Abstract
Xiao, Daliao, Xiaohui Huang, Lawrence D. Longo, and Lubo Zhang. PKC regulates α1-adrenoceptor-mediated contractions and baseline Ca2+ sensitivity in the uterine arteries of nonpregnant and pregnant sheep acclimatized to high alttude hypoxia. High Alt. Med. Biol. 11:153–162, 2010.—Chronic hypoxia has a profound effect on uterine artery adaptation to pregnancy. The present studies tested the hypothesis that pregnant kinase C (PKC) differentially regulates α1-adrenoceptor-mediated contractions and Ca2+ sensitivity in the uterine arteries of nonpregnant and pregnant sheep acclimatized to high altitude hypoxia. Uterine arteries were isolated from nonpregnant (NPUA) and near-term pregnant (PUA) ewes maintained at high altitude (3801 m, Pao2 ∼60 torr) for 110 days. Phorbol 12,13-dibutyrate (PDBu) decreased phenylephrine-induced contractions in PUA but not in NPUA, which was partly inhibited by the PKC inhibitor GF109203X. Additionally, GF109203X shifted the concentration–response curve of phenylephrine-induced contractions to the right in PUA. In β-escin-permeabilized arteries, Ca2+-induced increases in 20-kDa myosin light chain phosphorylation (MLC20-P) were similar in NPUA and PUA. However, Ca2+ produced a concentration-dependent increase in the ratio of tension to MLC20-P in PUA, as compared with NPUA. PKC inhibition decreased Ca2+-induced contractions in both NPUA and PUA. PDBu induced contractions of PUA in the absence of changes in MLC20-P, which was not affected by PD098059. There was a significant increase in the basal activity of PKCɛ in PUA, but not in NPUA, in hypoxic sheep, as compared with normoxic animals. The results demonstrate that the inhibitory effect of PKC on α1-adrenoceptor-mediated contractions of uterine arteries is preserved in pregnant sheep at high altitude. However, the PKC-mediated thin-filament regulatory pathway is upregulated, resulting in increased baseline Ca2+ sensitivity in the uterine artery during pregnancy at high altitude.
Key Words: uterine artery, pregnancy, PKC, Ca2+ sensitivity, high altitude hypoxia
Introduction
Chronic hypoxia during pregnancy is one of the most common insults to the maternal cardiovascular system and fetal development and is thought to be associated with an increased risk of preeclampsia and fetal intrauterine growth restriction (IUGR) (Magness and Rosenfeld, 1986; Zamudio et al., 1995a, 1995b; Keyes et al., 2003; Julian et al., 2008). However, in our high altitude-sheep animal model, chronic hypoxia alters maternal and fetal cardiovascular systems without significant IUGR. This suggests that there may be compensatory adaptations to chronic hypoxia in sheep, which perhaps are similar to those observed in well-adapted human populations, such as Tibetans and Andeans who exhibit lower IUGR (Moore et al., 2001). Many previous studies have examined the adaptation of uterine artery contractile and relaxation mechanisms to pregnancy (Magness and Rosenfeld, 1986; Ford, 1995; Rosenfeld, 2001; Xiao and Zhang, 2002; Bird et al., 2003; Xiao and Zhang, 2005), which is critical for maternal cardiovascular well-being and normal fetal development. However, the effects of chronic hypoxia on the adaptation of uterine artery contractility to pregnancy are not fully understood.
Among other mechanisms, protein kinase C (PKC) plays an important role in the regulation of contractility in uterine and other arteries. Our previous studies have demonstrated that ovine uterine artery vascular tone is regulated through both Ca2+ mobilization and the Ca2+ sensitivity of the contractile process (Xiao et al., 2006a). Although we did not determine the exact proportion of the uterine artery contraction that is mediated by Ca2+ sensitivity in normal pregnancy, our previous studies demonstrated that Ca2+ sensitivity-mediated contractions are significantly attenuated in pregnant uterine arteries, compared with nonpregnant ones (Xiao et al., 2006b). We have demonstrated that the pregnancy-associated decrease in the vascular tone of uterine artery is caused primarily by a downregulation of the PKC signaling pathway (Xiao and Zhang, 2002, 2005; Xiao et al., 2006a) and that this is mediated by a decrease in PKC-mediated baseline Ca2+ sensitivity in a thin-filament-dependent pathway (Xiao and Zhang, 2002; Xiao et al., 2004, 2005; Xiao and Zhang, 2005; Xiao et al., 2006b). Consistently, it has been shown that pregnancy is associated with the attenuated arterial PKC activity (Magness et al., 1991; Farley and Ford, 1992; Ford, 1995; Kanashiro et al., 2000). Our recent studies demonstrated that PKC interacted with α1-adrenoceptors and modulated α1-adrenoceptor-mediated contractions of the uterine arteries (Zhang et al., 2006; Zhang and Zhang, 2007, 2008). Whereas in the uterine artery of nonpregnant sheep activation of PKC significantly enhanced phenylephrine-induced contractions, in pregnant animals, PKC inhibited the phenylephrine-induced contractions (Zhang et al., 2006). This is likely to be important in maintaining low sympathetic reactivity of the uterine vasculature during pregnancy. However, it is not known whether and to what extent this pregnancy-induced adaptation is altered in uterine arteries of the animals acclimatized to high altitude hypoxia. In addition, we have shown an increase in PKC-mediated baseline Ca2+ sensitivity in uterine arteries of pregnant sheep acclimatized to high altitude hypoxia (Chang et al., 2009). However, it remains unknown whether and to what extent the thin-filament-mediated pathway, that is, independent of changes in myosin light chain phosphorylation, is involved in the adaptation of Ca2+ sensitivity in animals at high altitude.
The goal of the present study is twofold. First, we investigated the regulatory effects of PKC on α1-adrenoceptor-mediated contractions in uterine arteries of nonpregnant and near-term pregnant sheep acclimatized to high altitude hypoxia (3801 m, Pao2 ∼60 torr) for 110 days. By comparing with the results obtained in the uterine arteries of normoxic animals in our previous studies (Zhang et al., 2006), we tested the hypothesis that chronic hypoxia resulted in changes in the PKC coupling to α1-adrenoceptor-mediated contractions in uterine arteries of nonpregnant and pregnant sheep. Second, we determined the role of the thin-filament pathway in the enhanced baseline Ca2+ sensitivity in uterine arteries of pregnant animals at high altitude. By clamping Ca2+ concentrations and measuring myosin light chain phosphorylation simultaneously, we tested the hypothesis that the increased PKC-induced baseline Ca2+ sensitivity in uterine arteries of pregnant sheep at high altitude was mediated primarily by a thin-filament mechanism.
Methods
Tissue preparation
Nonpregnant and time-dated pregnant (30 days of gestation) sheep were obtained from the Nebeker Ranch in Lancaster, California (altitude ∼300 m; maternal Pao2 100 ± 2 torr). For the long-term hypoxic exposure, animals were transported to the Barcroft Laboratory, White Mountain Research Station, Bishop, California (altitude 3801 m; maternal Pao2 60 ± 2 torr) and maintained at high altitude for ∼110 days, as previously described (Xiao and Zhang, 2004). Animals then were transported to the laboratory at Loma Linda University during which they experienced normoxia for about 4 to 6 h. Shortly after arrival, we placed a tracheal catheter in the ewe, through which N2 flowed at a rate to maintain Pao2 at ∼60 torr, and this was maintained until the time of the experimental study. Ewes were anesthetized with thiamylal (10 mg/kg), and anesthesia was maintained on 1.5% to 2.0% halothane in oxygen throughout surgery. The uterine arteries were removed and placed in a modified Krebs solution (pH 7.4) of the following composition (in mmol/L): 115.21 NaCl, 4.7 KCl, 1.80 CaCl2, 1.16 MgSO4, 1.18 KH2PO4, 22.14 NaHCO3, 0.03 EDTA, and 7.88 dextrose, oxygenated with a mixture of 95% O2–5% CO2. After removal of the tissues, animals were killed with an overdose of the proprietary euthanasia solution Euthasol (pentobarbital sodium, 100 mg/kg, and phenytoin sodium, 10 mg/kg; Virbac, Ft. Worth, TX, USA). All procedures and protocols were approved by the Institutional Animal Care and Use Committee guidelines.
Contraction studies
The fourth-generation branches (∼0.8 mm in external diameter) of main uterine arteries were separated from the surrounding tissue and cut into 2-mm ring segments. The small branches of uterine arteries were chosen, because they are much closer in characteristics to arterioles and play a substantial role in vascular resistance. Isometric tension was measured in the Krebs solution in a tissue bath at 37°C, as described previously (Xiao and Zhang, 2005). Briefly, each ring was equilibrated for 60 min and then stretched gradually to the optimal resting tension, as determined by the tension that developed in response to 120 mmol/L KCl added at each stretch level. Because the length–tension relationship was similar in both pregnant and nonpregnant vessels (Xiao and Zhang, 2005), we kept the same optimal resting tension in current studies. Tissues were then stimulated with cumulative additions of phenylephrine in approximate one-half log increments to generate a concentration–response curve, and contractile tensions were recorded with an online computer. After phenylephrine was washed away, tissues were relaxed to the baseline and recovered at the resting tension for 30 min. The second concentration–response curves of phenylephrine-induced contractions were then repeated in the absence or presence of a PKC activator phorbol 12,13-dibutyrate (PDBu, 100 nmol/L, for 10 min) and/or a PKC inhibitor, GF109203X (1 μmol/L, for 20 min). EC50 values for the agonist in each experiment were taken as the molar concentration at which the contraction—response curve intersected 50% of the maximum response and were expressed as pD2 (−logEC50) values.
Measurement of baseline Ca2+ sensitivity
Two main Ca2+ buffer solutions were used as previously described (Xiao et al., 2006b). The zero Ca2+ relaxing solution contained (in mmol/L) 110 potassium acetate, 5 ethylene glycol tetraacetic aid (EGTA), 5 adenosine triphosphate, 6 magnesium acetate, 1 dithiothreitol, 0.01 leupeptin, 20 imidazole, and 20 HEPES, at pH 6.8 (titrated with KOH). The maximum Ca2+ solution contained 1 mmol/L Ca2+ in addition to those components in the zero Ca2+ buffer. As described in the previous study (Xiao et al., 2006b), Ca2+ buffer solutions were prepared by solving the multiequilibrium equations for interactions among the different ions, and solutions containing intermediate free Ca2+ concentrations were prepared by mixing appropriate amounts of the zero Ca2+ relaxing solution and the maximum Ca2+ buffer solutions, titrated to pH 7.0 with 1 mol/L KOH. The arterial rings were attached to isometric force transducers and bathed in phosphate saline solution (PSS) at 37°C. After 60 min of equilibration, each ring was stretched to the optimal resting tension, as determined by the tension developed in response to 120 mmol/L KCl added at each stretch level. The arterial rings were then placed into the zero Ca2+ relaxing solution and were permeabilized, as previously described (Xiao et al., 2006b). Briefly, chemical permeabilization was achieved by adding 40 μmol/L β-escin to the relaxing solution and allowing the arteries to incubate for 20 min at 25°C. The permeabilized solution was then replaced with the relaxing solution containing 1 μmol/L A23187 to deplete Ca2+ from the sarcoplasmic reticulum. Concentration–response curves to Ca2+ were obtained by cumulative increases of Ca2+ concentrations in approximately one-half log increments in β-escin-permeabilized arterial rings. In certain experiments, the concentration–response curves to Ca2+ were conducted in the presence or absence of a PKC activator or inhibitor.
Measurement of MLC20 phosphorylation
Phosphorylation of 20-kDa myosin light chain (MLC20-P) and contractile tensions were determined simultaneously in the same tissues, as previously described (Je et al., 2001; D'Angelo and Adam, 2002; Xiao et al., 2004; Xiao and Zhang, 2005). Tensions developed were continuously recorded with an online computer, and arterial rings were snap-frozen with liquid N2-cooled clamps at the indicated times and rapidly immersed in a Dry Ice–acetone slurry containing a 10% trichloroacetic acid (TCA) and 10 mmol/L dithiothreitol (DTT) mixture. Tissues were stored at −80°C until the analysis of MLC20-P. To measure MLC20-P, tissues were brought to room temperature in a Dry Ice–acetone–TCA–DTT mixture and then washed 3 times with ether to remove the TCA. Tissues then were extracted in 100 μL of sample buffer containing 20 mmol/L tris base and 23 mmol/L glycine (pH 8.6), 8.0 mol/L urea, 10 mM DTT, 10% glycerol, and 0.04% bromophenol blue, as previously described. Samples (20 μL) were electrophoresed at 12 mA for 2.5 h after a 30-min prerun in 1.0-mm minipolyacrylamide gels containing 10% arcelamide–0.27% bisacrylamide, 40% glycerol, 8.0 mol/L urea, and 20 mmmol/L tris base (pH 8.8). Proteins were transferred to nitrocellulose membranes and subjected to immunoblot with a specific MLC20 antibody (1:500). Goat antimouse IgG conjugated with horseradish peroxidase was used as a secondary antibody (1:2000). Bands were detected with enhanced chemiluminscence (ECL), visualized on Hyperfilm (General Electric Health Services, Piscataway, NJ, USA) and analyzed with the Kodak (Rochester, NY, USA) 1D image analysis software. Moles of phosphate per mole of MLC20 (mol Pi/mol MLC20, fractional light chain phosphorylation) were calculated by dividing the density of the phosphorylated band by the sum of the densities of the phosphorylated plus the unphosphorylated bands.
Immunoblot analysis
Tissues were homogenized in ice-cold homogenization buffer A containing tris-HCl 20 mmol/L, sucrose 250 mmol/L, EDTA 5 mmol/L, EGTA 5 mmol/L, β-mercaptoethanol 10 mmol/L, benzamidine 1 mmol/L, phenylmethylsulfonyl fluoride (PMSF) 1 mmol/L, leupetin 50 μmol/L, dithiothreitol 1 mmol/L, and aprotinin 2 μg/mL, pH 7.5. The homogenates were centrifuged at 100,000 × g for 20 min at 4°C, and the supernatants were collected as the cytosolic fraction. The pellets were resuspended in homogenization buffer A containing 1% triton X-100 by stirring overnight at 4°C, diluted with the buffer A to a final concentration of 0.2% triton X-100, and then centrifuged at 100,000 × g for 20 min at 4°C. The supernatants were collected and referred to as the particulate fraction. Samples of cytosolic and particulate fractions with equal protein were subjected to electrophoresis on 7.5% (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were incubated at room temperature for 1 h in tris-buffered saline solution (TBS) containing 5% dried milk and 0.5% Tween 20, followed by incubation with primary antibodies for PKCα and PKCɛ (Santa Cruz Biotechnology, Santa Cruz, CA, USA), respectively, overnight at 4°C and secondary antibody for 1 h at room temperature. Bands were detected with enhanced chemiluminescence (ECL), visualized on Hyperfilm and analyzed with the Kodak 1D image analysis software. To normalize the loading variation of each sample, the corresponding actin level presented in each sample was determined by using monoclonal antiactin as primary antibody (Santa Cruz Biotechnology).
Data analysis
Concentration–response curves were analyzed by computer-assisted nonlinear regression to fit the data using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Results were expressed as means ± SEM obtained from the number (n) of experimental animals given. Differences were evaluated for statistical significance (p < 0.05) by ANOVA, followed by the Newman–Keuls post hoc test.
Results
Effect of PKC on phenylephrine-induced contractions
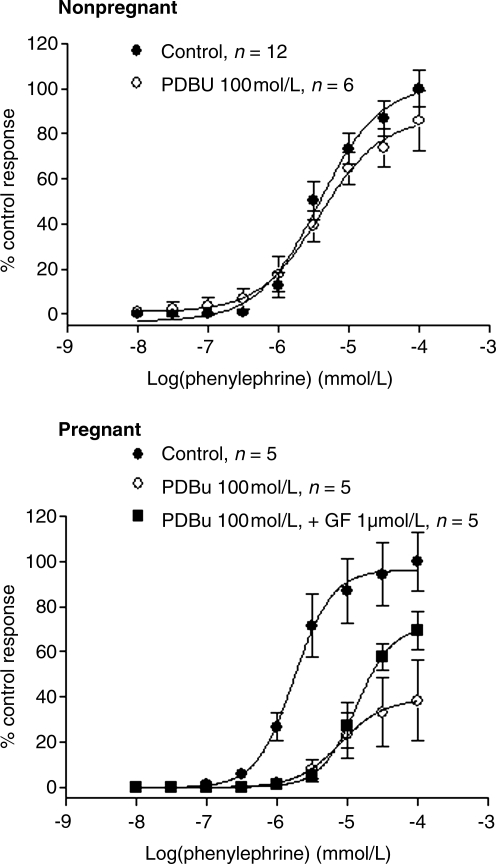
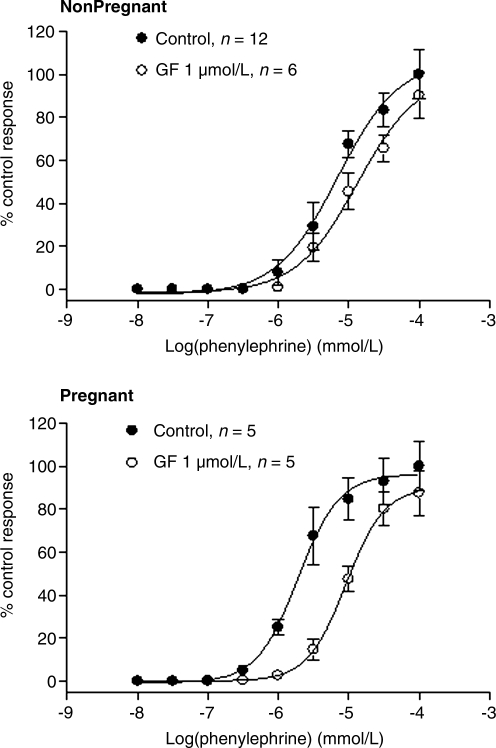
Figure 1 shows that phenylephrine produced concentration-dependent contractions in uterine arteries of both nonpregnant and pregnant ewes acclimatized to high altitude. In vessels of nonpregnant animals, PKC activator PDBu (100 nmol/L) had no significant effect on phenylephrine-induced contractions (Fig. 1, the upper panel). In contrast, PDBu significantly decreased both the pD2 value (5.1 ± 0.3 vs. 5.8 ± 0.1, p < 0.05) and maximal response (39.0 ± 10.3 vs. 96.5 ± 6.3, p < 0.05) in uterine arteries of pregnant ewes, which was reversed partly by the addition of PKC inhibitor GF109203X (Emax: 71.5 ± 6.6 vs. 39.0 ± 10.3, p < 0.05) (Fig. 1, the lower panel). As shown in Fig. 2, inhibition of PKC by GF109203X did not significantly affect phenylephrine-induced contractions in uterine arteries of nonpregnant sheep, but shifted the concentration–response curve to the right in pregnant animals (pD2 value: 5.0 ± 0.1 vs. 5.7 ± 0.1, p < 0.05). In our previous study, we demonstrated that phenylephrine-induced maximal contractile responses of uterine arteries are significantly higher in normoxic pregnant sheep than in normoxic nonpregnant sheep (Zhang et al., 2006). However, in high altitude hypoxic sheep, phenylephrine-induced maximal contractile responses in uterine arteries were not significantly different between pregnant and nonpregnant animals (Emax: 16.5 ± 0.7 g vs. 16.2 ± 0.6 g, p > 0.05).
FIG. 1.
Effect of PDBu on phenylephrine-induced contractions of uterine arteries in long-term, high-altitude hypoxic sheep. Phenylephrine-induced contractions were determined in the uterine arteries obtained from nonpregnant and pregnant ewes acclimatized to high altitude hypoxia in the absence or presence of PDBu (100 nmol/L, pretreatment for 10 min). GF109203X (1 μmol/L, pretreatment for 20 min) was added before PDBu in uterine arteries of pregnant animals. Data are means ± SEM of the tissues from 5 to 12 animals.
FIG. 2.
Effect of GF109203X on phenylephrine-induced contractions of uterine arteries in long-term, high altitude hypoxic sheep. Phenylephrine-induced contractions were determined in the uterine arteries obtained from nonpregnant and pregnant ewes acclimatized to high altitude hypoxia in the absence or presence of GF109203X (1 μmol/L, pretreatment for 20 min). Data are means ± SEM of the tissues from 5 to 6 animals.
Ca2+-induced myosin phosphorylation and contractions
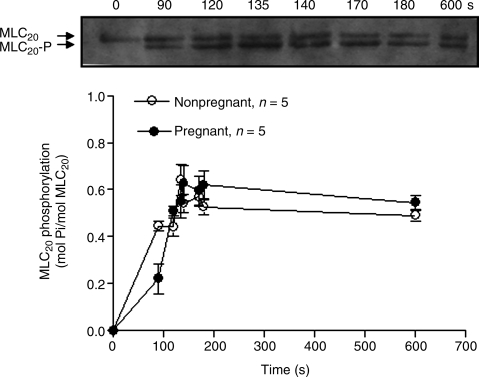
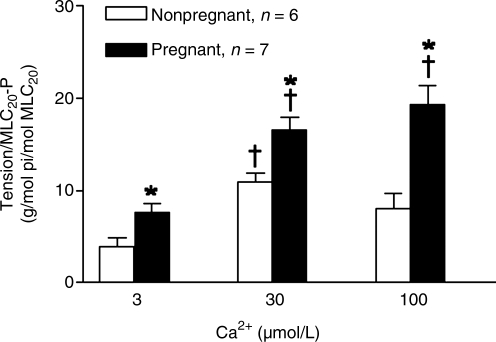
In a previous study, we demonstrated that Ca2+-induced contractions of the uterine artery significantly increased in pregnant, compared with nonpregnant, sheep acclimatized to high altitude hypoxia (Chang et al., 2009). To determine whether the significantly increased Ca2+-induced contractions were associated with changes of MLC20-P, we measured Ca2+-induced time-dependent changes in MLC20-P. Figure 3 shows the time course of Ca2+-induced increases in MLC20-P in permeabilized uterine arteries of nonpregnant and pregnant animals at high altitude. In both pregnant and nonpregnant vessels, Ca2+ produced time-dependent increases in MLC20-P. In contrast to the previous finding of significantly increased Ca2+-induced contractions (Chang et al., 2009), the Ca2+-induced increases in MLC20-P were similar in both nonpregnant and pregnant uterine arteries (two-way ANOVA test, p > 0.05) (Fig. 3). To further determine the thick filament, that is, MLC20-P, versus the thin filament, that is, MLC20-P -independent, mechanisms in the regulation of the baseline Ca2+ sensitivity in the uterine arteries of hypoxic animals, we measured simultaneously Ca2+-induced MLC20-P and contractions in the same tissues. Figure 4 shows that Ca2+ produced a concentration-dependent increase in the ratio of tension to MLC20-P in the uterine arteries, and this was significantly higher in the vessels of pregnant than in nonpregnant animals.
FIG. 3.
Effect of pregnancy on the Ca2+-induced MLC20-P in β-escin-permeabilized uterine arteries in long-term, high altitude hypoxic sheep. β-escin-permeabilized uterine arteries from nonpregnant and pregnant ewes acclimatized to high altitude hypoxia were stimulated with 10 μmol/L Ca2+. MLC20-P was detected by Western immunoblot (as described in METHODS). Representative immunoblot (top) shows unphosphorylated MLC20 and MLC20-P induced by 10 μmol/L Ca2+ at indicated time points in one of the uterine artery rings isolated from pregnant sheep. Data are means ± SEM of tissues from 5 animals.
FIG. 4.
Effect of pregnancy on the ratio of Ca2+-induced contractions to MLC20-P in β-escin-permeabilized uterine arteries in long-term, high altitude hypoxic sheep. β-escin-permeabilized uterine arteries from nonpregnant and pregnant ewes acclimatized to high altitude hypoxia were stimulated with increasing concentrations of Ca2+. MLC20-P and contractions were measured simultaneously in the same tissues. Data are means ± SEM of tissues from 6 to 7 animals. *p < 0.05 versus nonpregnant animals; †p < 0.05 versus 3 μmol/L Ca2+.
Effect of PKC on Ca2+-induced contractions
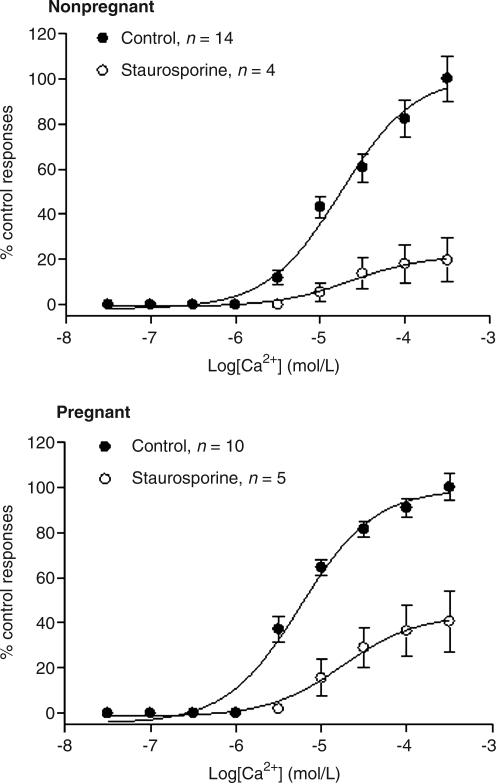
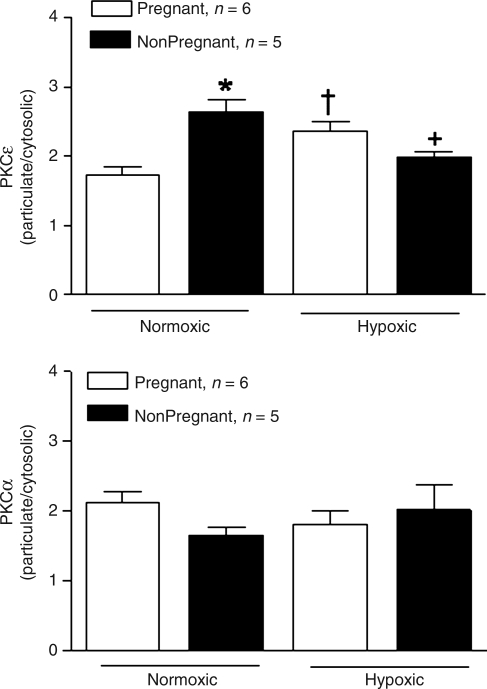
The PKC inhibitor staurosporine (0.5 μmol/Λ) significantly decreased the Ca2+-induced contractions of the uterine arteries in both nonpregnant and pregnant sheep at high altitude (Fig. 5). As shown in Fig. 6, there was a significant increase in the ratio of particulate to cytosolic fractions of PKCɛ in the uterine artery of nonpregnant, as compared with pregnant, sheep of normoxic animals. The ratio of PKCɛ was significantly increased in pregnant, but decreased in nonpregnant, animals acclimatized to high altitude (Fig. 6). In contrast, the ratio of particulate to cytosolic fractions of PKCα in the uterine artery did not differ significantly among the four groups of animals (Fig. 6).
FIG. 5.
Effect of staurosporine on Ca2+-induced contractions in β-escin-permeabilized uterine arteries in long-term high altitude hypoxic sheep. Ca2+-induced contractions were determined in β-escin-permeabilized uterine arteries obtained from nonpregnant and pregnant ewes acclimatized to high altitude hypoxia in the absence or presence of staurosporine (0.5 μmol/L, pretreatment for 20 min). Data are means ± SEM of tissues from 4 to 14 animals.
FIG. 6.
Effect of high altitude hypoxia on the baseline PKC activity in uterine arteries. PKCɛ (upper panel) and PKCα (lower panel) protein abundance was determined in the uterine arteries obtained from nonpregnant and pregnant ewes of normoxic control and high altitude hypoxic treatment. The baseline PKC activity is expressed as the ratio of the particulate to cytosolic fractions of PKC isozymes. Data are means ± SEM of tissues from 5 to 6 animals. *p < 0.05 versus pregnant animals; †p < 0.05 versus normoxic animals.
Effect of ERK1/2 on PKC-mediated contractions
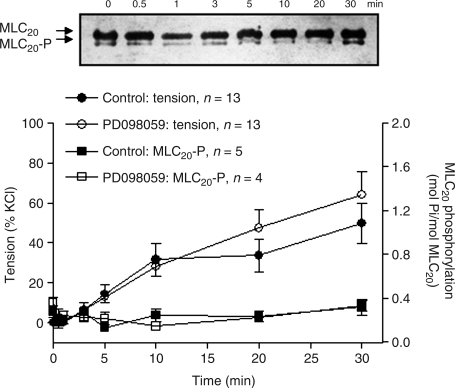
In uterine arteries of pregnant ewes acclimatized to high altitude hypoxia, PDBu produced a time-dependent increase in contractions without significant changes in MLC20-P (Fig. 7), demonstrating a primary thin-filament mechanism in PKC-induced contractions. In contrast to the finding in normoxic animals, in which the ERK1/2 inhibitor PD098059 significantly increased PDBu-induced contractions in uterine arteries of pregnant sheep in a MLC20-P independent manner (Xiao et al., 2004), PD098059 showed no significant effects on either PDBu-induced contractions or MLC20-P in the uterine arteries of pregnant ewes acclimatized to high altitude hypoxia (two-way ANOVA test, p > 0.05) (Fig. 7).
FIG. 7.
PDBu-induced MLC20-P and contractions of uterine arteries of pregnant sheep at high altitude. Uterine arteries from pregnant ewes acclimatized to high altitude hypoxia were stimulated with 10 μmol/L PDBu in the absence or presence of PD098059 (30 μmol/L, pretreatment for 20 min). Contractile tension and MLC20-P were measured simultaneously in the same tissues at indicated time points. Representative immunoblot (top) shows unphosphorylated MLC20 and MLC20-P induced by 10 μmol/L PDBu in the uterine artery rings isolated from pregnant sheep. Data are means ± SEM of tissues from 4 to 13 animals.
Discussion
The present study has demonstrated that the inhibitory effect of PKC on α1-adrenoceptor-mediated contractions of the uterine artery is maintained in pregnant sheep acclimatized to high altitude hypoxia. In addition, the increased PKC-induced baseline Ca2+ sensitivity in uterine arteries of pregnant sheep at high altitude is mediated primarily by a thin-filament mechanism. These findings suggest that sheep uterine arteries exhibit very important compensatory mechanisms to chronic hypoxia, that is, maintained low uterine vascular sympathetic reactivity counteracting increased PKC-induced baseline Ca2+ sensitivity. Although the increased PKC-induced baseline Ca2+ sensitivity and uterine arterial tone have the potential to reduce uterine blood flow, low uterine vascular sympathetic reactivity may be important in maintaining blood flow and protecting the fetus from IUGR in response to high altitude hypoxia.
Previous studies in normoxic animals have demonstrated that PKC activation increases α1-adrenoceptor-induced contractions in uterine arteries of nonpregnant sheep, but inhibits the contractions in uterine arteries of pregnant animals (Zhang et al., 2006). This suggests that PKC interacts with α1-adrenoceptors and modulates α1-adrenoceptor-mediated vascular contractions in uterine arteries. The reversed regulatory role of PKC on α1-adrenoceptor-mediated contractions from a potentiation in uterine arteries of nonpregnant ewes to an inhibition in pregnant animals is likely to play a role in maintaining the low uterine vascular sympathetic reactivity during pregnancy. The finding in the present study that the potentiation effect of PKC on α1-adrenoceptor-mediated contractions was abolished in uterine arteries of nonpregnant sheep acclimatized to high altitude hypoxia is intriguing and suggests that hypoxia mimics the effect of pregnancy and has a possible role in the adaptation of PKC–α1-adrenoceptors interaction in the regulation of uterine artery contractility during pregnancy. Consistent with this finding, the inhibitory effect of PKC on α1-adrenoceptor-mediated contractions in uterine arteries of pregnant ewes, observed in normoxic animals (Zhang et al., 2006), was preserved in pregnant animals acclimatized to high altitude hypoxia. Given that hypoxia increases the sympathetic activity and elevates circulating catecholamine, the maintained low uterine vascular sympathetic reactivity may be important in maintaining pregnancy at high altitude. The finding that PDBu-mediated responses were partially blocked by GF109203X suggests a causal relation between PKC activation and PDBu-mediated inhibitory effects in uterine arteries of pregnant sheep at altitude. The finding that both PKC activator and its inhibitor inhibited phenylephrine-induced contractions in pregnant uterine arteries of high altitude ewes is consistent with a previous report on ovine cerebral arteries (Longo et al., 2000). It suggests a dual role of PKC in modulating α1-adrenoceptor-mediated pharmacomechanical coupling.
In normoxic animals, pregnancy is associated with a significant decrease in the baseline uterine artery Ca2+ sensitivity (Xiao et al., 2006b). In contrast, the baseline Ca2+ sensitivity of the uterine artery was significantly higher in pregnant sheep as compared with nonpregnant animals at high altitude (Chang et al., 2009). The Ca2+ sensitivity of myofilaments in smooth-muscle cell is regulated through both thick- and thin-filament regulatory pathways (Horowitz et al., 1996; Amobi et al., 1999; Arner and Pfitzer, 1999; Somlyo and Somlyo, 2003). The finding in the present study that Ca2+-induced MLC20-P was similar in uterine arteries of nonpregnant and pregnant ewes at high altitude, despite increased Ca2+-induced contractions in pregnant animals, suggests that the increased baseline Ca2+ sensitivity of the uterine artery in pregnant sheep acclimatized to high altitude hypoxia results primarily from a thin-filament, that is, MLC20-P -independent, mechanism. This notion is further supported by determining directly the relation between Ca2+-induced MLC20-P and contractions of the uterine arteries in the same tissue, which revealed an increased ratio of tension development to MLC20-P in pregnant, as compared with nonpregnant, sheep acclimatized to high altitude. These findings are consistent with previous studies showing a key role of the thin-filament mechanism in the regulation of Ca2+ sensitivity and vascular tone in the uterine artery (Xiao et al., 2004; Xiao and Zhang, 2005; Xiao et al., 2006b).
In a previous study, we showed that PDBu potentiates Ca2+-induced contractions in uterine arteries of nonpregnant, but not pregnant, sheep at high altitude (Chang et al., 2009). Although this finding may suggest a role of PKC in the regulation of uterine artery Ca2+ sensitivity in nonpregnant animals, the present finding, that inhibition of PKC with staurosporine significantly decreased Ca2+-induced contractions of the uterine arteries in both nonpregnant and pregnant sheep, demonstrates the causal role of endogenous PKC in the regulation of the baseline Ca2+ sensitivity in the uterine artery. It has been demonstrated in the uterine artery that staurosporine inhibits PKC activity (Xiao and Zhang, 2002). The finding of decreased staurosporine-mediated inhibition in uterine arteries of pregnant ewes, as compared with nonpregnant animals, suggests an enhanced coupling of PKC to the baseline Ca2+ sensitivity in pregnant animals at high altitude. Both PKCα and PKCɛ have been implicated in vascular smooth-muscle contraction through increasing Ca2+ sensitivity (Khalil et al., 1992; Walsh et al., 1994, 1996; Horowitz et al., 1996; Littler et al., 2003). In the present study, we have demonstrated that chronic hypoxia significantly increases the ratio of particulate to cytosolic fractions of PKCɛ in the uterine artery of pregnant sheep, suggesting a role of increased basal activity of PKCɛ in the enhanced baseline Ca2+ sensitivity of the uterine artery in pregnant animals acclimatized to high altitude hypoxia. Translocation of PKC isozymes from cytosolic to membrane particulate fractions is the hallmark of PKC activation and has been widely used as a measure of PKC isoform activation in cells (Steinberg, 2008). In contrast, the basal activity of PKCɛ was significantly decreased in the uterine artery of nonpregnant sheep at high altitude, as compared with normoxic animals. Whereas the mechanisms of the opposite regulatory effects of hypoxia on the PKCɛ activity in the uterine artery between nonpregnant and pregnant animals remain to be determined, the decreased PKCɛ activity in nonpregnant animals is likely to contribute to the loss of potentiation effect of PDBu on α1-adrenoceptor-mediated contractions of the uterine arteries in nonpregnant sheep at high altitude. Unlike PKCɛ, the basal activity of PKCα was found not to differ significantly in the uterine arteries among all four groups of animals, demonstrating the heterogeneity of vascular PKC isozymes in the adaptation to pregnancy and hypoxia.
It has been suggested that PKC regulates the Ca2+ sensitivity via the thin-filament regulatory pathway in vascular smooth muscle, including the uterine arteries (Jiang and Morgan, 1989; Sato et al., 1992; Zamudio et al., 1995a; Xiao et al., 2004, 2005). Indeed, the present study demonstrated that PKC activation induced contractions of the uterine arteries independent of changes in MLC20-P in pregnant sheep at high altitude, supporting the notion that thin-filament regulatory mechanisms contribute to the hypoxic-induced upregulation of PKC-mediated baseline Ca2+ sensitivity in the uterine artery. Previous studies have demonstrated that pregnancy-increased ERK1/2 acts as an upstream signal in suppressing PKC-mediated vascular tone in the uterine arteries, and this adaptation is inhibited by chronic hypoxia (Xiao and Zhang, 2002; Xiao et al., 2004, 2006a; Chang et al., 2009). In contrast to the previous finding in normoxic pregnant animals that inhibition of ERK1/2 with PD098059 increased PKC-mediated contractions without changing MLC20-P levels (Xiao et al., 2004), PD098059 had no effect on PDBu-induced contractions, as well as MLC20-P phosphorylation, in the uterine arteries of pregnant ewes at high altitude, suggesting that chronic hypoxia abolishes the pregnancy-induced inhibitory effect of ERK1/2 on the PKC-mediated thin-filament pathway in the uterine artery.
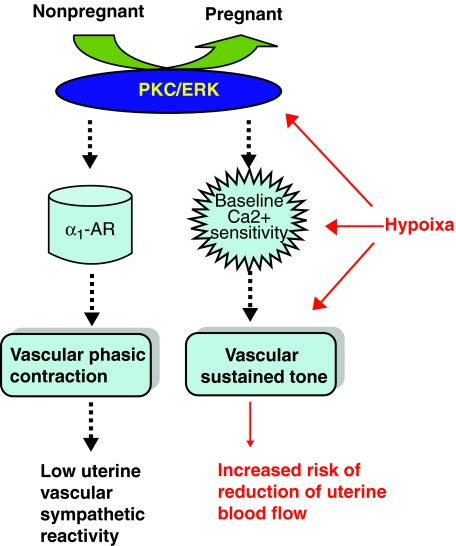
In summary, the present study has demonstrated the complexity of interaction effects between pregnancy and chronic hypoxia on uterine artery contractility (Fig. 8). Whereas the maintained inhibitory effect of PKC on α1-adrenoceptor-mediated contractions of the uterine artery in pregnant animals may be important for maintaining pregnancy at high altitude, the enhanced PKC-mediated Ca2+ sensitivity is likely to increase uterine vascular resistance and contribute to the increased risk of preeclampsia and fetal intrauterine growth restriction observed in pregnancy at high altitude (Zamudio et al., 1995b; White and Zhang, 2003; Julian et al., 2008). It appears that the ERK1/2–PKC-mediated thin-filament pathway is important in the hypoxic-induced increase in the baseline Ca2+ sensitivity. Given that estrogen and progesterone play an important role in the regulation of the ERK1/2–PKC pathway and uterine vascular adaptation to pregnancy (Byers et al., 2005; Xiao et al., 2009), further studies are needed to investigate the effect of hypoxia on the steroid hormones and their modulations of the thin-filament regulatory pathway in the uterine artery.
FIG. 8.
Effect of long-term, high altitude hypoxia on the adaptation of uterine artery contractions to pregnancy. Pregnancy inhibits the PKC/ERK1/2 signaling pathway, resulting in attenuated α1-adrenorecptor (α1-AR)-mediated phasic contractions and decreased baseline Ca2+ sensitivity and vascular tone in the uterine arteries. Long-term, high altitude hypoxia during pregnancy preserves the pregnancy-mediated, low vascular, sympathetic reactivity, but increases the baseline Ca2+ sensitivity and vascular tone via a PKC/ERK1/2-mediated thin-filament pathway. Broken arrows indicate downregulation. Solid arrows indicate upregulation.
Acknowledgments
This work was supported in part by NIH grants HD31226 and HL89012, by the Regents of the University of California TRDRP grant 18KT-0024, and by Loma Linda University School of Medicine.
Disclosures
The authors have no conflicts of interest or financial ties to disclose.
References
- Amobi N.I. Sugden D. Smith I.C. Pharmacomechanical coupling in rat vas deferens: effects of agents that modulate intracellular release of calcium and protein kinase C activation. Life Sci. 1999;65:145–156. doi: 10.1016/s0024-3205(99)00231-3. [DOI] [PubMed] [Google Scholar]
- Arner A. Pfitzer G. Regulation of cross-bridge cycling by Ca2+ in smooth muscle. Rev. Physiol. Biochem. Pharmacol. 1999;134:63–146. doi: 10.1007/3-540-64753-8_3. [DOI] [PubMed] [Google Scholar]
- Bird I.M. Zhang L. Magness R.R. Possible mechanisms underlying pregnancy- induced changes in uterine artery endothelial function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Byers M.J. Zangl A. Phernetton T.M. Lopez G. Chen D.B. Magness R.R. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J. Physiol. 2005;565:85–99. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. Xiao D. Huang X. Longo L.D. Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1840–H1849. doi: 10.1152/ajpheart.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G. Adam L.P. Inhibition of ERK attenuates force development by lowering myosin light chain phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H602–H610. doi: 10.1152/ajpheart.00221.2001. [DOI] [PubMed] [Google Scholar]
- Farley D.B. Ford S.P. Evidence for declining extracellular calcium uptake and protein kinase C activity in uterine arterial smooth muscle during gestation in gilts. Biol. Reprod. 1992;46:315–321. doi: 10.1095/biolreprod46.3.315. [DOI] [PubMed] [Google Scholar]
- Ford S.P. Control of blood flow to the gravid uterus of domestic livestock species. J. Anim. Sci. 1995;73:1852–1860. doi: 10.2527/1995.7361852x. [DOI] [PubMed] [Google Scholar]
- Horowitz A. Clement-Chomienne O. Walsh M.P. Morgan K.G. Epsilon-isoenzyme of protein kinase C induces a Ca2+-independent contraction in vascular smooth muscle. Am. J. Physiol. 1996;271:C589–C594. doi: 10.1152/ajpcell.1996.271.2.C589. [DOI] [PubMed] [Google Scholar]
- Je H.D. Gangopadhyay S.S. Ashworth T.D. Morgan K.G. Calponin is required for agonist-induced signal transduction: evidence from an antisense approach in ferret smooth muscle. J. Physiol. 2001;537:567–577. doi: 10.1111/j.1469-7793.2001.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.J. Morgan K.G. Agonist-specific myosin phosphorylation and intracellular calcium during isometric contractions of arterial smooth muscle. Pflugers Arch. 1989;413:637–643. doi: 10.1007/BF00581814. [DOI] [PubMed] [Google Scholar]
- Julian C.G. Galan H.L. Wilson M.J. Desilva W. Cioffi-Ragan D. Schwartz J. Moore L.G. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R906–R915. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanashiro C.A. Cockrell K.L. Alexander B.T. Granger J.P. Khalil R.A. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am. J. Physiol. 2000;278:R295–R303. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- Keyes L.E. Armaza J.F. Niermeyer S. Vargas E. Young D.A. Moore L.G. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr. Res. 2003;54:20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- Khalil R.A. Lajoie C. Resnick M.S. Morgan K.G. Ca2+-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am. J. Physiol. 1992;263:C714–C719. doi: 10.1152/ajpcell.1992.263.3.C714. [DOI] [PubMed] [Google Scholar]
- Littler C.M. Morris K.G. Fagan K.A. McMurtry I.F. Messing R.O. Dempsey E.C. Protein kinase C-epsilon-null mice have decreased hypoxic pulmonary vasoconstriction. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1321–H1331. doi: 10.1152/ajpheart.00795.2002. [DOI] [PubMed] [Google Scholar]
- Longo L.D. Zhao Y. Long W. Miguel C. Windemuth R.S. Cantwell A.M. Nanyonga A.T. Saito T. Zhang L. Dual role of PKC in modulating pharmacomechanical coupling in fetal and adult cerebral arteries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1419–1429. doi: 10.1152/ajpregu.2000.279.4.R1419. [DOI] [PubMed] [Google Scholar]
- Magness R.R. Rosenfeld C.R. Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. Am. J. Obstet. Gynecol. 1986;155:897–904. doi: 10.1016/s0002-9378(86)80047-3. [DOI] [PubMed] [Google Scholar]
- Magness R.R. Rosenfeld C.R. Carr B.R. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am. J. Physiol. 1991;260:E464–E470. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- Moore L.G. Young D. McCullough R.E. Droma T. Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am. J. Hum. Biol. 2001;13:635–644. doi: 10.1002/ajhb.1102. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C.R. Mechanisms regulating angiotensin II responsiveness by the uteroplacental circulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1025–R1040. doi: 10.1152/ajpregu.2001.281.4.R1025. [DOI] [PubMed] [Google Scholar]
- Sato K. Hori M. Ozaki H. Takano-Ohmuro H. Tsuchiya T. Sugi H. Karaki H. Myosin phosphorylation-independent contraction induced by phorbol ester in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1992;261:497–505. [PubMed] [Google Scholar]
- Somlyo A.P. Somlyo A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Steinberg S.F. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.P. Andrea J.E. Allen B.G. Clement-Chomienne O. Collins E.M. Morgan K.G. Smooth muscle protein kinase C. Can. J. Physiol. Pharmacol. 1994;72:1392–1399. doi: 10.1139/y94-201. [DOI] [PubMed] [Google Scholar]
- Walsh M.P. Horowitz A. Clement-Chomienne O. Andrea J.E. Allen B.G. Morgan K.G. Protein kinase C mediation of Ca2+-independent contractions of vascular smooth muscle. Biochem. Cell. Biol. 1996;74:485–502. doi: 10.1139/o96-053. [DOI] [PubMed] [Google Scholar]
- White M. Zhang L. Effects of chronic hypoxia on maternal vascular changes during pregnancy: invited review. High. Alt. Med. Biol. 2003;4:157–169. doi: 10.1089/152702903322022776. [DOI] [PubMed] [Google Scholar]
- Xiao D. Buchholz J.N. Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am. J. Physiol. Heart Circ. Physiol. 2006a;290:H2337–H2343. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- Xiao D. Huang X. Longo L.D. Pearce W.J. Zhang L. Regulation of baseline Ca2+ sensitivity in permeabilized uterine arteries: effect of pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2006b;291:H413–H420. doi: 10.1152/ajpheart.00103.2006. [DOI] [PubMed] [Google Scholar]
- Xiao D. Huang X. Yang S. Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: role of protein kinase C/extracellular signal-regulated kinase 1/2. Hypertension. 2009;54:352–358. doi: 10.1161/HYPERTENSIONAHA.109.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D. Longo L.D. Zhang L. α1-Adrenoceptor-mediated phosphorylation of MYPT1 and CPI-17 in the uterine artery: role of ERK/PKC. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2828–H2835. doi: 10.1152/ajpheart.01189.2004. [DOI] [PubMed] [Google Scholar]
- Xiao D. Pearce W.J. Longo L.D. Zhang L. ERK-mediated uterine artery contraction: role of thick and thin filament regulatory pathways. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1615–H1622. doi: 10.1152/ajpheart.00981.2003. [DOI] [PubMed] [Google Scholar]
- Xiao D. Zhang L. ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H292–H300. doi: 10.1152/ajpheart.2002.282.1.H292. [DOI] [PubMed] [Google Scholar]
- Xiao D. Zhang L. Calcium homeostasis and contractions of the uterine artery: effect of pregnancy and chronic hypoxia. Biol. Reprod. 2004;70:1171–1177. doi: 10.1095/biolreprod.103.024943. [DOI] [PubMed] [Google Scholar]
- Xiao D. Zhang L. Adaptation of uterine artery thick- and thin- filament regulatory pathway to pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H142–H148. doi: 10.1152/ajpheart.00655.2004. [DOI] [PubMed] [Google Scholar]
- Zamudio S. Palmer S.K. Dahms T.E. Berman J.C. Young D.A. Moore L.G. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J. Appl. Physiol. 1995a;79:15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- Zamudio S. Palmer S.K. Droma T. Stamm E. Coffin C. Moore L.G. Effects of altitude on uterine artery blood flow during normal pregnancy. J. Appl. Physiol. 1995b;79:7–14. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]
- Zhang H. Xiao D. Longo L.D. Zhang L. Regulation of α1-adrenoceptor-mediated contractions of the uterine artery by PKC: effect of pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2282–H2289. doi: 10.1152/ajpheart.00321.2006. [DOI] [PubMed] [Google Scholar]
- Zhang H. Zhang L. Regulation of alpha1-adrenoceptor-mediated contractions of the uterine artery by protein kinase C: role of the thick- and thin-filament regulatory pathways. J. Pharmacol. Exp. Ther. 2007;322:1253–1260. doi: 10.1124/jpet.107.124313. [DOI] [PubMed] [Google Scholar]
- Zhang H. Zhang L. Role of protein kinase C isozymes in the regulation of alpha1-adrenergic receptor-mediated contractions in ovine uterine arteries. Biol. Reprod. 2008;78:35–42. doi: 10.1095/biolreprod.107.063479. [DOI] [PMC free article] [PubMed] [Google Scholar]