Abstract
The nonstructural (NS) proteins of West Nile virus (WNV) have been associated with participation in evasion of host innate immune defenses. In the present study, we characterized immune response to an attenuated WNV strain, which has a P38G substitution in the NS4B protein. The WNV NS4B-P38G mutant induced a lower level of viremia and no lethality in C57BL/6 (B6) mice following a systemic infection. Interestingly, there were higher type 1 IFNs and IL-1β responses compared to mice infected by wild-type WNV. NS4B-P38G mutant-infected mice also showed stronger effector and memory T cell responses. WNV specific antibody responses were not different between mice infected with these two viruses. As a consequence, all mice were protected from a secondary infection with a lethal dose of wild-type WNV following a primary infection with NS4B-P38G mutant. Moreover, NS4B-P38G mutant infection in cultured bone-marrow derived dendritic cells (DCs) were shown to have a reduced replication rate, but a higher level of innate cytokine production than wild-type WNV, some of which were dependent on Myd88 signaling. In conclusion, the NS4B-P38G mutant strain induces higher protective innate and adaptive immune response in mice, which results in a lower viremia and no lethality in either primary or secondary infection, suggesting a high potential as an attenuating mutation in a vaccine candidate.
Keywords: West Nile virus, NS4B Protein, Immune Response, T cell
Introduction
West Nile virus (WNV), a mosquito-borne neurotropic pathogen, belongs to the family of Flaviviridae, the genus Flavivirus, a group of plus-sense, single-stranded RNA viruses [1–2]. It was originally isolated in Africa, and later caused epidemics with mainly febrile illness in humans in Europe, Africa, the Middle East, and parts of Asia. In 1999, a more virulent WNV strain was detected in New York City. Since then, it has rapidly spread throughout the continental United States, southern Canada, Mexico, Guatemala, the Caribbean and to several countries in South America. The virus has become a public health concern in North America [3]. WNV infection of the central nervous system (CNS, neuroinvasive disease) commonly presents as encephalitis, meningitis or acute flaccid paralysis. The overall mortality rate in persons who develop WNV neuroinvasive disease is about 10%, although the mortality rate increases significantly in the elderly and immunocompromised. Multiple approaches to WNV vaccines have been taken, including recombinant DNA [4], recombinant protein vaccines [5] and chimeric live attenuated [6]. Nevertheless, no vaccines have been approved for human use. Development of safe and effective vaccines against WNV remains as a high priority.
The WNV genome is a single-stranded, positive-sense RNA molecule, approximately 11,000 nucleotides in length that is translated into a single polypeptide, which is co- and post-translationally processed into ten proteins – three structural proteins (envelope (E), membrane, nucleocapsid) and seven nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) [1–2]. The NS proteins encode protease (NS3) and polymerase (NS5) activities and function as part of the replication complex. In addition, they contain important epitopes for mouse and human T cell recognition during WNV infection [7–8]. Several NS proteins have also been reported to be associated with evasion of host innate immune defenses [9–11], such as inhibiting interferon (IFN) signaling by the blockage of STAT1 and STAT2 activation [12–14]. Furthermore, a C102S mutation in NS4B protein of WNV was shown to attenuate the neuroinvasive and neurovirulence phenotypes in mice [15]. The NS4B P38 residue is conserved in both mosquito- and tick- borne flaviviruses, except for the Brazilian flavivirus Ilheus, which encodes an alanine at this residue. By utilizing site-directed mutagenesis of an infectious clone, we have recently identified an attenuated WNV NY99 strain with a substitution of P38G in NS4B protein. This P38G mutant has both temperature-sensitive and small-plaque phenotypes, and significantly reduced neuroinvasiveness in NIH Swiss outbred mice as compared to the parental wild-type NY99 WNV (Wicker JA, and Barrett AD. et. al. Manuscript submitted). In this study, we have evaluated the potential of NS4B-P38G mutation for incorporation in a vaccine candidate by further characterization of the immune response induced by infection with NS4B-P38G mutant in mice.
1. Materials and methods
1.1. Mice
6–10-week-old C57BL/6 (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Myeloid differentiation factor 88 deficient (Myd88−/−) mice were bred to the B6 background by backcrossing for 10 successive generations [16–17]. Groups were age and sex-matched for each experiment and were housed under identical conditions. All animal experiments were approved by the Animal Care and Use Committee at the University of Texas Medical Branch.
1.2. WNV Infection
Mice were inoculated intraperitoneally (i.p.) with 500 PFU of wild-type WNV NY99 infectious clone-derived virus, WNV NS4B P38G or NS4B P38A mutant strains that were produced utilizing site-directed mutagenesis. Full genomic sequencing of the WNV NS4B-P38G mutant revealed additional NS4B T116I and NS3 N480H substitutions, and these were likely compensatory mutations in response to the engineered attenuating P38G substitution (Wicker JA, and Barrett AD. et. al. Manuscript submitted). In some experiments, surviving mice were re- challenged with a lethal dose (LD100, 2000 PFU) of wild-type WNV NY99 infectious clone- derived virus at day 30 post-primary infection. Infected mice were monitored twice daily for morbidity. When mice showed signs of morbidity (obvious severe illness, unable to right self when tipped on side or back, obvious extreme weight loss), they were immediately collected and euthanized by CO2. WNV NY99-infectious clone-derived virus or WNV NS4B-P38G mutant were each passaged once in Vero cells to make a virus stock for cell culture. Bone-marrow derived dendritic cells (DCs) were generated as described previously [18]. Briefly, bone marrow cells from B6 mice were isolated and cultured for 6 days in RPMI-1640 supplemented with granulocyte-macrophage-colony stimulating factor, and interleukin-4 (Peprotech) to generate myeloid DC. Day 6-cultured DCs were infected with WNV NY99 infectious clone-derived virus or NS4B-P38G mutant passage 1 (MOI = 0.2) and harvested at 24 and 96 hr post-infection. Supernatants and cells were collected for measurement of viral load and cytokine production.
2.3. Quantitative PCR (Q-PCR) for viral load and cytokine production
RNA was extracted from WNV infected cells or tissues using RNAeasy extraction kit (Qiagen, Valencia, CA). RNA was used to synthesize complementary (c)DNA using the ProSTAR First-strand RT-PCR kit (Stratagene, Cedar Creek, TX). The sequences of the primer-probe sets for WNV envelope (WNVE) and cytokine gene cDNA and PCR reaction conditions were described previously [19–20]. The assay was performed in an iCycler (Bio-Rad, Hercules, CA). To normalize the samples, the same amount of cDNA was used in a Q-PCR for β-actin. The ratio of the amount of amplified gene compared with the amount of β-actin cDNA represented the relative levels in each sample.
2.4. Bioplex
Culture supernatant or sera were collected for analysis of cytokine production using a Bio- Plex Pro Mouse Cytokine Assay (Biorad).
2.5. Flow cytometry
Splenocytes were stained with antibodies for cell surface markers, including CD4 or CD8, (e-Biosciences). After staining, cells were fixed with 0.5% paraformaldehyde in PBS and examined using a C6 Flow Cytometer (Accuri cytometers, Ann Arbor, MI). To measure intracellular cytokine production, splenocytes from WNV-infected mice or controls were isolated and stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Sigma-Aldrich) for 4 h or WNV-specific NS3 and E peptides (RRWCFDGPRTNTILE and PVGRLVTVNPFVSVA, respectively, [21]) for CD4 T cells or WNV specific NS4B and E peptides (SSVWNATTA and IALTFLAV, respectively, [22–23]) for CD8 T cells for 5h at 37°C. Golgi-plug (BD Biosciences) was added at the beginning of stimulation. Cells were harvested, stained with Abs for CD4 or CD8, fixed in 2% paraformaldehyde and permeabilized with 0.5% saponin before adding PE-conjugated anti-IFN-γ, or control PE-conjugated rat IgG1 (BD Biosciences).
2.6. ELISA
Microtiter plates were coated with recombinant WNV-E protein expressed in Drosophila melanogaster S2 cells [24] overnight at 4°C at 100 ng/well in coating buffer [0.015 M Na2CO3, 0.03 M NaHCO3, and 0.003 M NaN3 (pH 9.6)]. Sera from infected mice were diluted from 1/40 or 1/100 in PBS with 2% BSA, added to the duplicate wells, and incubated for 1 h at room temperature. Plates were washed with PBS-Tween (PBST). Alkaline phosphatase-conjugated goat anti-mouse IgG or IgM (Sigma-Aldrich, St. Louis, MO) at a dilution of 1/1000 in PBS-T was added for 1h at room temperature. After washing with PBS-T, color was developed with p-nitrophenyl phosphate (Sigma-Aldrich) for 10 min and intensity determined at an absorbance of 405 nm using a spectrophotometer.
2.7. Plaque assay
Vero cells were seeded in 6-well plates in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO) 24h before infection. Serial dilutions of sera from infected mice were added and incubated for 1h. Subsequently, DMEM containing 2% FBS and 1% low-melting-point agarose were added and the plates were incubated for 4 days. A second overlay of 2.5ml 1% agarose-medium containing 0.01% neutral red was added to visualize plaques. Virus concentrations were determined as PFU/ml. The limit of detection of this assay was 25 PFU/ml.
2.8. Plaque reduction neutralization tests
Neutralizing antibody titers were determined by plaque reduction neutralization assay using a 50% neutralization cutoff (PRNT50). Serum was diluted 1:5 in PBS and heat inactivated at 56 °C for 30 min before preparing two-fold dilutions in 100 μl of maintenance medium. The parental virus was diluted to 200 PFU/100 μl and 100 μl of virus was added to 100 μl of serially diluted serum, mixed and placed at 4 °C overnight. One hundred microliters of the serum/virus mixture was added to Vero cells in 6 well plates. Plaques were counted and the PRNT50 was determined.
2.9. Statistical analysis
Data analysis was performed using Prism software (Graph-Pad) statistical analysis. Values for phenotype analysis, viral burden, and cytokine production experiments were presented as means ± SEM. P values of these experiments were calculated with a non-paired Student’s t test. Statistical significance was accepted at P < 0.05.
3. Results
3.1. A NS4B-P38G WNV mutant strain induces a lower level of viremia and causes no lethality in mice following i.p. infection
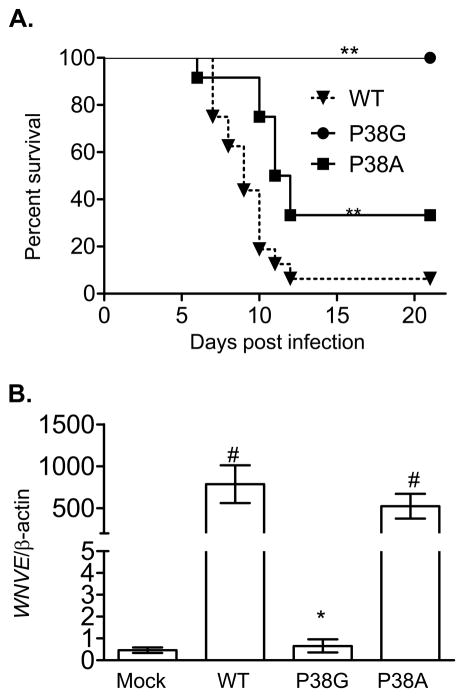
The murine model has been used as an effective in vivo experimental model to investigate host immunity to WNV infection in humans [25–26]. Recent work has shown that a NS4B-P38G WNV mutant strain confers attenuation of the neuroinvasiveness in female outbred NIH swiss mice whereas the NS4B-P38A mutant does not (Wicker JA, and Barrett AD. et. al. Manuscript submitted). To further characterize the NS4B-P38G mutant phenotype in vivo, we undertook studies in inbred B6 mice by comparing wild-type infectious clone derived-WNV NY99, and NS4B-P38G and NS4B-P38A mutant strains following i.p. inoculation of 500 PFU of each virus. All mice (100%) infected with the NS4B-P38G mutant survived a three-week infection period compared to 13% survival in wild-type WNV strain-infected mice (Fig. 1A, P < 0.01), while mice infected with WNV NS4B-P38A mutant also showed an increased survival rate (33%) compared to wild-type strain (P < 0.01). Examination of viremia by Q-PCR on day 3 post-infection (Fig. 1B) showed that the NS4B-P38G mutant replication was more than 1000-fold lower than that in mice infected with wild-type WNV (P < 0.05). Furthermore, viremia in mice infected with the NS4B-P38A mutant was not significantly different from those of wild type WNV (P > 0.05). These results suggest mutation of the P38 residue of NS4B protein, and in particular a P38G substitution, leads to a significant reduction in both viremia and lethality in mice.
Fig. 1.
Comparison of infection between WNV wild-type and NS4B mutant strains following i.p. infection. A. Survival rate. Mice were injected with 500 PFU of WNV strains and monitored twice daily. n = 16 for wild-type strain infected-mice (WT). n = 6 for P38G strain infected-mice (P38G). n = 12 for P38A mutant strain infected mice (P38A). B. Viral load was determined by Q-PCR in blood. The y-axis depicts the ratio of the amplified WNV-E cDNA to β-actin cDNA of each sample. **P < 0.01 or *P < 0.05 compared to wild-type strain infected-mice. # P < 0.05 compared to mock infected-mice. Data are presented as means ± SEM, n = 4.
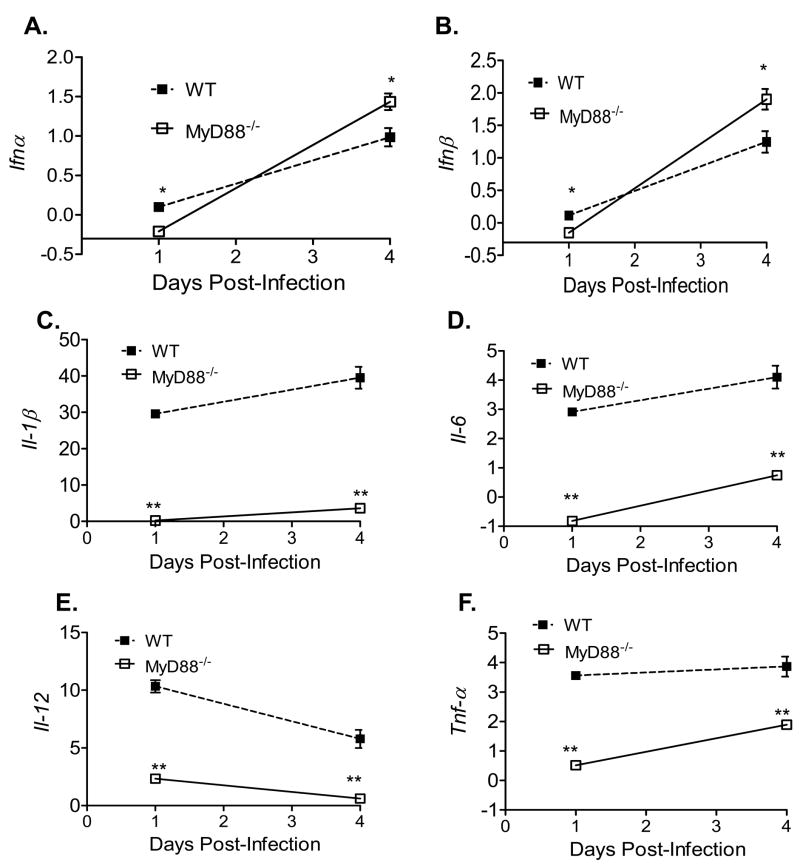
3.2. There is a higher innate cytokine response in P38G NS4B WNV mutant strain-infected mice
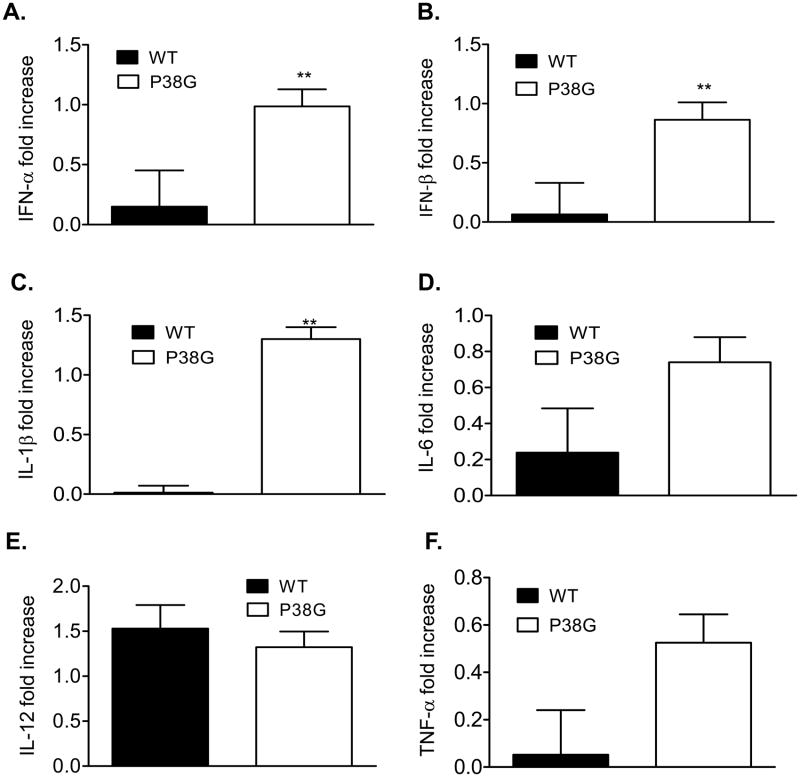
To further understand the role of NS4B protein in viral pathogenesis in this mouse model, we next focused on innate cytokine production following infection with either wild-type WNV or NS4B-P38G mutant. As shown in Fig. 2, type 1 IFNs (IFN-α and IFN-β) and proinflammatory cytokines (IL-1β, IL-6, IL-12, and TNF-α) were all induced in NS4B-P38G mutant-infected mice on day 3 post-infection. Among them, IFN-α, IFN-β and IL-1β levels were significantly increased in NS4B-P38G mutant strain -infected mice compared to wild-type WNV-infected mice (Figs. 2A, 2B and 2C, P < 0.01). This difference for the production of type 1 IFNs and IL-1β between WNV NY99 and NS4B-P38G mutant strain -infected mice was not observed on day 1 post infection (data not shown).
Fig. 2.
Cytokine production in mice following infection with WNV wild-type or P38G NS4B mutant strains. Cytokine levels in blood at day 3 post-infection were determined using Q-PCR (A-B, D-F) or Bioplex (C). Fold of increase compared to mock-infected group was shown. Data are presented as means ± SEM, n = 4. * P < 0.05 or **P < 0.01 compared to wild-type strain-infected mice.
3.3. NS4B-P38G mutant-infected mice showed stronger effector and memory T cell responses
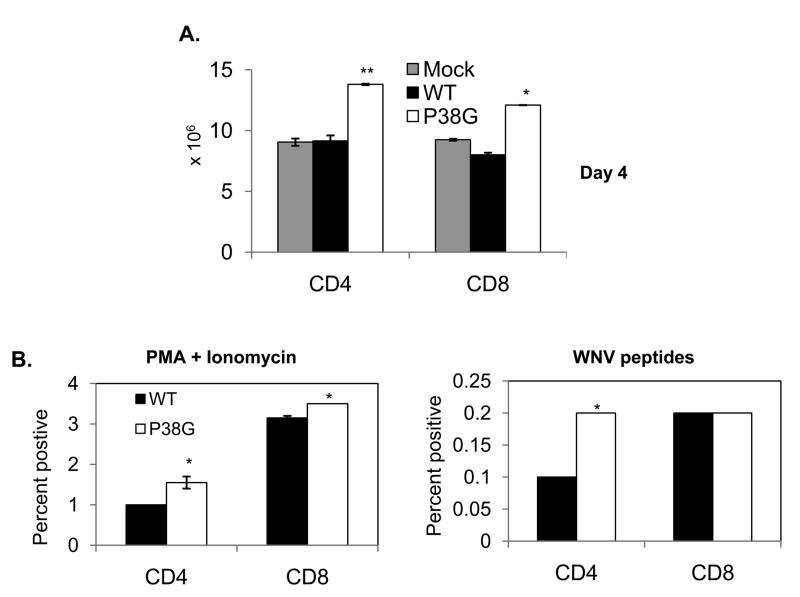
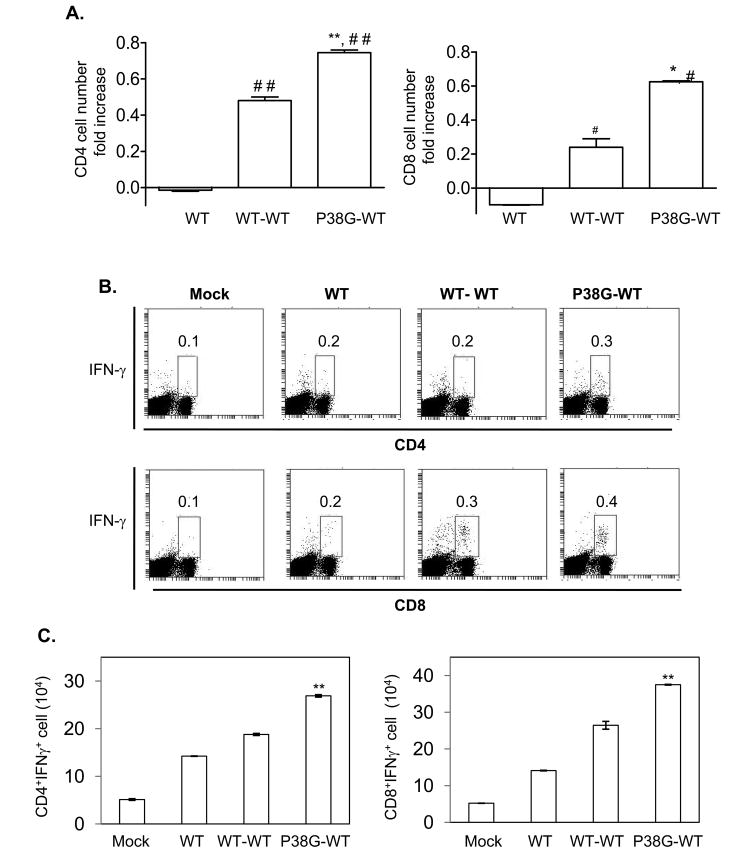
Both CD4+ and CD8+ αβ T-cells are important for host survival following WNV infection and might contribute to a long-lasting protective immunity [27–28]. Therefore, to assess the effect of NS4B-P38G mutant infection on cellular immunity, we studied CD4+ and CD8+ T cell responses following primary WNV infection with either wild-type WNV or NS4B-P38G mutant. On day 4 post-infection, there was approximately 50% more CD4+ or CD8+ T cell expansion in NS4B-P38G mutant-infected mice than those of wild-type WNV-infected mice (Fig. 3A, P < 0.01 or 0.05). We also analyzed IFN-γ production of splenic CD4+ and CD8+ T cells from WNV-infected mice using an ex vivo intracellular cytokine assay (ICS). We noted that the percentage of CD4+IFNγ+ splenocytes of NS4B-P38G mutant-infected mice was 55% or 100% higher than those of wild-type WNV-infected upon ex vivo stimulation with PMA and ionomycin or WNV peptides, respectively (Fig. 3B left and right panels, P < 0.05). The percentage of CD8+IFNγ+ splenocytes of NS4B-P38G mutant-infected mice was also slightly increased by 11% (Fig. 3B left panel, P < 0.05) with stimulation of PMA and ionomycin or remained at the same level as those of wild-type WNV-infected mice following treatment with WNV peptides (Fig. 3B right panel, P > 0.05). At the later stage of infection (day 21), the percentage of both CD4+IFNγ+ and CD8+IFNγ+ splenocytes in NS4B-P38G mutant-infected mice were higher than those of wild-type WNV-infected mice upon ex vivo stimulation with WNV peptides (Suppl. Fig. 1). To further understand the effect of NS4B-P38G mutant infection on memory T cell development, we next measured CD4+ and CD8+ T cell responses in mice that survived a primary infection by either wild-type WNV or NS4B-P38G mutant followed by a secondary lethal challenge with a LD100 of wild-type WNV. At day 4 post-secondary infection, CD4+ and CD8+ T cells of both groups expanded as compared to naïve mice or mice primarily infected with wild type WNV (Fig. 4A left and right panels, P < 0.01 or 0.05). In particular, mice that survived primary NS4B-P38G mutant strain infection had 18–30% more CD4+ and CD8+ T cell expansion upon re-infection than those of mice that survived from wild-type WNV infection (Fig. 4A left and right panels, P < 0.01 or 0.05). By using ICS analysis, we noted that about 40% higher numbers of CD4+IFNγ+ and CD8+IFNγ+ splenocytes in mice primarily infected with NS4B-P38G mutant than those with wild-type WNV (Figs. 4B & 4C left and right panels, P < 0.01). Overall, these data suggest that NS4B P38G mutant infection induces a higher T cell response in mice than wild-type WNV.
Fig. 3.
T cell responses following primary infection with WNV wild-type or P38G-NS4B mutant strains. A, Splenocytes were isolated at day 4 post-infection with mock, WNV wild-type or P38G NS4B mutant strains and stained for CD4 or CD8. Number of cells per mouse was shown. B. Splenocytes of wild-type or NS4B P38G mutant strains-infected mice at day 4 were cultured ex vivo with PMA plus ionomycin or WNV peptides, and stained for IFN-γ, CD4 or CD8. **P < 0.01 or *P < 0.05 compared to wild-type strain. n = 3–5 mice /group from three separate experiments.
Fig. 4.
T cell responses during a secondary infection with LD100 of wild-type WNV in mice survived from a primary infection with wild-type or NS4B P38G mutant strains. Mice were primarily infected with mock, wild-type WNV or NS4B P38G mutant followed by a secondary infection with LD100 wild-type WNV, designated as (WT, WT-WT, P38G-WT respectively). A, Splenocytes were isolated at day 4 post-re-infection and stained for CD4 or CD8. Fold increase on the number of CD4+ or CD8+ T cell compared to mock-infected mice was shown. B-C, Splenocytes were cultured ex vivo with WNV peptides, and stained for IFN-γ, CD4 or CD8. B, one representative experiment was shown. C, number of CD4+IFNγ+ (left panel) or CD8+IFNγ+ (right panel) splenocytes were shown. **P < 0.01 or *P < 0.05 compared to mice primarily infected with wild-type WNV followed by secondary infection with wild-type strain (WT-WT). ## P < 0.01, or # P < 0.05 compared to primarily wild-type strain-infected mice (WT). n = 3–5 mice /group pooled from two experiments.
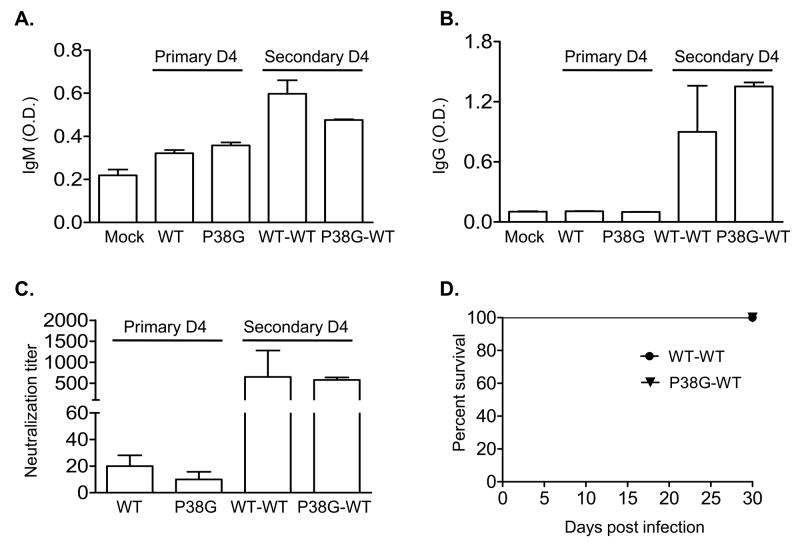
3.4. WNV specific antibody responses were not different between mice infected with these two viruses; all mice are protected from a secondary challenge with LD100 of wild-type WNV following primary infection with P38G mutant
B cell-mediated humoral immune responses are critical for the host defense against disseminated infection by WNV [29–31] and might also contribute to a protective memory response. To determine if WNV NS4B P38G mutant infection induces antibodies (Abs) to WNV during infection, we measured WNV-specific IgM (Fig. 5A) and IgG levels (Fig. 5B) by ELISA, and neutralization titers (Fig. 5C), in the sera from mice that were infected with wild-type WNV or NS4B-P38G mutant by a plaque reduction test. Ab responses in both groups of mice were similar at 4 days, an early interval post-infection. Further, we noted no differences in Ab responses to a secondary lethal challenge with wild-type WNV between mice that survived a primary infection by wild-type WNV or NS4B-P38G mutant (P > 0.05). These results indicate that infection by WNV P38G mutant and wild-type WNV induces a similar level of WNV specific humoral response. Moreover, we assessed survival rate of mice primarily immunized with NS4B P38G mutant or wild-type WNV during a secondary infection of LD100 of wild-type WNV. All mice (100%) primarily infected with either the NS4B-P38G mutant or wild-type WNV survived a four-week re-infection period by LD100 wild-type WNV (Fig. 5D, P > 0.05).
Fig. 5.
Humoral response following NS4B P38G infection. Sera were collected from mice either at day 4 (D4) during primary WNV infection or at D4 of secondary infection following WNV primary infection with either wild-type WNV or NS4B P38G mutant. The development of specific IgM (A) or IgG (B) antibodies to WNV was determined after incubating sera with absorbed purified r-WNV-E protein. C, Plaque reduction neutralization tests. n = 4–6 mice /group from two separate experiments, performed in duplicate. D. Survival rate. Mice survived from primary infection with wild-type WNV or NS4B-P38G were re-infected with 2000 PFU of wild-type WNV strain and monitored twice daily. n = 4 for wild-type strain primarily infected-mice (WT-WT). n = 5 for P38G strain primarily infected-mice (P38G-WT).
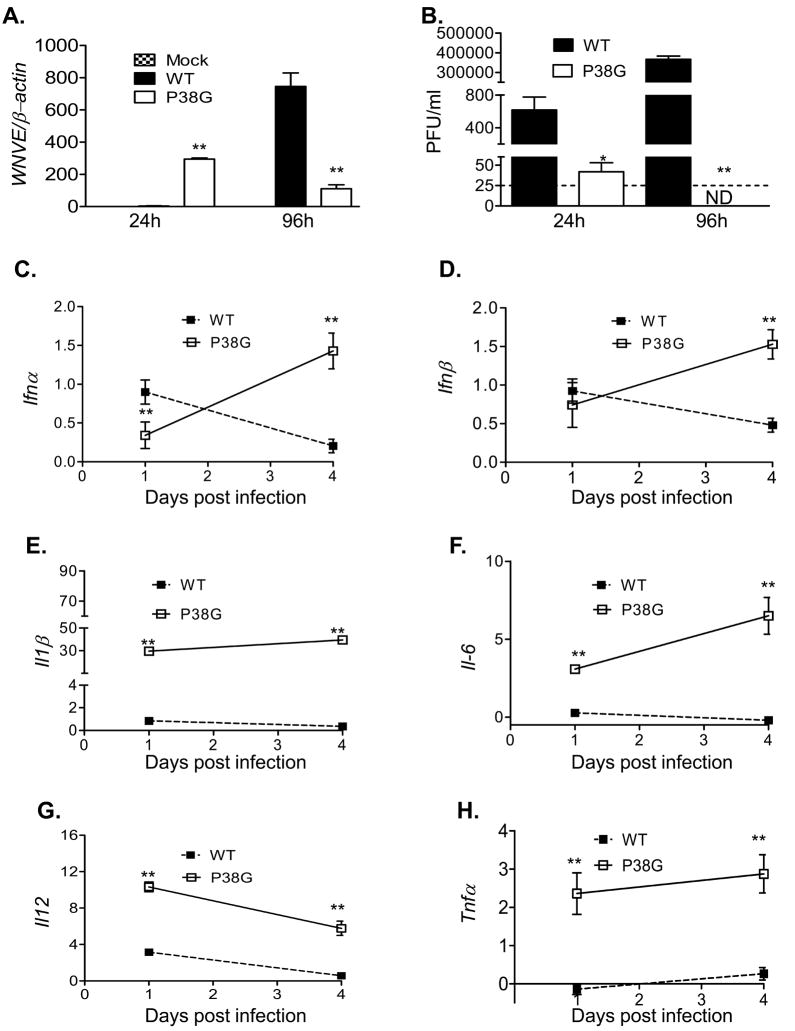
3.5. NS4B-P38G mutant infection in primary DCs shows a reduced replication rate, but a higher level of innate cytokine production than wild-type virus partially dependent on Myd88 signaling
DCs represent the most important antigen presenting cells (APCs) exhibiting the unique capacity to initiate primary T cell responses. They are innate immune cells that are permissive to WNV infection. Viral load in NS4B-P38G mutant infected-primary DCs was greatly reduced at day 4 post-infection compared to wild-type virus as measured by Q-PCR and plaque assay (Figs. 6A & 6B). Interestingly, on day 1 post-infection, an increase in viral load of NS4B-P38G mutant infected DCs was observed by Q-PCR analysis while infectivity measurement by the plaque assay showed the opposite results where infection by NS4B-P38G mutant was significantly lower than wild-type virus at this time point, indicating lack of productive replication occurred with the NS4B-P38G mutant. Furthermore, the expression of IFN-α and IFN-β in NS4B-P38G mutant-infected DCs on day 4 post-infection was increased by two- to six- fold compared to those of wild-type virus-infected cells (Figs. 6C & 6D, P < 0.05). Levels of proinflammatory cytokines in DCs, including IL-1β, IL-6, IL-12, and TNF-α were all significantly higher (about 10- 100 fold) in NS4B-P38G mutant infected DCs at days 1 and 4 post-infection (Figs. 6E- 6H, P < 0.01), compared to those of wild-type WNV-infected DCs. DCs express a collection of pattern recognition receptors on the surface that can specifically interact with pathogen-associated molecular patterns (PAMPS), including toll-like receptors (TLRs). TLR play an essential role in triggering the signals that provide DC maturation and the initiation of adaptive immune responses against pathogens [32]. The core TLR signaling pathway utilizes Myd88 as the primary adaptor [33]. Myd88-mediated innate immune responses are known to be protective against wild-type WNV infection in mice [17, 34]. To understand the role of MyD88 signaling in induction of immune response during NS4B-P38G mutant infection, we measured cytokine production in wild-type B6 and Myd88−/ −DCs following WNV infection. Type 1 IFNs production following NS4B-P38G mutant infection in Myd88−/− DCs showed only a small difference compared to wild-type DCs, slightly lower or higher on days 1 and 4 post-infection (Figs. 7A & 7B, P < 0.05). In comparison, the production of proinflammatory cytokines by NS4B-P38G mutant, including IL-1β, IL-6, and IL-12 and TNF-α, was consistently reduced on days 1 and 4 post-infection (> 3–30 fold) in Myd88−/− DCs (Fig. 7C- F). Type 1 IFNs production by the wild type WNV infection showed a minimal or no difference between wild-type B6 and Myd88−/− DCs. The same pattern was observed in proinflammatory cytokine production, except an enhanced IL-12 production was seen in Myd88−/− DCs on day 4 post-infection (data not shown). Taken together, these results suggests that NS4B-P38G mutant infection has a lower replication rate in DCs, but induced a higher innate immune response than wild-type virus, and was partially dependent on Myd88 signaling.
Fig. 6.
Infection in primary DCs by WNV wild-type strain or P38G NS4B mutant strain. AB. Viral load was determined using Q-PCR (A) or Plaque assay (B) at the indicated hours post-infection. The dotted line represents the limit of detection of the plaque assay as 25 PFU/ml. C- H. Cytokine expression was determined using Q-PCR. Data are presented as fold of increase compared to mock infected. n = 3. * P < 0.05 or **P < 0.01 compared to wild-type strain- infected mice.
Fig. 7.
Myd88 signaling is partially involved in induction of higher innate cytokine production and during NS4B P38G mutant infection. A- F. Cytokine levels in primary DCs were determined using Q-PCR. Data are presented as fold of increase compared to mock infected. n = 3. * P < 0.05 or **P < 0.01 compared to wild-type strain mice.
4. Discussion
The development of safe and effective vaccines against WNV remains a high priority. An ideal candidate WNV vaccine requires greatly reduced potential for neurovirulence, neuroinvasiveness, and restricted virus replication, while still having the ability to induce a robust protective immune response. Live attenuated vaccines have traditionally induced the best protective immune responses, but most have been derived empirically. Several approaches to attenuate WNV have been reported, including a capsid deletion mutation attenuated strain [35] and mutations in E and NS proteins for chimeric vaccine [36]. Further characterization of immune response to the attenuated WNV strains, including both innate and adaptive immunity are important steps in understanding their protective mechanisms. We recently identified a NS4B P38G mutant strain which had a significantly reduced neuroinvasiveness in NIH Swiss outbred mice as compared to the parental wild-type NY99 WNV (Wicker JA, and Barrett AD. et. al. Manuscript submitted). In this study, we have shown that this NS4B P38G mutant had a lower viremia and no lethality in mice following systemic infection in B6 inbred mice. Nevertheless, there was a higher level of type 1 IFN and IL-1β production following infection by NS4B P38G mutant. Both CD4 and CD8 T cell responses were also significantly enhanced in NS4B mutant-infected mice; whereas WNV specific Abs were induced at the same level as those by wild-type WNV. Finally, despite of the lower viremia and no lethality during primary infection of P38G NS4B mutant, all surviving mice were protected from a secondary challenge with LD100 of wild-type WNV. NS4B P38G mutant infection in all in vitro and in vivo infection studies were consistently shown to have small-plaques and non-neuroinvasive phenotypes. Reversion at NS4B-38 was never detected, suggesting genetic stability. Overall, these results suggest that the NS4B P38G mutation has a high potential for contribution to a future vaccine candidate.
Distinct inflammatory cytokines act directly on naive CD4+ and CD8+ T cells to provide a third signal, synergize with signals from the TCR and co-stimulatory receptors, to optimally activate differentiation and clonal expansion. IL-1 was shown to increase proliferation of CD4+ T cells in response to Ag and IL-2, which is consistent with effects on in vivo priming of CD4+ T cells [37]. The rules that dictate which signal 3 cytokine drives CD8+ T cell responses to microbial infection remain uncertain [38]. The two main candidate signal 3 cytokines produced for CD8+ T cell in response to intracellular pathogens have been reported to be type 1 IFNs and IL-12 [39–41]. During an acute viral infection, such as WNV, T cells go through three distinct phases involving initial activation and expansion (effector T cell), a contraction or death phase, and the establishment and maintenance of memory (memory T cell) [42]. In this study, NS4B-P38G mutant infection in primary DCs was shown to induce increased levels of type 1 IFNs and proinflammatory cytokines, such as IL-1β, and IL-12. Further, we found that higher CD4+ and CD8+ T cell responses occurred in NS4B-P38G mutant-infected mice at the early stage of infection (effector T cells) and the later stage of infection or during a secondary infection when memory T cells started to develop or had been developed. Therefore, we speculate that the increased levels of innate cytokines induced by APC following a primary infection of NS4B-P38G mutant, such as type 1 IFN, IL-12 and IL-1β, may contribute to a more efficient T cell activation and differentiation, which could ultimately lead to a higher level of memory T cell response. Future investigation will continue to focus on the role of the individual cytokine in memory T cell development during WNV infection.
The NS4B and NS5 proteins of flaviviruses have been shown to be IFN antagonists [9–11]. In this study, we have shown that a NS4B-P38G mutant infection induced a higher level of type 1 IFNs production compared to wild-type WNV. This suggests that P38 residue may serve as an important determinant in antagonizing IFN signaling during WNV infection. Flavivirus NS proteins are known to antagonize IFN signaling via multiple mechanisms, including the delay of PRR activation, inhibition of IFN gene transcription, and interference of Janus kinase (JAK) and signal transducers and activators of transcription (STAT) signaling pathways [12, 43–45]. Here, we found that type 1 IFNs production by wild-type WNV virus was not different between wild-type and Myd88−/− DCs. This is consistent with recent reports by others [17, 34]. Moreover, we noted minimal differences in IFN production between wild-type and Myd88−/− DCs infected by NS4B P38G mutant. Combined together, these data suggest that induction of type 1 IFN by either WNV strain may be Myd88-independent. Furthermore, we noted that NS4B-P38G mutant infection induced a significant increase in proinflammatory cytokine production compared to wild-type WNV, and this response was significantly reduced in Myd88−/− DCs. This is consistent with the hypothesis that Myd88 signaling may be required for induction of proinflammatory cytokine production by NS4B-P38G mutant infection.
In conclusion, our work has shown that a NS4B WNV mutant strain infection in mice induces higher innate cytokine production and enhanced CD4+ and CD8+ T cell responses with a lower viremia and no lethality, thereby demonstrating a high potential for contribution of this mutation in a future vaccine candidate. These results may provide critical insights for new strategies in improving the efficiency of future attenuated flavivirus vaccines to generate long-lasting protective immune responses
Supplementary Material
T cell response at the later stage of WNV infection. Splenocytes from day 21 WNV wild-type or NS4B P38G mutant strains-infected mice were cultured ex vivo with WNV peptides, and stained for IFN-γ, CD4 or CD8. Cells were gated on total splenocytes and percentage of CD4+ IFNγ+ (top panel) or CD8+IFNγ+ (bottom panel) were shown. Data presented are representative of two similar experiments.
Acknowledgments
This work was supported in part by NIH grant R01AI072060 (to T.W.) and the Clayton Foundation for Research (ADTB). We thank Drs. Shizuo Akira (Osaka University, Japan) and Richard Flavell (Howard Hughes Medical Institute, Yale University School of Medicine, New Haven) for providing Myd88−/− mice.
Abbreviations used in this paper
- APC
antigen presenting cell
- B6
C57BL/6
- CNS
central nervous system
- E
envelope
- IFN
interferon
- i.p
intraperitoneally
- LD
lethal dose
- Myd88
myeloid differentiation factor 88
- NS
Nonstructural
- Q-PCR
quantitative PCR
- WNV
West Nile virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, et al. Isolation of West Nile virus from mosquitoes, crows, and a Cooper’s hawk in Connecticut. Science. 1999;286(5448):2331–3. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 2.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286(5448):2333–7. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 3.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2(9):519–29. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 4.Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75(9):4040–7. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Anderson JF, Magnarelli LA, Wong SJ, Koski RA, Fikrig E. Immunization of mice against West Nile virus with recombinant envelope protein. J Immunol. 2001;167(9):5273–7. doi: 10.4049/jimmunol.167.9.5273. [DOI] [PubMed] [Google Scholar]
- 6.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci U S A. 2006 Apr 25;103(17):6694–9. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill AB, Mullbacher A, Parrish C, Coia G, Westaway EG, Blanden RV. Broad cross-reactivity with marked fine specificity in the cytotoxic T cell response to flaviviruses. J Gen Virol. 1992 May;73( Pt 5):1115–23. doi: 10.1099/0022-1317-73-5-1115. [DOI] [PubMed] [Google Scholar]
- 8.McMurtrey CP, Lelic A, Piazza P, Chakrabarti AK, Yablonsky EJ, Wahl A, et al. Epitope discovery in West Nile virus infection: Identification and immune recognition of viral epitopes. Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):2981–6. doi: 10.1073/pnas.0711874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puig-Basagoiti F, Tilgner M, Bennett CJ, Zhou Y, Munoz-Jordan JL, Garcia-Sastre A, et al. A mouse cell-adapted NS4B mutation attenuates West Nile virus RNA synthesis. Virology. 2007 Apr 25;361(1):229–41. doi: 10.1016/j.virol.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi SL, Fayzulin R, Dewsbury N, Bourne N, Mason PW. Mutations in West Nile virus nonstructural proteins that facilitate replicon persistence in vitro attenuate virus replication in vitro and in vivo. Virology. 2007 Jul 20;364(1):184–95. doi: 10.1016/j.virol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, et al. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J Virol. 2010 Apr;84(7):3503–15. doi: 10.1128/JVI.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu WJ, Wang XJ, Mokhonov VV, Shi PY, Randall R, Khromykh AA. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J Virol. 2005 Feb;79(3):1934–42. doi: 10.1128/JVI.79.3.1934-1942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, et al. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005 Jul;79(13):8004–13. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans JD, Seeger C. Differential effects of mutations in NS4B on West Nile virus replication and inhibition of interferon signaling. J Virol. 2007 Nov;81(21):11809–16. doi: 10.1128/JVI.00791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicker JA, Whiteman MC, Beasley DW, Davis CT, Zhang S, Schneider BS, et al. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology. 2006 Jun 5;349(2):245–53. doi: 10.1016/j.virol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998 Jul;9(1):143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 17.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009 Feb;30(2):242–53. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daffis S, Samuel MA, Suthar MS, Keller BC, Gale M, Jr, Diamond MS. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J Virol. 2008 Sep;82(17):8465–75. doi: 10.1128/JVI.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, et al. Rapid detection of West Nile virus from human clinical specimens, field- collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38(11):4066–71. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004 Dec;10(12):1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 21.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008 Dec 15;181(12):8568–75. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007 Jul;37(7):1855–63. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 23.Purtha WE, Myers N, Mitaksov V, Sitati E, Connolly J, Fremont DH, et al. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur J Immunol. 2007 Jul;37(7):1845–54. doi: 10.1002/eji.200737192. [DOI] [PubMed] [Google Scholar]
- 24.Wong SJ, Demarest VL, Boyle RH, Wang T, Ledizet M, Kar K, et al. Detection of human anti-flavivirus antibodies with a West Nile virus recombinant antigen microsphere immunoassay. J Clin Microbiol. 2004 Jan;42(1):65–72. doi: 10.1128/JCM.42.1.65-72.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296(1):17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- 26.Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16(3):259–78. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha B, Samuel MA, Diamond MS. CD8+ T Cells Require Perforin To Clear West Nile Virus from Infected Neurons. J Virol. 2006 Jan;80(1):119–29. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Lobigs M, Lee E, Mullbacher A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol. 2003 Dec;77(24):13323–34. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001 Dec;951:286–97. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- 30.Price WH, Thind IS. The mechanism of cross-protection afforded by dengue virus against West Nile virus in hamsters. J Hyg (Lond) 1972 Dec;70(4):611–7. doi: 10.1017/s0022172400022476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003 Feb;77(4):2578–86. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004 Oct;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 33.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003 Jan 22;85(2):85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 34.Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M, Jr, et al. The innate immune adaptor molecule MyD88 restricts West Nile replication and spread in neurons of the central nervous system. J Virol. 2010 Sep 29; doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlick P, Kofler RM, Schittl B, Taucher C, Nagy E, Meinke A, et al. Characterization of West Nile virus live vaccine candidates attenuated by capsid deletion mutations. Vaccine. 2010 Aug 16;28(36):5903–9. doi: 10.1016/j.vaccine.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 36.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010 Jan 8;28(3):632–49. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 37.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999 Mar 15;162(6):3256–62. [PubMed] [Google Scholar]
- 38.Tam MA, Wick MJ. MyD88 and interferon-alpha/beta are differentially required for dendritic cell maturation but dispensable for development of protective memory against Listeria. Immunology. 2009 Nov;128(3):429–38. doi: 10.1111/j.1365-2567.2009.03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999 Feb 1;189(3):521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008 Feb;8(2):107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 41.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006 Jun;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 42.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001 May;2(5):415–22. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriyama M, Matsumura H, Nirei K, Arakawa Y, Yamagami H, Ogawa M, et al. Factors influencing treatment efficacy of 24-week combination therapy with interferon alpha-2b plus ribavirin for chronic hepatitis C. Dig Dis Sci. 2007 Sep;52(9):2418–26. doi: 10.1007/s10620-006-9693-0. [DOI] [PubMed] [Google Scholar]
- 44.Wilson JR, de Sessions PF, Leon MA, Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J Virol. 2008 Sep;82(17):8262–71. doi: 10.1128/JVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredericksen BL, Smith M, Katze MG, Shi PY, Gale M., Jr The host response to West Nile Virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J Virol. 2004 Jul;78(14):7737–47. doi: 10.1128/JVI.78.14.7737-7747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T cell response at the later stage of WNV infection. Splenocytes from day 21 WNV wild-type or NS4B P38G mutant strains-infected mice were cultured ex vivo with WNV peptides, and stained for IFN-γ, CD4 or CD8. Cells were gated on total splenocytes and percentage of CD4+ IFNγ+ (top panel) or CD8+IFNγ+ (bottom panel) were shown. Data presented are representative of two similar experiments.