Abstract
Stress interacts with addictive processes to increase drug use, drug seeking, and relapse. The hippocampal formation (HF) is an important site at which stress circuits and endogenous opioid systems intersect and likely plays a critical role in the interaction between stress and drug addiction. Our prior studies demonstrate that the stress-related neuropeptide corticotropin-releasing factor (CRF) and the delta-opioid receptor (DOR) colocalize in interneuron populations in the hilus of the dentate gyrus and stratum oriens of CA1 and CA3. While independent ultrastructural studies of DORs and CRF receptors suggest that each receptor is found in CA1 pyramidal cell dendrites and dendritic spines, whether DORs and CRF receptors colocalize in CA1 neuronal profiles has not been investigated. Here, hippocampal sections of adult male and proestrus female Sprague-Dawley rats were processed for dual label pre-embedding immunoelectron microscopy using well-characterized antisera directed against the DOR for immunoperoxidase and against the CRF receptor for immunogold. DOR-immunoreactivity (-ir) was found presynaptically in axons and axon terminals as well as postsynaptically in somata, dendrites and dendritic spines in stratum radiatum of CA1. In contrast, CRF receptor-ir was predominantly found postsynaptically in CA1 somata, dendrites, and dendritic spines. CRF receptor-ir frequently was observed in DOR-labeled dendritic profiles and primarily was found in the cytoplasm rather than at or near the plasma membrane. Quantitative analysis of CRF receptor-ir colocalization with DOR-ir in pyramidal cell dendrites revealed that proestrus females and males show comparable levels of CRF receptor-ir per dendrite and similar cytoplasmic density of CRF receptor-ir. In contrast, proestrus females display an increased number of dual-labeled dendritic profiles and increased membrane density of CRF receptor-ir in comparison to males. We further examined the functional consequences of CRF receptor-ir colocalization with DOR-ir in the same neuron using the hormone responsive neuronal cell line NG108-15, which endogenously express DORs, and assayed intracellular cAMP production in response to CRF receptor and DOR agonists. Results demonstrated that short-term application of DOR agonist SNC80 inhibited CRF-induced cAMP accumulation in NG108-15 cells transfected with the CRF receptor. These studies provide new insights on opioid-stress system interaction in the hippocampus of both males and females and establish potential mechanisms through which DOR activation may influence CRF receptor activity.
Keywords: Opioids, Hippocampus, Hormones, Estrogen, Stress, Corticotropin releasing hormone, NG108-15, cAMP, SNC80
Introduction
Relapse to drug taking following abstinence is a major impediment to the treatment of addiction. Clinical studies in human addicts reveal that exposure to environmental stimuli associated with drug taking behavior elicits craving and can promote relapse (O’Brien et al., 1998; Bossert et al., 2005; Crombag et al., 2008). Moreover, several brain areas involved in learning and memory processes, including the hippocampal formation (HF), are activated in imaging studies during craving induced by drug-associated cues in human subjects (Kilts et al., 2001; Schneider et al., 2001; Risinger et al., 2005). Preclinical studies also demonstrate an important role for the HF in reinstatement of drug seeking behavior (Fuchs et al., 2005; Fuchs et al., 2007; Bossert et al., 2007; Rogers et al., 2008; Atkins et al., 2008) and learning in response to stress (McEwen & Milner, 2007; Bangasser & Shors, 2007; Dalla et al., 2009; McLaughlin et al., 2009). Stress has been shown to interact with addictive processes to increase drug use, drug seeking, and relapse (Shaham et al., 2000; Stewart, 2003; Saal et al., 2003; Sinha, 2007) and the relationship between stress and relapse to drug seeking behavior is particularly pronounced in females (Rubin et al., 1996; McKay et al., 1996; Elman et al., 2001). Thus, the role played by the hippocampal formation in the interaction between stress and drug addiction, particularly in females, requires further inquiry.
While several studies investigating the impact of stress on relapse vulnerability to opiate abuse have focused on interactions between endogenous opioid systems and the stress neurohormone corticotropin releasing factor (CRF) receptor in the locus coeruleus (Curtis et al., 2006; Valentino & Van Bockstaele, 2008; Reyes et al., 2008; Van Bockstaele et al., 2010), few studies have explored the relationship between these two systems in the HF. Prior studies in male rats indicate that CRF receptor mRNA is abundant in CA1 pyramidal neurons (Van et al., 2000; Justice et al., 2008). CRF receptor immunoreactivity (ir) also is present in CA1 pyramidal neurons in the rodent hippocampus (Chen et al., 2000; Hermann & Lutz, 2005) and ultrastructural studies using post-embedding electron microscopy revealed CRF receptor-ir concentrated at asymmetric postsynaptic densities of CA1 pyramidal cell dendritic spines (Chen et al., 2004). Similarly, studies in rats demonstrate that delta opioid receptor (DOR)-ir is found postsynaptically on pyramidal cell dendrites and dendritic spines in CA1 (Commons & Milner, 1997; Williams et al., 2011) and DOR mRNA is present in CA1 (George et al., 1994; Stumm et al., 2004). Although mu opioid receptors (MORs) are also found in the CA1, these receptors are exclusively localized to interneurons and not pyramidal cell dendrites and dendritic spines (Drake & Milner, 1999; Drake & Milner, 2002). Functionally, both DORs (Piguet & North, 1993; Bramham & Sarvey, 1996; Svoboda et al., 1999; Drake et al., 2007; Bao et al., 2007) and the CRF receptor (Aldenhoff et al., 1983; Wang et al., 1998; Blank et al., 2002; Schierloh et al., 2007) affect excitatory transmission and the induction of synaptic plasticity in the hippocampus. Reports also indicate that the CRF receptor (Shaham et al., 1997; Brown et al., 2009; Shalev et al., 2010) and DOR (Marinelli et al., 2007; Marinelli et al., 2009) play a role in reinstatement of drug seeking behavior in animal models of addiction. Interestingly, in vivo administration of CRF is neuroprotective in CA1 neurons and improves spatial memory via a mechanism involving DOR activation (Charron et al., 2008a; Charron et al., 2009). Thus, the relationship between DORs and CRF receptors in the CA1 merits direct study.
As prior reports suggest that the CRF receptor and the DOR may localize to similar neuronal populations and subcompartments within CA1 lamina, the present study sought to confirm these observations in males and extend them, where applicable, to females. Dual label immunoelectron microscopy was employed to assess CRF receptor-ir and trafficking in CA1 pyramidal neurons of male rats in comparison to normal cycling proestrus female rats, as circulating estrogen levels are highest in proestrus (Belanger et al., 1981). Prior studies in our laboratory and others, particularly regarding ovarian steroid modulation of opioids and opioid receptors in the hippocampus, confirm that morphological changes induced by exogenously supplied estradiol reflect changes observed during proestrus in normal cycling females (Woolley & McEwen, 1992; Wilson et al., 2002; Torres-Reveron et al., 2008; Torres-Reveron et al., 2009a; Torres-Reveron et al., 2009b). Furthermore, as seven-transmembrane domain G protein coupled receptors (GPCRs), DORs and CRF receptors couple to distinct G proteins and demonstrate opposing effects on adenylyl cyclase activity and resulting cAMP production (Quock et al., 1999; Eckart et al., 2002). Thus, we further examined the functional consequences of CRF receptor colocalization with DORs in the same neuronal population using the hormone responsive neuronal cell line NG108-15, which endogenously express DORs (Barg et al., 1984; Kieffer et al., 1992), and assayed intracellular cAMP production in response to CRF receptor and DOR agonists.
Materials and methods
Animals and estrous cycle determination
Adult male (275 - 325 g; upon arrival approximately 60 days old) and female (225 - 250 g; upon arrival approximately 60 days old) Sprague Dawley rats from Charles River Laboratories (Wilmington, MA) were pair-housed with ad libitum access to food and water and with 12:12 light/dark cycles (lights on 0600 - 1800). All procedures were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. Female rats were allowed to acclimate for one week after which estrous cycle phase was determined using vaginal smear cytology (Turner & Bagnara, 1971; Marcondes et al., 2002). Only female rats that showed two consecutive, regular, 4-5 day estrous cycles were included in the study. Animals in the proestrus phase of the estrous cycle were analyzed in comparison to males. While vaginal smear cytology was the main method used to determine estrous cycle phase, phases were further verified by measuring uterine weights and plasma estradiol levels from blood samples collected from the heart immediately prior to the perfusion procedure. Plasma serum levels of estradiol were determined by radioimmunoassay using a Coat-A-Count kit from Diagnostics Products Corporation (Los Angeles, CA) with a sensitivity of 8 pg/ml for estradiol. The proestrus females used in the current study have been used in prior studies with previously reported estradiol levels and uterine weights (Williams et al., 2011).
Antisera
A goat polyclonal antibody directed against the 425-444 amino acid sequence of the carboxy terminus of the CRF receptor precursor of human origin, which is identical to the corresponding sequence in rat (C-20 sc-1757, Santa Cruz Biochemical, CA), was used in dual label immunoelectron microscopy studies. Preadsorption with the antigenic peptide sequence resulted in absence of labeling in immunoblots (Chen et al., 2000; Bangasser et al., 2010) and tissue sections (Sauvage & Steckler, 2001; Reyes et al., 2006) and no detectable labeling was observed for this antisera in CRF receptor knockout mice (Waselus et al., 2009), indicating specificity. A rabbit polyclonal antibody raised against N-terminal amino acids 3-17 of the DOR (Chemicon, Temecula, CA) also was used in dual label immunoelectron microscopy studies. This antibody has been previously used for Western blot, confocal, as well as ultrastructural studies and preimmune sera and preadsorption controls resulted in no detectable labeling (Commons & Milner, 1996; Commons & Milner, 1997; Persson et al., 2000; Persson et al., 2005; Saland et al., 2005)(Supplemental Fig. 1). For immunofluorescence studies, a guinea pig polyclonal antiserum raised against amino acids 34-48 of the DOR was used, with previously characterized specificity by immunoblot, preadsorption, and immunocytochemical controls (Cheng et al., 1995; Svingos et al., 1995; Commons & Milner, 1996). A rabbit monoclonal antibody recognizing the FLAG peptide sequence in N- or C-terminal FLAG fusion proteins was purchased from Sigma-Aldrich (F2555; Saint Louis, MO) for use in immunofluorescence studies.
Section preparation
Rats were deeply anesthetized with sodium pentobarbital (150 mg/kg) in the morning (between 9:30 and 11:30 am) and their brains fixed by aortic arch perfusion with 3.75% acrolein and 2% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.6) (Milner & Veznedaroglu, 1992; Milner et al., 2001). The brains were removed from the skull and cut into 5 mm coronal blocks using a brain mold (Activational Systems, Inc.), and postfixed for 30 minutes in 2% paraformaldehyde in PB. The brains were sectioned on a Leica Vibratome (40 μm thick) and stored in cryoprotectant (30% sucrose and 30% ethylene glycol in PB) until immunocytochemical processing. Prior to immunocytochemistry, coronal sections of all groups were rinsed in PB, coded with hole-punches and pooled into single crucibles to insure identical exposure to immunoreagents (Pierce et al., 1999). Sections then were treated with 1% sodium borohydride in PB for 30 minutes to neutralize free aldehydes and rinsed in PB.
Electron microscopic immunocytochemistry
For dual label electron microscopic localization of the DOR with the CRF receptor, sections were labeled for the DOR using immunoperoxidase and the CRF receptor using immunogold through methods that have been described previously (Towart et al., 2003; Torres-Reveron et al., 2009b). To enhance reagent penetration, sections were soaked in a cryoprotectant solution (25% sucrose and 3.5% glycerol in 0.05M PB) for 15 min, and rapidly freeze-thawed by sequential immersion in liquid chlorodifluoromethane (Freon, Refron Inc., NY), liquid nitrogen, and PB at room temperature. Sections then were processed for immunocytochemistry using the avidin-biotin complex (ABC) - peroxidase technique (Hsu et al., 1981). For this, sections from each group were rinsed in Tris-buffered saline (TS; pH 7.6) and incubated in 0.5% bovine serum albumin (BSA) in TS for 30 min. Sections then were incubated in an antisera cocktail of rabbit polyclonal DOR (1:1500) with goat polyclonal CRF receptor (1:100) in 0.1% BSA in TS for 72 hrs at 4°C and then processed through 1) a 1:400 dilution of biotinylated donkey anti-rabbit immunoglobulin (IgG) (Jackson Immunoresearch Labs, Inc., West Grove, PA), 30 min; 2) a 1:100 dilution of peroxidase-avidin complex (Vectastain Elite Kit, Vector Laboratories, Burlingame, CA), 30 min and 3) 3,3′-diaminobenzidine (DAB; Sigma, St. Louis, MO) and H2O2 in TS for 4-6 minutes. All incubations were separated by washes in TS.
To visualize the CRF receptor, sections next were processed with the silver-enhanced immunogold technique (Chan et al., 1990). For this, sections were rinsed in TS and incubated in a 1:50 dilution of donkey anti-goat IgG conjugated to 1-nm gold particles (Electron Microscopy Sciences, EMS, Washington, PA) in 0.001% gelatin and 0.08% BSA in PBS overnight at 4°C. Sections were rinsed in PBS, postfixed in 1.25% glutaraldehyde in PBS for 10 min, rinsed again in PBS followed by 1.2% sodium citrate buffer (pH 7.4). The conjugated gold particles were enhanced by incubation in silver solution (IntenSE; Amersham) for 3-5 min. Sections were fixed 1 hr in 2% osmium tetroxide, dehydrated in ascending concentrations of ethanols and propylene oxide, and embedded in EMBed 812 (EMS) between two sheets of Aclar plastic (Honeywell, Pottsville, PA). Ultra-thin sections (70-72 nm thick) through the dorsal CA1 (AP −3.6 to −4.0 from Bregma (Swanson, 2000)) were cut on a Leica UCT ultratome. Sections were counterstained with uranyl acetate and Reynold’s lead citrate and examined with a Philips CM10 transmission electron microscope equipped with an Advanced Microscopy Techniques digital camera (Danvers, MA).
Electron microscopic data analysis
Ultra-thin sections from 6 rats in proestrus and 5 male rats were analyzed. Immunoperoxidase labeling for the DOR was distinguished as an electron-dense reaction product precipitate. Silver-intensified immunogold (SIG) labeling for the CRF receptor appeared as intense black electron-dense particles located within cytoplasmic compartments or on the plasma membrane. Profiles were considered dual labeled if they contained electron-dense reaction product and at least two SIG particles. To circumvent problems due to differences in antibody penetration, images were taken from the plastic-tissue interface (i.e., the most superficial portion of the tissue section). Micrographs containing CRF receptor SIG particles at magnifications of 10,500× to 19,000× were analyzed in a total tissue area of 6,050 μm2 per animal in stratum radiatum of CA1. Profiles containing CRF receptor SIG particles and DOR-ir were classified as neuronal processes (dendrites, dendritic spines, axon terminals) or astrocytes based on criteria described in (Peters et al., 1991).
As dual labeling was particularly abundant in dendritic profiles, further quantitative analysis of CRF receptor SIG particles in DOR-labeled proximal dendrites (average diameter > 1.0 μm) was carried out to determine the distribution and density of CRF receptor-SIG particles in plasmalemmal and cytoplasmic compartments. For this, CRF receptor-immunogold particle localization was recorded as either cytoplasmic, plasmalemmal or “near plasma membrane” (i.e., particles within 50 nM, but not touching, the plasma membrane). Several ratios were calculated based on these designations: 1) plasma membrane SIG particles to total number of SIG particles (PL:Total); 2) near plasma membrane SIG particles to total number of SIG particles (Near:Total); and 3) cytoplasmic SIG particles to total number of SIG particles (CY:Total). Morphological parameters that were used as indirect measures of dendrite size, including surface area (perimeter), cross-sectional area, and average diameter, were measured using Microcomputer Imaging Device software (MCID, Imaging Research Inc, Ontario, Canada). These measurements were used to determine the number of plasmalemmal CRF receptor-SIG particles in a profile / profile perimeter (PL:μm) and the number of cytoplasmic CRF receptor-SIG particles in a profile / profile cross-sectional area (CY:μm2).
Cell culture and plasmid expression constructs
NG108-15 cells obtained from American Type Culture Collection were cultured in 5% CO2 atmosphere at 37°C and maintained in Dulbecco’s modified eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS; Sigma-Aldrich) and 1% PenStrep (100 U/ml penicillin G sodium and 100 μg/ml streptomycin sulfate; Invitrogen). For transfections, cells were seeded in DMEM supplemented with 5% FBS.
The plasmid construct (sp)FL-r/CRFr1 was provided by Dr. Greti Aguilera at the National Institute of Child Health and Human Development, National Institutes of Health, MD (Young et al., 2007). The construct contains the full-length 1.1 kb rat CRFr1 cDNA with a FLAG (DYKDDDDK) epitope tag inserted after the endogenous N-terminal signal peptide (sp) sequence at amino acid 31 and was used as a template for further subcloning. First, to generate the expression construct (ppt)4×FL-r/CRFr1-myc, PCR primers were designed to amplify the CRFr1 cDNA from the FLAG sequence to the stop codon with the endogenous stop codon intentionally eliminated by a point mutation. The amplified product was then cloned into the vector pCR4-TOPO (Invitrogen) to be validated with bi-directional sequencing. The FL-r/CRFr1 sequence was then subcloned into the expression vector 3×FL-CMV25-myc (Sigma-Aldrich). This vector contains an N-terminal pre-pro-trypsin (ppt) leader sequence followed by three copies of the FLAG epitope tag. Furthermore, 3′ of the cloning site is a C-terminal myc epitope tag coding sequence followed by an exogenous stop codon. Altogether, four FLAG-tags therefore precede the r/CRFr1 coding sequence and one myc-tag follows the coding sequence. The final construct, (ppt)4×FL-r/CRFr1-myc, was transfected into NG108-15 cells used in immunocytochemical studies.
Second, to generate the (sp)FL-r/CRFr1-eGFP expression construct, the original Aguilera template was used again, but this time, the forward PCR primer included the endogenous signal peptide sequence and the inserted FLAG sequence. The (sp)FL-r/CRFr1 sequence was amplified again without the stop codon and cloned into pCR4-TOPO (Invitrogen) for sequence validation. The CRF receptor was then subcloned in-frame into pEGFP-N1 (Clontech, Mountain View, CA) for enhanced green fluorescent protein (eGFP). The final construct, (sp)FL-r/CRFr1-eGFP, was transfected into NG108-15 cells used in intracellular cAMP assays.
Immunofluorescence labeling of NG108-15 cells
NG108-15 cells were plated on poly-L-lysine-coated 18 mm coverslips in 12 well plates at a density of 4 × 103 cells per well for differentiation as previously described (Akama & McEwen, 2003). Twenty-four hrs after plating, cells were washed in warm PBS and media replaced with differentiating medium, consisting of Neurobasal medium supplemented with B27 (Invitrogen) and 1 mM dibutyryl cAMP (D0627; Sigma-Aldrich). Cells were allowed to differentiate for 5 d before transfection with the (ppt)4×FL-r/CRFr1-myc construct in Opti-MEM (Invitrogen) using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocols and allowed to grow at 37°C for 3 d. These differentiated NG108-15 cells demonstrate distinct neuronal morphology of arborized projections that contact extensions from other neurons (Akama & McEwen, 2003). For immunocytochemistry, transfected and untransfected NG108-15 cells were fixed in 4% PFA in PBS for 10 min, rinsed in PBS and incubated in: 1) 0.1% triton (TX) in PBS, 1 min; 2) 5% normal goat serum (NGS) in PBS for 1 hr; 3) a combination of guinea pig polyclonal DOR (1:1500) with rabbit monoclonal FLAG (1:3000) antisera in 5% NGS in PBS for 24 hrs at 4°C; and 4) a 1:600 dilution of goat anti-guinea pig Cy5 IgG (Jackson Immunoresearch Labs) and a 1:600 dilution of goat anti-rabbit Alexa Fluor 488 (Invitrogen) in 5% NGS in PBS for 1 hr at room temperature. All incubations were separated by washes in PBS. ProLong Gold antifade reagent with DAPI (Invitrogen) was used to mount NG108-15 coverslips on slides. As controls, these immunocytochemical procedures were utilized on cells with the omission of the primary or secondary antisera. Immunofluorescence images were acquired sequentially using a Nikon H550L microscope equipped with a Nikon Eclipse 90i camera. Alexa Fluor 488 (FLAG) was pseudocolored green and Cy5 (DOR) was pseudocolored red.
Cyclic AMP assays
Intracellular cAMP content was measured with the cAMP XP Assay Kit (4339; Cell Signaling Technology, Inc., Danvers, MA). Cells were seeded in 96-well plates at a density of approximately 104 cells per well in 100 μl media and allowed to grow for 24 hr at 37°C. The (sp)FL-r/CRFr1-eGFP construct was transfected into cells in Opti-MEM (Invitrogen) using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocols and cells were allowed to grow for 36-48 hrs at 37°C. On the day of an experiment, plates were washed in warm PBS and cells were incubated in 200 μl DMEM containing 0.5 mM 3-isobutyl-1-methylxanthine (IBMX; EMD Chemicals, In., Newark, NJ), an inhibitor of cyclic nucleotide phosphodiesterase, 30 min at 37°C to prevent the breakdown of accumulated cAMP. Cells then were incubated in 100 μl DMEM containing 0.5 mM IBMX ± 10 nM (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80; Tocris Bioscience, Ellisville, MO), a highly selective non-peptide DOR agonist (Calderon et al., 1994; Bilsky et al., 1995), for 30 min at 37°C. 100 μl DMEM containing 10 μM Forskolin (Tocris) or 100 nM CRF (Tocris) in 0.5 mM IBMX ± 10 nM SNC80 was added to each well and the incubation continued at 37°C for 15 min, at which time cells were washed in ice cold PBS, 100 μl lysis reagent was added to each well, and the plate was shaken on a microplate shaker for 10-15 min. From each sample, 50 μl from the cell culture plate was transferred into the appropriate well of the immunoassay microtiter plate. cAMP was measured using the manufacturer’s Enzyme Immuno Assay Kit Protocol (Cell Signaling Technology). Calculations were performed using SoftMax Pro 5.3 Software (Molecular Devices, Inc.) and data are expressed as a percentage of forskolin-stimulated cAMP levels. Samples were measured in duplicate or triplicate in three separate experiments (n=3).
Statistical Analysis and data presentation
Profile frequency was analyzed by Student’s t-test while CRF receptor-SIG particle density and distribution in DOR-ir dendritic profiles were analyzed by Kruskal-Wallis and Mann-Whitney U nonparametric statistical tests as these data were not normally distributed (Shapiro-Wilk p < 0.05) using SPSS for Windows V. 11.0 (SPSS). Intracellular cAMP production was analyzed by ANOVA. Pictures were formatted and adjusted for sharpness, brightness and contrast using Adobe Photoshop CS4 software (Adobe Systems, Inc., San Jose CA) and prepared in Microsoft Office PowerPoint 2007. Graphs were prepared with Graph Pad Prism 5.03 (Graph Pad Software, Inc., San Diego CA). All ultrastructural data in bar graphs are presented as mean ± SEM.
Results
CRF receptors colocalize with DORs in CA1 pyramidal cell dendrites
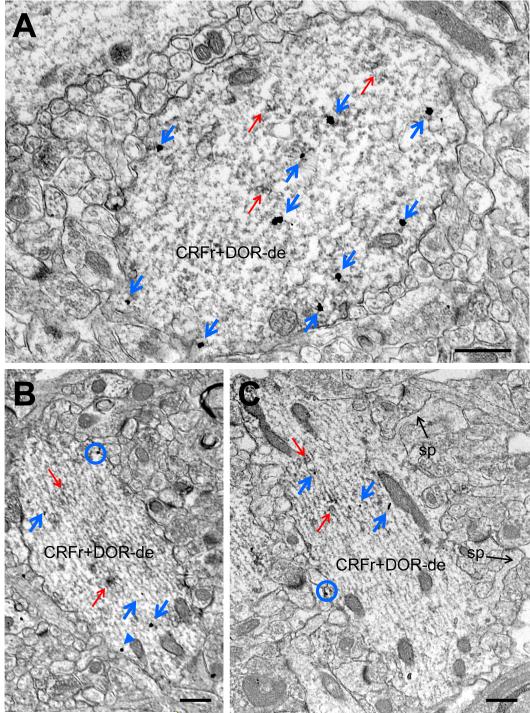
In agreement with previous studies in rats (Commons & Milner, 1997; Williams et al., 2011), DOR-ir was present in CA1 somata, dendrites and dendritic spines, and axon terminals. CRF receptor-ir was present in CA1 dendrites and dendritic spines, consistent with prior reports in rodents (Chen et al., 2000; Chen et al., 2004; Hermann & Lutz, 2005). In stratum radiatum of CA1, CRF receptor-ir was particularly abundant in DOR-labeled dendritic profiles (Fig. 1). Shafts of dual-labeled dendrites in stratum radiatum were usually oriented perpendicular to the pyramidal cell layer, received few synapses on the dendritic shaft, and were often visibly spiny (Fig. 1C). As these ultrastructural features are consistent in morphology with dendrites of pyramidal cells rather than interneurons (Harris et al., 1992), CRF receptor-ir colocalized with DOR-ir in pyramidal cell dendrites. Qualitatively, most DOR-labeled dendritic profiles contained CRF receptor-ir. Frequency analysis of dual-labeled dendritic profiles revealed that proestrus females had an increased number of DOR-labeled dendrites containing CRF receptor-ir in comparison to males (t=2.560, d.f.=9, p=0.031; Fig. 2A).
Fig. 1.
CRF receptor-ir is found in DOR-labeled dendrites in stratum radiatum of CA1. Representative electron micrographs from a male (A) and proestrus female (B, C) demonstrate CRF receptor-labeling in both cytoplasmic and plasmalemmal sites in spiny DOR-ir dendrites similar in morphology to pyramidal cells using the immunogold-silver technique. CRF receptor-SIG particles are found along the plasma membrane (blue circles), near the plasma membrane (blue arrowhead), or in the cytoplasm (blue arrows) of DOR-immunoperoxidase labeled (red arrows) dendrites. CRFr+DOR−De = dual-labeled dendrite, sp = spine. Scale bars = 500 nm.
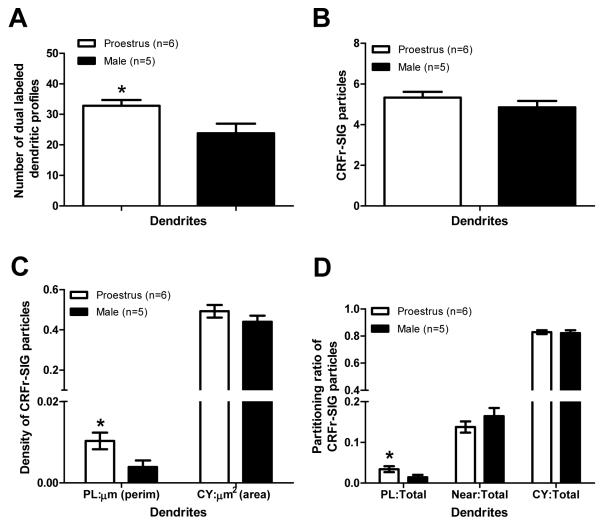
Fig. 2.
Ovarian hormones influence the distribution of CRF receptor-SIG particles in CA1 stratum radiatum dendrites containing DORs. (A) Proestrus females display an increased number of DOR-labeled dendrites containing CRF receptor-ir in comparison to males (*p<0.05). (B) The number of CRF receptor-SIG particles per DOR-labeled dendrite is similar in males and proestrus females. (C) Proestrus females have increased plasma membrane density of CRF receptor-SIG particles on DOR-labeled dendrites in comparison to males (*p<0.05). (D) The number of CRF receptor-SIG particles is increased on the plasma membrane of DOR-labeled dendrites in proestrus females in comparison to males (*p<0.05).
Ovarian hormones influence CRF receptor subcellular distribution in DOR-labeled pyramidal cell dendrites in SR of CA1
In CA1 stratum radiatum dendrites, most CRF receptor-labeling was in the cytoplasmic compartment with less than 20% of CRF receptor-SIG particles directly associated with the plasma membrane in males and proestrus females (Fig. 1, Fig. 2D). Quantitative ultrastructural analysis revealed that females had similar numbers of CRF receptor-SIG particles per dendrite in comparison to males (Mann-Whitney U Test, z=0.978, p=0.328; Fig. 2B). Moreover, proestrus females displayed similar density and number of CRF receptor-SIG particles in the cytoplasm in comparison to males (CY:μm2 z=0.482, p=0.630; CY:Total z=0.134, p=0.893; Fig. 2C, D). In contrast, proestrus females and males demonstrated significant differences in CRF receptors on the plasma membrane of DOR-labeled dendrites. Specifically, proestrus females displayed increased density of CRF receptor-SIG particles on the plasma membrane (z=2.210, p=0.027; Fig. 2C) as well as an increased number of CRF receptor-SIG particles on the plasma membrane (z=2.174, p=0.030; Fig. 2D) in comparison to males.
Inhibition of CRF receptor mediated signaling by DOR activation
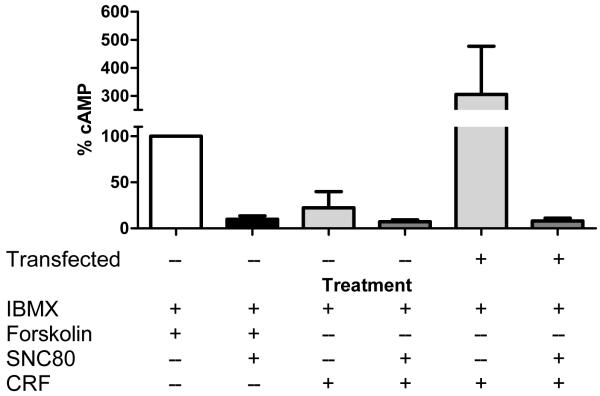
CRF-induced activation of CRF receptors leads to production of cAMP through stimulation of adenylyl cyclase by Gαs (De Souza, 1995; Eckart et al., 2002). Conversely, the DOR has been reported to inhibit adenylyl cyclase through a Gαi/o-mediated mechanism (Quock et al., 1999). A possible site of interaction between the CRF and opioid systems may therefore center upon cAMP production. NG108-15 cells are a useful in vitro model for specifically studying DOR interactions with other receptors as well as signal transduction mechanisms (Bergsbaken et al., 1993). We analyzed the effects of DOR and CRF receptor agonists in NG108-15 cells transfected with a CRF receptor FLAG fusion construct. As demonstrated in Fig. 3, transfected cells express both DORs and CRF receptors whereas untransfected cells only express DORs. Statistical analysis of cAMP assay results revealed a trend for differences among agonist treatments in untransfected and transfected NG108-15 cells (F(5,12) = 2.827, p = 0.0651). Specifically, transfected CRF receptors were functional, as shown by their ability to increase intracellular cAMP levels in NG108-15 cells stimulated with CRF (Fig. 4). CRF stimulation of untransfected NG108-15 cells produced little appreciable increase in intracellular cAMP levels. Activation of DORs, by the selective non-peptide agonist SNC80, attenuated forskolin-increased intracellular cAMP levels in untransfected (Fig.4) and transfected (not shown) cells. Moreover, DOR activation reduced CRF-induced intracellular cAMP levels in transfected NG108-15 cells (Fig. 4).
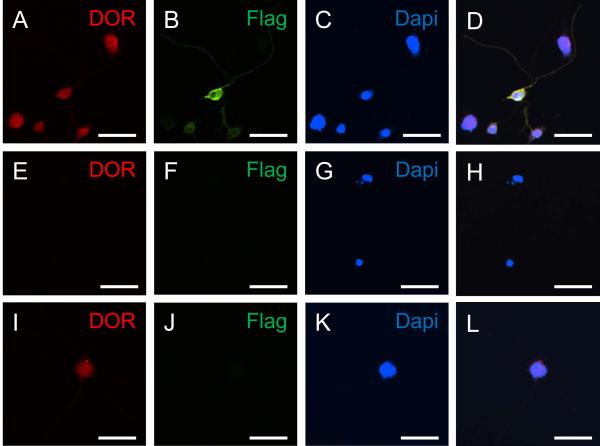
Fig. 3.
Immunofluorescent images illustrating colocalization of DOR (A, E, I) with CRF receptor (B, F, J) in NG108-15 cells. (A-D) NG108-15 cells transfected with the (ppt)4×FL-r/CRFr1-myc fusion construct display CRF receptor colocalization with DOR-labeled cells, where A, B, and C are unmerged images of D. (E-H) Omission of primary antisera in immunocytochemical processing of NG108-15 cells transfected with the (ppt)4×FL-r/CRFr1-myc fusion construct results in no detectable DOR or CRF receptor (FLAG) labeling, where E, F, and G are unmerged images of H. (I-L) Untransfected NG108-15 cells display DOR-labeling only, where I, J, and K are unmerged images of L. DOR-labeling is red. CRF receptor (FLAG)-labeling is green. DAPI is blue. Dual-labeled cells appear yellow. Scale bars = 50 μm.
Fig. 4.
CRF-induced increases in intracellular cAMP levels are mediated by the CRF receptor and attenuated by DOR activation. The cAMP levels were quantified in IBMX-pretreated transfected and untransfected cells after forskolin (10 μM), SNC80 (10 nM), and CRF (100 nM) treatment. Values are the mean of determinations made in duplicate or triplicate in three separate experiments and expressed as a percentage of forskolin-induced intracellular cAMP levels in untransfected NG108-15 cells.
Discussion
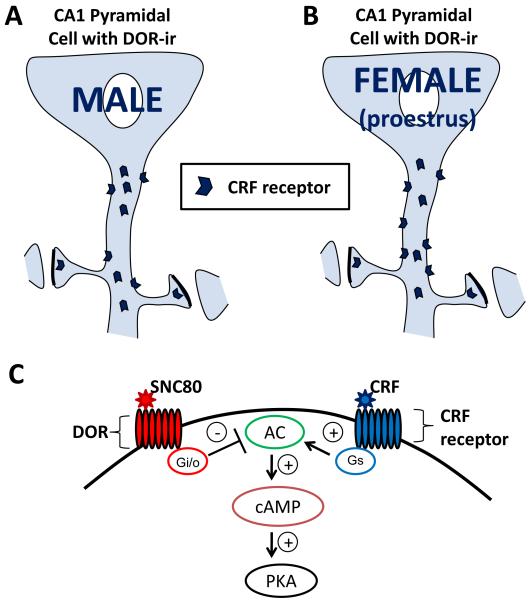
This study is the first to demonstrate that CRF receptors colocalize with DORs in hippocampal CA1 pyramidal cell dendrites. In addition, in comparison to males, proestrus females have an increased number of dendrites co-labeled with CRF receptor- and DOR-ir as well as increased density and number of CRF receptors localized on the plasma membrane of dual labeled dendrites (Fig. 5A-B). In vitro results indicate that activation of DORs attenuates CRF receptor mediated increases in intracellular cAMP levels, suggesting DOR modulation of CRF receptor signaling when both receptors are present in the same neuron (Fig. 5C).
Fig. 5.
Schematic diagram summarizing ovarian hormone influences on CRF receptor distribution in DOR-labeled dendrites within stratum radiatum of CA1. (A) In males, CRF receptors are frequently found in the cytoplasm and infrequently on the plasma membrane of DOR-labeled pyramidal cell dendrites. (B) In comparison to males, proestrus (high estrogen) females display increased number and density of CRF receptors on the plasma membrane of DOR-labeled dendrites. Taken together, these results suggest that when estrogen levels are high in females, CRF receptor distribution to the dendritic plasma membrane is greater than their male counterparts and may result in enhanced sensitivity to CRF. (C) A schematic representation showing in vitro inhibition of CRF receptor mediated cAMP production by DOR activation, one possible site of opioid and CRF system interaction. AC = adenylyl cyclase, PKA = protein kinase A.
Methodological considerations
The pre-embedding immunogold method was chosen to localize CRF receptor immunoreactivity at the ultrastructural level as it maintains morphological preservation while providing discrete subcellular localization of the antigen of interest (Leranth & Pickel, 1989). This method is more appropriate than post-embedding methods for localization of immunoreactivity at extrasynaptic sites, and thus is suitable for quantifying the regional distribution of CRF receptors (Lujan et al., 1996). Although pre-embedding immunogold labeling can produce lower estimates of receptor number than immunoperoxidase labeling, due to reduced reagent penetration (Leranth & Pickel, 1989), this limitation was not likely to affect comparisons of CRF receptor density between groups as (a) tissue was pooled and processed together to facilitate relative comparisons (Pierce et al., 1999), and (b) ultra-thin sections were collected from the plastic-tissue interface where immunoreagent access is maximal (Leranth & Pickel, 1989).
The current study used the hormone responsive neuroblastoma NG108-15 cell line to assay intracellular cAMP production in response to CRF receptor and DOR agonists. This well established in vitro model system has demonstrated batch-to-batch consistency and neuronal homogeneity (Hamprecht et al., 1985; Yano et al., 1998) and is characterized by the presence of DORs and the absence of other opioid receptor subtypes (Barg et al., 1984; Kieffer et al., 1992). As such, NG108-15 cells are useful for specifically studying DOR interactions with other receptors as well as signal transduction mechanisms as they are readily amenable to transfection (Bergsbaken et al., 1993). These cells also demonstrate more neuronally differentiated features, including the formation of processes reminiscent of axons and dendrites, in response to all-trans retinoic acid (Beczkowska et al., 1997). Our previous studies as well as those of others have shown that NG108-15 cells contain estrogen receptors, are estrogen responsive, and form spine-like processes in culture that respond to estrogen similarly to what has been observed in vivo (Woolley et al., 1990; Akama & McEwen, 2003; Aizawa & Yamamuro, 2008). Thus, these cells are useful for demonstrating DOR agonist effects on intracellular signaling, as described in the current study, and the modulatory effects of estrogen on the same signaling mechanisms as planned for future studies.
Ovarian hormones alter CRF receptor distribution in DOR-labeled pyramidal cell dendrites
In addition to an increased number of dual labeled dendritic profiles in proestrus females compared to males, ultrastructural studies demonstrated increased CRF receptor density and localization to the plasma membrane in proestrus females (Fig. 5A-B). Such altered CRF receptor distribution in males and females may have functional consequences given previous reports in the locus coeruleus (LC), a key site of opioid and CRF system intersection, which demonstrate sex differences in stress responsiveness of LC neurons that are attributable to altered CRF receptor signaling and/or trafficking in females (Curtis et al., 2006; Bangasser et al., 2010). Specifically, in response to stress, females demonstrate CRF receptor recruitment to the plasma membrane (and impaired internalization) while males demonstrate CRF receptor internalization (Bangasser et al., 2010). Thus, increased CRF receptor localization to the plasma membrane of pyramidal cell dendrites of proestrus females could result in enhanced sensitivity to CRF and, if CRF receptor trafficking in the CA1 follows the same pattern observed in the LC in response to stress, a decreased ability to adapt to excessive CRF. However, while ultrastructural studies in the LC have demonstrated that roughly 20% or greater reductions in CRF receptor distribution to the plasma membrane correspond well with electrophysiological changes in neuronal activation in response to CRF administration (Bangasser et al., 2010), it is unknown what magnitude of CRF receptor redistribution to or away from the plasma membrane of hippocampal neurons at the ultrastructural level is biologically meaningful. Future studies using stress to evoke hippocampal CRF release will determine whether small changes in CRF receptor plasma membrane localization, like the ~2-3% difference noted between unstressed proestrus females and males in the present study, produce or contribute to functional outcomes.
Interestingly, studies suggest a pivotal role for the CRF receptor in hippocampal dendritic spine dynamics and, ultimately, dendritic integrity in response to stress (Maletic-Savatic et al., 1999; Chen et al., 2006; Chen et al., 2008). For example, exposure to CRF provokes spine loss and dendritic regression in hippocampal organotypic cultures and selective blockade of the CRF receptor has the opposite effect (Chen et al., 2008). Such derangement of spine dynamics is a plausible candidate mechanism for stress-evoked dendritic atrophy and associated synaptic dysfunction (Brunson et al., 2005) as dendritic spines are thought to be involved in the acquisition and retention of associative memories and stressful encounters can alter their density in the HF (Shors et al., 2001; Leuner & Shors, 2004; Segal, 2005; Parducz et al., 2006). In male rats, acute stress increases CA1 spine density and increases associative learning (Servatius & Shors, 1994; Shors & Servatius, 1997; Shors et al., 2001). In female rats, exposure to the same stressor decreases CA1 spine density and decreases associative learning (Shors et al., 1998; Shors et al., 2001; Bangasser & Shors, 2004). Thus, ovarian hormones, particularly estrogens which also influence spine density in the hippocampal CA1 region, appear to play a role in these sexually dimorphic responses to stress (Gould et al., 1990; Woolley & McEwen, 1992; McLaughlin et al., 2005; Cooke & Woolley, 2005; Luine et al., 2007). Indeed, emerging evidence suggests that estrogens modulate several overlapping cellular pathways in the hippocampus to regulate CA1 spine formation and plasticity. One model suggests that estrogens activate the phosphotidylinositol 3-Kinase (PI3-K) pathway, resulting in Akt activation, LIM kinase activation, and increased PSD-95 expression, leading to actin remodeling and spine formation (Akama & McEwen, 2003; Znamensky et al., 2003; Spencer et al., 2008). Hence, estrogen actions through the CRF receptor could also mediate spine turnover. The present observation of increased CRF receptor density on the CA1 pyramidal cell dendritic plasma membrane of proestrus (high estrogen) females in comparison to males could therefore contribute both to normal fluctuations in CA1 spine density during the rodent estrous cycle (Woolley & McEwen, 1992) and the differential effect of acute stress on CA1 morphology, as proestrus females may be more sensitive to the effects of stress-evoked CRF release (Chen et al., 2004).
However, as the current studies only examined proestrus females in comparison to males, it is unknown whether the present findings are (1) a result of true sexual dimorphism at the level of the CRF receptor (i.e., fundamentally and permanently different in males and females regardless of estrous cycle phase (McCarthy & Konkle, 2005)), as has been suggested for the CRF receptor in the LC (Bangasser et al., 2010), or (2) a response modulated by changes in adult circulating gonadal hormones given the observation of fluctuating CA1 spine dynamics across the rodent estrous cycle (Woolley & McEwen, 1992). A first step to addressing whether our findings are a result of organizational versus activational effects of ovarian hormones will be to examine the subcellular distribution of the CRF receptor in normal cycling females in other phases of the estrous cycle, namely estrus (high progesterone) and diestrus (low estrogen and progesterone), in comparison to proestrus females in future studies. Additional ultrastructural studies will focus on stress-evoked CRF receptor trafficking in the CA1 of males and normal cycling females.
DOR activation influences CRF receptor-mediated signaling
The CRF receptor is a GPCR linked to adenylyl cyclase through Gαs protein activation. Subsequent cAMP production leads to activation of protein kinase A (PKA), a mechanism thought to be responsible for many of the known effects of CRF in the rodent hippocampus (Chen et al., 1986; Battaglia et al., 1987). For example, exogenous CRF application produces long-lasting enhancement of synaptic efficacy in the dentate gyrus (Wang et al., 1998; Wang et al., 2000), reversibly increases spiking of pyramidal cells (Aldenhoff et al., 1983), and enhances the amplitude of CA1 population spikes evoked by stimulation of the Schaffer collateral pathway (Hollrigel et al., 1998) in the rat hippocampal formation. However, reports indicate that CRF receptors also have the ability to interact with other G-protein systems, including Gαq (Grammatopoulos et al., 2001; Hillhouse & Grammatopoulos, 2006). Indeed, it is now well established that CRF receptors can activate a number of intracellular pathways which include but are not limited to protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) (Hillhouse & Grammatopoulos, 2006). Furthermore, selective involvement of PKC and extracellular signal-regulated kinase (ERK), a member of the MAPK family, have been demonstrated in PKA-independent CRF receptor mediated effects on neuronal excitability and stress-associated learning tasks in the hippocampal formation (Blank et al., 2003; Sananbenesi et al., 2003; Sheng et al., 2008). Such promiscuity of the CRF receptor with multiple signal transduction effectors (1) suggests that the net effect of CRF receptor activation is dependent on the balance between the possible signaling cascades it may activate, rather than the effect of CRF upon one pathway exclusively (Gallagher et al., 2008) and (2) emphasizes the importance of identifying the contribution of other GPCRs, like DORs, to shared signal transduction mechanisms.
It is well established that most DOR-mediated events are dependent on the activity of pertussis toxin-sensitive G proteins (Gαi/o) and that DOR selective ligands inhibit intracellular cAMP levels and modulate the activity of voltage-gated calcium and potassium channels (Quock et al., 1999). However, DORs also modulate a variety of protein kinases, including PKC and MAPK (Quock et al., 1999). Given the anatomical and functional observations of the current study, namely (1) colocalization of CRF receptor-ir with DOR-ir in pyramidal neurons and (2) DOR-mediated inhibition of CRF receptor induced elevations in intracellular cAMP levels, DORs are ideally positioned to modulate CRF receptor signaling. While DOR activation can inhibit or attenuate CRF signaling through cAMP pathways via Gαs, DOR activation could also enhance or facilitate CRF signaling through PKC and MAPK pathways via Gαq. Such receptor cross-talk between the opioid and CRF system in the hippocampal formation may underly the neuroprotective properties of the DOR and CRF receptor observed during ischemic or excitotoxic events.
Substantial data has demonstrated that DOR activation is protective against ischemic stress in both in vitro and in vivo models of brain injury (Ma et al., 2005; Narita et al., 2006; Charron et al., 2008a; Charron et al., 2008b; Charron et al., 2009; Gao et al., 2010). Among other mechanisms posited for DOR-mediated neuroprotection, including enhanced antioxidant ability, stabilized ion homeostasis, and inhibiting caspase activity, are activation of MAPK family member ERK (Hayashi et al., 2002; Feng et al., 2009; Gao et al., 2010) and inhibition of glutamate release (Johnson & Turner, 2010). The role of CRF and its receptor in neurotoxicity is more complex, as a dichotomy appears to exist between in vivo and in vitro studies. For example, in vitro studies suggest CRF is neuroprotective via mechanisms involving MAPK activation (Elliott-Hunt et al., 2002; Pedersen et al., 2002) while in vivo work suggests that CRF is involved in the pathogenesis of neuronal damage in the hippocampus via mechanisms involving cAMP-dependent hyperexcitability of pyramidal neurons resulting in a net increase in glutamate release (Hollrigel et al., 1998; Elliott-Hunt et al., 2002). The discrepancy between in vitro and in vivo findings may stem from CRF induced activation of diverse intracellular signaling pathways present in vivo. One possibility supported by the findings of the current study could be that DOR activation inhibits CRF receptor-associated cAMP-PKA signaling pathways but promotes CRF receptor-associated MAPK pathways. Such synergy between GPCRs has been proposed for cannabinoid receptor - CRF receptor interactions in the rodent cortex and is supported by recent in vivo studies demonstrating neuroprotective effects of CRF administration in CA1 neurons contingent on DOR activation (Charron et al., 2008a; Charron et al., 2009). While results in the current report show DOR attenuation of CRF signaling via cAMP, whether DORs also enhance CRF receptor-associated MAPK pathways will need to be addressed in future studies.
Importantly, ligand activation of DORs is dependent upon DOR presentation at the plasma membrane (Pradhan et al., 2009). Although the subcellular distribution of DORs within CRF receptor-labeled dendrites has not yet been examined and will be in future experiments, prior ultrastructural studies of CA1 pyramidal cell dendrites labeled for DOR-ir suggest that DORs are primarily found intracellularly rather than on the plasma membrane (Commons & Milner, 1997; Williams et al., 2011). Thus, the ability of DORs to influence CRF receptor signaling in vivo will largely depend on stimuli promoting DOR recruitment to the plasma membrane. Reports indicate that such stimuli include inflammation (Cahill et al., 2003; Cahill et al., 2007), stress (Commons, 2003), and chronic opioid exposure (Cahill et al., 2001; Ma et al., 2006; Bie et al., 2009). Consequently, the present observations of (1) increased CRF receptor-ir colocalization in dendrites containing DOR-ir and (2) increased CRF receptor-ir at the plasma membrane of these dendrites in drug naïve, unstressed proestrus females compared to males would suggest potentially increased unopposed CRF receptor signaling in females rather than increased opportunity for DOR regulation of CRF receptor signaling. Future studies will address trafficking of DORs and CRF receptors in CA1 pyramidal cell dendrites of normal cycling females and males in response to stress and opiate exposure to identify the in vivo physiological conditions facilitating DOR interaction with the CRF receptor.
Clinical implications
Our findings provide new anatomical evidence of CRF receptor colocalization with DORs in hippocampal CA1 pyramidal neurons, supporting and extending the growing body of literature investigating the interaction between endogenous opioid and CRF systems. In particular, they suggest that DORs influence CRF receptor signaling through cAMP-PKA pathways. In addition, the number and density of CRF receptors on the plasma membrane of pyramidal cell dendrites is slightly greater in high estrogen females than in males. The increased presence of the CRF receptor on the neuronal surface for response to CRF is consistent with compelling evidence of sexual dimorphism in stress reactivity and the increased prevalence of stress-related psychiatric disorders in women (Kessler et al., 1993; Kessler et al., 1995; Marcus et al., 2005; Marcus et al., 2008). Previous reports have proven the efficacy of DOR antagonists in blocking the reinstatement of drug seeking behavior (Ciccocioppo et al., 2002; Marinelli et al., 2009; Kotlinska et al., 2010) whereas CRF precipitates reinstatement of drug-seeking behavior (Shaham et al., 1997; Brown et al., 2009; Shalev et al., 2010) in male animal models of addiction. Furthermore, excessive elevation of hippocampal CRF peptide levels in vivo may contribute to abnormal neuronal excitation and neuronal damage (Hollrigel et al., 1998; Roe et al., 1998) particularly when DORs are blocked (Charron et al., 2008a; Charron et al., 2009). Thus, tight regulation of CRF levels and CRF receptor activity, particularly those signal transduction mechanisms mediating hyperexcitability and neuronal damage, may be important for both normal and pathological activation of the hippocampal circuit. Research focused on DOR regulation of hippocampal CRF receptor activity, as both stress and opiate exposure promote trafficking of DORs to the plasma membrane, may therefore highlight new pharmacological targets to counteract heightened stress sensitivity and relapse proclivity in women.
Supplementary Material
Fig. 1. Western blot testing of the rabbit polyclonal DOR antisera from Chemicon (AB1560). No detectable labeling for the DOR was observed in COS-7 cell lysates (lanes 1-5) as these cells do not endogenously express DORs, whereas the expected 36 kDa band was observed in NG108- 15 cell lysates (lanes 7-11) which have been shown to endogenously express DORs (Barg et al., 1984; Kieffer et al., 1992; Persson et al., 2005).
Acknowledgements
This work was supported by National Institutes of Health grants DA08259, HL18974, HL096571, DA028072, NIH-MSTP grant GM07739, the American Psychological Association Diversity Program in Neuroscience, and the UNCF-Merck Science Initiative. We are also thankful for hippocampal tissue provided by Dr. Annelyn Torres-Reveron and the technical assistance of Ms. Jeanette Chapleau and Ms. Louisa Thompson.
GRANT SUPPORT: NIH grants DA08259 (TAM), HL18974 (TAM), HL096571 (TAM), DA028072 (TJW), and NIH MSTP grant GM07739 (TJW)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aizawa S, Yamamuro Y. Estradiol regulates alternative splicing of estrogen receptor-alpha mRNA in differentiated NG108-15 neuronal cells. Life Sci. 2008;82:692–698. doi: 10.1016/j.lfs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J.Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol.Biochem.Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol.Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Acute stress impairs trace eye blink conditioning in females without altering the unconditioned response. Neurobiol.Learn.Mem. 2004;82:57–60. doi: 10.1016/j.nlm.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat.Neurosci. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao G, Kang L, Li H, Li Y, Pu L, Xia P, et al. Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology. 2007;32:1738–1749. doi: 10.1038/sj.npp.1301308. [DOI] [PubMed] [Google Scholar]
- Barg J, Levy R, Simantov R. Up-regulation of opiate receptors by opiate antagonists in neuroblastoma-glioma cell culture: the possibility of interaction with guanosine triphosphate-binding proteins. Neurosci.Lett. 1984;50:133–137. doi: 10.1016/0304-3940(84)90475-0. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Webster EL, De Souza EB. Characterization of corticotropin-releasing factor receptor-mediated adenylate cyclase activity in the rat central nervous system. Synapse. 1987;1:572–581. doi: 10.1002/syn.890010610. [DOI] [PubMed] [Google Scholar]
- Beczkowska IW, Gracy KN, Pickel VM, Inturrisi CE. Detection of delta opioid receptor and N-methyl-D-aspartate receptor-like immunoreactivity in retinoic acid-differentiated neuroblastoma × glioma (NG108-15) cells. J.Neurosci.Res. 1997;47:83–89. [PubMed] [Google Scholar]
- Belanger A, Cusan L, Caron S, Barden N, Dupont A. Ovarian progestins, androgens and estrogen throughout the 4-day estrous cycle in the rat. Biol.Reprod. 1981;24:591–596. doi: 10.1095/biolreprod24.3.591. [DOI] [PubMed] [Google Scholar]
- Bergsbaken CL, Sommers SL, Law PY. Effect of forskolin and isobutylmethylxanthine on delta-opioid receptor activity in neuroblastoma × glioma NG108-15 cells. J.Pharmacol.Exp.Ther. 1993;264:1474–1483. [PubMed] [Google Scholar]
- Bie B, Zhu W, Pan ZZ. Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses. J.Pharmacol.Exp.Ther. 2009;329:290–296. doi: 10.1124/jpet.108.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, et al. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J.Pharmacol.Exp.Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J.Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J.Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur.J.Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J.Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Sarvey JM. Endogenous activation of mu and delta-1 opioid receptors is required for long-term potentiation induction in the lateral perforant path: dependence on GABAergic inhibition. J.Neurosci. 1996;16:8123–8131. doi: 10.1523/JNEUROSCI.16-24-08123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. J.Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol.Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J.Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon SN, Rothman RB, Porreca F, Flippen-Anderson JL, McNutt RW, Xu H, et al. Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(alphaR)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3- methoxybenzyl]-N,N-diethylbenzamide (SNC 80): a highly selective, nonpeptide delta opioid receptor agonist. J.Med.Chem. 1994;37:2125–2128. doi: 10.1021/jm00040a002. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J.Neurosci.Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron C, Frechette S, Proulx G, Plamondon H. In vivo administration of corticotropin-releasing hormone at remote intervals following ischemia enhances CA1 neuronal survival and recovery of spatial memory impairments: a role for opioid receptors. Behav.Brain Res. 2008a;188:125–135. doi: 10.1016/j.bbr.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta 1-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008b;93:502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Charron C, Schock SC, Proulx G, Thompson CS, Hakim AM, Plamondon H. Protection conferred by Corticotropin-releasing hormone in rat primary cortical neurons against chemical ischemia involves opioid receptor activation. Brain Res. 2009;1257:117–127. doi: 10.1016/j.brainres.2008.12.053. [DOI] [PubMed] [Google Scholar]
- Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res. 1986;381:49–57. doi: 10.1016/0006-8993(86)90688-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J.Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J.Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fenoglio KA, Dube CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol.Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, et al. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J.Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Commons KG. Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J.Comp Neurol. 2003;464:197–207. doi: 10.1002/cne.10788. [DOI] [PubMed] [Google Scholar]
- Commons KG, Milner TA. Cellular and subcellular localization of delta opioid receptor immunoreactivity in the rat dentate gyrus. Brain Res. 1996;738:181–195. doi: 10.1016/s0006-8993(96)00774-3. [DOI] [PubMed] [Google Scholar]
- Commons KG, Milner TA. Localization of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. J.Comp Neurol. 1997;381:373–387. [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J.Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos.Trans.R.Soc.Lond B Biol.Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Dalla C, Whetstone AS, Hodes GE, Shors TJ. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci.Lett. 2009;449:52–56. doi: 10.1016/j.neulet.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog.Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 1999;849:203–215. doi: 10.1016/s0006-8993(99)01910-1. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Eckart K, Jahn O, Radulovic J, Radulovic M, Blank T, Stiedl O, et al. Pharmacology and biology of corticotropin-releasing factor (CRF) receptors. Receptors.Channels. 2002;8:163–177. [PubMed] [Google Scholar]
- Elliott-Hunt CR, Kazlauskaite J, Wilde GJ, Grammatopoulos DK, Hillhouse EW. Potential signalling pathways underlying corticotrophin-releasing hormone-mediated neuroprotection from excitotoxicity in rat hippocampus. J.Neurochem. 2002;80:416–425. doi: 10.1046/j.0022-3042.2001.00712.x. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am.J.Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chao D, He X, Yang Y, Kang X, Lazarus LH, et al. A novel insight into neuroprotection against hypoxic/ischemic stress. Sheng Li Xue.Bao. 2009;61:585–592. [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur.J.Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur.J.Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao CJ, Li JP, Wang W, Lu BC, Niu L, Zhu C, et al. Effects of intracerebroventricular application of the delta opioid receptor agonist [D-Ala2, D-Leu5] enkephalin on neurological recovery following asphyxial cardiac arrest in rats. Neuroscience. 2010;168:531–542. doi: 10.1016/j.neuroscience.2010.02.025. [DOI] [PubMed] [Google Scholar]
- George SR, Zastawny RL, Briones-Urbina R, Cheng R, Nguyen T, Heiber M, et al. Distinct distributions of mu, delta and kappa opioid receptor mRNA in rat brain. Biochem.Biophys.Res.Commun. 1994;205:1438–1444. doi: 10.1006/bbrc.1994.2826. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J.Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J.Neurochem. 2001;76:509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Hamprecht B, Glaser T, Reiser G, Bayer E, Propst F. Culture and characteristics of hormone-responsive neuroblastoma × glioma hybrid cells. Methods Enzymol. 1985;109:316–341. doi: 10.1016/0076-6879(85)09096-6. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J.Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Tsao LI, Su TP. Antiapoptotic and cytotoxic properties of delta opioid peptide [D-Ala(2),D-Leu(5)]enkephalin in PC12 cells. Synapse. 2002;43:86–94. doi: 10.1002/syn.10019. [DOI] [PubMed] [Google Scholar]
- Hermann H, Lutz B. Coexpression of the cannabinoid receptor type 1 with the corticotropin-releasing hormone receptor type 1 in distinct regions of the adult mouse forebrain. Neurosci.Lett. 2005;375:13–18. doi: 10.1016/j.neulet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr.Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J.Histochem.Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Turner SM. Protecting motor networks during perinatal ischemia: the case for delta-opioid receptors. Ann.N.Y.Acad.Sci. 2010;1198:260–270. doi: 10.1111/j.1749-6632.2010.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J.Comp Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J.Affect.Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch.Gen.Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc.Natl.Acad.Sci.U.S.A. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch.Gen.Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kotlinska JH, Gibula-Bruzda E, Pachuta A, Kunce D, Witkowska E, Chung NN, et al. Influence of new deltorphin analogues on reinstatement of cocaine-induced conditioned place preference in rats. Behav.Pharmacol. 2010;21:638–648. doi: 10.1097/FBP.0b013e32833e7e97. [DOI] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic preembedding double-labeling methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical Tract-Tracing Methods. Vol. 2. InPlenum Press; New York: 1989. pp. 129–172. [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol.Neurobiol. 2004;29:117–130. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J.Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur.J.Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol.Pharmacol. 2006;69:1137–1145. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J.Biol.Chem. 2005;280:16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz.J.Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, et al. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr.Psychiatry. 2008;49:238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, et al. Gender differences in depression: findings from the STAR*D study. J.Affect.Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, Le AD. Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur.J.Neurosci. 2009;30:671–678. doi: 10.1111/j.1460-9568.2009.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur.J.Neurosci. 2007;26:2815–2823. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res.Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J.Nerv.Ment.Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol.Neurobiol. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J.Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Veznedaroglu E. Ultrastructural localization of neuropeptide Y-like immunoreactivity in the rat hippocampal formation. Hippocampus. 1992;2:107–125. doi: 10.1002/hipo.450020204. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, et al. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J.Neurochem. 2006;97:1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J.Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, et al. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J.Neurosci. 2002;22:404–412. doi: 10.1523/JNEUROSCI.22-02-00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Eriksson PS. Comparison of immunoblotted delta opioid receptor proteins expressed in the adult rat brain and their regulation by growth hormone. Neurosci.Res. 2005;52:1–9. doi: 10.1016/j.neures.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Persson PA, Thorlin T, Ronnback L, Hansson E, Eriksson PS. Differential expression of delta opioid receptors and mRNA in proliferating astrocytes during the cell cycle. J.Neurosci.Res. 2000;61:371–375. doi: 10.1002/1097-4547(20000815)61:4<371::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system. Oxford University Press; New York: 1991. [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Piguet P, North RA. Opioid actions at mu and delta receptors in the rat dentate gyrus in vitro. J.Pharmacol.Exp.Ther. 1993;266:1139–1149. [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS.One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quock RM, Burkey TH, Varga E, Hosohata Y, Hosohata K, Cowell SM, et al. The delta-opioid receptor: molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol.Rev. 1999;51:503–532. [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur.J.Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Roe SY, McGowan EM, Rothwell NJ. Evidence for the involvement of corticotrophin-releasing hormone in the pathogenesis of traumatic brain injury. Eur.J.Neurosci. 1998;10:553–559. doi: 10.1046/j.1460-9568.1998.00064.x. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A, Stout RL, Longabaugh R. Gender differences in relapse situations. Addiction. 1996;91(Suppl):S111–S120. [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saland LC, Hastings CM, Abeyta A, Chavez JB. Chronic ethanol modulates delta and mu-opioid receptor expression in rat CNS: immunohistochemical analysis with quantitiative confocal microscopy. Neurosci.Lett. 2005;381:163–168. doi: 10.1016/j.neulet.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J.Neurosci. 2003;23:11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Schierloh A, Deussing J, Wurst W, Zieglgansberger W, Rammes G. Corticotropin-releasing factor (CRF) receptor type 1-dependent modulation of synaptic plasticity. Neurosci.Lett. 2007;416:82–86. doi: 10.1016/j.neulet.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am.J.Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat.Rev.Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats. Behav.Neurosci. 1994;108:1101–1106. doi: 10.1037//0735-7044.108.6.1101. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res.Brain Res.Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J.Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Zhang Y, Sun J, Gao L, Ma B, Lu J, et al. Corticotropin-releasing hormone (CRH) depresses n-methyl-D-aspartate receptor-mediated current in cultured rat hippocampal neurons via CRH receptor type 1. Endocrinology. 2008;149:1389–1398. doi: 10.1210/en.2007-1378. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J.Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol.Learn.Mem. 1997;68:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr.Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am.J.Addict. 2003;12:1–17. [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Schulz S, Hollt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J.Comp Neurol. 2004;469:107–118. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Cheng PY, Clarke CL, Pickel VM. Ultrastructural localization of delta-opioid receptor and Met5-enkephalin immunoreactivity in rat insular cortex. Brain Res. 1995;700:25–39. doi: 10.1016/0006-8993(95)00977-x. [DOI] [PubMed] [Google Scholar]
- Svoboda KR, Adams CE, Lupica CR. Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J.Neurosci. 1999;19:85–95. doi: 10.1523/JNEUROSCI.19-01-00085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. 2nd ed Academic Press; San Diego, CA: 2000. [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Drake CT, McEwen BS, et al. Ovarian steroids modulate leu-enkephalin levels and target leu-enkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Res. 2008;1232:70–84. doi: 10.1016/j.brainres.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, et al. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009a;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, Drake CT, et al. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp.Neurol. 2009b;219:319–327. doi: 10.1016/j.expneurol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J.Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Turner CD, Bagnara JT. General Endocrinology. W.B. Saunders; Philadelphia: 1971. [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur.J.Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res. 2010;1314:162–174. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van PK, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J.Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wang HL, Tsai LY, Lee EH. Corticotropin-releasing factor produces a protein synthesis--dependent long-lasting potentiation in dentate gyrus neurons. J.Neurophysiol. 2000;83:343–349. doi: 10.1152/jn.2000.83.1.343. [DOI] [PubMed] [Google Scholar]
- Wang HL, Wayner MJ, Chai CY, Lee EH. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur.J.Neurosci. 1998;10:3428–3437. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol.Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA. Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol.Learn.Mem. 2011 doi: 10.1016/j.nlm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci.Lett. 2002;328:160–164. doi: 10.1016/s0304-3940(02)00481-0. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J.Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J.Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- Young SF, Griffante C, Aguilera G. Dimerization between vasopressin V1b and corticotropin releasing hormone type 1 receptors. Cell Mol.Neurobiol. 2007;27:439–461. doi: 10.1007/s10571-006-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J.Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. Western blot testing of the rabbit polyclonal DOR antisera from Chemicon (AB1560). No detectable labeling for the DOR was observed in COS-7 cell lysates (lanes 1-5) as these cells do not endogenously express DORs, whereas the expected 36 kDa band was observed in NG108- 15 cell lysates (lanes 7-11) which have been shown to endogenously express DORs (Barg et al., 1984; Kieffer et al., 1992; Persson et al., 2005).