Abstract
Aim
To evaluate whether the avidity of serotype-specific IgG to pneumococcal serotypes is enhanced by an increased number of doses of the 7-valent pneumococcal conjugate vaccine (PCV) in infancy or by a 12 month 23-valent pneumococcal polysaccharide vaccine (23vPPS) booster, and /or subsequent re-exposure to a small dose of pneumococcal polysaccharide antigens (mPPS) at 17 months.
Methods
Fijian infants aged 6 weeks were recruited, stratified by ethnicity and randomized to 8 groups to receive 0, 1, 2, or 3 doses of PCV, with or without booster 23vPPS at 12 months. All children received mPPS at 17 months of age. Avidity of serotype-specific IgG for PCV serotypes in the first 12 months and for all 23vPPS serotypes thereafter was assessed by EIA after sodium thiocyanate elution.
Results
At one month post primary series, the 2 and 3 PCV dose groups demonstrated similar avidity, with the single dose group tending to have lower avidity. However, by age 9 months, the single dose group had similar avidity to the 2 and 3 PCV groups for most serotypes. The 23vPPS booster enhanced affinity maturation for most serotypes and this was most marked in those groups that received a single PCV dose. There was little further increase following the mPPS.
Conclusions
By 9 months of age, similar avidity can be induced following one, 2 or 3 doses of PCV. A 23vPPS booster at 12 months enhanced affinity maturation with an increase in antibody avidity for most serotypes. Subsequent re-challenge with mPPS at 17 months did not further enhance the avidity of serotype-specific response in the 12 month 23vPPS groups.
Introduction
Invasive pneumococcal disease (IPD) is an important cause of morbidity and mortality, particularly in the very young and the elderly (1). A recent review estimated that over 14 million episodes of serious pneumococcal disease occurred worldwide in the year 2000, with over 800,000 deaths in children under 5 years (2). At least 40 serogroups comprising of over 90 serotypes of pneumococcus have been identified (3, 4). In all regions of the world, serotypes 1, 5, and 14 account for 28–43% of IPD in under 5 year olds (5). The 7-valent pneumococcal conjugate vaccine (PCV, Prevnar™, Wyeth Vaccines) includes only serotypes 4, 6B, 9V, 14, 18C, 19F, 23F and covers a smaller proportion of the pneumococcal serotypes causing disease in children in low income countries compared to more affluent countries.
Immunological memory should be demonstrated for the licensure of new pneumococcal conjugate vaccine formulations (6). Immunological memory can be demonstrated by increased serotype-specific IgG titers following administration of a booster dose of pneumococcal polysaccharide (PPS) or conjugate vaccine, or by enhanced antibody avidity (6). An important feature of a memory response is the maturation of antibody avidity. Measurement of antibody avidity following PCV immunisation provides information on both the development of B cell memory (7) and the functional activity of antibodies (8, 9). One method of demonstrating immunological priming is to assess the ability of PPS to boost the immune response and elicit antibodies with increased affinity. Evaluation of affinity maturation has been used to assess priming capacity of PCV (10–13) and efficacy of reduced dose schedules (14). In vitro, higher avidity antibody is associated with greater opsonophagocytic capacity (11, 15). Furthermore, findings from a study assessing the contribution of avidity, antibody concentration, and IgG subclass to opsonophagocytic activity demonstrated that lower amounts of high avidity antibody were sufficient for killing of bacteria whereas higher amounts of low avidity antibody were required for effective killing activity (11). While the importance of avidity in determining protection from disease is unclear, studies of Haemophilus influenzae type b (Hib) conjugate vaccine have demonstrated that antibody avidity is strongly associated with functional activity of anti-Hib polysaccharide antibodies (16, 17), and anti-Hib polysaccharide antibodies of high avidity have been found to have protective efficacy in experimental Hib infection (18).
Due to the cost and the limited serotype coverage of PCV, some countries use reduced dose primary PCV series schedules with a 23-valent pneumococcal polysaccharide booster (23vPPS). In Fiji the 7 serotypes included in PCV would only cover 55% of IPD episodes in children under 5 year (19), so that the 23vPPS would potentially provide additional coverage against common disease associated serotypes. The aims of this study were to describe the kinetics of the avidity of serotype-specific IgG following 1) a reduced dose primary PCV series compared with a 3 dose primary series; 2) a booster of 23vPPS at 12 months; and 3) a small re-challenge dose of 23vPPS (mPPS, comprising 20% of the 23vPPS dose) at 17 months of age in infants who had or had not received the 23vPPS at 12 months of age.
Methods
1. Study participants
A single blind, open-label randomized Phase II vaccine trial was completed in Suva, Fiji documenting the safety, immunogenicity and impact on pneumococcal carriage of various pneumococcal vaccination schedules combining one, 2, or 3 doses of PCV in infancy followed by a booster of 23vPPS. A small 23vPPS re-challenge dose was given to all children at 17 months of age. Healthy infants aged between 6 and 8 weeks of age were eligible for enrolment. Details of the selection criteria, randomisation procedure and immunogenicity results (serum levels of serotype-specific IgG) have been reported elsewhere (20–22). This manuscript reports findings for avidity of serotype-specific IgG.
The study was approved by the Fiji National Research Ethics Review Committee and the University of Melbourne Human Research Ethics Committee and was conducted according to Good Clinical Practice.
2. Study procedures and vaccines
Infants were stratified by ethnicity and randomised into one of 8 groups. Infants received 1, 2, or 3 doses of the 7-valent CRM197 protein-polysaccharide conjugate vaccine (PCV) containing polysaccharide antigen from pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, 23F (Prevnar ™, Wyeth Vaccines). The vaccine contains 2 μg/serotype, except serotype 6B at 4μg. The 3 dose group received PCV at 6 weeks, 10 weeks, and 14 weeks of age. The 2 dose group received PCV at 6 and 14 weeks of age and the one dose group received PCV at 14 weeks of age. Vaccines were given a minimum of 25 days apart. Routine vaccines (Hiberix™ mixed with Tritanrix™-HepB™, GlaxoSmithKline) and oral polio were given at 6, 10, and 14 weeks of age. The children in all primary series groups were further randomised to receive a 23vPPS (23vPPS: Pneumovax™, Merck & Co., Inc., which consists of a purified mixture of 25μg of capsular polysaccharide from 23 pneumococcal serotypes) or no 23vPPS at 12 months of age in addition to Measles-Rubella vaccine. A small re-challenge dose of 20% of the 23vPPS (mPPS) was administered to all groups at 17 months of age.
3. Laboratory procedures
Table 1 presents the design of the study and the timing of blood draws. All children except the group randomised to receive no PCV or 23vPPS had blood drawn at 18 weeks and 12 months of age. In addition, those children who were not randomized to receive the 12 month 23vPPS had blood drawn at 9 months of age. Children who received the 12 month 23vPPS had additional bloods drawn 14 days post-23vPPS. All children had blood drawn at 17 months of age (prior to mPPS) and at 18 months (one month post-mPPS). Sera was separated by centrifugation in the health centre, kept chilled and transported to the Colonial War Memorial Hospital laboratory, Suva where it was divided into aliquots and stored at −70°C on the same day, until transported on dry ice to the Pneumococcal Laboratory, Murdoch Childrens Research Institute, Melbourne, for analysis.
Table 1.
Randomization and timing of vaccination and blood draws for each of the 8 groups
| A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|
| n=71 | n=65 | n=76 | n=80 | n=62 | n=66 | n=63 | n=69 | |
| 6 weeks | PCV | PCV | PCV | PCV | ||||
|
| ||||||||
| 10 weeks | PCV | PCV | ||||||
|
| ||||||||
| 14 weeks | PCV | PCV | PCV | PCV | PCV | PCV | ||
|
| ||||||||
| 18 weeks | B | B | B | B | B | B | B | |
|
| ||||||||
| 9 months | B | B | B | |||||
|
| ||||||||
| 12 months | B | B 23vPPS |
B | B 23vPPS |
B | B 23vPPS |
B 23vPPS |
|
|
| ||||||||
| 12 1/2 months | B | B | B | B | ||||
|
| ||||||||
| 17 months | B mPPS |
B mPPS |
B mPPS |
B mPPS |
B mPPS |
B mPPS |
B mPPS |
B mPPS |
|
| ||||||||
| 18 months | B | B | B | B | B | B | B | B |
PCV: 7-valent pneumococcal conjugate vaccine
B: Blood test
23vPPS: 23-valent pneumococcal polysaccharide vaccine
mPPS: Micro-dose (20%) of the 23vPPS
Specific IgG was measured using a modified 3rd generation ELISA based on current WHO recommendations (23) previously validated in our laboratory (24). Avidity of serotype-specific IgG antibodies to all 23 pneumococcal serotypes in 23vPPS was determined by EIA (25) which uses a 0.5 OD unit cut-off, with & without NaSCN, as the reference point for calculation of avidity. This assay was validated against an alternative method for measurement of avidity using a standardized IgG titer of 1 ug/ml as the reference point for calculation of avidity (14) and equivalent avidity index (AI) results were observed (data not shown). Initial steps were as for the ELISA method described. After incubation of serum samples and washing, sodium thiocyanate (NaSCN) in F-PBS was added to dissociate weak antibody-antigen complexes. NaSCN or F-PBS were incubated for 15 minutes on a shaker at room temperature, after which the plates were washed with PBS/T and antibody binding was detected by the addition of horseradish peroxidase-conjugated anti-human IgG. The colour was developed by the TMB Peroxidase Substrate system. Absorbance at 450 nm, reference filter 620 nm was read on an EIA reader. Control serum was added to each plate to assess reproducibility. The CV was less than 20% for all serotypes. Laboratory staff members were blinded to the group allocation of each serum sample. Results are expressed as an AI, assigned as the percentage of antibodies that remained bound to the antigens after NaSCN elution. The AI was calculated for each serotype assayed for each sample by dividing the end point titre of the serum sample with NaSCN treatment by the end-point titre of the sample without NaSCN treatment and multiplying by 100.
4. Statistical analysis
Cleaned data were exported to Stata version 9.0 (Stata Corporation, College Station, Texas) for analysis. PCV serotypes were serotypes contained within PCV (4, 6B, 9V, 14, 18C, 19F, 23F). Non-PCV serotypes were those not contained in PCV but contained in 23vPPS (1, 2, 3, 5, 7F, 8, 9N, 10A, 11A, 12F, 15B, 17F, 19A, 20, 22F, 33F). For analyses of serotype-specific increases in avidity to non-PCV serotypes following the 12 month 23vPPS booster, comparisons of avidity were performed by pooling data from the 4 groups that received the12 month 23vPPS and were compared with pooled data from the 4 groups that did not receive the 12 month 23vPPS. The results are expressed as a serotype-specific median AI (the median percentage of serotype-specific IgG that is avid) at each time point for each treatment group. To test for differences in distributions, between group comparisons of the median serotype-specific AI were performed using the Rank sum test, and paired comparisons of the median serotype-specific AI within groups were performed using the Sign rank test. A p-value of <0.01 was considered statistically significant.
Results
There were 552 infants enrolled in the study. The number of infants randomized to each group is shown in Table 1. The characteristics of children have been reported elsewhere (20, 22). There were 90 (16.3%) withdrawals. No participants were withdrawn due to an adverse event attributable to any of the vaccines.
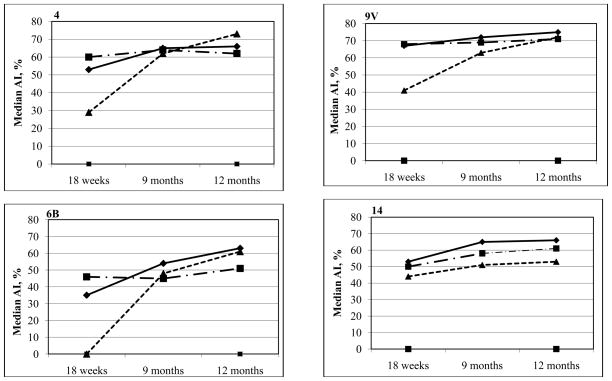
Figure 1 shows the kinetics of the median AI for each PCV group 4 weeks post primary series (18 weeks of age), and at 9 and 12 months of age. The median AI were significantly higher (each p<0.01) in the 2 PCV dose group as compared to the 3 PCV dose group at 18 weeks for 4 of 7 PCV serotypes (4, 6B, 18C, 19F) and significantly lower for serotype 23F (p<0.001). By 9 months of age these differences were no longer evident. At 12 months of age the median AI were significantly higher in the 3 PCV dose group as compared to the 2 dose group for 3 of 7 serotypes - serotypes 6B (p<0.001), 14 (p=0.002), and 23F (p<0.001). When comparing the 3 PCV dose group with the single dose group, the median AI in the 3 dose group were significantly higher for all PCV serotypes post primary series (each p≤0.001). Nevertheless, by 9 and 12 months of age these differences were no longer present, with similar median AI in the 2 groups for the majority of serotypes, except that the 3 PCV dose group had a higher median AI for serotype 23F (p 0.001) at 9 months, and serotypes 14 (p<0.001) and 23F (p<0.001) at 12 months, as compared to the single PCV dose group. These findings show that while 2 or 3 doses of PCV provide greater increases in the median percentage with high antibody avidity as compared with a single PCV dose in the early stages post primary series, the avidity of serotype-specific IgG in the single PCV dose group increases over time and are similar to those in the 2 and 3 PCV dose groups by 9 months of age.
Figure 1.
Kinetics of the median percentage of serotype-specific-IgG that is avid for the PCV serotypes taken 4 weeks following the PCV primary series, and at 9 and 12 months of age
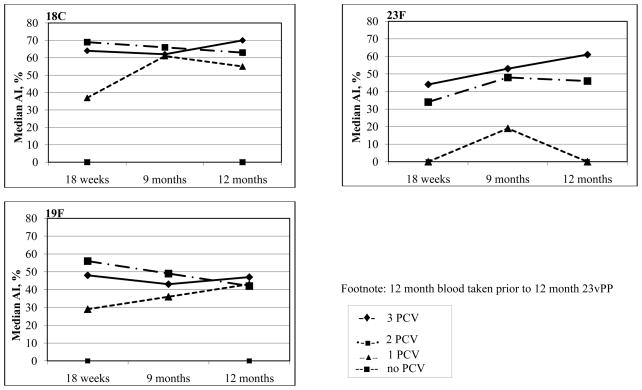
To assess the effect of 23vPPS booster on avidity, the serotype-specific AI immediately prior to and 2 weeks following 23vPPS booster were compared. In all PCV dose groups, the 23vPPS booster resulted in a significant increase in median AI for the majority of PCV serotypes. The increase in the median percentage with high avidity following 23vPPS was higher in the single PCV group as compared to the 2 or 3 PCV groups for several of the PCV serotypes. There were no significant differences in the median AI post-23vPPS for all PCV serotypes when comparing the 3 and 2 PCV dose groups. There were no significant differences in the median AI 2 weeks following the 23vPPS between the 3 and single PCV dose groups for 5 of 7 PCV serotypes with serotypes 4 (p<0.001) and 9V (p=0.004) being significantly higher in the single dose group. This suggests that a 23vPPS booster administered at 12 months results in similar increase in post immunization serotype-specific IgG avidity for the majority of serotypes irrespective of whether infants received one, 2 or 3 PCV doses in their primary series. Furthermore, a single PCV dose followed by 23vPPS appeared to provide a greater increase in serotype-specific IgG avidity when compared to the 3 dose PCV/23vPPS group for at least some serotypes. It has been reported previously that a single PCV dose resulted in higher serotype-specific IgG post- 23vPPS for 5 of 7 PCV serotypes (21) and in this study 4 of these 5 serotypes also demonstrated higher avidity when compared to the 2 or 3 PCV dose groups.
By 17 months of age, for those who had received 3 PCV doses, there were no significant differences in the median percentage with high serotype-specific IgG avidity between those who had or had not received the 23vPPS at 12 months of age for any serotypes except 19F (p=0.002). Similar results were found for the 2 PCV dose group. In the single PCV dose group, the median percentage with high avidity antibody at 17 months was significantly higher in those that had received the 12 month 23vPPS compared with those that had not received the 12 month 23vPPS for serotypes 6B (p=0.004), 18C (p<0.001), 19F (p<0.001), and 23F (p<0.001). For those groups that had received the 23vPPS at 12 months of age, there were no significant increases in avidity following the re-challenge with mPPS at 17 months of age (comparing the pre and post-mPPS sera within each PCV group) for all PCV serotypes except serotype 9V (p=0.001) in the 2 PCV/23vPPS group. For those groups that had not received the 23vPPS at 12 months, there were significant increases in avidity following the re-challenge dose (comparing pre and post-mPPS sera within each PCV group) for all PCV groups except serotypes 4 (p=0.731) in the 3 PCV dose group, and serotype 19F (p=0.914) in the no PCV dose group.
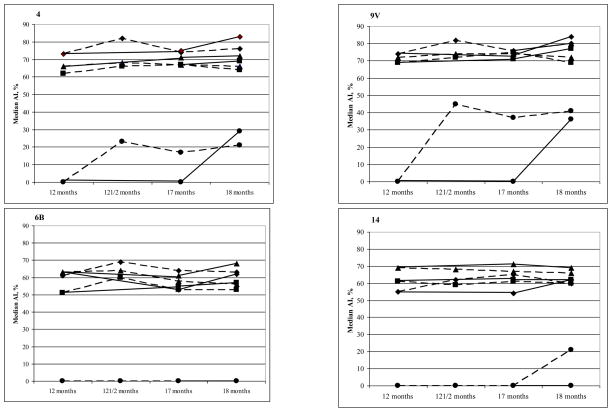
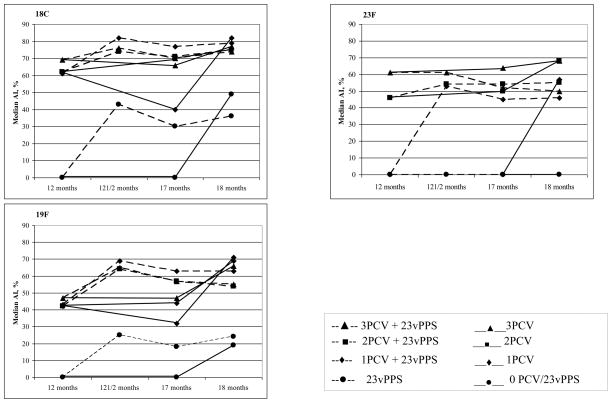
Figure 2 shows the kinetics of the serotype-specific IgG avidity following the 23vPPS at 12 months of age for each PCV serotype. These findings suggest that although a 23vPPS booster at 12 months induces a marked increase in avidity, a similar increase is achieved with mPPS administered at 18 months. Indeed, the median percentage with high avidity serotype-specific IgG were significantly higher at 18 months for many serotypes in the children who did not receive a prior 23vPPS at 12 months as compared to those who did.
Figure 2.
Kinetics of the median percentage of serotype-specific IgG that is avid for the PCV serotypes pre and post 12 month 23vPPS and pre and post 18 month mPPS in those that did or did not receive the 12 month 23vPPS
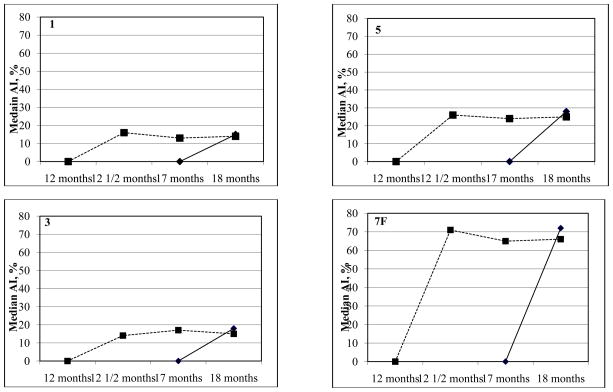
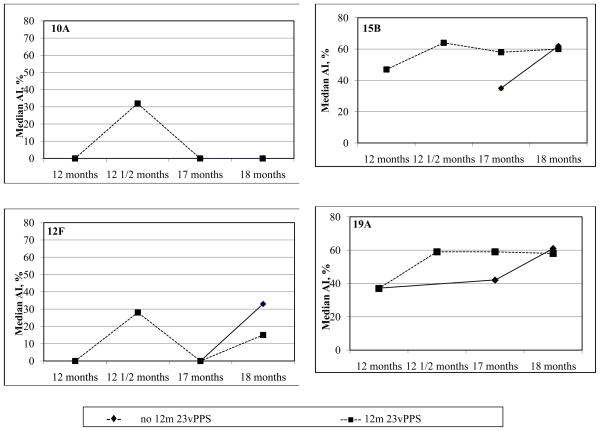
For the non-PCV serotypes, the median AI at 2 weeks following the 12 month 23vPPS were significantly higher in the groups that received the 12 month 23vPPS when compared with those that had not received the 12 month 23vPPS (each p<0.001). These differences between the groups that received the 12 month 23vPPS and those that did not receive the 23vPPS persisted to 17 months of age for all non-PCV serotypes (each p<0.01). At 18 months, following the mPPS booster, the median AI for all non-PCV serotypes were no longer significantly different between those groups that received the 23vPPS and those that did not for 9 of 16 non-PCV serotypes. Furthermore, there were no significant increases in median AI following the re-challenge mPPS dose in the groups that had received the 12 month 23vPPS for the majority of serotypes. The kinetics of the serotype-specific IgG avidity to 8 of the non-PCV serotypes are shown in Figure 3.
Figure 3.
Kinetics of the median percentage of serotype-specific IgG that is avid for selected non-PCV serotypes in those that did or did not receive the 12 month 23vPPS
Discussion
This is the first comprehensive study describing the kinetics of the avidity of serotype-specific IgG for all 23vPPS serotypes following different pneumococcal vaccination schedules. This study has shown that in the first 12 months of life, a 2 or 3 PCV dose primary series in infancy increased the median percentage with high avidity for PCV serotypes. Surprisingly however, although the single PCV dose group induced minimal increases in the median percentage with serotype-specific IgG immediately post primary series, levels of high avidity serotype-specific IgG increased progressively thereafter so that levels were similar to those observed for the 2 and 3 dose groups by 9 months, with the exception of serotype 23F. Consistent with our findings, a study from the United Kingdom trialing reduced PCV dose schedules together with a booster at 12 months of age (14) found that induction of high avidity serotype-specific IgG against the 3 serotypes tested (6B, 14, 23F) were similar for those receiving 2 or 3 PCV doses and increased significantly for both groups in the period following priming and post boosting (14).
From 12 to 17 months of age, the 12 month 23vPPS booster increased the median percentage with high avidity for most PCV serotypes and all non-PCV serotypes. At 17 months these differences persisted as there were significantly higher median AI for all non-PCV serotypes in the 23vPPS group compared to the group that had not received the 23vPPS at 12 months of age. Avidity may be seen as a surrogate measure of the induction of immunological memory for conjugate vaccines (7), so these findings indicate that one, 2, or 3 PCV doses prime infants to a similar degree. Furthermore, the single PCV dose group had at least similar or higher avidity for all PCV serotypes post the 12 month 23vPPS except for serotypes 14 and 23F as compared to 2 or 3 PCV dose groups, suggesting better priming following a single PCV dose. We have previously demonstrated similar trends for serum titers of serotype-specific IgG (21). Others have found that a conjugate vaccine booster is better than a PPS booster (11, 12, 14, 26). Infants primed with PCV and who received a PCV booster, but not PPS booster, had an increase in the amount of high avidity serotype-specific IgG (12) which suggested that the response to PCV was T cell dependent, while the T cell-independent PPS only activated existing memory B cells. A study in infants immunized at 2, 4, and 6 months of age with one of 4 different conjugate vaccines, and boosted at 14 months with the homologous conjugate or PPS found that while the induction of high avidity serotype-specific IgG against serotypes 6B, 14, 19F and 23F increased with age, only a booster dose of conjugate further increased avidity whereas a PPS booster did not (11). Similarly, a reduced dose UK study found that a booster with PCV induced significantly higher avidity responses against the 3 serotypes tested (6B, 14, 23F) compared to a 23vPPS booster, and induction of high avidity serotype- specific IgG against 3 serotypes tested in Ghanaian children was higher in those who had received PCV as compared to 23vPPS (26).
In this study, following a re-challenge with mPPS, there were no further increases in avidity in the groups that received the 12 month 23vPPS. In contrast, there was a significant increase in avidity induced by mPPS in the groups that had not received the 23vPPS at 12 months. Nevertheless, at the end of the study there was little difference in avidity between any of the groups that were primed with PCV that had or had not received the 12 month 23vPPS. We have demonstrated previously that prior receipt of 23vPPS causes immunological hyporesponsiveness to a 23vPPS re-challenge, as assessed by serum levels of serotype-specific IgG (22). Although the children who had received the 12 month 23vPPS had higher circulating antibody concentrations at 17 months of age, the response to a re-challenge dose demonstrated a lack of secondary response to all 23 serotypes irrespective of the pre-existing antibody level. In contrast, those who had not received the 12 month 23vPPS could clearly mount a response to mPPS (22). Our data (for PCV serotypes at least) suggests that hyporesponsiveness is not related to deletion of high avidity B cell clones as avidity does not decrease following the 12 month 23vPPS. The clinical relevance of this finding is unknown, as is the clinical relevance of the finding in this current study of no increase in antibody concentration or avidity in the group that had received the 12 month 23vPPS prior to mPPS. Moreover, all vaccinated groups had similar serotype-specific IgG at the end of the study.
The kinetics of avidity following vaccination varied for individual serotypes. The amount of high avidity serotype-specific IgG against serotype 6B increased post primary series following 2 or 3 PCV doses but not following a single PCV dose. However by 9 months of age, amounts of high avidity serotype-specific IgG were similar for serotype 6B across all PCV dose groups. In contrast to the avidity for serotype 6B, the avidity for serotype 23F did not increase by 9 months. Nevertheless, following the 12 month 23vPPS the increase in avidity for serotypes 6B and 23F increased with similar kinetics as observed for all other PCV groups. As expected, there was no significant increase in the avidity for the majority of non-PCV serotypes (except 15B and 19A) from 9 months to 12 months of age, prior to 23vPPS, and 12 month 23vPPS resulted in an increase in avidity for all non-PCV serotypes compared to those that did not receive the vaccine. As this is the first study to document avidity for non-PCV serotypes there are no other studies to compare results with.
Antibody avidity is an expression of the functional antibody affinity and is considered to affect the protective efficacy of antibodies. In vitro, higher avidity antibody is associated with greater opsonophagocytic capacity (11, 15). Avidity is therefore expected to correlate with specific IgG and OPA results (27), however this has not been consistently demonstrated. We have previously assessed the correlation between OPA titer, specific IgG, and avid IgG and have demonstrated a good correlation between OPA titer and specific IgG, and OPA titer and avid IgG for 5 of 6 PCV serotypes (4, 6B, 9V, 18C, 23F) both post-PCV and 23vPPS (28). However no relationship was found between the OPA titer and avid IgG for the non-PCV serotypes 1 and 5 (28). Other researchers have shown no association between antibody avidity and opsonophagocytic capacity however there was a tendency to negative correlation between the antibody concentration needed for opsonophagocytosis and relative avidity, suggesting that lesser amounts of high avidity antibody than of low avidity antibody were needed for bacterial killing (27). One caveat of our study is that avidity assays have not been standardized. However, our current findings for antibody avidity correlate with our OPA data for the same infants (29), supporting the validity of these findings. Two weeks following the 12 month 23vPPS, there were no significant differences in OPA for all serotypes between the single PCV dose group and the 3 dose group (29). Nevertheless, there are slight differences between these avidity results and previously published OPA (29) and our serotype-specific IgG antibody concentration data (20) for infants from this study during the first 12 months of life. We had found that 3 or 2 PCV doses elicited higher serotype-specific IgG antibody concentrations for most serotypes than a single dose, following 23vPPS (20). Cross reactivity between heterologous serotypes has been demonstrated (30) and may account for these differences.
In summary, this is the first large study to evaluate and compare functional antibody responses following various immunization schedules with PCV and 23vPPS. An important finding was that the functional antibody (avidity) following a single PCV dose was similar to that observed following 2 or 3 PCV doses in the primary series. Furthermore, we have shown that following 23vPPS, the increase in avidity seemed to be higher when preceded by a single dose of PCV as compared to 2 or 3 PCV doses, which is consistent with our previous findings in this cohort (21). In this study, we have also shown that the increase in avidity following a 23vPPS at 12 months of age was similar to that following a 17 month mPPS alone. Taken together, these findings support the use of abbreviated schedules in developing countries, either “2+1” or perhaps even a “1+1” schedule. However, there are several caveats to this approach: firstly, the value of a single PCV/23vPPS schedule is undermined by our current finding of immunological hyporesponsiveness post re-challenge (22). Secondly, it is uncertain whether significant protection from a single PCV dose administered early in infancy would persist throughout the “at risk” period. Nevertheless, based on our current findings, the early administration of a booster at 6 or 9 months of age (“1+1” schedule) would be worthy of further investigation for use in developing countries.
Acknowledgments
The authors wish to sincerely thank all the FiPP staff and families participating in the study, and the many other people who contributed to the study including: Jane Nelson, Amanda O’Brien, Kathryn Bright, Samantha Colquhoun, Amy Bin Chen, Timothy Gemetzis, Amy Auge, Katherine Gilbert, Evan Willis, Philip Greenwood, Beth Temple, Vanessa Johnston, Loretta Thorn, Porter Anderson, Brian Greenwood, George Siber, David Klein, Elizabeth Horigan, and Farukh Khambaty, and the members of the DSMB. Funding was provided by NIAID and the National Health and Medical Research Council, Australia. Pneumovax™ was kindly donated by CSL Biotherapies, Australia. The co-administered Tritanrix™-HepB™ and Hiberix™ vaccines were kindly donated by GlaxoSmithKline. Clinical trials number NCT00170612.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec. 2007 Mar 23;82(12):93–104. [PubMed] [Google Scholar]
- 2.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Jin P, Kong F, Xiao M, Oftadeh S, Zhou F, Liu C, et al. First Report of Putative Streptococcus pneumoniae Serotype 6D among Nasopharyngeal Isolates from Fijian Children. J Infect Dis. 2009 Nov 1;200(9):1375–80. doi: 10.1086/606118. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007 Apr;45(4):1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GAVI’s PneumoADIP, Department of International Health, Health. JHBSoP. Pneumococcal Regional Serotype Distribution for Pneumococcal AMC TPP; 2008 Contract No.: Document Number|.
- 6.Jodar L, Butler J, Carlone G, Dagan R, Goldblatt D, Kayhty H, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003 Jul 4;21(23):3265–72. doi: 10.1016/s0264-410x(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 7.Goldblatt D, Vaz AR, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. 1998 Apr;177(4):1112–5. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 8.Granoff DM, Anderson EL, Osterholm MT, Holmes SJ, McHugh JE, Belshe RB, et al. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr. 1992 Aug;121(2):187–94. doi: 10.1016/s0022-3476(05)81186-2. [DOI] [PubMed] [Google Scholar]
- 9.Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999 May;67(5):2366–70. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wuorimaa TK, Dagan R, Bailleux F, Haikala R, Ekstrom N, Eskola J, et al. Functional activity of antibodies after immunization of Finnish and Israeli infants with an 11-valent pneumococcal conjugate vaccine. Vaccine. 2005 Nov 16;23(46–47):5328–32. doi: 10.1016/j.vaccine.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Anttila M, Eskola J, Ahman H, Kayhty H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999 Apr 9;17(15–16):1970–7. doi: 10.1016/s0264-410x(98)00458-7. [DOI] [PubMed] [Google Scholar]
- 12.Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998 Jun;177(6):1614–21. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 13.Ekstrom N, Ahman H, Verho J, Jokinen J, Vakevainen M, Kilpi T, et al. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect Immun. 2005 Jan;73(1):369–77. doi: 10.1128/IAI.73.1.369-377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006 Apr;25(4):312–9. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 15.Lee LH, Frasch CE, Falk LA, Klein DL, Deal CD. Correlates of immunity for pneumococcal conjugate vaccines. Vaccine. 2003 May 16;21(17–18):2190–6. doi: 10.1016/s0264-410x(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 16.Schlesinger Y, Granoff DM. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992 Mar 18;267(11):1489–94. [PubMed] [Google Scholar]
- 17.Granoff DM, Lucas AH. Laboratory correlates of protection against Haemophilus influenzae type b disease. Importance of assessment of antibody avidity and immunologic memory. Ann N Y Acad Sci. 1995 May 31;754:278–88. doi: 10.1111/j.1749-6632.1995.tb44461.x. [DOI] [PubMed] [Google Scholar]
- 18.Lucas AH, Granoff DM. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. J Immunol. 1995 Apr 15;154(8):4195–202. [PubMed] [Google Scholar]
- 19.Russell FM, Carapetis JR, Tikoduadua L, Paeds D, Chandra R, Seduadua A, et al. Invasive pneumococcal disease in Fiji: clinical syndromes, epidemiology, and the potential impact of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010 Sep;29(9):870–2. doi: 10.1097/INF.0b013e3181ec7ae2. [DOI] [PubMed] [Google Scholar]
- 20.Russell FM, Balloch A, Tang ML, Carapetis JR, Licciardi P, Nelson J, et al. Immunogenicity following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2009 Sep 18;27(41):5685–91. doi: 10.1016/j.vaccine.2009.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell FM, Licciardi PV, Balloch A, Biaukula V, Tikoduadua L, Carapetis JR, et al. Safety and immunogenicity of the 23-valent pneumococcal polysaccharide vaccine at 12 months of age, following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine in infancy. Vaccine. 2010 Apr 19;28(18):3086–94. doi: 10.1016/j.vaccine.2010.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell FM, Carapetis JR, Balloch A, Licciardi PV, Jenney AW, Tikoduadua L, et al. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial. Vaccine. 2010 Apr 26;28(19):3341–9. doi: 10.1016/j.vaccine.2010.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahm MH, Goldblatt D. Training manual for enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA) http://www.vaccine.uab.edu/ELISA%20Protocol.pdf. Journal [serial on the Internet]. 2006 Date.
- 24.Balloch A, Licciardi PV, Leach A, Nurkka A, Tang ML. Results from an inter-laboratory comparison of pneumococcal serotype-specific IgG measurement and critical parameters that affect assay performance. Vaccine. Feb 3;28(5):1333–40. doi: 10.1016/j.vaccine.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Wuorimaa T, Dagan R, Vakevainen M, Bailleux F, Haikala R, Yaich M, et al. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J Infect Dis. 2001 Nov 1;184(9):1211–5. doi: 10.1086/323648. [DOI] [PubMed] [Google Scholar]
- 26.Goldblatt D, Akoto A, Ashton L, et al. Immunogenicity and the generation of immune memory following 9-valent pneumococcal conjugate vaccination in Ghanaian infants with sickle cell disease; 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 Sept 24–27; Toronto, Ontario, Canada. [Google Scholar]
- 27.Anttila M, Voutilainen M, Jantti V, Eskola J, Kayhty H. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin Exp Immunol. 1999 Dec;118(3):402–7. doi: 10.1046/j.1365-2249.1999.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balloch A, Licciardi P, Russell F, Burton R, Nahm M, Mulholland K, et al. Does serotype-specific IgG and avidity to Streptococcus pneumoniae following infant immunisation correlate with functional opsonophagocytic activity?. 6th International Symposium on Pneumococci and Pneumococcal Diseases; 2008 June 8–12; Reykjavik, Iceland. 2008. [Google Scholar]
- 29.Russell FM, Carapetis JR, Burton RL, Lin J, Licciardi PV, Balloch A, et al. Opsonophagocytic activity following a reduced dose 7-valent pneumococcal conjugate vaccine infant primary series and 23-valent pneumococcal polysaccharide vaccine at 12 months of age. Vaccine. Jan 10;29(3):535–44. doi: 10.1016/j.vaccine.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Licciardi PV, Balloch A, Russell FM, Mulholland EK, Tang MLK. Antibodies to serotype 9V exhibit novel serogroup cross-reactivity following infant pneumococcal immunization. Vaccine. 2010 May 14;28(22):3793–800. doi: 10.1016/j.vaccine.2010.03.033. [DOI] [PubMed] [Google Scholar]