1. Introduction
Our vision is an invaluable gift that allows us to navigate the world that surrounds us. Through vision we learn and recognize each other. More than 80% of information that we receive from the outside world, is obtained through vision. Thus, maintaining a good vision is critical for our survival in the ever-changing world, while its deterioration causes many problems ranging from a minor nuisance to an insurmountable obstacle. The health of ocular surface is critical for acute vision and, therefore, needs to be monitored, maintained, or restored in case of an ocular disease or a developing pathological condition. One such condition is dry eye (DE) [1] There is no doubt that DE is a potentially debilitating condition (or disease) whose symptoms range from minor to severe, in which case the patients are facing constant difficulties in everyday living. The onset of DE is invariably linked to a quick deterioration of an ocular surface structure called tear film (TF). Normally, TF is a continuous, complex, multilayered structure (Figure 1) composed of water, inorganic salts, carbohydrates, lipids, and proteins that covers the entire exposed ocular surface and fulfils the protective, lubricatory, nutritional, and antimicrobial roles [2], [3]. One of the main functions of TF is to keep the delicate corneal, conjunctival, and epithelial cells moist. Being a very thin structure, TF of an open eye is relatively unstable, and within several seconds breaks up thus exposing the underlaying ocular structures such as cornea and conjunctiva (Figure 2, TBUT our pictures). In healthy individuals with no ocular surface pathologies, TF is stable for 10 s or more, while in DE patients its intactness lasts for less than 6 s [4] [5]. This parameter is routinely called TF break-up type (TBUT or, sometimes, TFBUT) and is a common diagnostic tool regularly used in ophthalmic practice to diagnose DE. Short TBUT leave the ocular surface exposed to the air, which irritates the cornea and causes excessive blinking and tearing. In severe cases the ocular surface desiccates and an irreversible damage to the cornea may occur [6].
Figure 1.
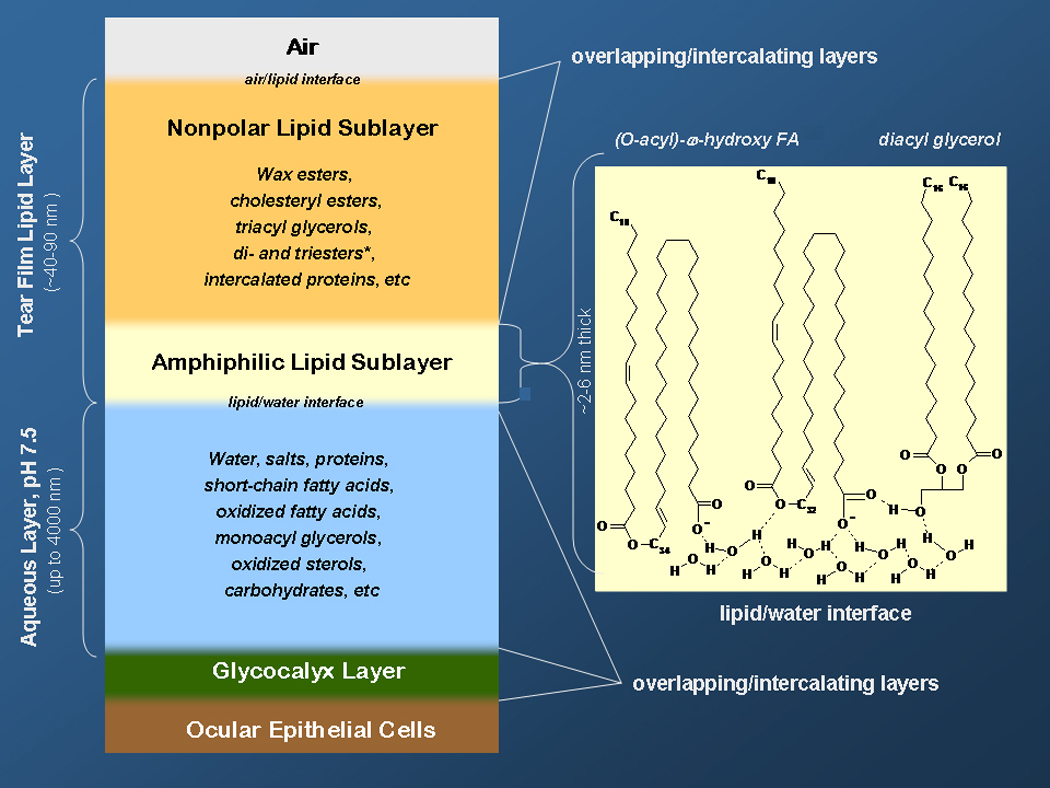
Tear film and tear film lipid layer (reprinted from [43] with permission)
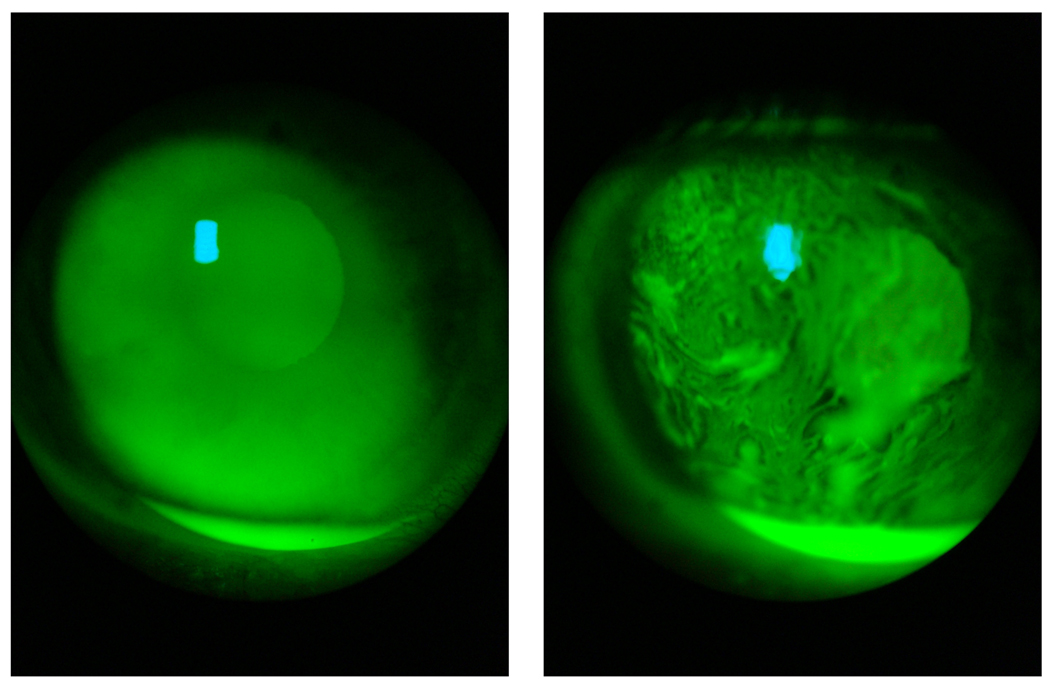
Figure 2.
Measuring of tear film breakup time using fluorescein staining and a slit lamp.
Panel A: uniform staining of the tear film after a blink.
Panel B: irregular staining developed as the result of tear film deterioration caused by non-blinking
TF originates primarily from two different sources – lacrimal (or lachrymal) glands that produce aqueous tears (AT) and meibomian glands (MG) which are also known as the palpebral glands, tarsal glands, or tarsoconjunctival glands. MG are a variety of sebaceous glands located in the eyelids of humans and animals (Figure 3A). The glands easily secrete a lipid secretion (MGS, or meibum [7]) on their own, or upon applying a gentle pressure with Q-tips (Figure 3B). In 1666, Heinrich Meibom was the first to report the existence of MG in humans [8], but it was not until 1897 when Orlando Pes provided the first clues with regard to the chemical composition of their secretion [9]. Per Pes, the oily MG secretion was a mixture of fats (presumably, triglycerides, TAG), fatty acids (FA), and cholesterol (Chl). Considering the rudimentary state of the lipid analysis at the time, and a minuscule size of the samples available for the analyses, the extraordinarity of this observation cannot be overstated. The further progress in our understanding of the biochemistry of MGS started in the late fifties and early sixties of the 20th century with the work of Linton, Curnow, and Riley [10]. Interestingly, Linton et al. disagreed with Pes on the presence of Chl and free (non-esterified) FA (FFA) in MGS: the former group could not find any of these in their preparations of MGS. However, “neutral” fats were detected in both of the studies. Notably, large amounts of unidentified lipid material were reported by Linton et al. No amino-containing lipids, carbohydrates, and proteins were observed in MGS and a conclusion was drawn that MGS consisted solely of a lipid staining material. A few years later, Ehlers [11] published results of a comprehensive investigation on the structure of the precorneal film, in which considerable attention was paid to its lipids. A crude separation of lipids present in the human MGS and in the precorneal film, nevertheless, demonstrated that cholesteryl esters (CE) were their major components, while FFA, free Chl, and putative triacylglycerols (TAG) and amino-containing phospholipids (amino-PL) were detected only as minor constituents. Also, Ehlers concluded that CE were most likely comprised of several species differing in their FA moieties. This surprising disagreement between Linton et al and Ehlers, was, apparently, the first documented dispute on the lipid composition of MGS.
Figure 3.
Visible light microscopy evaluation of meibomian glands (panel A) and manual expression of meibomian gland secretions from a lower eyelid of a volunteer using two cotton swabs.
Panel A. Meibomian glands are marked with black arrows.
Panel B. Expressed meibum is marked with a black arrow.
In the following years, considerable efforts have been undertaken to elucidate the details of the lipid composition of human and animal MGS. To learn the details of the earlier research, the reader is advised to study earlier comprehensive reviews on the topic, in which an overwhelming amount of information was presented and interpreted [2, 12]. Thus, in this paper I will discuss only the major steps in those efforts that concern human MGS, and will concentrate on the recent developments in the area that have happened during the last decade or so, most of which are related to the advancements in analytical techniques, their diversification, and the use of new approaches to modeling TF in vitro.
Most of this review is concerned with human MGS. The subject of animal MGS is broad and deserves a separate discussion as, despite obvious well documented similarities between the human and the animal MGS, there are profound differences not only between humans and animals, but between different animals, too [13]. Thus, direct extrapolation of the results obtained in the experiments with animals onto the human subjects would most certainly lead to erroneous results and conclusions, and generally should be avoided. However, a few remarks about animal Meibomian glands and animal meibum will be made in this paper, where appropriate.
2. Anatomy of the meibomian gland
The very first description of multiple sebaceous glands that populate both the upper and the lower eyelids of humans was provided by Heinrich Meibom, a German physician and anatomist [8]. The glands, that are also known as tarsal glands, were later named the Meibomian glands. There are between 30 and 40 glands in the upper eyelid and 20 to 30 in the lower eyelid [14]. However, depending on the age of subjects, and their ocular health conditions, the number of active glands can change. For example, in dry eye patients the number of fully functional (and observable) glands can substantially decrease [15]. This would inevitably lead to a hypo-production of meibum, and the subsequent deterioration of the tear film. The Meibomian gland central ducts that deliver the Meibomian gland secretions onto the ocular surface, run perpendicularly to the eyelid margins. They can be directly observed in a living human being using an IR-sensitive camera [16], or by dissecting the tarsal plates ex vivo [17]. Figures 4A and 4B clearly demonstrate the potential of the non-contact IR imaging technique for evaluation of structure and number of MG in normal and dry eye patients, which makes it a very useful scientific and diagnostic tool. The central duct of a MG is connected to lateral alveoli that are populated with lipid-producing cells. The lipids that are produced by Meibomian gland secretory cells are formed in massive quantities, which can be easily observed after histochemical staining the tissues with lipid-specific dyes ([18] and Figure 5). The amount of lipid bodies (or droplets) that are formed in the secretory cells increases inversely proportional to their distance to the Meibomian gland duct, into which they eventually release the lipid-enriched secretion by a holocrine mechanism. The glands constantly produce and excrete meibum, whose accumulation in meibomian ducts was beautifully illustrated by Linton et al. [10], while regular blinking spreads meibum across the ocular surface. Blinking also believed to help in removing the older lipid layers. Thus, TF and TFLL are believed to be replenished and/or replaced during each blinking cycle [19], though this has not been verified yet. Thus, we could have expected to detect typical cell membrane lipids (such as relatively polar PL and sphingomyelins, SM) to be found in MGS; however, as we will see later on, this is not the case: many typical cell membrane lipids are either not detected in meibum, or are seen as just a very minor pool of lipids, the majority of which are of overwhelmingly nonpolar nature.
Figure 4.
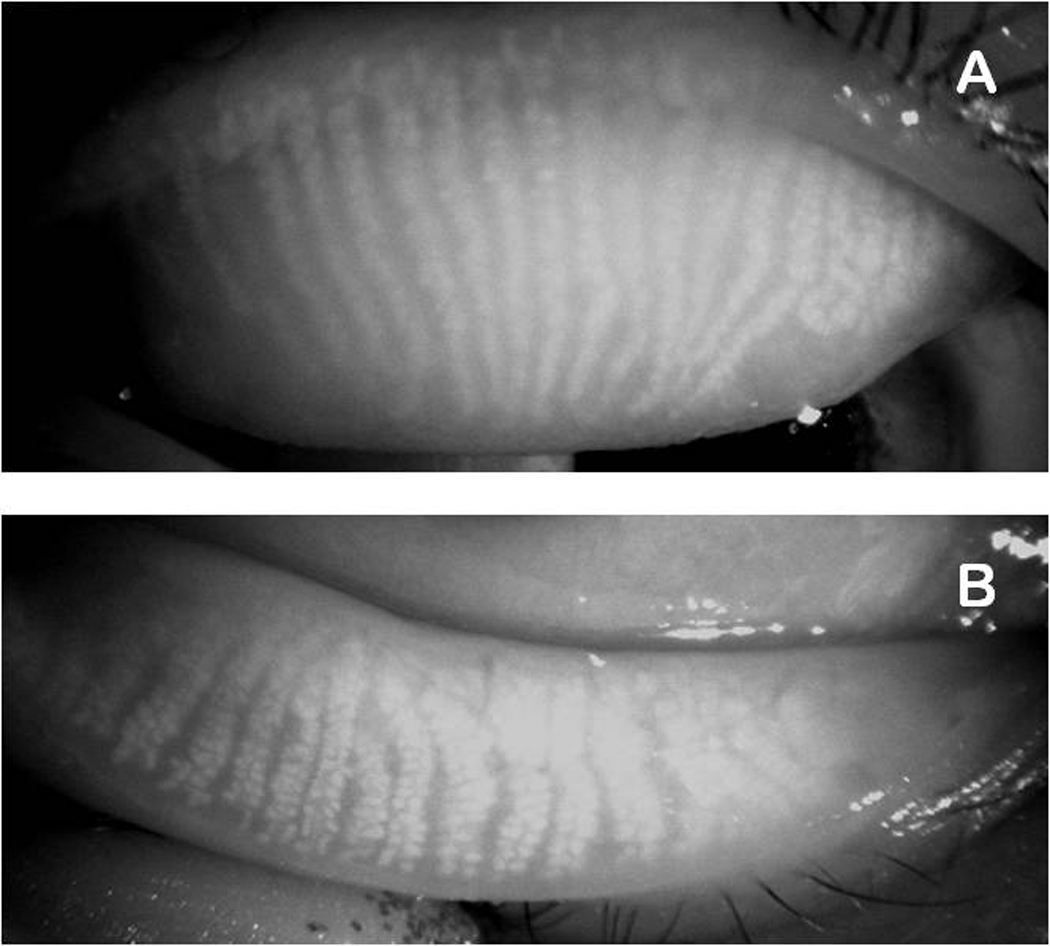
Infrared photographs of meibomian glands of a young healthy female volunteer (28 years old) (courtesy of Dr. R. Arita). The glands are visible as wavy white structures.
Panel A. Upper eyelid.
Panel B. Lower eyelid.
Figure 5.
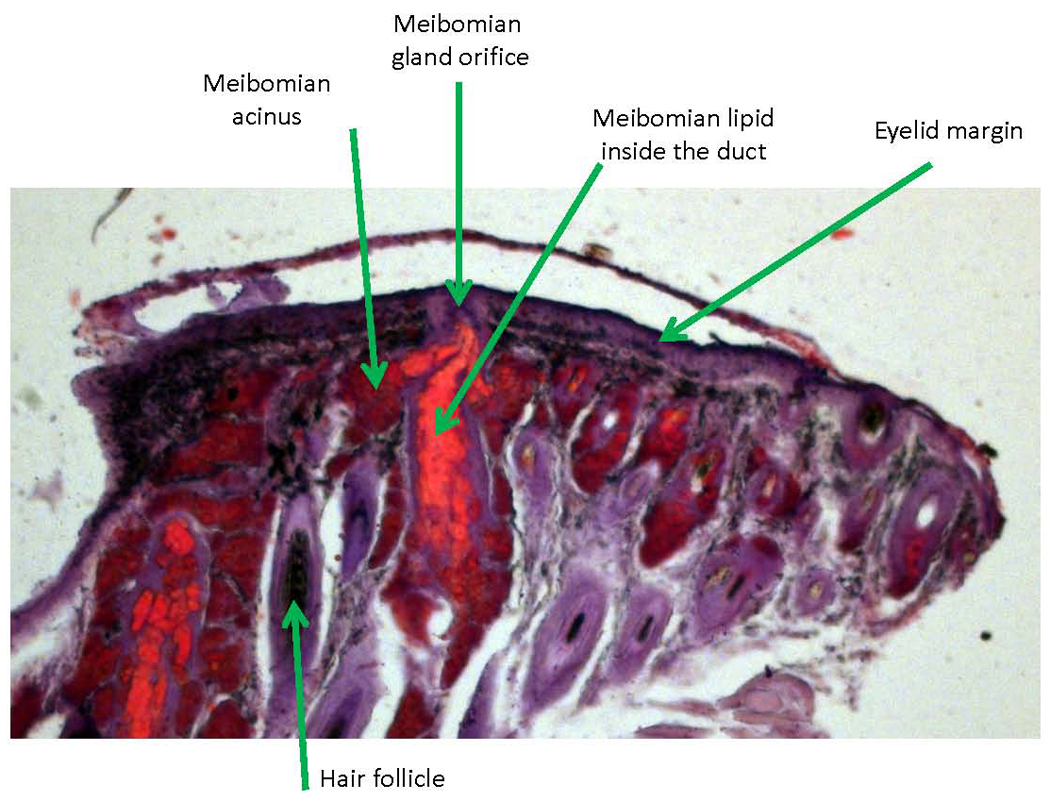
Histochemical staining of meibomian glands. An upper mouse meibomian gland is shown. The tissue lipids were stained with Oil Red O and counter-stained with hematoxylin. Notice accumulation of large amount of stained lipids (bright red) in the main (central) duct of the gland.
Meibomian gland morphology has been described in many studies. For visual information on the histology of meibomian glands and on their ultrastructural characteristics, the reader is advised to refer to previous studies, e.g. an earlier paper by Jester et al [18], where the details of the anatomy of meibomian gland and the intracellular organization of meibocytes are beautifully illustrated. Highly detailed microphotographs of meibomian glands, albeit of mice, were published by Gorgas and Volkl [20]. The large lipid droplets were found to be surrounded by a massive number of hexagonally packed peroxisomes and (smooth) endoplasmic reticulum. Similar microphotographs can be found in the paper by Jester et al [18]. Gorgas and Volkl [20] proposed that it was the peroxisomes where the biosynthesis of the precursors for major lipid classes, such as very long chain fatty acids for wax esters (WE), occurred.
3. Analytical procedures
Since Pes [9], it’s been universally recognized that meibum is an exceptionally complex mixture of various lipids. Many of the typical lipid classes were reported to be found in meibum [7, 12]. Those included hydrocarbons (HC), WE, CE and Chl, TAG and diacyl glycerols (DAG), PL, SM, ceramides (Cer), (O-acyl)-omega-hydroxy FA (OAHFA) and their steryl esters, FFA, fatty acid amides (FAm), and many other compounds (Scheme 1). A rough estimate of the number of major individual compounds that have been observed and identified in recent studies is over 100, while the number of minor components and compounds yet to be identified could be in thousands [12]. Indeed, even for the relatively simple compounds of WE family multiple isoforms of the same molecular mass have been reported [21]. The presence of such isobaric positional, geometrical, and/or chiral isomers can easily increase the number of lipid species many fold from hundreds to thousands. This enormous diversity of meibum constituents, in combination with the small size of a typical sample (e.g., in a pioneering study of Linton et al., 539 volunteers donated only 200 mg of meibum, combined [10]) calls for state-of-the-art technology if one hopes to limit the number of volunteers, or wants to evaluate the inter-donor variations in the lipid profiles collected from individual donors. However, the current state of affairs is such that such a perfect individual procedure does not exist, and one needs to utilize several experimental approaches to characterize MGS to, at least, a minimally acceptable degree of certainty. Yet, some methods have been found to be more suitable for such analyses than the others. Below is a brief comparison of the strengths and weaknesses of various techniques made on the bases of the author’s personal experience with the subject as well as analysis of available literature.
Scheme 1.
Four major analytical methods currently stand out as the most suitable for lipidomic analysis of MGS: high pressure liquid chromatography (HPLC), gas chromatography (GC), mass spectrometry (MS), and nuclear magnetic resonance spectroscopy (NMR). Infrared spectroscopy (IR), Raman spectroscopy (RS), and fluorescence spectrometry (FS), though useful in conformational analyses of lipids and in detecting certain lipid classes [22, 23] are not generally considered a proper analytical tool for unequivocal identification of a compound. Indeed, infrared absorption spectra can be helpful in identifying the key functional group of a pure compound, but IR is never used as the sole analytical tool, especially when it comes to identification of individual components of a complex mixture of similar compounds: the IR spectra simply do not have enough information to distinguish between a series of homologous compounds, and their quantitation as individual species. However, quantitation of lipids as a whole group by Fourier transform IR, though tricky, is not impossible [24]. Other experimental techniques such as open column silicic acid chromatography (OCC) and thin layer chromatography (TLC) with or without selective lipid staining were shown to be useful for separation of certain lipid classes, but are currently used less and less frequently because of the advancements in HPLC techniques. These and other aspects of a range of analytical techniques suitable for the analysis of MGS are discussed below. Please consider that the results of earlier experiments mentioned below are discussed within the framework of the knowledge and methodology that existed at the time.
4. Chronology of human meibomian gland secretions studies
4.1. Seeding studies in the sixties to mid-seventies of the 20th century
TLC was, apparently, the first separation technique used for evaluation of human MGS. In 1961, Linton et al. [10] used the paper disc chromatography in a series of eluents with subsequent (semi)selective lipid staining to evaluate the lipid composition of pooled samples of human MGS. They reported that MGS melted from 35–40°C and had a substantial presence of “neutral” fats, plasmalogens, choline lipids, phospholipids, and a large portion of unidentified lipids. Neither of the reported lipid classes was studied in further detail. At the same time, Linton et al. stated that Chl, FFA, and amino lipids were not detected. Thus, the authors were not able to confirm the much earlier observation of Pes [9] with regard to the presence of substantial amounts of FFA and Chl in human MGS. The whole samples were also analyzed by elemental micro-analysis and IR. The elemental analysis demonstrated that C (80.6%), H (12.56%), and O (6.19%) were the major elements leaving ≤0.65% for other elements. IR analysis revealed bands of carbonyl (1,736 cm−1) and double bonds (3,000 cm−1).
The TLC results reported by Linton et al. deserve some elaboration. First, it is apparent that the group of “neutral fats” included co-migrating under the chosen conditions CE, TAG, WE, and, possibly, other classes of lipids. Chl, being a more polar compound than the “neutral fats”, could be easily separated in a paper chromatography experiment [25], but was not detected in MGS [10]. Considering that Chl content of normal MGS is typically less than 1% [21, 26, 27], this amount of Chl in MGS was, most likely, below the low limit of detection of paper chromatography and the implemented staining technique. The group of amino lipids (i.e. lipids carrying a free amino group that can be detected in the reaction with ninhydrin) theoretically could include phosphatidylethanolamine (PE), phosphatidylserine (PS), some sphingosine-, sphinganine-, and ornithine-containing lipids, conjugates of fatty acids with peptides, etc. However, one must realize that the ninhydrin reaction was developed to detect primary amines (such as α–amino acids) only, and that ninhydrin does not readily react with secondary and ternary amino groups of compounds like lysine, arginine, ornithine, citrulline and histidine [28, 29]. Thus, neither choline-containing phospholipids, nor ceramides and their derivatives could be detected in such a reaction. Choline-containing lipids, on the other hand, were detected after K2Cr2O7 – diphenylcarbazide treatment. However, it is not clear how their presence was estimated to be “less than 10% of the total secretion”, as this conclusion could not be derived from the data of elemental analyses which showed no nitrogen or phosphorous in the samples. No FFA were observed, either. Plasmalogens (i.e. compounds with at least one ether bond between, e.g., a fatty alcohol and glycerol), on the other hand, were observed in the samples after 2,4-dinitrophenyl hydrazine staining. Thus, Linton et al. concluded that MGS differed markedly from skin sebaceous gland secretions evaluated earlier by Horacek and Cernikova [25].
A few years later, a similar, but much more systematic, TLC-based approach was used by Ehlers [11]. The major difference between his experiments and those of Linton et al. was the use of silica gel TLC plates. Compared to paper chromatography, silica gel TLC offers (theoretically) a much higher resolution and sensitivity, and allowed for the use of a much broader range of staining techniques as silica gel does not interfere with the stains as much as paper does, and allows plates to be charred to visualize the separated lipids. The higher sensitivity of silica gel TLC versus paper disk TLC is related primarily to a less pronounced dilution of a sample in case of a silica gel plate where the sample is typically moving in just one direction and is diluted only due to the radial diffusion of its components, while in the paper disk chromatography the sample moves from the center of the disk toward its outer rims forming rings of components which get progressively more and more diluted proportionally to Rf2. When the human MGS lipids were developed and stained with a range of chromogenic reagents, an array of lipids was observed. The detected lipids were identified as lecithin (a phosphatidyl choline, or PC), cephalins (PE and PS), SM, FFA, Chl, CE, and TAG in the following order of apparent abundance: CE>>Chl=FFA=PE, PS=PC>SM=TAG. No actual quantitation of the above compounds that would involve calibration curves for specific lipid classes or individual lipid species was performed. Ehlers’ search for glycolipids in human MGS produced no positive results. However, some of the observed lipid TLC spots were not attributed to any particular lipid classes and remained unidentified. In summary, Ehlers observed at least four classes of lipids (CE, Chl, FFA, and amino lipids) that had not been detected in paper disk TLC experiments of Linton et al. [10], but confirmed the seeding report of Pes [9] with regard to Chl and FFA. The side-by-side comparison of these three pioneering publications clearly demonstrates the role of methodology in human MGS studies.
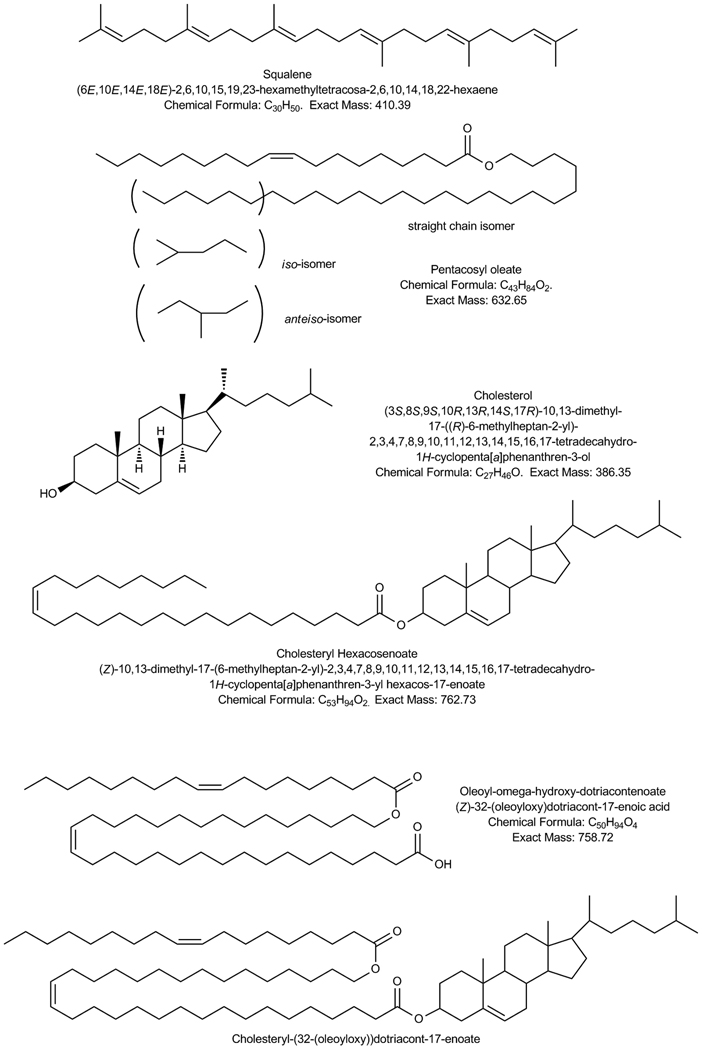
This newly developed interest in studying human MGS was picked up by Nicholas Nicolaides [30] with whom the quick progress in our understanding of the chemistry of human MGS studies began in the eighties of the 20th century (see below). In his first paper on the topic, Nicolaides made a fundamental observation that the major lipid components of MGS were WE and CE, and that the lipids of MGS were strikingly different from those of sebum [7]. By using a combination of TLC and OCC, Nicolaides et al. corroborated the observation of Pes [9] and Ehlers [11] of only small amounts of FFA and TAG in human MGS, and added that he detected only minimal amounts of DAG, monoacyl glycerols (MAG), and squalene (Sql). Importantly, Nicolaides et al. [7, 31–33] reported the existence of a group of lipids which he called “diesters” – complex compounds based on α–or ω-hydroxy-FA (R1) whose carboxylic group is esterified to a fatty alcohol (FAl) or Chl (R3), while α–hydroxy moiety is used to form an ester bond with another FA (R2):
[R2-C(O)-O]-[R1-C(O)-O]-R3
However, no actual quantitation of the detected lipids of MGS was performed in the study, though a note was included in the paper that “By our sampling technique the nonpolar lipids constitute nearly all of the lipids of … human Meibomian glands” [30]. Later on, in a groundbreaking series of publications which started in 1979 and ended abruptly in 1989, Nicolaides et al revealed the very complex nature of human and animal MGS [7, 18, 30, 33–40] (see below).
Keith [41] studied normal and seborrhoeic blepharo-kerato-conjunctivital MGS by TLC. Normal sebum lipids were used as controls. He found that, meibomian lipids were mostly composed of WE and CE, with very little Chl, Sql, TAG, and FFA.
The next milestone in the meibomian lipid research was a publication of Andrews [42]. Apparently, this was the first study in which, in addition to already traditional TLC and OCC techniques, a modern experimental approach (namely, vapor-phase chromatography) was used to characterize the individual components of human MGS. The samples of MGS were expressed from the investigator’s eyelids using cotton swabs and a lid conformer, and a glass rod was used to collect the secretions – an approach that is still in use. Though the actual amount of the sample was not stated, one can assume that it was on a par with the yields achieved by Linton et al. [10] and Nicolaides et al. [7], i.e. below 1 mg of MGS from one subject. TLC experiments with selective staining of Chl-containing species demonstrated the large quantities of CE that were present in tested samples. Also observed were major quantities of wax esters similar to those found in beeswax, while HC, FFA, Chl, and TAG were detected only in insignificant amounts, which clearly differentiated MGS from sebum. A preliminary evaluation of the polar or phospholipid fraction of MGS lipids (namely, lecithin, PE, and SM) allowed Andrews to conclude that they might be present in human MGS, but in small quantities, which corroborated the data of Ehlers. It is not clear from the Andrews’ publication whether polar lipids and phospholipids were considered one and the same group of compounds.
The most interesting part of the study was the chemical evaluation of the meibomian esters. To achieve this goal, the nonpolar meibomian lipids were initially separated from the more polar fractions by TLC and then saponified in a KOH/MeOH mixture to hydrolyze the esters to FFA and FAl. The resulting mixture of sterols, FFA, and FAl was selectively extracted to separate alcohols from fatty acids. The alcohol pool was analyzed by vapor-phase chromatography either as is, or as silylated derivatives with or without prior hydrogenation. FFA were converted in methyl esters and analyzed in the same fashion with and without hydrogenation. The unknowns in the samples were compared with available standard FA and FAl and the structural assignments of the unknowns were made based on the retention times of the peaks. Note that not every unknown had its standard. Therefore, some extrapolations had to be made. This approach clearly demonstrated that human MGS had long chain and very long chain FA (VLCFA) and FAl as major constituents of meibomian nonpolar esters. The carbon chain lengths of FAl varied between C20 and C28, while FA were in the range of C15 to C30. The most abundant FAl were C24 and C26, while the most abundant FA was found to be oleic acid. The relative ratio of normal to iso- to anteiso-isomers of FAl and FA was also evaluated, and a very complex pattern of the isomers was observed.
Importantly, based on the data of Andrews [42], the combined Chl and CE fraction was estimated to be about 36 mole % of MGS, while the esters of FAl comprised about 55 mole% of the mixture. The rest 9 mole % of the lipids remained unidentified. Among the FA, oleic acid dominated the pool (~26 mole %), with C26 and C25 acids being a distant second and third (8 and 7.5 mole %, correspondingly).
The study accentuated a problem with the chemical analysis of human MGS, namely the difficulties in assigning particular structures (i.e. specific combinations of FA and FAl) to the starting intact molecules that had existed in MGS before the lipids were hydrolyzed. Indeed, Andrews [42] noted that ”The principal mixed ester fraction raises a number of questions, chiefly on the composition of the ester subfraction versus the wax subfraction…” due to difficulties in separating these two pools of lipids. This problem persisted for many years after the publication of Andrews, which prompted us to call it “The Meibomian Puzzle” [43].
In 1973, Cory et al. conducted a study of human MGS collected from normal donors and rosacea patients [44]. One of the merits of this study was that the investigators evaluated interdonor variability of MGS samples. The samples were analyzed by silica gel TLC only with subsequent charring of the separated analytes. The investigators were able to detect several classes of lipid including monoesters (WE and CE, about 60% of the entire MGS), diesters (about 17%), and smaller proportions of TAG, FFA, and free sterols. The authors concluded that neither the production of MGS, nor their lipid compositions were affected in rosacea patients compared with normal volunteers. However, one should note that the study did show a somewhat higher proportion of free sterols and TAG in the rosacea patients. Interestingly, PL were not observed in the study samples.
Further progress in the area should be credited to John Tiffany [45], who undertook a systematic effort to elucidate the lipid composition of human MGS by means of TLC and gas-liquid chromatography (GLC, a variant of GC): the lipid classes were studied using TLC, while the pools of FA and FAl were analyzed by GLC. Tiffany cautiously noted that for the analyses of lipid classes, the minimal amount of meibomian lipids needed was 0.5 mg, while for GLC analyses of FA and FAl even the smallest samples sufficed. Thus, not every sample was analyzed in every assay.
The study by Tiffany [45] is interesting in many ways. Firstly, a very wide variation between samples for all lipid classes was observed. Secondly, Tiffany warned the readers about the inadequacy of the charring technique as its efficacy, and thus our ability to quantitate the lipids, depends on the nature of the lipids. A good example was that of hydrocarbons. HC (including Sql) were found in some of the samples in very large quantities (25 to 36%), but were missing in the others. Tiffany stated that the response of Sql to charring was much higher than that of other HC, perhaps because of its higher degree of unsaturation. Thus, the results of their quantitation were doubted by the author himself. Thirdly, all tested samples contained a very large pool of TAG (between 11 and 43%), very little DAG, and even less MAG. Fourthly, Chl and FFA were found in a few samples in the range 1–2 and 7–24% (!), respectively. Fifthly, WE and CE were always present as major constituents, but varied widely between the samples. Lastly, PL were detected in some of the samples in amounts of 0.8 to 5%. The last observation was explained by Tiffany as a consequence of softer squeezing of the eyelids in his experiments vs. the earlier studies, which resulted in lesser expression of cellular debris.
Tiffany paid considerable attention to the FA and FAl composition of MGS. Six individual samples were analyzed by GLC. Tiffany corroborated the earlier observation of Andrews that FA and FAl were a complex mixture of saturated and unsaturated straight-chain, iso- and antesio-isomers. These were characterized by Tiffany in terms of their equivalent chain lengths (or ECL) – and empirical approach based on the retention times of the analytes in an GC or GLC experiment, that allows a researcher to describe complex mixtures for which only a few standard compounds exist. Again, widely different numbers were obtained for different individual samples. However, the range of detected FA was between C9 and C30, while the alcohols ranged were between C12 and C30. Another cautionary warning made by Tiffany was that compounds beyond C30 were impossible to detect because of the peak broadening which made them indistinguishable from the baseline and impossible to integrate. The intersample variability for FA and FAl was too great to describe in this paper, but generally FA and FAl peaked out at C22–C27. Interestingly, according to Tiffany, “… very little unsaturated fatty acids were present… in marked contrast to the findings of Andrews [42]…, where a large amount of oleic acid was detected”. Another observation made by Tiffany was the estimated ratio of branched-chain–to–straight-chain FA and FAl: for both the groups it ranged between 0.13–0.15 to 0.36–0.40. Such a high degree of branching ought to have physiological significance, which indeed was hypothesized by Tiffany to be to reduce the melting point of MGS to near body temperature. Tiffany argued that the melting point of straight-chain analogues of the same compounds would have been 20–40°C higher that of the branched ones, and “… the lipid would be unable to spread on the lid margin or tear film surface”. This is a very important consideration, as it will be discussed below.
Summarizing the results of these earlier pioneering studies, we should note that they laid a foundation for the future advances in the area of biochemistry, biophysics, and physiology of human Meibomian glands. They also highlighted a range of problems many of which we deal with even today. As the observations presented in these earlier papers were used as arguments and/or trampolines in countless studies that have been performed in the subsequent decades, their influence on the field cannot be overestimated. However, as I will discuss below, these earlier efforts also showed the limitations of the then-current technologies, which made it necessary to verify the earlier findings with newer, more sensitive and accurate techniques – a cycle which has not ended even today.
4.2. Late-seventies to late nineties of the 20th century
Undoubtedly, the most comprehensive studies of the time were conducted by Nicolas Nicolaides et al. [7, 18, 33–36, 38–40, 46–48]. In fact, the very term “meibum” was, for the first time, introduced by Nicolaides and Santos [7]. Coming from the dermatological field, Nicolaides applied analytical approaches that had been tested on much more readily available skin lipid samples, to MGS, with groundbreaking results. In these studies conducted over a decade-long period, Nicolaides et al. evaluated the lipid composition of human MGS and that of selected animal models. Working with animal models (e.g. steer MGS [33]) allowed for large amounts of specimens to be collected. In that comparative study of human and steer MGS, the steer lipids were collected in the quantities approaching one gram of total lipid material. This made it possible to conduct gravimetric analyses of the individual lipid fractions – an accomplishment virtually impossible to achieve with human MGS. The large sample size also facilitated the parallel use of a wide range of experimental techniques, including TLC, OCC, GC/GLC, and mass spectrometry (MS). Apparently, a study of McFadden et al. [40] was the first one in which HPLC in combination with MS was implemented to evaluate meibomian lipids.
The major step in the exploration of human MGS was the observation of several very complex types of meibomian lipids which Nicolaides et al. called “diesters” and “triesters” [33]. The two (backbones) cores of these compounds are very long chain hydroxy-FA (HFA) and α,ω-diols. Two structural isomers of HFA were described: α-HFA and ω-HFA. Overall, HFA were found to comprise about 10% of the entire FA pool of MGS, regardless of their origin [39]. It was reported that ω-HFA were present in the highest ratio (up to 85% of the HFA pool). Both ω-HFA and α,ω-diols were of extremely long chain C29–C38 variety. Per Nicolaides and Santos [33], these compounds esterified to Chl and to other FA comprised up to 10% (w/w) of total steer meibomian lipids. The other 90% of the lipids were more common WE, CE, TAG, etc. HC were observed in human samples, but were classified mostly as exogenous material. Notably, they contained no Sql [7]. If we were to make a critical comment about this work, this would have been that most of the analyses of MGS were done with animal samples, and then the results were extrapolated onto the human samples. However, considering the limited availability of human specimens, and the state of the analytical techniques available in the early-to-mid eighties of the 20th century, this was an understandable and justifiable decision.
Along with characterization of lipid classes, Nicolaides et al. evaluated the structures of FA and FAl they were composed of. Corroborating the earlier results of Andrews, FA and FAl were found to be highly diverse. A large proportion of acids and alcohols were of iso- and anteiso-varieties, and so were α,ω-diols [38]. Most of the detected FA and FAl were very long chain compounds with the number of carbons in the excess of C20 and up to C30, while HFA were even longer (C30 to C38). Importantly, in a later publication, Nicolaides et al demonstrated that another common ocular abnormality – chalazia – was biochemically very different from MGS, as it had a very large proportion of PL and SM, a much smaller pool of CE, and virtually no WE. Also, the FA of its lipids were mostly of straight-chain type, unlike those of the lipid pool of MGS. Thus, chalazion was proposed to be formed mostly of membranes of phagocytes, whose composition they closely resembled.
The further progress in the lipid analysis of human MGS is associated with the numerous studies of dry eye syndrome (DES). In 1986, Dougherty and McCulley compared MGS of normal controls and those of patients with six different types of chronic blepharitis [49]. TLC and GLC were employed to evaluate the samples. Lipids were initially separated into classes by TLC, to give a combined fraction of WE and CE, and individual fractions of TAG, DAG, MAG, FFA, sterols, free FAl, and of what they called “origin material”. Then, FFA were analyzed by GLC. A range of saturated and unsaturated straight chain, iso-, and anteiso-FFA was detected starting with C12 and ending at C29, of which almost 50% were C16:0, C18:0, and C18:1. Some differences between the patients were reported.
A year later, Harvey et al published a very comprehensive study on the overall FA and FAl content of rat and human MGS [50]. The total MGS were converted in trimethylsilyl derivatives, or nicotinates, or picolinyl derivatives of FFA and FAl, and analyzed by flame-ionization (FI) GC (FI-GC) and/or GC-MS. No attempts to separate lipids into classes prior to their derivatization were made. More than 200 chromatographic peaks were detected. This study reconfirmed the great complexity of the lipidome of MGS regardless the source.
An attempt to characterize WE and CE of human MGS was made in 1989 when Osgood et al [51] compared samples of normal donors and those of chronic blepharitis patients using TLC and FI-GLC. The compounds were analyzed using the concept of ECL. At least twelve WE were detected with ECL of 33.6, 35.4, 36.1, 37.3, 38.2, 39.2, 40.1, 41.2, 42.1, 43.2, 44.9, and 45.7, while CE produced ECL values of 19.1, 20.0, 21.1, 22.0, and 23.2. Note that for WE the ECL values for the entire molecules were calculated, while for CE only the ECL values of their FA residues were determined. Thus, the reported ECL values of WE are related to their full molecular weights (FMW), though uncertainty remains with regard to their unsaturation and/or branching. One can estimate the FMW of CE by adding the molecular weight of CE species by adding the projected molecular weights of their FA to 368 [the FMW of (Chl−H2O)]. A range of differences between six clinical groups of chronic blepharitis patients and normal controls were detected.
In a subsequent paper from the same laboratory, Dougherty et al. [52] described a more detailed characterization of FA of a combined transmethylated fraction of WE and CE. The authors reported that almost 60 individual species of FA were observed, more than 30 of which were positively identified. Those were various straight and branched FA of both saturated and unsaturated varieties with their lengths ranging from C12 to C29. Yet another publication from the same group [53] described the discovery of two types of healthy human donors, namely those with and those without CE in their MGS. The FAl of human WE were also analyzed [54]. Most of the FAl (~66%) were reported to be of the straight chain variety, while about 17% were iso-branched. Interestingly, the authors reported a large presence of epoxy-FA in MGS, namely 9,10-epoxy C18:0 (as a minor component) and 11,12-epoxy C20:0 (a major component). When TAG pool of MGS was analyzed by TLC and GC-MS, interesting observations were made [55]. First, TAG were found in quantities that allowed their structural evaluation. Second, most of their FA were of normal straight chain type (70 to 79%), while iso- and anteiso-isomers comprised 11–16 and 7–10%, correspondingly. The lengths of FA residues ranged from C12 to C28. Characteristically, oleic acid C18:1 was the major FA ~43% for normal donors and between 32 and 48% for various types of dry eye patients. Other Cn:1, Cn:2 and Cn:3 unsaturated FA were reported, too. A few previously unreported FA were detected in the TAG group, among which were dimethylated FA, e.g.ω-2,ω-4-dimethyl-FA. No ω-HFA, α,ω-diols, diesters and triesters were observed and/or reported in this or any other study from this group.
In a paper published in 1998, Mathers and Lane reported the lipid composition of human normal MGS to be as follows (in weight %): CE (39.4±3.1), WE (45.2±3.4), short chain WE (5.9±1.1), diesters (2.3±0.8), TG (3.1±2.2), FFA (2.8±1.3), Chl (1.2±0.5) [56].
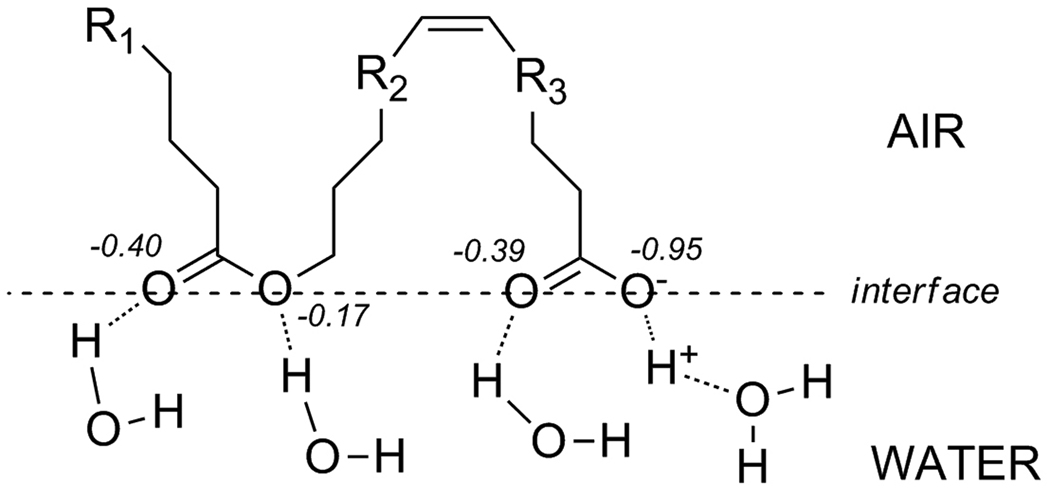
Despite the consensus on the important role of polar lipids in the TFLL stabilization, they had received comparatively less attention than their non-polar counterparts [7, 35, 57–75]. Undoubtedly, this was caused by their relatively minor presence in already very small samples. In the earlier studies discussed above, a few PL and SM were reported to be present in human MGS [42, 62, 63, 66, 71]. In 1973, Holly emphasized and summarized the view which would dominate the field for years to come on the role of polar lipids in the tear film as an interface between the aqueous subphase and the layer of nonpolar lipids which comprise the bulk of meibum [75].
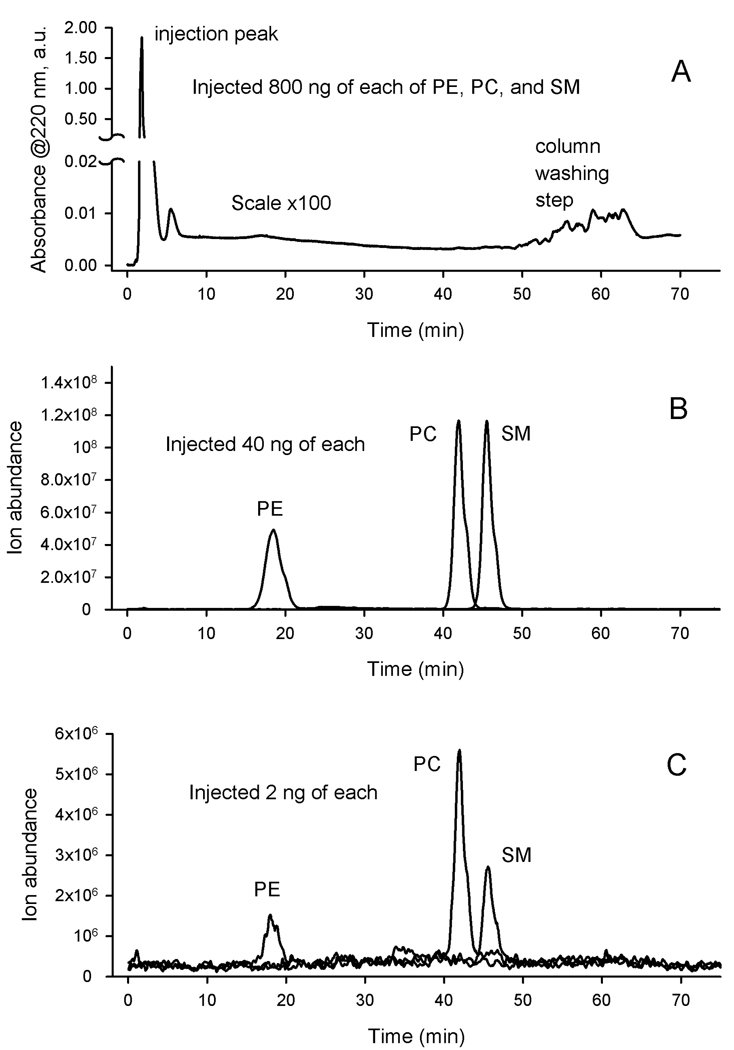
However, it was not until the late 1990’s that polar lipids started to be targetly analyzed and the model of TFLL was detailed [72, 76]. A range of polar lipids was detected, four of which were identified as PE, SM, PC and cerebrosides (Crb) [71]. Another six polar lipids remained unidentified, but three of them were classified as “unknown phospholipids”. The authors studied three groups of human donors: normals, and two clinically different groups of dry eye patients, namely chronic blepharitis patients without and with keratoconjunctivitis sicca (CB and CB-KCS, correspondingly). The authors reported that the sum of PE and SM detected in MGS correlated with the type of the disease: the CB group was closer to normals, while CB-KCS was different from both the normal and the CB groups. The CB-KCS group produced meibum samples with significantly (P<0.05) lower relative amounts of PE and SM compared to other polar lipids than MGS of both normal and CB groups. A conclusion was made that PE and SM are critical for maintaining the optimal structure of TFLL, the deterioration of which led to the onset of CB-KCS. The MGS samples were first separated by TLC, after which the polar lipids were further separated by HPLC with the UV detection of the analytes at 220 nm. Thus, the analyses were classified on the basis of their retention times only. It is worth noting that the authors used no calibration curves and quantified none of the detected lipids: the data were presented only as relative amounts of polar lipids in percentage points, apparently after integrating their corresponding HPLC peak areas recorded at 220 nm, so that the sum of all HPLC peaks equaled 100% (this approach will be discussed below in some details). No information on the molecular structures of the detected lipids was provided.
In a related publication, the same authors evaluated the content of oleic acid in human MGS [77]. It was re-confirmed that oleic acid was the major unsaturated FA of MGS detected in WE, CE, and TAG, and as a FFA. The entire pool of FFA in MGS was observed to be ~0.5–1% (presumably, weight %), WE+CE – 84%, and TAG – 6%. The rest of the lipids (about 9%) remained unknown. The reported data on the individual lipid classes and their changes in patients with different ocular abnormalities were reported without any statistical analysis. Thus, one cannot independently evaluate whether the reported differences in the composition of the samples were statistically significant or not. However, the authors concluded that MGS of patients with meibomian seborrhea were richer in oleic acid than those of controls, which, in turn, were richer in oleic acid than MGS of patients with meibomian KCS.
4.3. Recent studies (2000 – present)
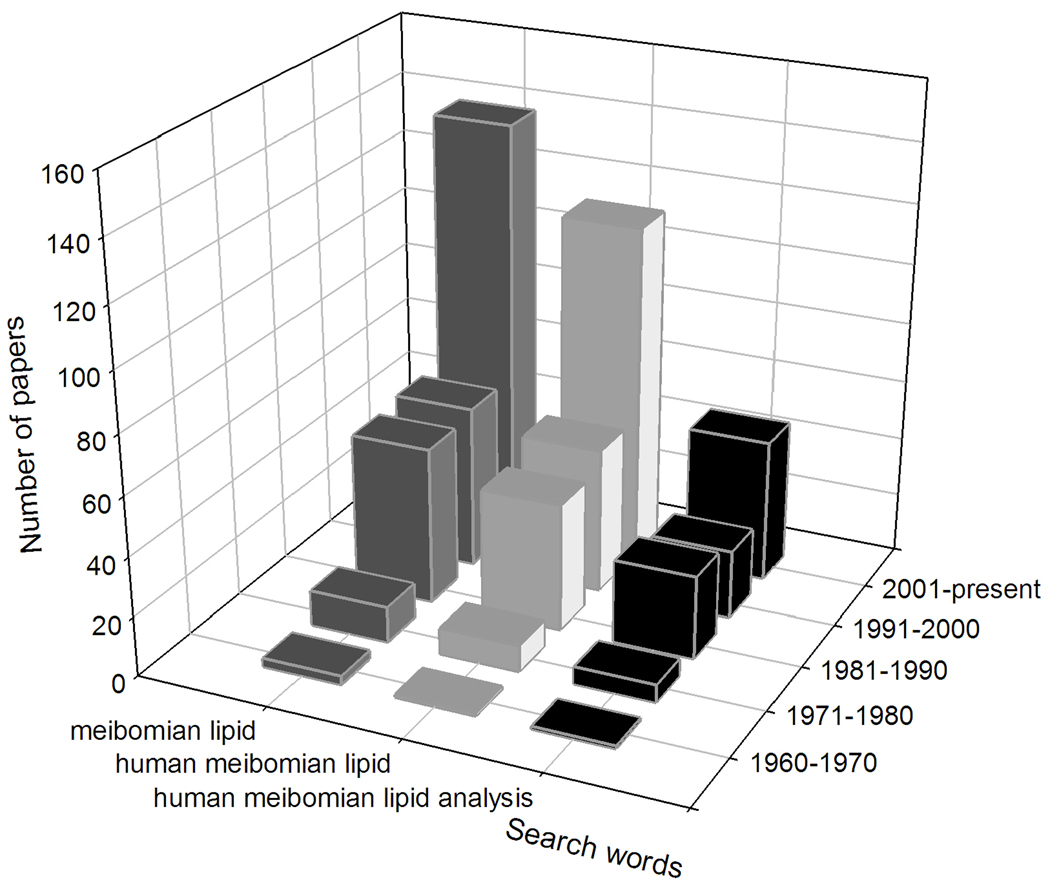
The 21st century brought a rapid increase in the number of papers on the topic – close to 50% of all the papers on meibomian lipids and their role in ocular physiology and pathology to date have been published in the first decade of this century (Figure 6).
Figure 6.
Publications on the topic of meibomian lipids since 1960 (data from PubMed; as of January, 2011).
This decade also witnessed the publication of the first papers on hormonal regulation of meibomian lipids biosynthesis, specifically by androgens [68, 78–89]. In fact, the very first report on the androgen regulation of the meibomian gland was published by the group in 1998 [89]. However, in that brief report the changes in the lipid profile of rabbit and human MGS were described in a very little detail, and only qualitatively. Nevertheless, the authors reported that under conditions of androgen deficiency human meibomian lipid profiles were altered, especially with regard to Chl and DAG. Many clinical symptoms also pointed toward the compromised functions of meibomian glands, and a conclusion was made about the impaired androgen hormonal status and meibomian gland dysfunction (or MGD).
In subsequent publications [85, 86], Sullivan and colleagues reported that meibomian glands are controlled by androgens, which influence the lipid profile of MGS. When antiandrogen treatment was used on human subjects, significant alterations in the neutral lipids were reported. The analyzed lipids included CE, WE, TAG, and DAG. The analyses were performed by LC-MS. Intriguingly, neither chromatograms, nor complete mass-spectra of the samples and the standard lipids were provided, and the attribution of the observed MS peaks raises some questions (a detailed discussion of these results is presented below). The authors interpreted some of their data as an “evidence of apparent changes in saturation or epoxidation, both in terms of elongation…and truncation… with peaks falling on integer multiples of m/z 14”. Sullivan et al. found that many of the lipid changes seemed to be “all” or “none”. However, in a later study, no epoxides were found in normal human MGS [13].
Later, the same group published observations on the changes in the lipid profiles of human MGS for patients with of complete androgen insensitivity syndrome (CAIS) [68]. LC-MS along with direct injection MS were the analytical tools of choice. Both polar and nonpolar groups of MGS lipids were targeted. Complex differences were observed between normal controls and CAIS patients. However, none of the detected lipids was identified with any degree of certainty, no structural analyses of the detected species were performed, and the compounds were reported only as ions with particular m/z values. To much surprise, the reported differences in nonpolar lipids detected in LC-MS experiments included MS peaks with m/z ratios between 100 and 447, which were too small to be true intact lipid species. The MS signals of polar lipids were also in an unrealistically low 262 to 570 range. The direct injection experiments performed to analyze only polar lipids produced a more cohesive body of data, with detected compounds ranging from m/z 269 to 902. Still, ions with m/z values in the 200 to 400 range could not be true intact polar lipids (with the possible exception of FFA), while a possible polar lipid with m/z 762 [most likely, a ubiquitous 1-palmitoyl-2-stearylphosphatidylcholine (PSPC) with a theoretical m/z 762] was found only in some of the samples, and produced very weak signals. Indeed, PC, PE, and SM had been previously reported as major polar lipids found in human MGS [66], and POPC is a quintessential and arguably the most abundant structural PL that is expected to be in virtually any animal tissue. Still, very little of it was observed by Sullivan et al. In the same paper Sullivan et al referred to ions m/z 637 and 654 as PC, which is odd as no typical PC is normally observed in that mass range. Thus, it seems that these observations on the androgen regulation of MG functions need further clarification and independent confirmation before they become a proven fact. A few years later, Sullivan et al. returned to the topic and published two papers on androgen regulation of meibomian glands in mice [90–92].
A targeted study of polar lipids of human MGS by Shine and McCulley published in 2003 [65, 66] concerned the detection and identification of PL, SM, and other sphingolipids in both normal sample donors, and CB patients. The secretions were collected with a traditional spatula method, and the collected lipids were analyzed by TLC, HPLC-UV, and GC-MS. Initially, the lipids were separated by TLC, and then the polar lipid fractions were subjected to HPLC-UV on an aminopropyl silica gel column. The UV detection of analytes was conducted at 220 nm. The authors identified individual polar lipids by their retention times by comparing the latter with those of polar lipid standards. The authors reported that PL comprised ~70% of the total polar lipid fraction, while Cer and CRB accounted for the rest of polar lipids. Among observed PL, the identified lipids were PC (38%), PE (16%), SM (7%) and about 39% of unknowns. Sphingolipids were presented by two sub-classes – Cer (30%) and Crb (70%). Very wide variations between normal MGS and CB patients and between six groups of CB patients were reported. The study, however, raises a few questions. First, the results are presented in a way that precludes evaluation of the interdonor variability of any of the tested sample. Second, it seems that no actual quantitation of any of the detected lipid was attempted as there was no calibration curves was used/reported in the study. Third, the UV detection was performed at 220 nm, which is not recommended for PL analysis as the lipids do not absorb UV light in that region. A more adequate analytical wavelength would have been 205 nm (suggested in an earlier paper [93]), and even that study the UV detection was not a recommended procedure as the UV absorptivity of different lipids varies greatly with their structure, and generally is too low for the practical purposes of the MGS analysis. Fourth, the polar lipids were classified by Shine and McCulley only on the basis of their retention times, which precludes their positive characterization as there are chances that a limited number of the arbitrarily chosen lipid standards could provide false positives. Finally, no sample chromatograms of MGS with or without lipid standards were provided. The same cautionary considerations apply to the subsequent paper on the topic published a year later [63]. Thus, one cannot independently evaluate the TLC, HPLC or GC-MS results reported in those studies.
The next relevant paper on the lipid composition of human MGS appeared in 2007 [94]. The authors collected samples of MGS from 16 normal donors using microcapillaries. The average sample was ~5 nanoliters (or 5 micrograms or less) of MGS. The samples were stored in Eppendorf tubes in frozen state until they were redissolved in chloroform-methanol solvent mixture to be analyzed by electrospray ionization time-of-flight tandem MS (ESI-Q-TOF MS). A range of previously unreported compounds was observed. Most importantly, the authors described various FAm and FFA in the C14 to C22 range, with oleamide being the most prominent species detected by ESI-Q-TOF. Its structure, along with the structures of other FAm observed in the samples of MGS, was established in MS fragmentation experiments where the sample lipids were compared with FAm standards. The authors proceeded further and proposed a signaling and/or a maintenance role of FAm in TF. Intriguingly, FAm of the same type have been shown to be common plasticizers that are used in chemical industry to manufacture various plastics, including polyethylene and polypropylene – the same materials used to manufacture Eppendorf tubes [95]. Thus, considering the minute size of the samples collected and analyzed by Nichols et al, and a very unfortunate combination of storage vessels (plastic Eppendorf tubes) and of a organic solvent of choice (chloroform), any exogenous contaminations, such as plastic extractives, could have prominently manifested themselves in the mass spectra of the samples. This possibility was pointed out in a brief letter [96] published in response to the paper of Nichols et al. in 2007. Later, oleamide was indeed shown to be one of the most common plastic extractives that contaminated biological samples that were in contact with plastic ware [95] [97]. Considering the potentially important regulatory and/or structural role of these compounds in vivo, further investigation into FAm was warranted.
The same year, Butovich et al published two papers on the lipidomic analyses of normal human MGS [27]. The authors collected MGS from healthy, non-dry eye volunteers using the spatula method. The samples averaged between 0.5 and 1 mg. The samples were analyzed “as is” without any prior modification by normal phase HPLC (NP-HPLC) in combination with ion trap MS (IT-MS). Two ionization procedures were employed – atmospheric pressure chemical ionization (APCI) and ESI. Initially, a range of lipid standards was tested to optimize the conditions of the HPLC separation and the detection of different lipid classes. Those included CE, WE, TAG, DAG, MAG, Chl, FFA, FAm, Cer, PL, and SM. Then, MGS were analyzed. Among the lipid classes positively identified in MGS as major lipid constituents were WE, CE, and Chl. Very little TAG was detected, while DAG, MAG, Cer, and FAm were absent. PL and SL were virtually absent with the exception of an extremely small pool of PC and SM, which (combined) accounted for not more than 0.05% (w/w) of the total lipid pool. Special attention was paid to the detection of oleamide. However, it appeared that if extreme care had been exercised not to contaminate the samples with plastic extractives, oleamide was not detected in any of the samples. Thus, a conclusion was made that the family of FAm reported by Nichols et al [94] was a sample contamination by plastic extractives. Among the positively identified lipids, oleic acid-based WE were one of the major groups. The compounds were characterized in fragmentation experiments alongside with authentic lipid standards. Unsaturated WE were shown to be mostly oleic acid esters of long chain and very long chain saturated FAl of the C18 to C30 family. Small amounts of C18:2- and C18:3-based WE were also found. However, the other structural features of WE, such as FA and FAl branching, have not been evaluated.
The differences in the nonpolar lipids between MGS and aqueous tears were independently evaluated by HPLC-MS [97]. Aqueous tear lipidome has been shown to be more complex than that of MGS, with a larger presence of lower molecular weight compounds, such as Chl and WE. Minute amounts of Sql were observed, mostly in the aqueous tear samples. Compounds tentatively identified as TAG, DAG, and other esters were also observed. Importantly, no Cer or FAm (including oleamide of biogenic origin) were detected in any of the tested samples.
The fatty acid composition and branching of MGS lipids have been most recently evaluated by Joffre et al. [98] and Souchier et al. [99]. These authors analyzed the FA composition of the combined transmethylated pool of all meibomian lipids by GC-FID and GC-MS. Detected FA ranged from C14 to C26. The major FA, as expected, was oleic acid C18:1 ω -9 and C18:1 ω-7 a (~33 and 6% of all FA, respectively). The other major monounsaturated FA were C16:1 ω-9 (7%) and C16:1 ω -7 (~4%). A C18:2 acid (apparently, linoleic acid) accounted for 4% of all FA, while C18:3 made up less than 1.5%. Straight-chain saturated FA (24.6% total) were represented mostly by C16:0 FA (12.6%) and C18:0 (7.6%), with the rest being C14:0, C17:0, C22:0 and C24:0. Branched-chain FA (20.2%) were of both iso- and anteiso-varieties ranging from C16:0 to C26:0, among which longer-chain species prevailed. Some differences between normal samples and those collected from dry eye patients were observed, particularly in terms of FA saturation and branching.
Realizing that knowledge of the overall FA composition of MGS is important, but limited, targeted analysis of individual lipid classes was deemed to be necessary. In original works of Andrews [42], Nicolaides [7, 30, 33–36, 38, 39], Tiffany et al [45], Harvey [50], Osgood [51] and Dougherty [49, 52], FA and FAl compositions of MGS were evaluated by GC-FID and GC-MS, however the intact lipid species were not possible to detect because of the limitations of the techniques employed. To characterize the intact lipids without their prior derivatization, HPLC-MS proved to be very instrumental [40]. The pool of unsaturated WE in human MGS has been shown to be based mainly on oleic acid [13, 27], while CE contained a wide range of FA starting with C16 and going up to C32–C34 [26]. The pool of CE was reported to be dominated by very long chain and extremely long chain FA (VLC-FA and ELC-FA) of both saturated and monounsaturated varieties. Very little of di- and tri-unsaturated derivatives was found. The overall ratio of saturated to unsaturated FA of CE had been recently determined to be roughly 4:1 [26]. Yet in another publication from the same laboratory, another class of meibomian lipids, namely OAHFA, was described [13]. Again, as with CE, OAHFA were a depot of VLC-FA and ELC-FA of approximately the same length, but unlike CE their HFA residues were mostly monounsaturated. These results will be discussed in more detail in the next section of this paper. Moreover, FA branching has not been evaluated for detected lipid species in any of these studies.
An interesting study by Borchman et al. concerned spectroscopic evaluation of human meibomian and tear lipids [100, 101]. The authors used Fourier transform IR (FT-IR) and FS spectroscopies to study conformations of meibomian lipids at different temperatures. Though not an analytical study per se (the data provided by FT-IR and FS data are not sufficient to identify a compound with any degree of certainty), the results of Borchman et al. demonstrated some differences between MGS and tear lipids. In particular, the FT-IR spectrum of MGS was found to be surprisingly similar to that of a wax palmityloleate [101]. The authors were very cautious in claiming that MGS had much less PL than the lipids extracted from aqueous tears, which suggests their different lipid compositions. Notably, Borchman et al. tentatively assigned an IR band 1043 cm−1 observed in aqueous tears sample to a (phospholipid) diester C-O-P-O-C stretch, but did not detect it in MGS [101]. Similarly, no signals of SM in the fingerprint region of the MGS spectra were detected by FT-IR. All in all, it seemed that the FT-IR technique was/is better suited for studying the structural re-arrangements, such as trans⇄gauche transformations, than for evaluating the composition of complex lipid mixtures as the IR band assignments are prone to errors if attempted for complex mixtures of unknown compounds. It is also worth mentioning that IR spectroscopy is never used by chemists for positive identification of a compound as the only analytical procedure, even when the compound is in pure form: the IR must be used in combination with other analytical methods and is better suited for conformational analyses and confirmation of the presence of certain types of chemical bonds and groups.
A few years later, the same authors characterized meibum using Raman spectroscopy (RS) [23]. As with FT-IR, interpretation of the results obtained by RS is not straightforward. Pertinent to this comment is the observation of “carotenoid-like compounds” in meibum of human donors, and the inverse correlation of their content with age: the younger the donors were, the more carotene-like compounds were observed in their meibum. The amount of these compounds for a young donor was estimated to be either 90µg/g or 88 mg/g, depending on the part of the paper. The relevance of this observation will be discussed below. Unlike FT-IR, the RS was shown to be not suitable for conformational analyses of lipids because it was prone to experimental artifacts caused by heating the samples with laser beams. Indeed, heating a sample from the room temperature to 35°C by the laser beam of the Raman spectrometer caused its disordering which rendered its trans,gauche analyses impossible to carry out. However, the authors were able to measure the saturation level of meibum (i.e. its CH2/=CH ratio), which was reported to be either ~3, or 12, again depending on the page of the paper.
5. Chemical composition of human meibomian gland secretions
The brief review of the history of the meibomian lipids studies presented above leads to a legitimate question “What is the actual chemical composition of the meibomian lipidome?”. There is no simple and definitive answer to this question. Indeed, with the extraordinary diversity of the meibomian lipids, even on the level of their classes, unavailability of many of much-needed lipid standards for structural evaluation and quantitation of all meibum constituents, and quite possible inter-donor and inter-sample variations, the exact lipid composition of MGS is still a work in progress. Nevertheless, one can compare the data reported in the most relevant papers focused strictly on lipidomic analyses of human MGS, but before we do so, a few comments on the methodology of lipidomic analyses are warranted.
5.1. The choice of analytical procedures
First and foremost, the rule of thumb in choosing a method is an old British saying “Horses for courses”. Often, a researcher is tempted to use a method that is either cheaper, or simpler, or more available at the moment, instead of using a technique which provides better results, albeit at the expense of time, convenience, or higher costs. When analyzing MGS, one can chose between a range of analytical techniques. The choice of TLC, OCC, GC-FID, GC-MS, HPLC-UV, HPLC-MS, NMR, IR, RS, or FS (among other available techniques) should be dictated by the final goal of the experiment. No technique used alone can provide enough information to positively identify a compound, especially if the analyte is present in small amounts and in a complex mixture. Each of the techniques listed above has its own strengths and limitations, some of which have been discussed in an earlier review on the topic [43].
TLC and OCC, being older methods, show their age and, despite their convenience and simplicity, should be used cautiously. Briefly, TLC is suitable for crude separation of lipid classes, but typically cannot separate series of homologous compounds, and its visualization techniques (i.e. staining) lack sensitivity and selectivity. Also, it is prone to exogenous contaminations [7, 45]. However, TLC provides a simple and effective tool in separating two classes of lipids which are hard to separate otherwise – WE and CE [102] – though it typically does not resolve the members of the individual classes. After scrapping of the corresponding lipid fractions off a TLC plate, one can use GC-FID or GC-MS to analyze the corresponding pools of lipids for their individual constituents. OCC is best suited for preparative separation of lipid classes and purification of the samples from non-lipid contaminants as a preliminary step before actual analysis.
An intrinsic limitation of GC is the low volatility of underivatized intact lipids. Complex lipids, for example, need to be hydrolyzed to their corresponding building blocks such as FA, FAl, Chl, etc, and further derivatized through, for example, methylation or silylation. Thus, the original structure becomes scrambled and difficult to reconstruct, particularly if the lipids were present as a complex mixture to begin with. The detection of lipids by GC-FID is rather nonspecific and completely depends on the accurate measurements of the retention times of analytes. Then, the only way to identify an analyte is to compare its retention time with those of known standards hoping that there will be a perfect match. Otherwise, extrapolation and interpolation of the data will be required to ascribe a probable structure to the unknown. GC-MS is more informative in this respect, but similarly to GC-FID is still limited by the necessity to hydrolyze and derivatize lipids to make them volatile.
To overcome this requirement, one needs to employ HPLC. Earlier, HPLC was used in combination with UV detectors (HPLC-UV), whose sensitivity was limited by the low UV absorptivity of common lipids which typically do not have UV chromophores in their structures [notable exclusions are oxidized unsaturated lipids (Figure 7) and other compounds with conjugated double bonds, such as carotenoids]. Some lipids can be labeled with chromophores before the analysis, but this method is unsuitable for many lipid classes as they do not have proper chemically reactive groups that can be modified by chromophores or fluorophores (for HPLC-FS). Additionally, there is no single chromophore that can be attached to all lipids in the mixture as, for example, it is done in amino acid analysis with OPA or PITC. Another popular detection technique – evaporative light scattering (ELS) – is nonselective and insufficiently sensitive for samples as small and complex as MGS are, and also relies on the retention times as the only analytical parameter.
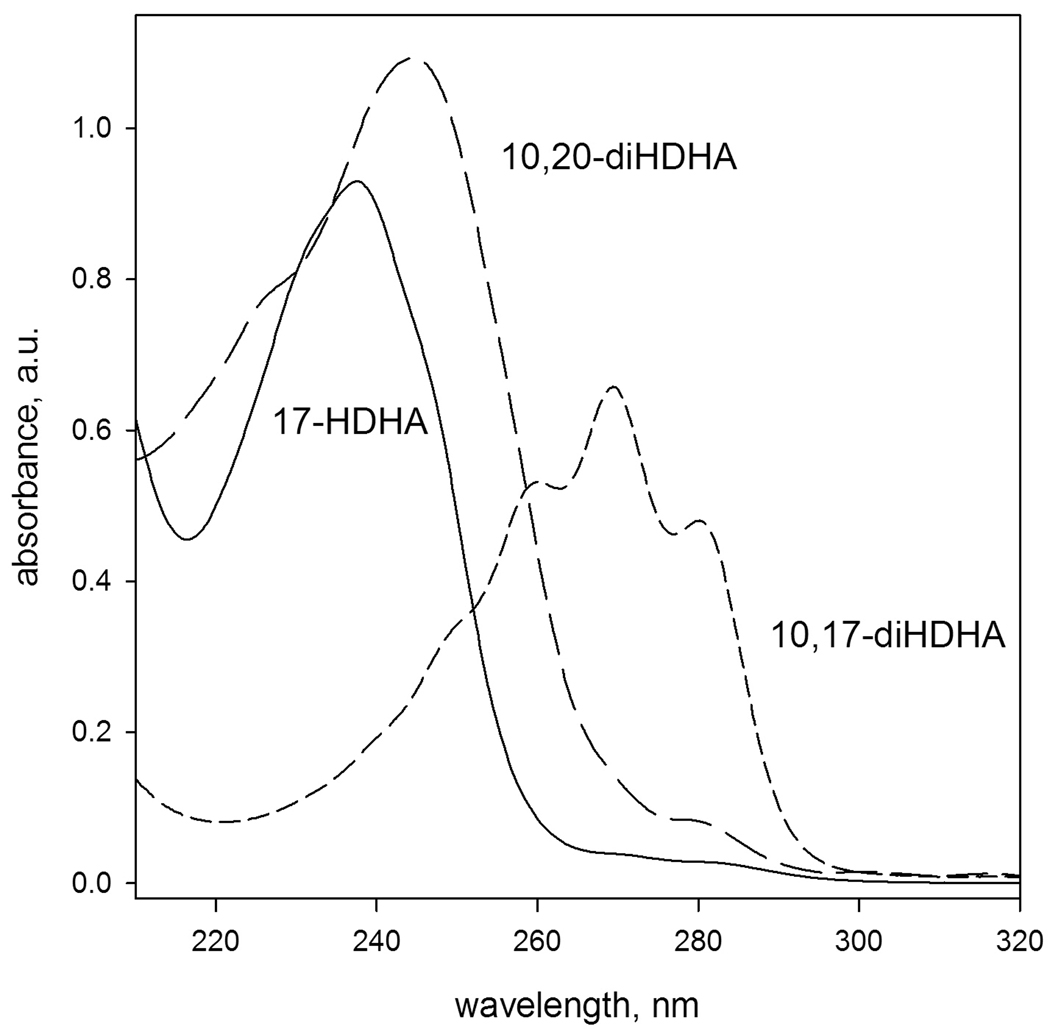
Figure 7.
Ultraviolet spectra of three oxidized derivatives of docosahexaenoic acid – 17-hydro(per)oxy-DHA (solid), non-conjugated dioxygenated 10,20-dihydroxy-DHA (long dashes), and a conjugated deoxygenated 10,17-dihydroxy-DHA (short dashes) in hexane-iso-propanol mixture (1:1, v/v)
NMR is very instrumental when one needs to study geometrical features of compounds and confirm the presence of particular groups in the molecule, but is much more difficult to use for evaluation of complex mixtures, and is hardly quantitative. However, in a recent report [103] NMR was successfully used to quantify skin lipids, and the method in general is gaining popularity [104]. However, its sensitivity is low compared with MS, which can be currently overcome only by increasing the length of the analysis. To keep the length of an NMR analysis reasonable, the recommended concentration of a sample is in millimolar range, or above, sometimes approaching tens of milligrams of a sample per ~0.5–0.7 mL of a solvent (for carbon spectra). This makes analyzing minor MGS constituents very problematic, if not impossible. Among the available techniques, 1H-NMR seems to be the most sensitive, but the proton spectra it produces are most difficult to analyze and interpret, especially for mixtures of compounds. 13C-NMR, on the other hand, produces simpler spectra, but its sensitivity is much lower because of the lower natural abundance of 13C isotopes.
The application of IR spectroscopy for MGS studies has received lately a lot of attention [22, 57, 101]. The method is nondestructive and is easy to carry out. However, it is not considered by analytical chemists a proper sole technique for product characterization, especially if the analytes are present in the form of complex mixtures of various, often homologues, compounds [13]. IR can be instrumental as a supplementary technique to confirm the presence of a particular functional group in a compound, and in the conformational studies, but IR cannot provide any information on the molecular weights of the analytes, cannot distinguish between close homologues, and generally is poorly suited for analyzing complex mixtures. Its sensitivity and resolution remain a lot to be desired, and a margin of error in quantitative experiments is very high. Additionally, a lot of assumptions must be made when ascribing certain bands to certain bonds, and when deconvoluting complex spectra with overlapping signals, thus opening a door for misinterpretation of the data. Thus, the results obtained with this technique only should be treated cautiously and be confirmed in independent studies using other analytical techniques.
RS generally has the same strengths and weaknesses as IR. The scope of conventional FS is even narrower because of the very limited number of lipids that posses natural fluorescence (such as carotenes), and is mostly used for fluorescent lipid analogs when studying lipid aggregation, lipid membranes, etc. Thus, IR, RS, and FS are not viable choices for lipidomic analysis of MGS.
HPLC-MS, on the other hand, is the current golden standard when it comes to analyzing complex lipid mixtures. The reasons for this are many. For the vast majority of analytes, MS detectors are orders of magnitude more sensitive than the UV and FS ones. The analytes can be analyzed “as is” without the need to make them either volatile (a requirement in GC analyses), or UV- or FS-visible (for HPLC-UV and HPLC-FS). Unlike all other methods of detection, MS can provide accurate information about the molecular masses of the analytes, critically important for the correct identification of compounds. In fragmentation experiments, many of a compound’s functional groups can be identified, and locations of its double bonds (if any) can be established [105–107]. Very useful in this respect is the use of lipid standards, especially of their isotopically labeled derivatives [108, 109], which greatly facilitate the analysis and quantitation of analytes. All these, in combination with a wide variety of HPLC columns and solvents, make it possible to identify a compound with high degree of probability, and quantify it even in a complex lipid mixture. The most popular at the time are ESI and APCI techniques in combination with triple-quadrupole (TQ), IT (also known as MSn), or Q-TOF). Matrix assisted laser desorption TOF (MALDI-TOF) MS – also a popular choice – is not a chromatographic technique, and as such lacks the ability to separate lipids spatially prior to their analysis. It is also a very soft ionization technique which is poorly suited for fragmentation studies. Additionally, the samples need to be pre-mixed with a matrix, which often leads to the inhomogeneity of the mixtures, and, as a consequence, a spotty reproducibility of the analyses.
Another popular choice of the introduction of samples into a mass spectrometer is the “shotgun” (SG) approach [110–113]. The main idea behind SG-MS approach is to save time by forgoing the prior chromatographic separation of the analytes, with an additional benefit of keeping the analytes undiluted by the eluent and, thus, at their highest concentration. In human meibomian lipid studies this approach was tested by Chen et al [58]. The authors were able to detect the compounds that had been already detected and evaluated in the preceding HPLC-MS studies [13, 21, 26, 27, 43, 58, 61, 97], and those evaluated in even earlier GC and GC-MS studies of Nicolaides et al [7, 30, 33–36, 38, 39], albeit only after the complete hydrolysis and transesterification of complex lipids, which scrambled the structures of starting compounds. However, SG-MS proved to be limiting as many of complex lipids present in meibum underwent spontaneous in-source fragmentation releasing their fragments (such as free Chl and FA), which then were confused for free Chl and FA present in intact meibum. This disadvantage of the shotgun approach could be easily overcome by using HPLC separation as the initial step of the analysis, thus separating free Chl and FFA from more complex lipids before they enter the MS detector [73].
However, HPLC-MS is not without its flaws. It is known that, under the same conditions, some lipids ionize better than the others. Thus, it might be necessary to run experiments in different conditions, or with different ionization techniques and in different MS polarity modes (positive or negative), to detect all the components in a mixture. Being relatively soft ionization techniques, ESI and MALDI are inferior to the electron impact (EI) MS (used in combination with GC) in terms of fragmentation of compounds: many types of nonpolar molecules, e.g. saturated HC, are easier to detect using EI MS than any other MS technique. GC-EI MS is arguably the best tool for analyzing homologous FFA, FAl, sterols, and their derivatives, as it provides unsurpassable separation of these compounds, which is difficult, if not impossible, to achieve using HPLC. Thus, ESI and, especially, MALDI are better suited for the detection of polar molecules such as PL, peptides, and proteins. The APCI technique was developed with less polar compounds in mind, and thus is closing the gap between ESI and EI, though it is still not as effective as the latter when the analytes do not have polar groups and/or double bonds in their structures. ESI and APCI are not particularly well suited for evaluating carbon chain branching – again, EI is far more effective in this respect. However, APCI, ESI, and MALDI are well suited for correct molecular weight determinations, evaluation of structural features of complex lipids (e.g. their FA, FAl, and sterol composition), for fingerprinting complex lipid mixtures or individual lipid components (in fragmentation experiments).
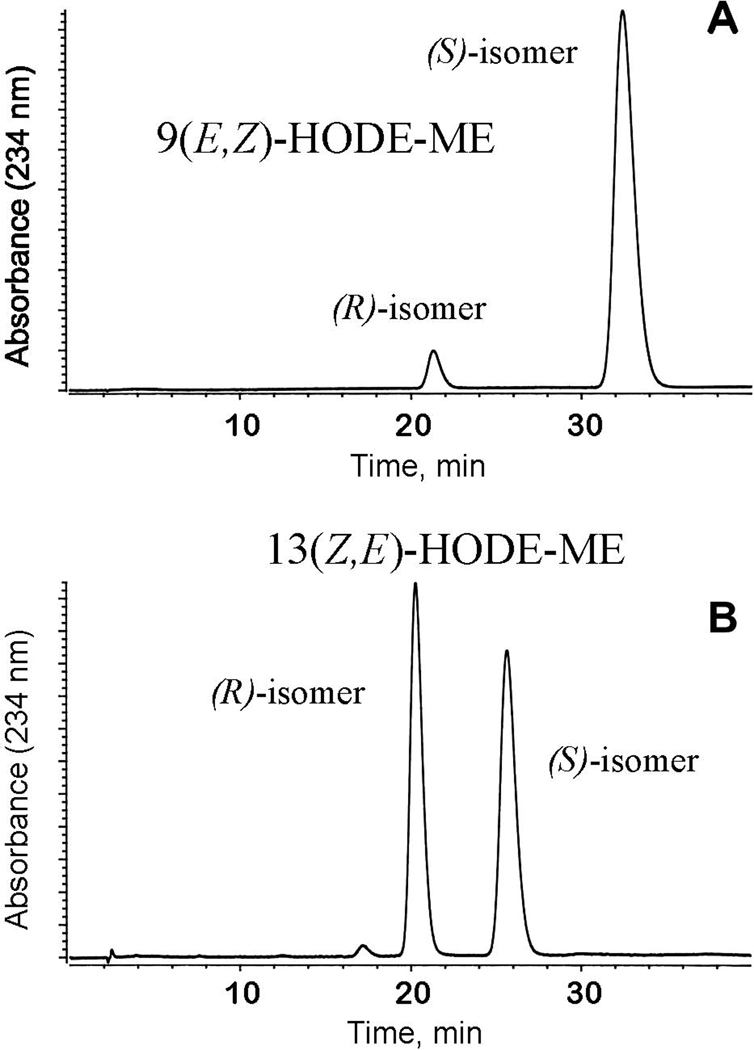
Another area where stand-alone MS and HPLC-MS are somewhat lacking is conformational analysis of molecules. While cis,trans geometry of compounds can be evaluated through the differences in the HPLC retention times of different geometric isomers (provided the proper standards are available), NMR should be employed to do structural assignment of the chromatographically pure isomers. MS as a standalone procedure is hardly an answer. One can argue that it is possible to use specific fragmentation patterns [114], or silver ion HPLC [115], to distinguish between cis and trans isomers of the analytes, still the final proof of the geometrical features can be provided only by NMR and, to certain extent, by IR. Similarly to cis,trans isomerism, (R,S) stereochemistry of compounds can be evaluated in HPLC-MS experiments if using chiral columns (Figure 8) [116], but the attributions can be made only on the basis of the retention times. For GC-FID or GC-MS, more straightforward, albeit much more elaborate, protocols have been published which allowed unambiguous stereo assignments to be made [117, 118].
Figure 8.
Separation of chiral and positional isomers of monohydroxylated linoleic acid on a chiral column Chiralcel OD-H (reprinted from [116] with permission).
Summarizing, one can conclude that a set of various tools is needed to complete the full structural characterization of MGS lipidome: HPLC-MS – for molecular weight determination of complex intact lipids, their structural and chiral analyses; GC-MS – for in-depth structural analysis of individual FA, FAl, and sterols (as free entities, or after hydrolysis of complex lipids); NMR – for evaluation of the elemental composition of a compound or a mixture, for cis,trans conformational analysis, and for verification of structural assignments made in MS and HPLC experiments; IR – for direct confirmation of the presence of particular types of chemical bonds, their geometry, and lipid packing (with some reservations). Other techniques, such as FS and RS, at present are niche techniques, and are of limited interest at present time, though this may change in the future.
5.2. Lipid classes and individual lipid species of MGS
5.2.1. Wax esters
Up to one-third of human meibum (w/w) is believed to be represented by WE [7]. This estimate is based on the early studies where samples of MGS collected from individual donors were pooled, lipid classes were separated by TLC and/or OCC, and then, apparently, measured gravimetrically. In a comprehensive study on the topic, Nicolaides et al reported that, based on GLC studies, the FA residues if human WE ranged from C12 to C29, whereas the number of double bonds in their structures ranged from 0 to 2, with the only tri-unsaturated FA detected being C18:3. The most common FA were C18:1ω-9 (44% of all FA), C18:1ω-7 (12%), C16:1ω-7 (8%), anteiso-C17:0 (10%), iso-C16:0(5%), with the rest of individual FA being below 2%. The overall ratio of saturated to unsaturated FA was calculated to be about 1:4. The corresponding FAl of human MGS started with a C18 and were limited to C31 as the longest species detected. Four species stood out: iso-C26:0 (21%), iso-C24:0 (15%), anteiso-C25:0 (13%), and anteiso-C27:0 (6%). Interestingly, the overall ratio of saturated to the unsaturated FAl was the opposite (4:1). Because of the limitations of GLC, the exact combinations of these FA and FAl in the intact WE were impossible to determine. However, with the advancement of the alternative techniques, such as HPLC-MS, direct observation, evaluation, and quantitation of intact WE molecules became feasible.
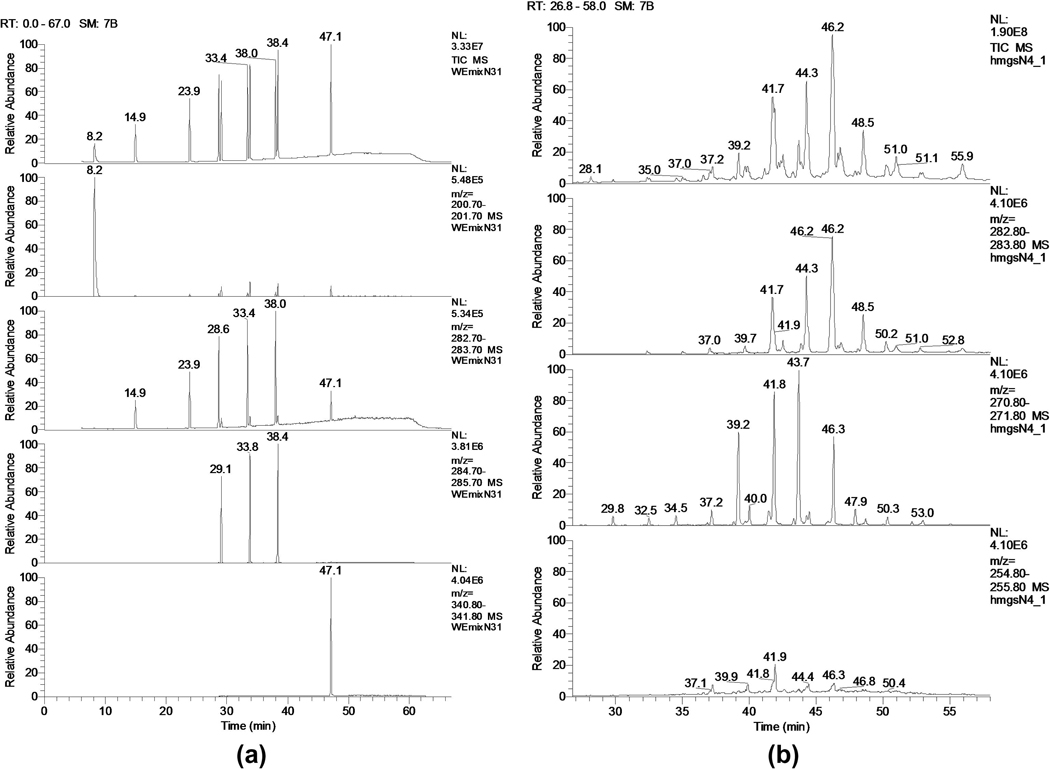
The most prominent unsaturated WE species detected in normal human MGS were oleic acid-based WE [7, 13, 27, 61], though some amounts of palmitoleates have been reported [58]. However, the latter compounds, in our hands, were the less prominent members of the WE family, and their relative amounts rarely exceeded 10% of their C18:1-FA-based counterparts [119]. The major unsaturated WE detected had C24:0, C25:0, and C26:0 FAl components in them. Thus, the three most common WE species of normal human WE are tetra-, penta- and hexadocosanyl oleates. The geometrical features of detected WE (such as iso- and anteiso geometry) could not be effectively evaluated in the HPLC-MS as there were no authentic standards available. However, these three major WE species (unlike some other WE reported in the same study) produced just one HPLC peak each, which indicated their HPLC homogeneity. If the assignments of FA structures made by Nicolaides [7, 38, 39] were correct, then the alcohol moieties of tetra- and hexadocosanyl oleates of human meibum are mostly of iso-nature, while in the pentadocosanyl oleate it is of anteiso one. This needs to be verified in GC-MS experiments. Currently, such experiments are underway in our laboratory. Preliminary direct GC-MS analysis of unmanipulated meibomian samples showed that the major saturated meibomian WE are based on C17:0-FA esterified to a wide range of very long chain alcohols (Figure 9), with smaller amounts of straight chain C16:0 FA-based WE also present. Fragmentation of their molecular ions clearly showed that the C17-FA was of anteiso-type: all the expected fragments (M-29) and (M-57) for intact WE, and related characteristic fragments m/z 241 (M-29) and m/z 213 (M-57) for 14-methyl-hexadecanoic acid (or anteiso-heptadecanoic acid) were clearly visible (Butovich, unpublished).
Figure 9.
Direct GC-MS analysis of a test mixture of 10 underivatized wax esters (Panel A) and of a sample of human meibum collected from a healthy volunteer (Panel B). Elution of the analytes from a GC column was performed using a temperature gradient.
Panel A. Ten WE were (in the order of elution): myristyl laurate (m/z 396), lauryl oleate (m/z 450), palmityl oleate (m/z 506), stearyl oleate (m/z 534), stearyl stearate (m/z 536), arachidyl oleate (m/z 562), arachidyl stearate (m/z 564), behenyl oleate (m/z 590), behenyl stearate (m/z 592), and behenyl behenate (m/z 648). Five chromatograms presented here depict (from top to bottom): 1) total ion chromatogram of the mixture; 2) elution profile of myristyl laurate; 3) elution profile of a common product ion m/z 283 (a proton adduct of oleic acid); the ion is generated due to spontaneous fragmentation of parent WE ions and allows all WE that contain oleic acid to be detected; 4) elution profile of a common product ion m/z 285 (a proton adduct of stearic acid); the ion is generated due to spontaneous fragmentation of parent WE ions and allows all WE that contain stearic acid to be detected; 5) elution profile of a unique for this mixture ion m/z 340 (a proton adduct of behenic acid) generated spontaneously from behenyl behenate.
Panel B. Human meibum was analyzed similarly the test mixture of WE described above. From top to bottom, four chromatograms are shown: 1) total ion chromatogram of normal human meibum; 2) elution profile of ion m/z 283 (C18:1-FA-based WE); 3) elution profile of ion m/z 271 (WE based on a C17:0-FA); 4) elution profile of ion m/z 255 (WE based on a C16:1-FA). The last three chromatograms show that the apparent abundance of C16:1-FA-based WE is much lower than that of C18:1- and C17:0- FA-based WE.
Despite the presence of large amounts of possibly epoxidizable unsaturated oleic acid-based WE, and a few previous reports on the presence of epoxidized lipids in human meibum [54], no epoxy-WE was found when normal human MGS was analyzed by HPLC-MS, and proper standards of epoxidized lipids were tested alongside the human samples [13].
The chemical nature of meibomian WE makes them extremely hydrophobic and, despite the presence of unsaturated C18:1 FA residue as the major FA component, relatively high melting. Their melting points, when measured in pure state, are expected to be well above physiological temperatures [120]; [121]. However, structural isomers such as iso- and anteiso-WE melt easier than their straight-chain analogs [122], and mixing of WE with other types of lipids may facilitate melting of even long chain WE [121]. Thus, the relatively low melting point of the whole human meibum (around 32–33°C, [97]) can be explained, at least in art, by these factors.
Importantly, the molar ratio of WE demonstrated a very low inter-donor variability [123] which was against the earlier claims about the high changeability of the lipid composition of MGS [45].
5.2.2. (O-acyl)-omega-hydroxy fatty acids
A related class of lipids, which are interesting from their structural viewpoint, is OAHFA (Figure 10). These compounds have a dual nature of being WE and FFA at the same time. Their existence in human meibum was first implied, but never claimed or confirmed, by Nicolaides and Santos [33]. Clearly, these compounds are either precursors, or degradation products, of more complex compounds of di- and tri-ester nature (see below). OAHFA is a diverse class of lipids. Their MS spectra, and the corresponding fragmentation patterns, were originally presented in 2007 [27]], though their unequivocal structural characterization required making authentic standards [such as (O-oleoyl)-16-hydroxypalmitate)] which were not available until two years later [13, 43].
Figure 10.
HPLC-MS analysis of OAHFA (reprinted from [21] with permission).
Panel A. Elution profile of free OAHFA as detected by RP HPLC-APCI MS in negative ion mode. Three most intense HPLC peaks are formed of three major OAHFA species present in human meibum (m/z 729, 757, and 785).
Panel B. Elution profile of OAHFA m/z 757
Panel C. Averaged mass spectrum of OAHFA eluted between 6 and 16 min into the experiment.
Panel D. Fragmentation mass spectrum of ion m/z 757 and its proposed structure
Panel E. RP-HPLC profile of a synthetic OAHFA, (O-oleoyl)-16-hydroxypalmitic acid (m/z 535).
Panel F. Zoom scan mass spectrum of authentic (O-oleoyl)-16-hydroxypalmitic acid
Panel G. Fragmentation spectrum of (O-oleoyl)-16-hydroxypalmitic acid
A key feature of OHFA is the nature of their omega-hydroxy FA (OHFA): unlike the acylating FA of OAHFA, which are mostly of mildly long C16 and C18 variety, the vast majority of OHFA are of extremely long chain nature. The major OHFA range from monounsaturated C28:1 to C34:1 [13], while the minor members of this family can considerably extend this range in both directions [58],[119]. The normally observed acylating FA are unsaturated oleic and palmitoleic ones, in this particular order of abundance. However, one cannot rule out the possibility of presence of a group of other FA that are esterified to OAHFA. Given the diversity of extremely long chain FA (ELC FA) in nature, the exact location of the double bonds in the meibomian OHFA molecules cannot be claimed with certainty at this time. However, 21-cis-triacontenoic (30:1 ω-9) and 23-cis-triacontenoic (30:1 ω-7) acids appear to be the most common C30:1 FA described in literature. Similarly, dotriacontenoic (C32:1) acids could be of (ω-7) and (ω-9) nature, too, and so could be the tetratriacontenoic (C34:1) acids. Interestingly, (ω -9)-isomers with cis geometry of the double bond dominate this lipid class, at least in plants [124].
Despite having a very long chain C28–C32 FA in their structure, OAHFA are relatively mobile under the conditions of RP HPLC and tend to have much shorter retention times than WE with the same number of carbon atoms [13], [119]. For example, while the RT of octacosenyl oleate (a WE with MW of 672 Da) in a RP HPLC experiment is around 14 min, the RT of (O-oleoyl)-omega-hydroxy-octacosenoate (MW 704) – a compound with the same number of carbon atoms and double bonds, but with two extra atoms of oxygen – is around 9 min (Figure 11), which confirms its much higher hydrophilicity [Butovich, unpublished]. This mobility is, undoubtedly, due to the presence of an additional free carboxyl group in the structure of OAHFA, which makes the compound much more hydrophilic. Considering that the typical RP HPLC experiments were conducted in acidified solvents, where carboxylic groups do not dissociate, alkalinization of the solutions, which would facilitate dissociation of the OAHFA carboxyls, would certainly make them even more hydrophilic and HPLC mobile. The melting points of OAHFA have not been reported yet.
Figure 11.
Reverse phase chromatograms of an OAHFA (m/z 703) and a WE (m/z 673). Note that the compounds have the same number of carbons in their structures, but their retention times differ due to two additional oxygen atoms in OAHFA, which makes it much more hydrophilic that the corresponding WE.
The putative physiological role of OAHFA might be directly related to two key features: their ability to ionize and the presence of double bonds in their structures. The very long carbon chains, interrupted in the middle by double bonds, bend. The double bonds of –CH=CH– type are also more hydrophilic than the saturated –CH2–CH2– group because the vinyl bond is slightly electronegative and can accept protons to form a proton adduct. This is well illustrated by the fact that, for example, under the conditions of MS experiment, unsaturated WE form proton adducts much more readily than their saturated analogs, proving the role of the double bond in the formation of proton adducts. Thus, OAHFA, when at the air/water interface, may assume a configuration that will ensure multiple points of contact with aqueous surface (Figure 12). This seems to be especially true with regard to the carboxyl groups of OAHFA, whose hydrophilicity is greatly potentiated by their pKa (between 4 and 5 for a typical short chain carboxylic acid, and somewhat higher for long chain FA) which is sufficiently low for the OAHFA to partly or completely dissociate at a physiological pH of about 7.4. In acidic solutions these compounds are electroneutral, and very hydrophobic. However, the negative charge that the OAHFA assume at a physiological pH places them strictly in the category of amphiphilic anionogenic compounds which could fulfill a role of the separating and stabilizing interfacial layer between very hydrophobic WE and CE of meibum and the aqueous layer of the tear film.
Figure 12.
Proposed orientation of an OAHFA at the air/water interface. Hydrophilic oxygen atoms are forming hydrogen and electrostatic bonds with water molecules and hydroniums, respectively. Electronegative oxygen atoms are labeled with their negative partial atomic charges (computed in a MM2/MMP2 molecular modeling experiment in MMX parametrization)
5.2.3. Cholesteryl esters
Except for two publications ([10], [53]), steryl esters in general, and CE in particular, have been consistently found in all human meibomian samples tested [7, 21, 26, 44, 86]. Recently, CE were analyzed using HPLC-APCI MSn [21, 26] and ESI SG-MS [58]. The nature of the sterol component of CE was investigated using multistage fragmentation of precursor ions in MSn-capable ion trap experiments [[27]]. The fragmentation pattern of the precursor ion m/z 369 (M – H2O + H+) detected in MGS samples was identical to that of authentic Chl and synthetic CE. A wide range of CE was detected. The vast majority of CE was of very long chain nature, with C18:1-CE being only a minor component of MGS. This is unlike other human and animal tissues where much shorter CE with C16 to C22 FA is a norm with C16 and C18 FA being the most common acyl components of CE [http://lipidlibrary.aocs.org/Lipids/cholest/index.htm]. The lengths of the human meibomian CE FA residues varied from C18 to, at least, C32. Most of the FA (up to 80 mol %) were of saturated nature, while the rest were mostly monounsaturated. The most abundant CE species were present in the following order:
C26:0>C25:0>C24:0>C27:0>C24:1≈C18:1≈C20:0> rest of CE [26].
Later, Chen et al. [58] re-confirmed these observations in mostly qualitative ESI SG-MS experiments. Interestingly, most of the unsaturated CE had FA residues with even number of carbons (i.e. C18:1, C20:1, C22:1 etc.) while saturated CE were of both (even and odd) types. It remains to be seen whether these CE are branched or straight-chain. Contrary to the previous reports, the inter-donor variability of CE studied by HPLC-MSn has been found to be relatively low [26]. This discrepancy, as in many other cases, is most likely caused by the deficiencies of older experimental techniques that required a lot of manipulations with the samples, and were not best suited for the analyses of intact lipid species. The overall amount of CE in dry meibum was estimated in quantitative HPLC-MS experiments and was found to be between 30 and 33% (w/w) [21, 26]. It appears that the observation of some meibum donors not having CE in their MGS [53] was made in mistake, and CE are indeed the prominent and obligatory components of human TF.
5.2.4. Minor lipids
It seems that the lipid classes discussed above represent, at least, 60% of the entire meibomian lipidome, and, therefore, should be considered as major lipid classes. The rest ≤40% of meibum are formed from lipids that can be considered as minor lipid classes, and which include FFA, free FAl, FAm, TAG, DAG, MAG, Cer, PL, Sql, HC, and, possibly, some other species. Note that OAHFA and their esters have not been quantified so far because of unavailability of their authentic standards.
5.2.4.1. Acyl glycerols
Meibomian TAG were reported on multiple occasions [7, 44, 55, 85, 86, 99, 125]. Nicolaides et al estimated TAG to comprise ~4% of human meibum [7], while Tiffany suggested a much different value of ~40% [45]. The latter number would place TAG in the category of major meibomian lipid classes. However, the data of Nicolaides [7], Chen et al. [58], and our own observations [27, 97] showed that this is not the case. Interestingly, Chen et al estimated the overall presence of TAG to be 0.05% of total meibomian lipids. Further quantitative studies of meibomian TAG are warranted to resolve such a huge discrepancy in the quantitation of TAG in meibum.
Structural elucidation of meibomian TAG [7] demonstrated that the most abundant FA residues of TAG were straight-chain C18:1 (oleic), C16:1 (palmitoleic), and C16:0 (palmitic) ones. Interestingly, only trace amounts of VLCFA were observed in the TAG fraction. In 1996, Shine and McCulley evaluated the FA content of TAG fraction of MGS of normal (healthy) volunteers and dry eye patients with different forms of the disease using GC-MS [55]. Again, straight chain FA dominated all the TAG samples (70 to 79% of the entire FA pool derived from TAG), while iso- and anteiso-FA were present in the amounts of 10–16% and 7–10%, correspondingly. The major FA found in all the TAG samples was OA. On average, in normal donors ~44% of all FA was OA, ~4% - stearic acid, and ~2% - iso-stearic acid. Each of the rest of the reported individual FA was less than 1% of the FA pool. Interestingly, palmitic and palmitoleic acids were not observed, evaluated, and/or reported as individual FA. The authors neither stated what the molar (or mass) ratio of TAG to other lipids in meibum was, nor did they report the full molecular structures of meibomian TAG species – undoubtedly, a limitation of GC-MS discussed above. Later on, Sullivan et al. [83, 84, 86] reported significant changes in the FA profiles of neutral meibomian lipids, including TAG, in response to antiandrogen treatment or as a result of androgen deficiency. The authors used HPLC-MS to monitor the lipid profiles. However, these papers lack detailed information on the implemented analytical procedures and standards used, and provided no description of the detected lipid species, which makes them hard to evaluate from the chemical standpoint. Similarly, in a clinical paper by Shine et al [125], the authors did not quantify the overall presence of TAG in human MGS, describing only relative changes in the lipid profiles of the dry eye patients that underwent minocycline therapy. However, statistically significant decreases in the presence of DAG and FFA were reported, which allowed the authors to conclude that minocycline inhibited lipolytic activity of enzymes present on the ocular surface, possibly lipases excreted by the ocular microflora. Later on, the same authors estimated the TAG content of meibum to be ~6% with oleic acid being their major FA (up to 48%) [77].
From the structural and quantitative standpoints, meibomian DAG remain extremely poorly understood, and even their existence in normal meibum has not been reliably proven yet. Shine et al [125] reported them in meibum of meibomianitis patients, and correlated their presence with the activity of pathogenic microflora. However, no comprehensive characterization of these lipids was performed and no structural information on DAG was presented in that paper. Similarly, Sullivan et al. [86] classified some of the detected lipids as DAG, though no chemical analysis of the analytes was performed. The reported signals with equal probability could have been attributed to WE or other lipid species, including much more complex lipid species that spontaneously fragmented in the ion source of the mass spectrometer.
To the best of the author’s knowledge, MAG have not been reliably detected and/or characterized in any of the published reports on human meibum. Nicolaides [30] and Tiffany [45] did list MAG in their publications. It is possible that some of the more polar, but yet unidentified components of meibum, especially those that have been detected in TLC experiments, could have belonged to the class of MAG. However, it remains to be seen whether they were compounds of biological origin, or were produced in situ as a result of inadvertent hydrolysis of more complex lipids.
5.2.4.2. Free fatty acids, alcohols, and amides
Meibomian FFA were discussed on numerous occasions as compounds that could possibly stabilize TFLL [7, 49, 58, 77, 94, 125, 126]. Nicolaides et al. [7]] estimated their content to be ~2% of whole meibum sample weight. A year later, McCulley et al [127] correlated the increased production of FFA in tears with the onset of meibomitis (or inflammation of eyelids) and keratoconjunctivitis sicca (or dry eye). Dougherty et al [126] considered FFA to be “toxic hydrolysis products” generated by microbial lipases from normal lipids that populate TFLL. The detrimental effects of the latter were proposed to be neutralized by antibiotics of the tetracycline group, either through inhibition of the microbial growth, or through inhibition of the production of lipases, or both. The FFA content of meibum in the study of Shine and McCulley [77] was in the range of 0.5 to 1%, a third of which was identified as oleic acid.
A surprising development was a report by Nichols et al. who conducted a ESI SG-MS study of human MGS and found very large amounts of FFA and FAm in every analyzed sample [94]. The authors proceeded further to claim that FAm were the major components in human MGS. A range of FFA and FAm was observed. These included compounds with FA chains ranging from C16 to about C22, some of which were of saturated nature, while the others were monounsaturated compounds (such as oleamide and erucamide). Noteworthy, the fragmentation MS experiments described by the authors provided a solid support to the idea that the detected compounds were indeed FFA and FAm. However, the MS spectra of meibomian samples produced by Nichols et al. did not have any traces of typical meibomian lipids that had been described on numerous occasions as major lipid species in humans and animals. For example, no signals for Chl, CE, WE, di- and tri-esters could be found in those spectra. Thus, it seemed that the authors either used inadequate experimental procedures, or misinterpreted their data. To verify these possibilities, we conducted a systematic study trying to detect and, if successful, quantify these FAm in normal human meibum [27, 61, 97]. Oleamide was indeed detected, but only in a handful of samples, and only in extremely low quantities, which were well below 0.05% (w/w). However, when an aliquot of a CHCl3:MeOH solution that was kept in an Eppendorf tube for 5 min was analyzed by HPLC-MS, a number of exogenous contaminations, including FFA and FAm, were detected (Figure 13). Thus, it appeared that oleamide and other FAm reported by Nichols et al were exogenous contaminants leached from plastic ware [95, 128] used in the experiments of Nichols et al [94].
Figure 13.
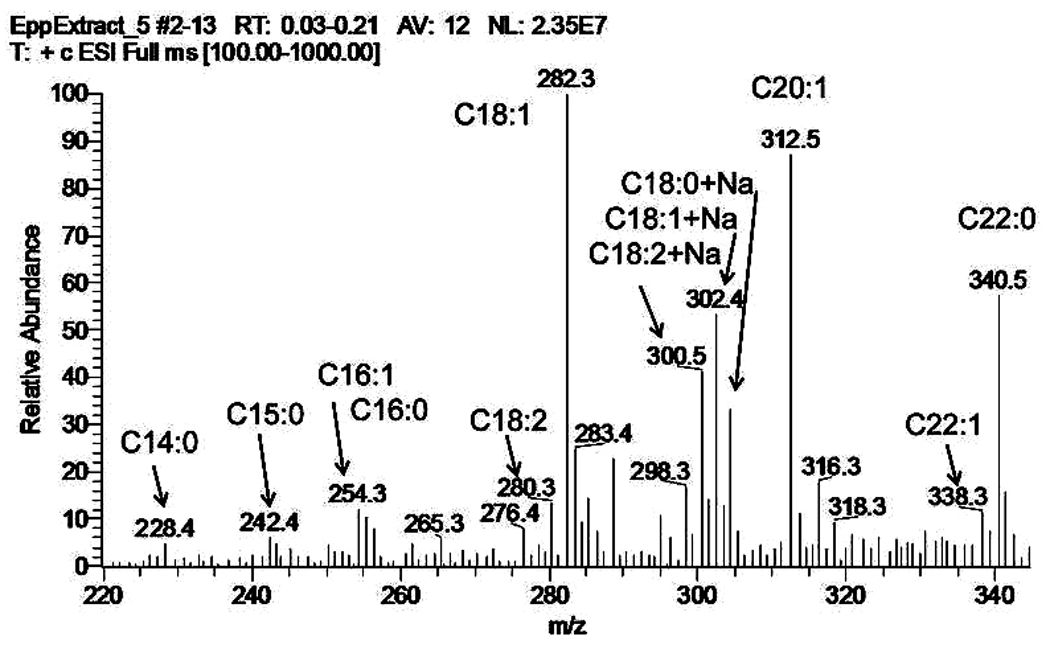
A positive ion mode ESI mass spectrum of a mixture of common plasticizers and plastic stabilizers extracted from an Eppendorf tube upon its exposure to a chloroform:methanol (1/1, v/v) solvent mixture. The signals of fatty acid amides are labeled according to the length of their carbon chains. The ions of both types, (M + H)+ and (M + Na)+, were detected. A very similar pattern was reported by Nichols et al [94], and was erroneously attributed to the lipids present in meibomian gland secretions.
Later, the same group of authors [58] published yet another paper on the lipid analysis of human meibum by ESI SG-MS, in which no references to oleamide and FAm were made. However, the authors again demonstrated very high intensity MS signals of FA in their samples (Figure 13, this paper, and Figures 2, 3, and 7 in reference [58]). These were classified as FFA of C16 to C32 nature, and estimated to account for 3% of meibum. Although seemingly a plausible possibility (after all, all these FA had indeed been found to be components of complex meibomian lipids), the observation by Chen et al. appears to be erroneous. The “shotgun” approach used by Chen et al, though fast and convenient, in this case, led the researchers to believe that the signals of FA originated exclusively from the FFA, while, in fact, they probably originated from more complex lipid species (such as OAHFA) that underwent spontaneous in-source fragmentation, and, thus, were experimental artifacts. To illustrate this point, let’s compare the “shotgun” MS data of Chen et al. [58], and our results obtained using HPLC-MS (Figure 13). It is clear that when HPLC is utilized prior the MS step, FFA are physically separated from more complex lipids, and cannot be any more confused with the in-source generated FA fragments of more complex lipids. Thus, their relative content can be easily evaluated, which, in this case, becomes much less impressive 0.1% (or less) compared with the claimed 3% in the experiments of Chen et al [58]. We cannot rule out a possibility that this situation can repeat itself in the shotgun experiments with other lipids.
The author is not aware of any credible evidence of the existence of a pool of free FAl in meibum, though these precursors and/or metabolites of WE and di- and tri-esters should be present in meibum, at least as minor components.
5.2.4.3. Phospholipids
Arguably, PL have been considered to be the most important class of polar lipids of MGS, whose role has been proposed to be the formation of the amphiphilic sublayer that separates the aqueous layer of the TF and the bulk of hydrophobic lipids that form the TFLL [72]. In this role, PL must be present in certain proportion to other, less polar, meibomian lipids, which should be easily achievable if PL were excreted from meibomian glands alongside with other lipids. The PL content of MGS was reported to be between 0,0015% (w/w) [129] and 4 to 15% [72] [7]. However, it is unclear how these numbers were obtained, or even how these lipid species were identified. In a series of papers, meibomian PL were analyzed using HPLC interfaced to a UV spectrophotometric detector [63, 71]. The UV detector was set to monitor PL at 220 nm, which was a disappointing choice of the analytical wavelength as normal (i.e. unoxidized) PL practically do not absorb light in this part of the UV spectrum: the minimally acceptable setting would have been 205 nm [93], and even then the poor UV absorptivity of PL (Figure 14) and the lack of any selectivity of the UV detector with PL analytes would be a serious problem when trying to detect, identify, and quantitate small amounts of PL in a lipid mixture as complex as meibum. These limitations had been extensively discussed in our previous publications ([43] and references cited therein), and the same concern was later mirrored in a paper by Chen et al [58]. It seems that the results on the meibomian PL and SM obtained by the HPLC-UV approach cannot be considered credible anymore.
Figure 14.
Comparison of HPLC-UV and HPLC-MS approaches to phospholipid analysis.
Panel A. An NP HPLC-UV method adopted from [63] was used to analyze a mixture of PE, PC, and SM. An aliquot of the mixture (800 ng of each lipid) was injected and the elution profile of the lipids was monitored at 220 nm. Note that there was no HPLC peaks of the phospholipids observed after the initial injection peak. A conclusion can be made that because of its insensitivity, the HPLC-UV approach is not suitable for analyzing complex lipid mixtures with small amounts of phospholipids, such as human meibum.
Panel B. The same mixture of three lipids was analyzed by NP HPLC-ESI MS in positive ion mode. Note that only a 40 ng aliquot of each lipid was injected. Three lipids were clearly visible with a negligible background noise.
Panel C. The same mixture of three lipids was analyzed by NP HPLC-ESI MS in positive ion mode. Note that this time only a 2 ng aliquot of each lipid was injected. Three lipids were still detectable albeit with a clearly declining signal-to-noise ratios.
Ham et al [130] attempted to detect PL and SM in human TF, with mixed results: there were only two lipids of this kind detected – dimyristoyl-PC (DMPC) and a compound tentatively identified as “sphingomyelin pyrophosphate” (SM-PP). These unexpected findings raise two inevitable questions: 1) was SM-PP correctly identified, as there had been no such compound found before in any tissue from any living being; and 2) is it even possible that meibum, known for its extremely complex lipid composition, had only one type of PC in its lipidome, namely a fairly uncommon DMPC? It seems that the only answer to both questions is “No”. It would have been much more realistic to expect meibum to have either very long chain PC (based on the FA found in other meibomian lipids), or to have a much more common C16-FA and C18-FA-based PC typically found in other human tissues. As no such compounds were reported by Ham et al., we should assume that either PL and SM are not normal components of human meibum, or their presence was below the low limit of detection (LLD) in those experiments. As no estimates of LLD and no proof of the structure of the putative DMPC and SM-PP were provided by Ham et al., their observations should not be used as a solid evidence of the existence of PL and SM pools in normal human meibum.
Noteworthy, our own results [27, 61] and a recent (largely confirmatory) paper by Chen et al. [58] also demonstrated that PL and SM could be found in normal human meibum only in miniscule amounts, if at all. In an attempt to detect and quantify PL and SM in meibum, we employed two different approaches, namely direct infusion (or “shotgun”) MS, and HPLC-MS [27]. A deuterated PC was used as an internal standard in the direct infusion experiments, which allowed us to estimate the combined presence of naturally occurred PC and SM in meibum to be less than 0.015% (w/w) [27], which was deemed too small to justify any further inquiries in this matter. An equally small amount of PC was observed in HPLC-MS experiments with external standards (a range of normal PC and SM species) [27]. Chen et al. [58] were not able to detect any PL and SM species in their samples at all, while Saville et al. estimated PC presence in meibum to be even smaller 0.0015% (w/w) [129]. It is also obvious that those PL were remnants of the cell membranes of matured and burst MG cells, that found their way into the secretion along with other components of the cells – denatured proteins, degraded DNA, etc. It is also obvious that such small amounts of PC may have only negligible effects on the TF and TFLL, as they are totally overwhelmed by other lipid molecules with the estimated ratio of PC to other lipid molecules to be about 1:70,000.
As discussed above, in earlier reports meibomian PL and SM were observed mostly in TLC experiments and were quite often confused with other “polar lipids”. Because of the limitations of those experiments, and the largely unsuccessful search for the evidence of meaningful quantities of PL and SM in human meibum using modern analytical techniques, it seems that both PL and SM have no structural role in the TF, or there is a source of PL and SM for the TFLL other than meibomian gland itself.
5.2.4.4. Other minor lipids
In nature, the observations of HC (i.e. paraffins) in living organisms are typically associated with plants and microorganisms. In the latter case HC often serve as nutrients. HC were also found in animal fur and human hair [131, 132]. Sporadically, HC were mentioned in the relation to human meibum [11] [42, 45]. However, it is more likely than not that HC that were found in the samples of human meibum were of exogenous origin and either contaminated the samples “post collection” [7], or were components of cosmetic and skin care products used by the meibum donors. It is also possible that some contamination could originate from plastic ware and organic solvents and chemical reagents used in the lipid analyses. Anyway, the only publication related to the topic of this review, in which HC were described with any degree of certainty, is the paper by Nicolaides et al [7], and still the authors concluded that detected HC were most likely a contamination from exogenous sources. Therefore, at present there is no any solid evidence of any HC present in human meibum except for squalene [30]; [45]; [97], and, possibly, carotenoids [23], though the latter report has yet to be confirmed by standard analytical techniques, such as MS. Depending on the part of the paper, Oshima et al. quoted “carotenoid-like compounds” to be present in meibum in either 90 µg/g, or 88 mg/g quantities. The latter number is, most likely, an error, as this amount of carotenes would turn meibum deep red or orange as their UV-Vis absorption maxima are between 400 and 500 nm. The first number, however, seems to be a more realistic estimate, considering that these compounds are effective as antioxidants if present in the microgram/milliliter quantities. However, carotenoids are not biosynthesized by humans, and need to be consumed with food (mainly, fruits and vegetables), which make this hypothetical accumulation of carotenes in meibum strictly diet-related. Thus, it remains to be seen if any paraffins other than Sql and, possibly, carotenoids are natural components of human meibum.
Cer are found in, basically, every human and animal tissue, where they play multiple roles, including structural and signaling ones. Skin cells in general, and those of eyelids in particular, are especially rich in simple and complex Cer [133]. These ubiquitous compounds fulfill multiple roles in the skin, one of the most important of which is the formation of the cutaneous permeability barrier. Cer are found in all layers of the skin, and, thus, can be considered a universal marker of the presence of skin cells in samples of biological origin. Thus, it seemed logical to expect Cer to be present in normal human meibum, and, indeed, Cer were reported on one occasions, along with PC, PE, SM, and Crb [66]. However, considering the inadequacy of the analytical method utilized (HPLC-UV) discussed above, this sole reported direct observation of Cer in human meibum should be verified by independent studies.
6. Biophysics of meibomian lipid films
Compared to the chemical composition of meibum, biophysical properties of TFLL remain an understudied area. Generally, to play a protective role in the tear film, meibomian lipids, once excreted from MG, need to form an (ideally) continuous film that would cover the entire ocular surface. The TFLL should form quickly, and be stable between the eye blinks. TFLL should be able to retard evaporation of water from the ocular surface beneath it. Also, it should be able to withstand repetitive, and quite substantial, mechanical stress caused by moving eyelids, either by maintaining its structure, or by restoring it quickly after the eyelids have moved. These properties of TFLL can potentially be modeled in vitro by recreating meibomian lipid films (MLF) from meibomian lipids by spreading them across an air-water interface. This line of thinking has its limitation, though, as meibomian glands might not be the only source of lipids to the TFLL [97].
So far, MLF have been studied using several experimental techniques, including the Langmuir trough (LT) [134–142], the pendant drop (PD) [139], and the sessile bubble [143] ones. The potential of the captive bubble technique [144], though very promising for TF studies, is yet to be fully exploited. Let’s consider the former three techniques in more detail.
In a recent paper, Svitova and Lin [143] studied interfacial rheology of tear film lipids extracted from worn lotrafilcon A contact lenses, using the sessile bubble technique. The sessile bubble was formed in a standard fashion. First, an aqueous buffer was placed into a glass cell. Then, a hydrophobicized glass capillary was immersed vertically into the aqueous buffer, and an air bubble at the immersed lower end of the capillary was formed by pumping a small amount of air through the capillary into the buffer. When the air bubble was formed, a calculated amount of lipid material dissolved in a toluene:isopropanol (5:1, v/v) solvent mixture was deposited directly onto the water/air interface from underneath the bubble with a microsyringe. Then, the mixture was stirred to distribute the lipids across the surface of the bubble and dissolve the solvent, the buffer that surrounded the bubble was replaced with a fresh aqueous solution (a rinsing step), and the deposited lipids were allowed to rest for 17 to 24 hrs without stirring before conducting any experiments. All the measurements were conducted in unthermostated apparatus at ambient temperature of 22±1°C. Svitova and Lin reported that, in quazi-equilibrium conditions achieved 16 to 24 hours into the experiment, the lipid films formed from the lipids extracted from worn contact lenses exerted a very high surface pressure π of ~50 mN/m and a low surface tension of ~22 mN/m. These numbers were close to those of PL and lung surfactants [150, 152], which implied the possible role of PL in the architecture of the TFLL and TF in general, as it was described by McCulley and Shine [72]. The equilibrium surface tension of the films was inversely proportional to their thickness, changing from 32 mN/m to 22 mN/m when the thickness changed from 2 nm to 27 nm. A typical TF protein lysozyme was shown to irreversibly translocate to, and bind with, the lipid films, without changing their equilibrium surface tension. Another property of MLF investigated in the study was their interfacial viscoelasticity. Again, in equilibrium the rheological storage modulus (or elasticity) of pure meibomian films was calculated to be ~25 mN/m. Adsorption of lysozyme did not change this number, demonstrating that the protein did not disturb (or penetrate into) the preformed lipid films.
However, this interesting study raises a few concerns that need to be answered before accepting the study’s major approaches and conclusions.
First, the lipids extracted from contact lenses worn for one month might not represent the actual TFLL composition, and, for that matter, its biophysical properties, as the lipids that had been accumulating on (or in) the lenses for a month might have undergone chemical alterations caused by their hydrolysis and/or oxidation. At the same time, the lenses could have accumulated material foreign to the TF lipids that were produced by the eyelid epithelium, the cornea, and/or the conjunctiva by rubbing the tissues against the contact lens. Alternatively, the samples could have been affected by the artificial enrichment of the accumulated lipid material with a particular lipid or a lipid class that had higher affinity to the lens than some other lipids, skewing the results of their rheological evaluation. Neither of these issues were explored in the paper, as no comparison of respective chemical compositions of meibum, tear film, and the extracted lipids were made.
Second, the necessity to “age” the deposited lipids for 16 to 24 hours in an aqueous buffer before measuring their surface properties could have a detrimental impact on their chemical composition. Neither of the two major mechanisms of lipid degradation – hydrolysis of complex lipids and oxidation of unsaturated compounds – was taken into account in the study. While the lipid hydrolysis would have been difficult to counteract, the lipid oxidation (either by molecular oxygen dissolved in the subphase, or by a photooxidation mechanism) could have been prevented by using an inert atmosphere and darkness (or, at least, dimmed light) throughout the experiments.
Third, considering minute amounts of lipids used to make the films (1 to 3 µg per 15 mm2 of the bubble surface to make a film ~10 nm thick) some of the more polar lipids could have been spontaneously dissolved in the aqueous subphase (~20 mL total) and/or washed away during the rinsing step. Thus, the overall hydrophilic-to-lipophilic balance of the meibomian lipid samples could have been altered, which would be inevitably reflected in their rheological behavior.
Fourth, the study was conducted at a room temperature of ~22 °C, which with all likelihood could not be very representative of the temperature, at which TFLL is formed and functions in vivo (a normal corneal temperature is about 32°C). As will be discussed below, changes in temperature cause dramatic alterations in the rheological properties of MLF [135].
The pendant drop technique is another related method of creating an air-aqueous interface suitable for meibomian film studies. This approach has been tested with bovine meibomian lipids by Miano et al [145] [139]. As no information on the experiments with human samples is available at this time, we will discuss the major observations made by Miano et al. keeping in mind that their results most likely cannot be automatically extrapolated onto the human meibum.
Initially, to demonstrate the validity of the pendant drop approach, the researchers compared the results obtained with this technique with a more traditional Langmuir trough approach. Importantly, the experiments with bovine meibum were conducted at 36°C, which should have helped with lipid melting and spreading. The surface area-surface pressure (π/A) plots obtained by both techniques were similar, though not identical. Both the approaches demonstrated the hysteresis of MLF, i.e. the difference between the compression and decompression parts of the dynamic π/A isotherms, which implied noticeable structural rearrangements within MLF. The maximum surface pressure πm, calculated for the surface density of lipid molecules approaching 15Å2/molecule, was found to be ~18 mN/m in the Langmuir trough studies, and ~24 mN/m in the pendant drop ones. Interestingly, Miano et al estimated an average molecular mass of a meibomian lipid to be 720, which is very close to 700 calculated for our experiments [135]. However, Miano et al. assumed that the average meibomian lipid is a C12 to C18-FA containing molecule with a polar group, similar to a phospholipid [139]. As we already discussed earlier, this is not the case, at least not for human meibum.
Then, a range of proteins typically found in biological fluids was tested to determine whether they can interact with the lipid layers. It was shown that some proteins (such as albumin) were not capable of intercalating the lipid layers, while the others (lactoferrin from human milk, lysozyme from chicken egg, sIgA from human colostrum) were. An even higher affinity to the lipid films was observed for human tear lipocalin and β-lactoglobulin. A higher surface pressure of 25 to 30 mN/m effectively excluded almost all tested proteins from the lipid film at the air/aqueous interface, with a notable exception of lipocalin, which had a very high affinity to lipids [146]. If one assumes that a rather random selection of the sources of these proteins had no impact on their ability to bind with bovine meibum, then one can expect that at least some of the proteins present in human tears will bind to (or penetrate into) the human TFLL, too.
An interesting experimental approach was tested by Petrov et al [147]. Bovine MLF were studied, in part, by the grazing incidence X-ray diffractometry (GIXD). The diffraction patterns were indicative of a rectangular lattice with the characteristic spacing of 3.57 × 4.16 Å. A similar pattern was observed for a synthetic lipid mixture composed of 70.2% (w/w) ethyl oleate, 21.1% cholesteryl stearate, 4.5% PC, 1.9% PE, 0.9% SM, 0.7% PS, 0.5% PI, and 0.2% of lyso-PC. Most of the PLs were of C16:0-FA variety. However, the fluorescence microscopy comparison of bovine MLF and the artificial lipid mixture revealed astonishingly different interfacial topographies of the two types of films. Despite the authors’ claim that the artificial lipid films were “reminiscent” of the bovine meibomian films (Fig. 4 in [147]), it is difficult to agree with the authors, especially considering that the microphotographs of both films were taken at different surface pressures. The (π/A) isotherms recorded for bovine samples and the artificial lipid mixtures were also dramatically different, which further highlights the differences between the samples, and implies that there are substantial differences in their structures and responses to lateral compression. These striking differences were most likely caused by the differences in the lipid compositions of the artificial lipid mixtures and meibum: the bovine meibum certainly does not have any ethyl oleate in it, and cholesteryl stearate most likely is not an average CE found in bovine meibum [7, 34], which forced the studied lipid mixtures to aggregate in totally different ways. Another questionable decision was the use of almost 9% (w/w) of various PLs in the mixture: in recent studies, PLs were observed in human meibum only in miniscule quantities, if at all. If bovine meibum resembled the human one, the amount of added PL in the tested artificial mixture should have been at least two orders of magnitude less than 0.9% used in the study of Petrov et al. However, these unfortunate choices of lipids made by the authors for artificial lipid mixtures studies, and their heavy reliance on the dubious data about the presence of large amounts of PL in meibum and on the dominance of the short-to-medium chain (<C19) FA in its lipids, were based on the information published in the literature at the time, and in no way they diminish the potential of the GIXD approach itself. It seems that the experiments should be expanded into the area of human meibomian lipid studies, and be strengthened by a better choice of model lipid mixtures.
The Langmuir trough (LT) technique has been the most frequently used technique in the meibomian film studies. The advantages of the LT technique are multifold. First, during the decades since its invention by Irving Langmuir in 1917, there has been a large amount of data accumulated that can be used in meibomian film studies. Second, Langmuir troughs are relatively simple and highly customizable experimental devices that can be constructed in various configurations, so choosing a proper Langmuir trough should not be a difficult choice. However, they require large amounts of samples (from 0.04 to 1 µg of meibum per each cm2 of the trough area, or between 3 and 80 µg of meibum per experiment for a typical mini-trough [135]). They also require large amounts of aqueous subphase (typically, a few dozen milliliters), which in case of complex subphases with expansive solutes (such as purified proteins and such) could become very costly. Thus, this approach is better suited for experiments that do not require expensive analytes, or when a large amount of a sample is available.
Kaercher et al. were, probably, the first who conducted a systematic study of the morphology and biophysical behavior of human meibum using a Langmuir trough in combination with surface potential measurements [148, 149] and Brewster angle microscopy ([148, 157] and references cited therein). Spectacular images of meibomian films, obtained using Brewster angle microscopy without staining samples with any sort of dyes, clearly demonstrated an extremely complex and ever-changing topography of the films. The observed topographical features of the films formed in a Langmuir trough depended on the surface pressure and temperature of the films. Several types of meibomian lipid phases were observed and described: a network-like phase composed of mobile lipid chains that surrounds dark areas of (quazi)liquid lipid room temperatures of ~18°C and low surface pressures (<0.5 mN/m); a dense, but still mobile, (quazi)homogeneous lipid layers at the same temperature and a slightly higher surface pressure π of 5mN/m; an immobile, dense, white lipid film at π>12mN/m; and many intermediate structures. At temperatures higher than 28°C, the films are fluid and neat meibum spreads quickly with a speed of at least 1 cm/sec, which is fast enough to have a physiological significance in the TFLL restoration after each blinking.
By measuring changes in the surface potential of the meibomian films during their compression-decompression in the Langmuir trough, Kaercher et al. detected a reproducible and fully reversible pattern: the potential jumped from ~50mV to ~300mV upon closing the Langmuir trough barriers, and returned back to ~50mV upon their opening. Such changes in the surface potential are indicative of the presence of amphiphilic molecules (dipoles) in meibum, which apparently change their surface aggregation and orientation from being dispersed and laying more or less flat on the aqueous subphase to becoming progressively condensed and assuming normal orientation to the interface. Kaercher et al. speculated that these amphiphiles could be molecules of PL (e.g. PC) and indeed demonstrated that PC caused similar changes in the surface potential. However, one could now argue that since PC could only be very minor components of meibum, their ascribed role is most likely played by OAHFA (see above).
It is important to notice that most of the experiments by Kaercher et al. were conducted at room temperatures that were well below the melting range of meibum. Thus, the properties of meibum at physiological temperatures might be somewhat different from those at physiological temperatures. Additionally, a potentially serious problem is the sample handling procedure described in the pioneering paper [149], which involved storing meibomian lipids on silicone strips for an unspecified period of time, and then dissolving the lipids in chloroform for spreading them on the surface of the aqueous subphase of the Langmuir trough. It is unclear from the paper whether the samples were protected from oxygen and humidity, any of which could quickly alter the chemical composition of samples. An even more serious problem comes from even a brief contact of chloroform with silicone: the former is notoriously known for its ability to quickly dissolve polymers, especially silicone. Thus, one cannot rule out a possibility that these extractives might have impacted the results of the Langmuir trough experiments.
In a recent paper, Leiske et al. [142] compared human and animal meibum. First, they evaluated the (π/A) isotherms of human, oxen, rabbit, and wallaby meibum samples using a Langmuir trough. Most of the animal samples resembled human meibum. Interestingly, the maximum surface pressure of meibomian films recorded in those experiments did not exceed 25 mN/m, with that of MLF being below 20 mN/m. The interfacial complex viscosity of these films was measured using an interfacial stress rheometer, and was shown to be dependent on the surface pressure. At low surface pressures, the complex viscosity was in the 10−3 to 10−2 mN × sec/m range, while upon compressing the film it exceeded 10−1 mN × sec/m. The ability of human MLF to resist extensional deformation (as measured in interfacial extensional rheology experiments) at low surface pressures was found to be minimal but increased upon compression of the film. The Brewster angle microscopy revealed a high degree of inhomogeneity of the films under all tested conditions. The authors concluded that the human meibomian films showed a gel-like behavior, which could be an important factor in the tear film stabilization in vivo.
Another quantitative approach to the evaluation of meibomian films in Langmuir trough experiments was tested in our recent publication [135]. Numeric differentiation of (π/A) isotherms allowed the researchers to determine the in-plane elasticity modulus Cs−1 (or rigidity) of the films in different conditions. The values of Cs1 were found to be dependent on the surface concentration of meibomian lipids, determined as lipid weight (in µg)/available trough area (in cm2), and temperature. The in-plane elasticity of the condensed meibomian film was in the range of 15 to 25 mN/m (close to the values determined by Leiske et al. ([142] and references cited therein) and by other groups, and increased with the decrease in the temperature, clearly because of the lipid solidification. The elasticity of meibum at physiological temperature was very close to the elasticity of a model compound – cholesteryl oleate – but very different from that of saturated and unsaturated TAG [135], Cer, Chl and their mixtures with meibum. A noticeable compression-decompression hysteresis ΔΔG, approaching 0.8 J/g meibum, was observed under all experimental conditions. This value was an order of magnitude lower than the value of hysteresis observed for tripalmitin (~6 J/g) [135] and placenta plasma membranes [150], but still indicated that the film underwent substantial structural re-arrangements upon its compression and decompression. Notable cooperativity effects were observed in melting meibum, which were described in terms of Hill cooperativity equation [http://en.wikipedia.org/wiki/Hill_equation]. For the bulk meibum melting, its T0.5 and Hill coefficient nH were calculated to be about 32.1°C and 19.3, respectively, while in thin, quazi-two-dimensional films formed on the surface of an aqueous subphase, T0.5 and nH were 19.7°C and 3. These experiments clearly demonstrated the effects of the presence of the aqueous subphase on the melting behavior of meibomian lipids, and emphasized the often overlooked differences between melting of meibum in thin films and in bulk.
7. Concluding remarks
It seems that despite impressive advances in the area of biochemical and biophysical characterization of MLF, we are just in the beginning of a long road that would eventually lead to a comprehensive understanding of how the tear film is organized and functions. The methods and results described above have provided valuable information on the lipid composition of human meibum, and on the topography and biophysical properties of the models of TFLL in vitro. Undoubtedly, one can expect many more interesting and innovative approaches and concepts tested in the nearest future, which will re-iterate our understanding of the tear film structure and physiology. However, new, more sensitive, non-invasive and safe approaches are needed to evaluate tear film in vivo (i.e. on the human ocular surface), determine its composition, structure, and dynamics in real time under controlled laboratory conditions, and in response to various environmental factors. Models of the tear film in vitro is a good starting point, but are limited by our abilities to mimic the exact chemical composition of the tear film and the cellular structure of the underlying ocular surface. Still, the basic information about the lipid-lipid and lipid-protein interactions can be obtained in these studies and be (cautiously) extrapolated onto the in vivo conditions. The tear film studies provide a unique opportunity to employ the latest chemical, biochemical, and biophysical experimental and theoretical approaches to a very challenging subject – human tear film, which exemplifies the need for a multidisciplinary approach to be successfully understood.
Acknowledgements
This work was supported in part by an NIH grant R0EY0119480 (to I.B.). The author wishes to express his sincere gratitude to Drs. T. Arita, A. McMahon, and J. C. Arciniega for the photographs of human and mouse meibomian glands, and the photographs of fluorescein-stained human tear film.
Abbreviations
- APCI
atmospheric pressure chemical ionization
- AT
aqueous tears
- CB
chronic blepharitis (or eyelid inflammation)
- CE
cholesteryl esters
- Cer
ceramides
- Chl
cholesterol
- Crb
cerebroside
- Cs−1
in-plane elasticity modulus
- DAG
diacyl glycerols
- DE
dry eye
- DES
dry eye syndrome
- ECL
equivalent chain length
- EI
electron impact
- ELC-FA
extremely long chain fatty acids (C28 or longer)
- ELS
evaporative light scattering
- ESI
electrospray ionization
- FA
fatty acids
- FAl
fatty alcohol
- FAm
fatty acid amides
- FFA
free (non-esterified) fatty acids
- FI
flame ionization
- FID
flame ionization detector
- FMW
full molecular weight
- FS
fluorescence spectroscopy
- GC
gas chromatography
- GLC
gas-liquid chromatography
- HC
hydrocarbons
- HPLC
high performance liquid chromatography
- IR
infrared spectroscopy
- IT
ion trap
- KCS
keratoconjunctivitis sicca
- LT
Langmuir trough
- MAG
monoacyl glycerols
- MALDI
matrix assisted laser desorption
- MG
meibomian glands
- MGS
meibomian gland secretions
- MS
mass spectrometry
- MW
molecular weight
- NMR
nuclear magnetic resonance spectroscopy
- NP-HPLC
normal phase HPLC
- OAHFA
(O-acyl)-omega-hydroxy fatty acids
- OCC
open column chromatography
- PC
phosphatidyl choline
- PE
phosphatidylethanolamine
- PL
phospholipids
- PS
phosphatidylserine
- Q-TOF MS
time-of-flight tandem mass spectroscopy
- RP HPLC
reverse phase HPLC
- RS
Raman spectroscopy
- RT
retention time
- SG
shotgun
- SM
sphingomyelin
- Sql
squalene
- ST
sterols
- TAG
triacyl glycerols
- TBUT
tear film breakup time
- TF
tear film
- TFLL
tear film lipid layer
- TLC
thin layer chromatography
- UV
ultraviolet
- VLC-FA
very long chain fatty acids (between C22 and C28)
- WE
wax esters
References
- 1.Kaercher T, Bron AJ. Classification and diagnosis of dry eye. Dev Ophthalmol. 2008;41:36–53. doi: 10.1159/000131069. [DOI] [PubMed] [Google Scholar]
- 2.Tiffany JM. The normal tear film. Dev Ophthalmol. 2008;41:1–20. doi: 10.1159/000131066. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. doi: 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 6.Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- 8.Meibom H. De vasis palpebrarum novis epistolae. Helmstadt: H. Muller; 1666. [Google Scholar]
- 9.Pes O. Ricerche microchimiche sulla secrezione delle ghiandole sebacee palpebrali. Arch Ottalmol. 1897;5:82–91. [Google Scholar]
- 10.Linton RG, Curnow DH, Riley WJ. The Meibomian Glands: An Investigation into the Secretion and Some Aspects of the Physiology. Br J Ophthalmol. 1961;45:718–723. doi: 10.1136/bjo.45.11.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers N. The Precorneal Film. Biomicroscopical, Histological and Chemical Investigations. Acta Ophthalmol Suppl. (SUPPL 81) 1965:1–134. [PubMed] [Google Scholar]
- 12.Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62. doi: 10.1016/b978-0-12-024922-0.50005-9. [DOI] [PubMed] [Google Scholar]
- 13.Butovich IA, Millar TJ. In Search for a Better Animal Model of Human Tear Film: Comparative Lipidomic Analysis of Human and Animal Meibum. Invest Ophthalmol Vis Sci. 2009;50:2545. E-Abstract. [Google Scholar]
- 14.Lawton AW. Structure and function of the eyelids and conjunctiva. In: Kaufman HE, Barron BA, McDonald MB, editors. The Cornea. 2-nd ed. Boston, Oxford, Johannesburg, Melbourne, New Delhi, Singapore: Butterworth-Heonemann; 1998. pp. 55–56. [Google Scholar]
- 15.McCann LC, Tomlinson A, Pearce EI, Diaper C. Tear and meibomian gland function in blepharitis and normals. Eye Contact Lens. 2009;35:203–208. doi: 10.1097/ICL.0b013e3181a9d79d. [DOI] [PubMed] [Google Scholar]
- 16.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21:S70–S74. doi: 10.1097/01.ico.0000263122.45898.09. [DOI] [PubMed] [Google Scholar]
- 18.Jester JV, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981;20:537–547. [PubMed] [Google Scholar]
- 19.Khanal S, Millar TJ. Nanoscale phase dynamics of the normal tear film. Nanomedicine. 2010;6:707–713. doi: 10.1016/j.nano.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Gorgas K, Volkl A. Peroxisomes in sebaceous glands. IV. Aggregates of tubular peroxisomes in the mouse Meibomian gland. Histochem J. 1984;16:1079–1098. doi: 10.1007/BF01002896. [DOI] [PubMed] [Google Scholar]
- 21.Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–513. doi: 10.1194/jlr.M800426-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borchman D, Foulks GN, Yappert MC, Kakar S, Podoll N, Rychwalski P, et al. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010;44:34–42. doi: 10.1159/000283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshima Y, Sato H, Zaghloul A, Foulks GN, Yappert MC, Borchman D. Characterization of human meibum lipid using raman spectroscopy. Curr Eye Res. 2009;34:824–835. doi: 10.3109/02713680903122029. [DOI] [PubMed] [Google Scholar]
- 24.Tsai JC, Lo YL, Lin CY, Sheu HM, Lin JC. Feasibility of rapid quantitation of stratum corneum lipid content by Fourier transform infrared spectrometry. Spectrosc-Int J. 2004;18:423–431. [Google Scholar]
- 25.Horacek J, Cernikova M. Examination of lipids in human sebum by disk chromatography. Biochem J. 1959;71:417–419. doi: 10.1042/bj0710417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010;75:726–733. doi: 10.1016/j.steroids.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776. doi: 10.1007/s11745-007-3080-2. [DOI] [PubMed] [Google Scholar]
- 28.Samejima K, Dairman W, Stone J, Udenfriend S. Condensation of ninhydrin with aldehydes and primary amines to yield highly fluorescent ternary products. II. Application to the detection and assay of peptides, amino acids, amines, and amino sugars. Anal Biochem. 1971;42:237–247. doi: 10.1016/0003-2697(71)90031-5. [DOI] [PubMed] [Google Scholar]
- 29.Samejima K, Dairman W, Udenfriend S. Condensation of ninhydrin with aldehydes and primary amines to yield highly fluorescent ternary products. I. Studies on the mechanism of the reaction and some characteristics of the condensation product. Anal Biochem. 1971;42:222–236. doi: 10.1016/0003-2697(71)90030-3. [DOI] [PubMed] [Google Scholar]
- 30.Nicolaides N. Skin Lipids. Ii. Lipid Class Composition of Samples from Various Species and Anatomical Sites. J Am Oil Chem Soc. 1965;42:691–702. doi: 10.1007/BF02540042. [DOI] [PubMed] [Google Scholar]
- 31.Fu HC, Nicolaides N. The structure of alkane diols of diesters in vernix caseosa lipids. Lipids. 1969;4:170–175. doi: 10.1007/BF02531942. [DOI] [PubMed] [Google Scholar]
- 32.Ansari MN, Fu HC, Nicolaides N. Fatty acids of the alkane diol diesters of vernix caseosa. Lipids. 1970;5:279–282. doi: 10.1007/BF02532480. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaides N, Santos EC. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 1985;20:454–467. doi: 10.1007/BF02534237. [DOI] [PubMed] [Google Scholar]
- 34.Nicolaides N, Santos EC, Smith RE, Jester JV. Meibomian gland dysfunction. III. Meibomian gland lipids. Invest Ophthalmol Vis Sci. 1989;30:946–951. [PubMed] [Google Scholar]
- 35.Nicolaides N, Flores A, Santos EC, Robin JB, Smith RE. The lipids of chalazia. Invest Ophthalmol Vis Sci. 1988;29:482–486. [PubMed] [Google Scholar]
- 36.Kolattukudy PE, Rogers LM, Nicolaides N. Biosynthesis of lipids by bovine meibomian glands. Lipids. 1985;20:468–474. doi: 10.1007/BF02534238. [DOI] [PubMed] [Google Scholar]
- 37.Nicolaides N, Santos EC, Papadakis K, Ruth EC, Muller L. The occurrence of long chain alpha, omega-diols in the lipids of steer and human meibomian glands. Lipids. 1984;19:990–993. doi: 10.1007/BF02534741. [DOI] [PubMed] [Google Scholar]
- 38.Nicolaides N, Santos EC, Papadakis K. Double-bond patterns of fatty acids and alcohols in steer and human meibomian gland lipids. Lipids. 1984;19:264–277. doi: 10.1007/BF02534454. [DOI] [PubMed] [Google Scholar]
- 39.Nicolaides N, Ruth EC. Unusual fatty acids in the lipids of steer and human meibomian gland excreta. Curr Eye Res. 1982;2:93–98. doi: 10.3109/02713688208997682. [DOI] [PubMed] [Google Scholar]
- 40.McFadden WH, Bradford DC, Eglinton G, Hajlbrahim SK, Nicolaides N. Application of combined liquid chromatography/mass spectrometry (LC/MS): analysis of petroporphyrins and meibomian gland waxes. J Chromatogr Sci. 1979;17:518–522. doi: 10.1093/chromsci/17.9.518. [DOI] [PubMed] [Google Scholar]
- 41.Keith CG. Seborrhoeic blepharo-kerato-conjunctivitis. Trans Ophthalmol Soc U K. 1967;87:85–103. [PubMed] [Google Scholar]
- 42.Andrews JS. The Meibomian secretion. Int Ophthalmol Clin. 1973;13:23–28. doi: 10.1097/00004397-197301310-00004. [DOI] [PubMed] [Google Scholar]
- 43.Butovich IA. The Meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009;28:483–498. doi: 10.1016/j.preteyeres.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973;89:25–27. doi: 10.1111/j.1365-2133.1973.tb01912.x. [DOI] [PubMed] [Google Scholar]
- 45.Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978;27:289–300. doi: 10.1016/0014-4835(78)90164-1. [DOI] [PubMed] [Google Scholar]
- 46.Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989;30:936–945. [PubMed] [Google Scholar]
- 47.Jester JV, Nicolaides N, Smith RE. Meibomian gland dysfunction. I. Keratin protein expression in normal human and rabbit meibomian glands. Invest Ophthalmol Vis Sci. 1989;30:927–935. [PubMed] [Google Scholar]
- 48.Robin JB, Jester JV, Nobe J, Nicolaides N, Smith RE. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. A clinical study. Ophthalmology. 1985;92:1423–1426. doi: 10.1016/s0161-6420(85)33848-4. [DOI] [PubMed] [Google Scholar]
- 49.Dougherty JM, McCulley JP. Analysis of the free fatty acid component of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:52–56. [PubMed] [Google Scholar]
- 50.Harvey DJ, Tiffany JM, Duerden JM, Pandher KS, Mengher LS. Identification by combined gas chromatography-mass spectrometry of constituent long-chain fatty acids and alcohols from the meibomian glands of the rat and a comparison with human meibomian lipids. J Chromatogr. 1987;414:253–263. doi: 10.1016/0378-4347(87)80051-8. [DOI] [PubMed] [Google Scholar]
- 51.Osgood JK, Dougherty JM, McCulley JP. The role of wax and sterol esters of meibomian secretions in chronic blepharitis. Invest Ophthalmol Vis Sci. 1989;30:1958–1961. [PubMed] [Google Scholar]
- 52.Dougherty JM, Osgood JK, McCulley JP. The role of wax and sterol ester fatty acids in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:1932–1937. [PubMed] [Google Scholar]
- 53.Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280. [PubMed] [Google Scholar]
- 54.Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:3515–3521. [PubMed] [Google Scholar]
- 55.Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15:340–346. doi: 10.1097/00003226-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360. doi: 10.1007/978-1-4615-5359-5_50. [DOI] [PubMed] [Google Scholar]
- 57.Borchman D, Foulks GN, Yappert MC. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr Eye Res. 2010;35:778–786. doi: 10.3109/02713683.2010.490895. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci. 2010;51:6220–6231. doi: 10.1167/iovs.10-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knop E, Knop N, Schirra F. Meibomian glands. Part II: physiology, characteristics, distribution and function of meibomian oil. Ophthalmologe. 2009;106:884–892. doi: 10.1007/s00347-009-2019-9. [DOI] [PubMed] [Google Scholar]
- 60.Butovich IA. Lipidomic analysis of human meibum using HPLC-MSn. Methods Mol Biol. 2009;579:221–246. doi: 10.1007/978-1-60761-322-0_11. [DOI] [PubMed] [Google Scholar]
- 61.Wojtowicz JC, Butovich IA, McCulley JP. Historical brief on composition of human meibum lipids. Ocul Surf. 2009;7:145–153. doi: 10.1016/s1542-0124(12)70309-9. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- 63.Shine WE, McCulley JP. Meibomianitis: polar lipid abnormalities. Cornea. 2004;23:781–783. doi: 10.1097/01.ico.0000133995.99520.1f. [DOI] [PubMed] [Google Scholar]
- 64.Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26:89–94. doi: 10.1076/ceyr.26.2.89.14515. [DOI] [PubMed] [Google Scholar]
- 66.McCulley JP, Shine WE. Eyelid disorders: the meibomian gland, blepharitis, and contact lenses. Eye Contact Lens. 2003;29:S93–S95. doi: 10.1097/00140068-200301001-00026. discussion S115–8, S92–4. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan BD, Cermak JM, Sullivan RM, Papas AS, Evans JE, Dana MR, et al. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjogren's syndrome. Adv Exp Med Biol. 2002;506:441–447. doi: 10.1007/978-1-4615-0717-8_62. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–1699. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- 69.Lozato PA, Pisella PJ, Baudouin C. The lipid layer of the lacrimal tear film: physiology and pathology. J Fr Ophtalmol. 2001;24:643–658. [PubMed] [Google Scholar]
- 70.Nagyova B, Tiffany JM. Components responsible for the surface tension of human tears. Curr Eye Res. 1999;19:4–11. doi: 10.1076/ceyr.19.1.4.5341. [DOI] [PubMed] [Google Scholar]
- 71.Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852. doi: 10.1001/archopht.116.7.849. [DOI] [PubMed] [Google Scholar]
- 72.McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88. discussion -93. [PMC free article] [PubMed] [Google Scholar]
- 73.Butovich IA. On the presence and role of polar lipids in meibum. Invest Ophthalmol Vis Sci. 2010;51:6908–6910. doi: 10.1167/iovs.10-6328. author reply 10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holly FJ. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515–525. doi: 10.1016/0014-4835(73)90064-x. [DOI] [PubMed] [Google Scholar]
- 75.Holly FJ. Formation and stability of the tear film. Int Ophthalmol Clin. 1973;13:73–96. [PubMed] [Google Scholar]
- 76.McCulley JP, Shine WE. Meibomian secretions in chronic blepharitis. Adv Exp Med Biol. 1998;438:319–326. doi: 10.1007/978-1-4615-5359-5_45. [DOI] [PubMed] [Google Scholar]
- 77.Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000;19:72–74. doi: 10.1097/00003226-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 78.Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:3993–4005. doi: 10.1167/iovs.09-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schirra F, Suzuki T, Dickinson DP, Townsend DJ, Gipson IK, Sullivan DA. Identification of steroidogenic enzyme mRNAs in the human lacrimal gland, meibomian gland, cornea, and conjunctiva. Cornea. 2006;25:438–442. doi: 10.1097/01.ico.0000183664.80004.44. [DOI] [PubMed] [Google Scholar]
- 80.Cermak JM, Krenzer KL, Sullivan RM, Dana MR, Sullivan DA. Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface? Cornea. 2003;22:516–521. doi: 10.1097/00003226-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Sullivan BD, Evans JE, Dana MR, Sullivan DA. Impact of androgen deficiency on the lipid profiles in human meibomian gland secretions. Adv Exp Med Biol. 2002;506:449–458. doi: 10.1007/978-1-4615-0717-8_63. [DOI] [PubMed] [Google Scholar]
- 82.Sullivan DA, Yamagami H, Liu M, Steagall RJ, Schirra F, Suzuki T, et al. Sex steroids, the meibomian gland and evaporative dry eye. Adv Exp Med Biol. 2002;506:389–399. doi: 10.1007/978-1-4615-0717-8_56. [DOI] [PubMed] [Google Scholar]
- 83.Krenzer KL, Dana MR, Ullman MD, Cermak JM, Tolls DB, Evans JE, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. J Clin Endocrinol Metab. 2000;85:4874–4882. doi: 10.1210/jcem.85.12.7072. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85:4866–4873. doi: 10.1210/jcem.85.12.7066. [DOI] [PubMed] [Google Scholar]
- 85.Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand. 2000;78:146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- 86.Rocha EM, Wickham LA, da Silveira LA, Krenzer KL, Yu FS, Toda I, et al. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol. 2000;84:76–84. doi: 10.1136/bjo.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sullivan DA, Wickham LA, Rocha EM, Krenzer KL, Sullivan BD, Steagall R, et al. Androgens and dry eye in Sjogren's syndrome. Ann N Y Acad Sci. 1999;876:312–324. doi: 10.1111/j.1749-6632.1999.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 88.Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, Toda I, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci. 1999;40:1261–1265. [PubMed] [Google Scholar]
- 89.Sullivan DA, Rocha EM, Ullman MD, Krenzer KL, Gao J, Toda I, et al. Androgen regulation of the meibomian gland. Adv Exp Med Biol. 1998;438:327–331. doi: 10.1007/978-1-4615-5359-5_46. [DOI] [PubMed] [Google Scholar]
- 90.Schirra F, Richards SM, Liu M, Suzuki T, Yamagami H, Sullivan DA. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp Eye Res. 2006;83:291–296. doi: 10.1016/j.exer.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 91.Schirra F, Richards SM, Sullivan DA. Androgen influence on cholesterogenic enzyme mRNA levels in the mouse meibomian gland. Curr Eye Res. 2007;32:393–398. doi: 10.1080/02713680701316674. [DOI] [PubMed] [Google Scholar]
- 92.Schirra F, Suzuki T, Richards SM, Jensen RV, Liu M, Lombardi MJ, et al. Androgen control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2005;46:3666–3675. doi: 10.1167/iovs.05-0426. [DOI] [PubMed] [Google Scholar]
- 93.Bernhard W, Linck M, Creutzburg H, Postle AD, Arning A, Martin-Carrera I, et al. High-performance liquid chromatographic analysis of phospholipids from different sources with combined fluorescence and ultraviolet detection. Anal Biochem. 1994;220:172–180. doi: 10.1006/abio.1994.1315. [DOI] [PubMed] [Google Scholar]
- 94.Nichols KK, Ham BM, Nichols JJ, Ziegler C, Green-Church KB. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48:34–39. doi: 10.1167/iovs.06-0753. [DOI] [PubMed] [Google Scholar]
- 95.McDonald GR, Hudson AL, Dunn SM, You H, Baker GB, Whittal RM, et al. Bioactive contaminants leach from disposable laboratory plasticware. Science. 2008;322:917. doi: 10.1126/science.1162395. [DOI] [PubMed] [Google Scholar]
- 96.Butovich IA, Uchiyama E, McCulley JP. On the Presence of Oleamide in Human Meibum: Quantification by LC/MS. Invest Ophthalmol Vis Sci. 2007 [Google Scholar]
- 97.Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49:3779–3789. doi: 10.1167/iovs.08-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joffre C, Souchier M, Gregoire S, Viau S, Bretillon L, Acar N, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92:116–119. doi: 10.1136/bjo.2007.126144. [DOI] [PubMed] [Google Scholar]
- 99.Souchier M, Joffre C, Gregoire S, Bretillon L, Muselier A, Acar N, et al. Changes in meibomian fatty acids and clinical signs in patients with meibomian gland dysfunction after minocycline treatment. Br J Ophthalmol. 2008;92:819–822. doi: 10.1136/bjo.2007.133900. [DOI] [PubMed] [Google Scholar]
- 100.Borchman D, Foulks GN, Yappert MC, Ho DV. Temperature-induced conformational changes in human tearlipids hydrocarbon chains. Biopolymers. 2007;87:124–133. doi: 10.1002/bip.20798. [DOI] [PubMed] [Google Scholar]
- 101.Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102. doi: 10.1016/j.chemphyslip.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Stewart ME, Downing DT. Separation of wax esters from steryl esters by chromatography on magnesium hydroxide. Lipids. 1981;16:355–359. doi: 10.1007/BF02534962. [DOI] [PubMed] [Google Scholar]
- 103.Robosky LC, Wade K, Woolson D, Baker JD, Manning ML, Gage DA, et al. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J Lipid Res. 2008;49:686–692. doi: 10.1194/jlr.D700035-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Pauli GF, Jaki BU, Lankin DC. Quantitative 1H NMR: development and potential of a method for natural products analysis. J Nat Prod. 2005;68:133–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- 105.Ermer J, Vogel M. Applications of hyphenated LC-MS techniques in pharmaceutical analysis. Biomed Chromatogr. 2000;14:373–383. doi: 10.1002/1099-0801(200010)14:6<373::AID-BMC29>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 106.Mitchell TW, Pham H, Thomas MC, Blanksby SJ. Identification of double bond position in lipids: from GC to OzID. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2722–2735. doi: 10.1016/j.jchromb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 107.Haynes CA, Allegood JC, Park H, Sullards MC. Sphingolipidomics: methods for the comprehensive analysis of sphingolipids. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2696–2708. doi: 10.1016/j.jchromb.2008.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quehenberger O, Armando A, Dumlao D, Stephens DL, Dennis EA. Lipidomics analysis of essential fatty acids in macrophages. Prostaglandins Leukot Essent Fatty Acids. 2008;79:123–129. doi: 10.1016/j.plefa.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lam PM, Marczylo TH, El-Talatini M, Finney M, Nallendran V, Taylor AH, et al. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Anal Biochem. 2008;380:195–201. doi: 10.1016/j.ab.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 110.Stahlman M, Ejsing CS, Tarasov K, Perman J, Boren J, Ekroos K. High-throughput shotgun lipidomics by quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2664–2672. doi: 10.1016/j.jchromb.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 111.Gross RW, Han X. Shotgun lipidomics of neutral lipids as an enabling technology for elucidation of lipid-related diseases. Am J Physiol Endocrinol Metab. 2009;297:E297–E303. doi: 10.1152/ajpendo.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han X, Gross RW. Shotgun lipidomics: multidimensional MS analysis of cellular lipidomes. Expert Rev Proteomics. 2005;2:253–264. doi: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- 113.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 114.Ji H, Voinov VG, Deinzer ML, Barofsky DF. Distinguishing between cis/trans isomers of monounsaturated fatty acids by FAB MS. Anal Chem. 2007;79:1519–1522. doi: 10.1021/ac061155d. [DOI] [PubMed] [Google Scholar]
- 115.Momchilova S, Nikolova-Damyanova B, Christie WW. Silver ion high-performance liquid chromatography of isomeric cis- and trans-octadecenoic acids: Effect of the ester moiety and mobile phase composition. Journal of Chromatography A. 1998;793:275–282. [Google Scholar]
- 116.Butovich IA, Reddy CC. Enzyme-catalyzed and enzyme-triggered pathways in dioxygenation of 1-monolinoleoyl-rac-glycerol by potato tuber lipoxygenase. Biochim Biophys Acta. 2001;1546:379–398. doi: 10.1016/s0167-4838(01)00162-5. [DOI] [PubMed] [Google Scholar]
- 117.Hamberg M, Samuelsson B. Stereochemistry in the formation of 9-hydroxy-10,12-octadecadienoic acid and 13-hydroxy-9,11-octadecadienoic acid from linoleic acid by fatty acid cyclooxygenase. Biochim Biophys Acta. 1980;617:545–547. doi: 10.1016/0005-2760(80)90022-3. [DOI] [PubMed] [Google Scholar]
- 118.Hamberg M. Steric analysis of hydroperoxides formed by lipoxygenase oxygenation of linoleic acid. Anal Biochem. 1971;43:515–526. doi: 10.1016/0003-2697(71)90282-x. [DOI] [PubMed] [Google Scholar]
- 119.Butovich IA. On the Presence of (O-acyl)-omega-hydroxy Fatty Acids and of Their Esters in Human Meibomian Gland Secretions. Invest Ophthalmol Vis Sci. 2011;52:639–641. doi: 10.1167/iovs.10-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iyengar BT, Schlenk H. Melting points of synthetic wax esters. Lipids. 1969;4:28–30. doi: 10.1007/BF02531790. [DOI] [PubMed] [Google Scholar]
- 121.Patel S, Nelson DR, Gibbs AG. Chemical and physical analyses of wax ester properties. J Insect Sci. 2001;1:4. doi: 10.1673/031.001.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ucciani E, SchmittRozieres M, Debal A, Comeau LC. Enzymatic synthesis of some wax-esters. Fett-Lipid. 1996;98:206–210. [Google Scholar]
- 123.Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, Prospective, Randomized, Double-masked, Placebo-controlled Clinical Trial of an Omega-3 Supplement for Dry Eye. Cornea. 2010 doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 124.Rezanka T, Sigler K. Identification of very long chain unsaturated fatty acids from Ximenia oil by atmospheric pressure chemical ionization liquid chromatography-mass spectroscopy. Phytochemistry. 2007;68:925–934. doi: 10.1016/j.phytochem.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 125.Shine WE, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res. 2003;76:417–420. doi: 10.1016/s0014-4835(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 126.Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–2975. [PubMed] [Google Scholar]
- 127.McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89:1173–1180. doi: 10.1016/s0161-6420(82)34669-2. [DOI] [PubMed] [Google Scholar]
- 128.Carrott MJ, Jones DC, Davidson G. Identification and analysis of polymer additives using packed-column supercritical fluid chromatography with APCI mass spectrometric detection. Analyst. 1998;123:1827–1833. [Google Scholar]
- 129.Saville JT, Zhao Z, Willcox MD, Ariyavidana MA, Blanksby SJ, Mitchell TW. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 130.Ham BM, Jacob JT, Cole RB. MALDI-TOF MS of phosphorylated lipids in biological fluids using immobilized metal affinity chromatography and a solid ionic crystal matrix. Anal Chem. 2005;77:4439–4447. doi: 10.1021/ac058000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gershbein LL, Krotoszynski BK, Singh EJ. Fractionation of hydrocarbons of human hair lipids by chromatographic and thermal diffusion methods. J Chromatogr. 1967;27:431–449. doi: 10.1016/s0021-9673(01)85900-8. [DOI] [PubMed] [Google Scholar]
- 132.O'Neill HJ, Gershbein LL. Analysis of fatty acid and alcoholic components of sebaceous lipid types. J Chromatogr Sci. 1976;14:28–36. doi: 10.1093/chromsci/14.1.28. [DOI] [PubMed] [Google Scholar]
- 133.Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res. 2010;51:1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mudgil P, Millar TJ. Surfactant properties of human meibomian lipids. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5445. [DOI] [PubMed] [Google Scholar]
- 135.Butovich IA, Arciniega JC, Wojtowicz JC. Meibomian lipid films and the impact of temperature. Invest Ophthalmol Vis Sci. 2010;51:5508–5518. doi: 10.1167/iovs.10-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50:140–151. doi: 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mudgil P, Millar TJ. Adsorption of apo- and holo-tear lipocalin to a bovine Meibomian lipid film. Exp Eye Res. 2008;86:622–628. doi: 10.1016/j.exer.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 138.Millar TJ, Tragoulias ST, Anderton PJ, Ball MS, Miano F, Dennis GR, et al. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100. doi: 10.1097/01.ico.0000164779.87795.3c. [DOI] [PubMed] [Google Scholar]
- 139.Tragoulias ST, Anderton PJ, Dennis GR, Miano F, Millar TJ. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea. 2005;24:189–200. doi: 10.1097/01.ico.0000138837.52694.37. [DOI] [PubMed] [Google Scholar]
- 140.Kaercher T, Honig D, Mobius D, Welt R. [Morphology of the Meibomian lipid film. Results of Brewster angle microscopy] Ophthalmologe. 1995;92:12–16. [PubMed] [Google Scholar]
- 141.Kaercher T, Honig D, Mobius D. Brewster angle microscopy. A new method of visualizing the spreading of Meibomian lipids. Int Ophthalmol. 1993;17:341–348. doi: 10.1007/BF00915741. [DOI] [PubMed] [Google Scholar]
- 142.Leiske DL, Raju SR, Ketelson HA, Millar TJ, Fuller GG. The interfacial viscoelastic properties and structures of human and animal Meibomian lipids. Exp Eye Res. 2010;90:598–604. doi: 10.1016/j.exer.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 143.Svitova TF, Lin MC. Tear lipids interfacial rheology: effect of lysozyme and lens care solutions. Optom Vis Sci. 2010;87:10–20. doi: 10.1097/OPX.0b013e3181c07908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schurch S, Green FH, Bachofen H. Formation and structure of surface films: captive bubble surfactometry. Biochim Biophys Acta. 1998;1408:180–202. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 145.Miano F, Calcara M, Millar TJ, Enea V. Insertion of tear proteins into a meibomian lipids film. Colloids Surf B Biointerfaces. 2005;44:49–55. doi: 10.1016/j.colsurfb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 146.Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40:3100–3107. [PubMed] [Google Scholar]
- 147.Petrov PG, Thompson JM, Rahman IB, Ellis RE, Green EM, Miano F, et al. Two-dimensional order in mammalian pre-ocular tear film. Exp Eye Res. 2007;84:1140–1146. doi: 10.1016/j.exer.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 148.Kaercher T, Mobius D, Welt R. Biophysical behaviour of the infant Meibomian lipid layer. Int Ophthalmol. 1994;18:15–19. doi: 10.1007/BF00919408. [DOI] [PubMed] [Google Scholar]
- 149.Kaercher T, Mobius D, Welt R. Biophysical characteristics of the Meibomian lipid layer under in vitro conditions. Int Ophthalmol. 1992;16:167–176. doi: 10.1007/BF00916437. [DOI] [PubMed] [Google Scholar]
- 150.Clop EM, Perillo MA. Langmuir films from human placental membranes: preparation, rheology, transfer to alkylated glasses, and sigmoidal kinetics of alkaline phosphatase in the resultant Langmuir- Blodgett film. Cell Biochem Biophys. 2010;56:91–107. doi: 10.1007/s12013-009-9073-4. [DOI] [PubMed] [Google Scholar]