) | 35 (34.3) | 49 (43.4) | 47 (43.5) | |
| RBBB | 67 (20.7) | 26 (25.5) | 24 (21.2) | 17 (15.7) | |
| RBBB with left anterior hemi-block | 111 (34.4) | 34 (33.3) | 34 (30.1) | 43 (39.8) | 0.094 |
| RBBB with left posterior hemi-block | 8 (2.5) | 2 (2.0) | 5 (4.4) | 1 (0.9) | |
| Alternating LBBB and RBBB | 5 (1.5) | 4 (3.9) | 1 (1.0) | 0 (0.0) | |
| RBBB with left anterior and posterior hemi-block | 1 (0.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | |
| PR interval (ms), mean ± SD | 187 ± 45 | 192.2 ± 54 | 198.9 ± 44.4 | 170 ± 33.4 | <0.001 |
| Structural heart disease, n (%) | 161 (49.8) | 48 (47.1) | 71 (62.8) | 42 (38.9) | 0.005 |
| Ischaemic heart disease | 59 (18.3) | 14 (13.7) | 31 (27.4) | 14 (13.0) | 0.007 |
| Valvular heart disease | 38 (11.8) | 13 (12.7) | 17 (15.0) | 8 (7.4) | 0.198 |
| Dilated cardiomyopathy | 23 (7.1) | 7 (6.9) | 14 (12.5) | 2 (1.9) | 0.009 |
| Hypertrophic cardiomyopathy | 13 (4.0) | 1 (1.0) | 8 (7.1) | 4 (3.7) | 0.071 |
| Hypertensive heart disease | 49 (15.2) | 19 (18.2) | 18 (15.9) | 12 (11.1) | 0.304 |
| Other | 7 (2.2) | 1 (1.0) | 3 (2.7) | 3 (2.8) | 0.608 |
| LVEF (%), mean ± SD | 56 ± 12 | 56 ± 12 | 52 ± 14 | 60 ± 10 | <0.001 |
| LVEF < 40%, n (%) | 26 (8.0) | 8 (7.8) | 16 (14.2) | 2 (1.9) | 0.004 |
| Atrial fibrillation, n (%) | 34 (10.5) | 16 (15.7) | 12 (10.8) | 6 (5.6) | 0.057 |
| Diabetes, n (%) | 72 (22.3) | 27 (26.5) | 28 (24.8) | 17 (15.7) | 0.128 |
| Hypertension, n (%) | 221 (68.4) | 71 (69.6) | 76 (67.3) | 74 (68.5) | 0.933 |
| Neurological disease, n (%) | 42 (13.0) | 15 (14.7) | 11 (9.7) | 16 (14.8) | 0.440 |
Demographic and clinical characteristics of the study population. The second column (total) represents all population; in the following columns, there are the data of patients who were diagnosed at Phase I, Phase II, or who entered at Phase III. LBBB, left bundle branch block; RBBB, right bundle branch block; LVEF, left ventricular ejection fraction.
Patients were followed for 19.2 ± 8.2 months (median 21.8; inter-quartile range 12.1).
Aetiological diagnosis
At the end of follow-up, an aetiological diagnosis was established in 267 patients (82.7%; Table 2), with the following diagnoses: bradyarrhythmia in 202 (paroxysmal A-V block documented at initial evaluation or by ILR in 88, abnormal infrahisian findings at EPS in 70, sick sinus syndrome or severe sinus bradycardia in 15, alternating BBB documented at initial evaluation in 4, and not specified in 25), CSS in 20, VT in 18, neurally mediated syncope in 9, orthostatic hypotension in 4, drug-induced in 3, secondary to cardiopulmonary disease in 2 (1 with severe aortic stenosis and 1 with pulmonary thrombo-embolism), SVT in 1, bradycardia–tachycardia syndrome in 1, and non-arrhythmic syncope in 7.
Table 2.
Diagnosis
| Diagnosis |
n | ||
|---|---|---|---|
| Analysed patients, n = 323 | |||
| Initial evaluation (Phase I), n = 102 | Bradyarrhythmia | A-VB | 52 |
| Alt BBB | 4 | ||
| SSS | 6 | ||
| NS | 13 | ||
| CSS | 6 | ||
| Neurally mediated | 9 | ||
| Orthostatic hypotension | 4 | ||
| Drug-induced | 3 | ||
| VT | 3 | ||
| Cardiopulmonary | 2 | ||
| EPS (Phase II), n = 113 | Bradyarrhythmia | Infrahisian abnormalities | 70 |
| SSS | 4 | ||
| NS | 12 | ||
| CSS | 14 | ||
| VT | 12 | ||
| SVT | 1 | ||
| ILR implantation (Phase III), n = 52 | Bradyarrhythmia | A-VB | 36 |
| SA | 5 | ||
| Non-arrhythmic | 7 | ||
| VT/VF | 3 | ||
| Brady/tachy | 1 | ||
| No diagnosis | 56 | ||
EPS, electrophysiological study; ILR, implantable loop recorder; A-VB, documentation of transient or advanced atrio-ventricular block, including type 2 second-degree atrio-ventricular block, with ECG, in hospital monitoring, Holter recording (Phase I), or ILR (Phase III); Alt BBB, alternating bundle branch block; SSS, sick sinus syndrome; NS, non-specified; CSS, carotid sinus syndrome; VT, ventricular tachycardia; SVT, supraventricular tachycardia; VF, ventricular fibrillation; SA, sinus arrest. Infrahisian abnormalities includes HV interval ≥70 ms or the presence of infrahisian block with atrial pacing or drug challenge.
Treatments
At the end of follow-up, a pacemaker was implanted in 220 (68.1%) patients, an ICD in 19 (5.8%), and radiofrequency catheter ablation was performed in 3 patients (Table 3). In the remaining patients who had other diagnoses, such as neurally mediated, orthostatic, or drug-related syncope, aetiology-specific measures were applied.
Table 3.
Treatments according to different phases
| Treatment | n | |
|---|---|---|
| Analysed patients, n = 323 | ||
| Initial evaluation (Phase I) | PMK | 82 |
| General measures/drug modification/PCM/other | 16 | |
| ICD | 2 | |
| RFA | 1 | |
| AVR | 1 | |
| EPS (Phase II) | PMK | 97 |
| ICD | 14 | |
| RFA | 1 | |
| No active treatment | 1 | |
| ILR implantation (Phase III) | PMK | 41 |
| ICD | 3 | |
| RFA | 1 | |
| No active treatment | 51 | |
EPS, electrophysiological study; ILR, implantable loop recorder; PMK, pacemaker implantation; ICD, implantable cardioverter defibrillator; RFA, radiofrequency catheter ablation; AVR , aortic valve replacement; PCM, physical counterpressure manoeuvres.
Endpoints
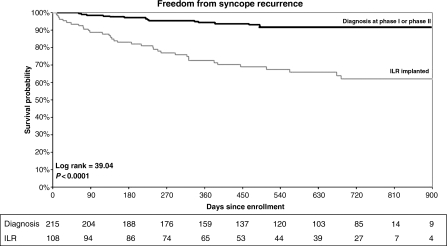
Of the 215 patients in whom diagnosis was achieved at Phase I or II and who were treated according to the findings, a syncopal recurrence was observed in 15 (7%). In contrast, syncope recurred in 36 of 108 (33%) patients in whom an ILR was implanted (P < 0.001; Figure 3).
Figure 3.
The Kaplan–Meier survival curve of syncopal recurrences in patients in which the diagnosis was achieved at Phase I or II and were treated according to the diagnosis (black line) and those in which an ILR was implanted after a negative work-up in Phase I or II. Patients diagnosed and treated had a significant reduction or syncopal recurrence when compared with those with an ILR implanted.
At follow-up, 5 of 14 (36%) patients in whom an ICD was implanted due to inducible VT at EPS had appropriate discharges.
The following arrhythmias were recorded with ILR (Table 2): A-V block (36 patients: 20 during a syncopal episode and 16 asymptomatic); asystole (5 patients); VT or ventricular fibrillation (3 patients), and an episode of rapid atrial fibrillation followed by sudden asystole in 1 patient.
Twenty patients (6%) died at follow-up: 6 due to no cardiac causes; 7 due to cardiac non-arrhythmic causes (5 due to heart failure and 1 due to acute aortic dissection); 3, all with implanted pacemakers, due to unknown causes; 1 had a sudden, undocumented syncope with a subdural hematoma, and 3 had sudden death (all 3 with ILR; in 2, the device was not interrogated, and in 1, a ventricular fibrillation was documented). There was no difference in mortality rate between patients diagnosed at Phase I or II, who received appropriate treatment, compared with those who had implanted ILR (6.0 vs. 6.5%, P = 0.878).
No differences were found in mortality or syncope recurrence with respect to the presence or absence of structural heart disease, or the type of BBB.
Discussion
The main finding in this study is that in patients with syncope, BBB, and preserved LVEF, the application of a systematic diagnostic strategy in accordance with ESC guidelines achieves a high rate of diagnosis (82.6%) with a low rate of mortality (6%), allowing clinicians to institute aetiology-specific treatment.
As expected, the most common cause of syncope in these patients was bradyarrhythmia, mostly due to paroxysmal A-V block. However, following this diagnostic strategy, other aetiologies of syncope were recognized in 17.6% of this population, such as CSS, neurally mediated, or drug-related syncope, or syncope secondary to VT, SVT, or cardiopulmonary disease. In addition, an arrhythmic cause could be ruled out in several patients who had a syncopal event documented by ILR.
The initial evaluation achieved a diagnosis in 25% of the studied population. Although the most frequent diagnosis at EPS was a bradyarrhythmia (76%), VT or SVT was induced in 14%. The role of EPS in patients with BBB and preserved LVEF has been discussed. The documentation of prolonged HV interval15 or infrahisian block with progressive atrial pacing16 or after drug challenge with class IC antiarrhythmic drugs17 has been identified as a marker for progression to A-V block, with an acceptable specificity, but with low sensitivity. In addition, the role of programmed ventricular stimulation in these patients is controversial. Although Englund et al.18 found that the induction of VT did not predict the occurrence of a ventricular arrhythmia at follow-up, Olshansky et al.19 found that inducibility of VT increased the risk of sudden death at follow-up and Link et al.20 found that the absence of inducibility, especially in patients with preserved LVEF, identified a group of patients with low risk of sudden death at follow-up. In our study population, the recurrence rate of syncope in patients treated according to the diagnoses achieved at Phases I and II was low, suggesting that those findings were specific. In addition, the rate of appropriate discharges in patients who received an ICD due to VT inducibility at EPS was similar to the discharge rate described in different published series in patients with ICD.21,22 This similarity suggests that in our study, inducibility identified the patients who were at risk of developing VT at follow-up. However, the suggestion that the sensitivity of EPS is relatively low was confirmed, in our study, by the fact that in 45% of the patients with a negative EPS, an arrhythmia was still documented by ILR. Again, ILR showed that bradyarrhythmia was the most common cause of syncope in these patients, but it allowed us to recognize some patients with VT, and also identified a non-arrhythmic cause of syncope in some patients who otherwise may never had been identified.
The potential risk of sudden death or severe cardiovascular events may be a concern associated following this strategy. In fact, several series suggested that the presence of abnormal ECG or severe structural heart disease and specifically depressed LVEF are risk factors for death or severe cardiovascular events in short-term follow-up,1,3–5,23 The relatively low mortality rate in this older population can be attributed to the fact that patients with depressed LVEF were probably not included and received an ICD. This explains why the mean LVEF is 56 ± 12, with very few patients with LVEF lower than 40%. In addition, most deaths were due to non-cardiac or non-arrhythmic causes. Some patients who received an implanted pacemaker died of unexplained causes, suggesting that bradyarrhythmia was not the cause of death in these patients. Admittedly, three patients died with an ILR implanted: in at least one of them, a ventricular fibrillation was documented that would not have been prevented by a pacemaker; in the other two, the cause of death remains unknown.
It can be argued that with a longer follow-up, some more patients would have syncopal recurrence or asymptomatic arrhythmias recorded by ILR, allowing clinicians to increase the number of patients with a final diagnosis.24–27 This is true, but in any case reinforces the value of this diagnostic strategy, encouraging clinicians to monitor these patients and not to initiate any treatment until a definite diagnosis is achieved.
The study was not designed to determine whether this diagnostic strategy was better than implanting a pacemaker in the majority of patients, and consequently, we cannot declare which option might be better. Owing to the low mortality and low syncopal recurrence rate observed when following this strategy in this specific population, only a controlled trial including a great number of patients would be able to answer this question.
Admittedly, some patients were excluded from the study because in those patients the suggested algorithm was not followed. As this was an observational study, investigators could not be forced to follow the protocol in all patients, and a selection bias cannot be excluded. However, demographic and clinical characteristics of excluded patients were similar to those included patients, decreasing the probability of a selection bias.
In conclusion, in patients with syncope, BBB, and relatively preserved LVEF, a diagnostic strategy consisting of initial clinical evaluation, followed by EPS and, if negative, implantation of an ILR, achieves a high rate of aetiological diagnosis. The high diagnosis rate in turn allows clinicians to select a specific treatment and to avoid unnecessary pacemaker implantation without a high rate of cardiovascular events or mortality. Whether this strategy is better than the strategy of implanting pacemakers into all patients with this clinical profile cannot be determined with current data.
Funding
This study was officially endorsed by the Spanish Society of Cardiology and was supported by a grant from Medtronic Europe. Funding to pay the Open Access publication charges for this article was provided by Medtronic, Inc.
Conflict of interest: X.N. is an employee of Medtronic Iberica.
Appendix
The following persons participated in the B4 study.
Coordinating Committee: R.G.-C. (Chair), A.M., F.A., M. Brignole, J.B., C.M., and X.N. Database electronic management: Remote Data Entry System, SL, Barcelona, Spain. Clinical monitors: M.P. López, G. Monzón, and N. Grovale; Statistical analysis: M. Martín, T. de Santo.
Centres and investigators (in the order of number of recruited patients): Ospedale S. Giuseppe, Empoli, Italy: A.D.R.; Hospital Clinic, Barcelona: J.B., L.M., I. Molina; Hospital General, Castellón, Spain: A.B.-N.; Hospital General, Albacete, Spain: J.G.-S.; Hospital Clínico, Valencia, Spain: R.G.-C., R.R.-G.; Ospedale Maggiore della Carità, Novara, Italy: M.B., E. Occhetta; Complejo Hospitalario de Ciudad Real, Ciudad Real, Spain: J. Benezet; Hospital Virgen de la Arrixaca, Murcia, Spain: J. Lacunza, A. García-Alberola; Arrhythmologic Centre, Ospedali del Tigullio, Lavagna, Italy: M. Brignole, F.C.; Hospital Vall d'Hebron, Barcelona, Spain: A.M., N. Rivas; Arcispedale Santa Maria La Nuova, Reggio Emilia, Italy: C.M.; Ospedale Grassi, Ostia, Italy: F.A.; Ospedale Grassi, Ostia, Italy: M. Santini; Hospital Municipal, Badalona, Spain: F. Planas; Hospital Virgen de las Nieves, Granada, Spain: M. Alvarez, L. Tercedor; Hospital Miguel Servet, Zaragoza, Spain: A. Asso; Hospital Xeral Cíes, Vigo, Spain: X. Beiras, E. García; Ospedale Umberto I, Mestre, Italy: F. Giada, A. Raviele; Hospital Lluis Alcañiz, Xativa, Spain: M. Rodriguez; Hospital Juan Ramón Jiménez, Huelva, Spain: R. Barba; Ospedale Santo Spirito in Sassia, Roma, Italy: L. Pandolfo, A. Porzio; Ospedale S. Camillo De Lellis, Rieti, Italy: S. Orazi; Hospital Puerta de Hierro, Madrid, Spain: I. Fernandez-Lozano, J. Toquero; Hospital Virgen del Rocío, Sevilla, Spain: G. Barón, F. Errázquin, A. Pedrote; Hospital Santa María, Lleida, Spain: J. Tomás; Clinica Cardiologica Universita de Padova, Padova, Italy: G. Buja, A. Folino; Ospedale San Gerardo del Tintori, Monza, Italy: S. De Ceglia, A. Vicenti; Hospital Clínico San Carlos, Madrid, Spain: J. Villacastín; Ospedale Careggi, Firenze, Italy: A. Ungar; Hospital General, Valencia, Spain: J. Roda, V. Palanca; Hospital La Paz, Madrid, Spain. J.L. Merino, R. Peinado; Hospital Ramón y Cajal, Madrid, Spain: A.H. Madrid; C. Moro; Hospital del Mar, Barcelona, Spain: J. Martí.
References
- 1.McAnulty JH, Rahimtoola SH, Murphy E, DeMots H, Ritzmann L, Kanarek PE, Kauffman S. Natural history of high risk bundle branch block: final report of a prospective study. N Engl J Med. 1982;307:137–143. doi: 10.1056/NEJM198207153070301. doi:10.1056/NEJM198207153070301. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Menozzi C, Moya A, Garcia-Civera R, Mont L, Alvarez M, Errazquin F, Beiras J, Bottoni N, Donateo P. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation. 2001;104:2045–2050. doi: 10.1161/hc4201.097837. doi:10.1161/hc4201.097837. [DOI] [PubMed] [Google Scholar]
- 3.Martin TP, Hanusa BH, Kapoor WN. Risk stratification of patients with syncope. Ann Emerg Med. 1997;29:459–466. doi: 10.1016/s0196-0644(97)70217-8. doi:10.1016/S0196-0644(97)70217-8. [DOI] [PubMed] [Google Scholar]
- 4.Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio) Study Investigators. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J. 2003;24:811–819. doi: 10.1016/s0195-668x(02)00827-8. doi:10.1016/S0195-668X(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 5.Del Rosso A, Ungar A, Maggi R, Giada F, Petix NR, De Santo T, Menozzi C, Brignole M. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1620–1626. doi: 10.1136/hrt.2008.143123. doi:10.1136/hrt.2008.143123. [DOI] [PubMed] [Google Scholar]
- 6.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. doi:10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 7.Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Granell RR, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. doi:10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabrizi F, Rosenqvist M, Bergfeldt L, Englund A. Time relation between a syncopal event and documentation of atrioventricular block in patients with bifascicular block: clinical implications. Cardiology. 2007;108:138–143. doi: 10.1159/000096038. doi:10.1159/000096038. [DOI] [PubMed] [Google Scholar]
- 9.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, Gasparini M, Linde C, Morgado FB, Oto A, Sutton R, Trusz-Gluza M European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2007;28:2256–2295. doi: 10.1093/eurheartj/ehm305. doi:10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- 10.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. J Neurol Sci. 1996;144:218–219. doi:10.1016/S0022-510X(96)00206-7. [PubMed] [Google Scholar]
- 11.Brignole M, Vardas P, Hoffman E, Huikuri H, Moya A, Ricci R, Sulke N, Wieling W. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11:671–687. doi: 10.1093/europace/eup097. doi:10.1093/europace/eup097. [DOI] [PubMed] [Google Scholar]
- 12.Moya A, Brignole M, Sutton R, Menozzi C, Garcia-Civera R, Wieling W, Andresen D, Benditt DG, Garcia-Sacristán JF, Beiras X, Grovale N, Vardas P. Reproducibility of electrocardiographic findings in patients with suspected reflex neurally-mediated syncope. Am J Cardiol. 2008;102:1518–1523. doi: 10.1016/j.amjcard.2008.07.043. doi:10.1016/j.amjcard.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 13.Krahn AD, Klein GL, Tee R, Skanes AC. Detection of asymptomatic arrhythmias in unexplained syncope. Am Heart J. 2004;148:326–332. doi: 10.1016/j.ahj.2004.01.024. doi:10.1016/j.ahj.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Ermis C, Zhu AX, Pham S, Li JM, Guerrero M, Vrudney A, Hiltner L, Lu F, Sakaguchi S, Lurie KG, Benditt DG. Comparison of automatic and patient activated arrhythmia recordings by implantable loop recorders in the evaluation of syncope. Am J Cardiol. 2003;92:815–819. doi: 10.1016/s0002-9149(03)00889-0. doi:10.1016/S0002-9149(03)00889-0. [DOI] [PubMed] [Google Scholar]
- 15.Scheinman MM, Peters RW, Morady F, Sauvé MJ, Malone P, Modin G. Electrophysiologic studies in patients with bundle branch block. Pacing Clin Electrophysiol. 1983;6:1157–1165. doi: 10.1111/j.1540-8159.1983.tb04453.x. doi:10.1111/j.1540-8159.1983.tb04453.x. [DOI] [PubMed] [Google Scholar]
- 16.Petrac D, Radić B, Birtić K, Gjurović J. Prospective evaluation of infrahisal second-degree AV block induced by atrial pacing in the presence of chronic bundle branch block and syncope. Pacing Clin Electrophysiol. 1996;19:784–792. doi: 10.1111/j.1540-8159.1996.tb03360.x. doi:10.1111/j.1540-8159.1996.tb03360.x. [DOI] [PubMed] [Google Scholar]
- 17.Englund A, Bergfeldt L, Rosenqvist M. Disopyramide stress test: a sensitive and specific tool for predicting impending high degree atrioventricular block in patients with bifascicular block. Br Heart J. 1995;74:650–655. doi: 10.1136/hrt.74.6.650. doi:10.1136/hrt.74.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Englund A, Bergfeldt L, Rehnqvist N, Astrom H, Rosenqvist M. Diagnostic value of programmed ventricular stimulation in patients with bifascicular block: a prospective study of patients with and without syncope. J Am Coll Cardiol. 1995;26::1508–1515. doi: 10.1016/0735-1097(95)00354-1. doi:10.1016/0735-1097(95)00354-1. [DOI] [PubMed] [Google Scholar]
- 19.Olshansky B, Hahn EA, Hartz VL, Prater SP, Mason JW. Clinical significance of syncope in the electrophysiologic study versus electrocardiographic monitoring (ESVEM) trial. The ESVEM Investigators. Am Heart J. 1999;137:878–886. doi: 10.1016/s0002-8703(99)70412-6. doi:10.1016/S0002-8703(99)70412-6. [DOI] [PubMed] [Google Scholar]
- 20.Link MS, Kim KM, Homoud MK, Estes NA, 3rd, Wang PJ. Long-term outcome of patients with syncope associated with coronary artery disease and a non diagnostic electrophysiologic evaluation. Am J Cardiol. 1999;83:1334–1337. doi: 10.1016/s0002-9149(99)00096-x. doi:10.1016/S0002-9149(99)00096-X. [DOI] [PubMed] [Google Scholar]
- 21.Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, Kralovec S, Sediva L, Ruskin JN, Josephson ME. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357:2657–2665. doi: 10.1056/NEJMoa065457. doi:10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamphuis HC, de Leeuw JR, Derksen R, Hauer RN, Winnubst JA. Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace. 2003;5:381–389. doi: 10.1016/s1099-5129(03)00078-3. doi:10.1016/S1099-5129(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 23.Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med. 1996;100:646–55. doi: 10.1016/s0002-9343(95)00052-6. doi:10.1016/S0002-9343(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 24.Krahn AD, Klein GJ, Yee R, Takle-Newhouse T, Norris C. Use of an extended monitoring strategy in patients with problematic syncope. Reveal Investigators. Circulation. 1999;99:406–410. doi: 10.1161/01.cir.99.3.406. [DOI] [PubMed] [Google Scholar]
- 25.Moya A, Brignole M, Menozzi C, Garcia-Civera R, Tognarini S, Mont L, Botto G, Giada F, Cornacchia D International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with isolated syncope and in patients with tilt-positive syncope. Circulation. 2001;104:1261–1267. doi: 10.1161/hc3601.095708. doi:10.1161/hc3601.095708. [DOI] [PubMed] [Google Scholar]
- 26.Solano A, Menozzi C, Maggi R, Donateo P, Bottoni N, Lolli G, Tomasi C, Croci F, Oddone D, Puggioni E, Brignole M. Incidence, diagnostic yield and safety of the implantable loop-recorder to detect the mechanism of syncope in patients with and without structural heart disease. Eur Heart J. 2004;25:1116–1119. doi: 10.1016/j.ehj.2004.05.013. doi:10.1016/j.ehj.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Menozzi C, Brignole M, Garcia-Civera R, Moya A, Botto G, Tercedor L, Migliorini R, Navarro X International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with heart disease and negative electrophysiologic test. Circulation. 2002;105:2741–2745. doi: 10.1161/01.cir.0000018125.31973.87. doi:10.1161/01.CIR.0000018125.31973.87. [DOI] [PubMed] [Google Scholar]