Abstract
Both physiological and behavioral studies provide evidence to suggest that deficits in frontal cortical control circuits may contribute to the risk for developing alcohol dependence. Event-related potential (ERP) and eye blink responses to startle and short delay prepulse-plus-startle stimuli, and psychiatric diagnoses were investigated in young adult (age 18-30 yrs) men (n=135) and women (n=205) Mexican Americans. Women displayed a significant increase in the amplitude of the eye blink response to both the startle and pre-pulse-plus-startle stimuli. None of the psychiatric diagnoses were associated with differences in eye blink responses. ERP responses to the startle and prepulse-plus startle stimuli included a negative polarity wave at approximately 400ms that was of the highest amplitude in the frontal leads (N4S). Women were found to have significantly higher amplitude N4S responses than men. Participants with alcohol dependence demonstrated significantly less inhibition and more facilitation of the N4S component by the pre-pulse stimuli. This finding was not associated with a diagnosis of: any other drug dependence disorder (including nicotine), anxiety or affective disorder, or conduct/antisocial personality disorder. The present study suggests that gender and a lifetime diagnosis of alcohol dependence may selectively contribute to this frontal late wave electrophysiological response to prepulse-plus-startle stimuli.
Keywords: Alcohol Dependence, ERPs, N400, frontal disinhibition, Prepulse Inhibition, Startle, Mexican Americans
1. Introduction
The identification of neurophysiological markers associated with psychiatric disorders in general and alcohol dependence in particular may help in determining the causal relationship between clinical phenomena associated with the disorder and basic molecular processes. Electrophysiological studies of individuals with alcohol dependence and subjects with a family history of alcoholism have demonstrated that deficits in a number of event-related potential (ERPs) components, with robust findings observed for late positivities (300-450 msec) (this literature is very large and there are a number of excellent reviews, see Begleiter and Porjesz, 1999, Porjesz and Begleiter, 2003, Porjesz et al., 2005, Campanella et al., 2009]) and more recently late negativities (300-650 msec) as well (see Roopesh et al., 2009, 2010).
Another psychophysiological measure that has been used to assess risk for and the consequences of alcohol use is the acoustic startle reflex (ASR) and prepulse inhibition of the startle (PPI). The startle reflex is actually a constellation of responses usually indexed by eye blink responses in humans but also by electrophysiological recordings from cortical areas (see Swerdlow et al., 1992, Ford et al., 1999). Prepulse inhibition of the startle (PPI) refers to the fact that if a weak stimulus is presented 30-500 msec prior to the presentation of the startle stimuli (prepulse) the behavioral response to the startle will generally be reduced in amplitude. It has been suggested that prepulse inhibition is an index of automatic sensorimotor gating (Geyer and Swerdlow, 2001). Whereas, prepulse facilitation (PPF) refers to the phenomena whereby the behavioral response to the startle is enhanced by weak stimuli (prepulses) presented at very short (less than 20 msec) or long (more that 500 ms) intervals prior to the startle stimuli. PPF has been suggested to reflect a combination of alerting, attention and/ or arousal (see Filion et al., 1998; Ludewig et al., 2003, Hsieh et al., 2006). The neuroanatomical substrates of the behavioral response to ASR/PPI/PPF have been extensively investigated in both humans and animals (see Swerdlow et al., 1994; Braff et al., 2001a, 2001b; Kumari et al., 2005). While most studies have focused on describing brain stem and midbrain contributions to the behavioral response to startle stimuli (see Fendt et al., 2001) it is clear that it involves a complex neural network extending from brainstem nuclei via the thalamus to higher order cortical areas that may regulate cognitive responses to startle (Schall et al., 1999, Fendt et al., 2001; Kumari et al., 2005; Campbell et al., 2007; Neuner et al., 2010). There is some evidence that the cognitive response to ASR/PPI may share a common underlying neurophysiology with some behavioral and clinical measures of cognition that require response inhibition (See Filion et al., 1999). For instance, both performance on the Wisconsin Card Sorting Task and PPI of startle have been suggested to reflect prefrontal cortical function and dysfunction (see Filion et al., 1999, Swerdlow and Geyer 1999).
Startle stimuli not only elicit a behavioral response but also generate a series of electrophysiological responses that can be averaged from the EEG and may be useful in the understanding of the cognitive responses to startle stimuli and its potential relationship to alcohol dependence. A number of studies have described startle ERP paradigms in humans that have been reported to generate N1, P2 and P3 and late wave components using scalp electrodes (see Roth et al., 1982, 1984; Putnam and Roth, 1990; Ford et al., 1999; Ornitz et al., 2001). Most studies have focused on the P300 component of the startle elicited ERP. The amplitude of the P300 startle ERP has been demonstrated to respond to both PPI and PPF, task determinants, as well as allocation of attention, changes in arousal, and emotional context. (see Roth et al., 1982,1984; Putnam and Roth 1987, 1990; Ford and Pfefferbaum, 1991; Suguwara et al., 1994; Hirano et al 1996; Schupp et.al., 1997, Cuthbert et al., 1998; Ornitz et al., 2001). The neural generators of the P300 to startling noises are not entirely known. It has been suggested that the neural circuits involved in blink responses to startle are different from those that generate the cortical ERP responses (Schupp et al., 1997; Ford et al., 1999). In addition, P300s generated by startle also appear to have different neural substrates than P300s elicited by standard auditory oddball targets (Ford et al., 1994). ERP responses to oddball targets have been suggested to arise from the temporal-parietal junction (Knight et al., 1989; Menon et al., 1997), whereas; it has been suggested that startle P300's most likely involve more frontal cortical structures (Ford et al., 1994,1999). Other ERP responses to startle include a negative-going late wave response (slow wave) in the 360-600 ms range that has been described in young normal controls (Putnam and Roth, 1990). This late wave response, was found to be maximal in frontal areas and was also found to show the greatest enhancement in amplitude due to changes in task requirements in that study (Putnam and Roth, 1990). This negativity may be an important index of cognitive responses to startle stimuli involving frontal cortical areas, however, it has not been extensively studied, especially in relation to psychiatric diagnosis.
Impairments in frontal lobe function and associated behaviors such as impulsivity and executive functioning have long been important theoretical constructs in the understanding of alcohol dependence (see Pfefferbaum et al., 1997; Begleiter and Porjesz, 1999; Crews and Boettinger, 2009; Campanella et al., 2009; Field et al., 2010). Behavioral responses (eye blinks) to a number of startle paradigms have been shown to be altered in patients with alcohol dependence. Increases in startle magnitudes have been observed during early withdrawal and abstinence (see Krystal et al., 1997; Saladin et al., 2002). Attenuated startle responses have also been observed in abstinent alcoholics when startle stimuli were associated with alcohol-related stimuli (Grusser et al., 2002). Additionally, reduced eye blink responses to startle stimuli associated with unpleasant stimuli has been seen in alcoholics with antisocial personality disorder (ASPD) (Miranda et al., 2003). Modulation of the startle by alcohol- associated cues has also been linked with relapse to drinking in alcohol dependent patients in treatment (Loeber et al., 2007).
Startle paradigms have also been incorporated into studies evaluating risk for alcohol dependence by evaluating offspring of alcohol dependent individuals. In one study, startle potentiation to negative stimuli was not found in participants with a family history of alcohol dependence as compared to individuals without such a family history (Miranda et al., 2002). In another study, responses to PPI but not to startle were found to be impaired in children with a parental history of alcohol dependence as compared to children of normal controls (Grillion et al., 1997). These two studies suggest that behavioral responses to some aspects of startle may represent a pre-existing or trait variable associated with risk for alcohol dependence. However, ERP studies that may index more of the cognitive responses to startle paradigms have not been recorded in participants with either a personal or family history of alcohol dependence, or other psychiatric disorders that are co-morbid with alcohol dependence.
The present investigation sought to explore the use of a startle paradigm, that uses startle and short delay prepulse-plus-startle stimuli, that elicit a large frontal negative slow wave. The study extends our initial studies of background EEG variants, and P300 ERP responses to facial expressions, in a population of young adult Mexican Americans at high risk for the development of alcoholism (see Criado and Ehlers, 2007; Ehlers and Phillips, 2007). The present study evaluated several new hypotheses. First, we sought to describe late wave frontal ERP component responses (designated the N4S) to startle and prepulse/startle stimuli in this population and determine if they differed by sex. Secondly, we evaluated whether the late wave N4S component to startle and prepulse/startle were altered as a function of the diagnosis of alcohol dependence and/or a family history of alcohol dependence. Thirdly, we also assessed whether any potential changes in N4S were seen in disorders previously found to be co-morbid with alcohol dependence in this population: antisocial personality disorder/conduct disorder (ASPD/CD) and affective/anxiety disorders (ANYAXAF) and other drug dependence (Gilder et al., 2007).
2. Materials and methods
2.1. Participants
Participants were recruited using a commercial mailing list that provided the addresses of individuals with Hispanic surnames in 11 zip codes in San Diego County that were identified as having a population that was over 20% Hispanic heritage and were within 25 miles of the research site. The mailed invitation stated that potential participants must be of Mexican American heritage, be between the ages of 18 and 30 years, be residing in the United States legally, and be able to read and write in English. Potential participants were requested to phone research staff for more information. During the phone interview potential participants were screened for the presence of the inclusion criteria as listed above and printed on the mailed invitation, and were excluded it they were: pregnant or nursing, currently had a major medical or neurological disorder, or a head injury that might bias the ERP testing. Participants were asked to refrain from alcohol or any other substance use for 24 hours prior to testing. On the test day, after a complete description of the study to the participants, written informed consent was obtained using a protocol approved by The Institutional Review Board of The Scripps Research Institute.
2.2. Psychiatric diagnoses
Each participant completed an interview with the face-to-face Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994), which was used to make substance use and other psychiatric disorder diagnoses according to DSM-III-R criteria. The SSAGA is a fully structured, polydiagnostic psychiatric interview that has undergone both reliability and validity testing (Bucholz et al., 1994; Hesselbrock et al., 1999). Family history of alcohol dependence was assessed using the Family History Assessment Module (FHAM) (Rice et al., 1995). Participants were eliminated from the current data analyses if they were taking psychoactive medication that may affect the ERP or had a positive breath-analyzer test on the day of the evaluation. Lifetime history of alcohol dependence, other drug dependence (marijuana, stimulants, sedatives, hallucinogens, opiods, nicotine), antisocial personality disorder/conduct disorder (ASPD/CD), major depressive disorder with impairment, and “any anxiety disorder” (social phobia, agoraphobia, panic disorder or obsessive compulsive disorder) in this population were defined by DSM-III-R criteria.
2.3. Startle ERP collection and analyses
Recordings were obtained from participants who were seated on a hospital bed in a sound-attenuated room. Acoustic startle stimuli were presented binaurally through headphones. The behavioral response to the startle (eye blink) is recorded using electrodes placed below and lateral to the eye as described by Braff et al. (2001b). The auditory stimuli consist of 45 trials. These trials include randomly presented startle stimuli (115 dB white noise burst for 40 msec n=30) and prepulse-startle stimuli (85 dB white noise burst for 20 msec-duration) immediately (<5 msec) followed by the startle (115 dB white noise burst for 40 msec n=15). Each individual startle and/or prepulse startle trial is separated by an interval of 15 seconds. Background white noise was presented for the entire session at a level of 60 dB. The behavioral variables assessed included: ASR magnitude on startle trials and prepulse trials as determined by quantification of the eye blink response as described below.
Seven channels of ERP data (FZ, CZ, PZ, F3, F4, F7, and F8, referenced to linked ear lobes with a forehead ground, international 10-20 system) were obtained using gold-plated electrodes with impedance held below 5 KΩ. Frontal electrodes were emphasized in the montage as previous data had suggested that ERP decrements in frontal areas distinguished subjects with a risk for alcohol dependence (see Bauer, 1997). An electrode placed left lateral infraorbitally and reference to the left earlobe was used to monitor both horizontal and vertical eye movements. ERP signals were amplified (time constant 0.3 s, 35 Hz low pass) using a Nihon Kohden EEG machine and were transferred on-line to a PC. The combined gain of the EEG amplifiers and the analog-to-digital multiplexer amplifier was 50K.
The eye blink and ERP trials were simultaneously digitized at a rate of 256 Hz (bandwidth 0.5-35 Hz). Individual trials where the EEG or eye blink exceeded ±250 microvolts (<5% of the trials) were eliminated before averaging. The N4S component of the ERP was quantified using a computerized peak detection routine that identifies baseline-to-peak amplitudes (in µV) within the specified latency window (350-500 msec). The eye blink amplitude was also assessed using this routine. The latency window for the eye blink was 50-120 msec. The baseline was determined by averaging the 150 ms of pre-stimulus activity obtained for each trial. The routine is user-driven and each peak detection must be verified by the user. All peaks were quantified by one investigator, and verified by a second investigator, both of whom were blind to participants' characteristics.
2.4. Data analysis
Data analyses focused on the three specific aims: (1) to describe late wave frontal ERP component responses (designated the N4S) to startle and prepulse/startle stimuli and determine if they differed by sex; (2) to evaluate whether the eye blink and/or N4S component responses to startle and prepulse/startle were altered as a function of the diagnosis of alcohol dependence and/or a family history of alcohol dependence; (3), to asses whether N4S responses were altered in other conditions typically co-morbid with alcohol dependence (e.g. antisocial personality disorder/conduct disorder (ASPD/CD) and affective/anxiety disorders (ANYAXAF) and other drug dependence).
To reduce the number of independent analyses performed on inter-related N4S data and to reduce the overall probability of Type 1 error, a principal component analysis (PCA) was performed. These analyses have been used previously in a number of different ethnic populations (see Bauer and Hesselbrock 1999a, 1999b, 2001, 2003; Ehlers et al., 2001). The N4S amplitudes for the seven electrode locations for the startle and prepulse startle stimuli were entered into the PCA. For each of the stimuli, varimax rotation yielded two components. The electrode sites loading on the first factor for the startle alone stimuli were: the frontal leads (FZ, F3, F4, F7, F8) (loadings= .861, .847, .882, .712, .844), and on the second factor for the startle alone stimuli were the two more posterior leads (CZ, PZ) (loadings= .790, .930). The percent of the variance explained by the first factor was 53% and the second factor was 30% for a total of 83% of the variance of the N4S amplitude to the startle explained by this model. The PCA for the prepulse plus startle stimuli revealed that the electrode sites loading on the first factor were: the frontal leads (FZ, F3, F4, F7, F8) (loadings= .853, .857, .870, .745, .823), and on the second factor for the prepulse plus startle stimuli were the two more posterior leads (CZ, PZ) (loadings= .779, .927). The percent of the variance explained by the first factor was 53% and the second factor was 30% for a total of 83% of the variance of the N4S amplitude to the prepulse plus startle being explained by this model.
N4S amplitudes were averaged across the electrode sites within each of the two identified components 1 = (FZ, F3, F4, F7, F8), 2 = (CZ, PZ) generating a mean for each of the two regions. These regionally averaged scores were generated for each stimulus condition (startle, prepulse-startle), generating mean amplitudes for each of the two component regions for each stimulus category for each individual. These regionally averaged N4S amplitudes were used as dependent variables.
In order to test hypothesis 1, the eye blink and regionally averaged N4S amplitude responses to startle and prepulse/startle stimuli were compared between men and women using ANOVA. To test hypothesis 2, eye blink and regionally averaged N4S component responses to startle and prepulse/startle were compared between those participants with a lifetime diagnosis of alcohol dependence and those without this diagnosis using ANCOVA (co-varying for sex). In a second set of analysis, eye blink and regionally averaged N4S component responses to startle and prepulse/startle were compared in participants with and without a first degree family history of alcohol dependence. These analyses were accomplished in a subset of the population that had no lifetime diagnoses of: alcohol dependence, antisocial personality disorder/conduct disorder (ASPD/CD), affective/anxiety disorders (ANYAXAF) or other drug dependence using ANCOVA (co-varing for sex). To test hypothesis 3, eye blink and regionally averaged N4S component responses to startle and prepulse/startle were compared between those participants with and without other psychiatric disorders commonly co-morbid with alcohol dependence: antisocial personality disorder/conduct disorder (ASPD/CD), affective/anxiety disorders (ANYAXAF) and other drug dependence using ANCOVA (co-varing for sex). Statistical significance was set at the 0.05 probability level.
3. Results
3.1. Descriptive data and sex differences in eye blink and N4S responses to startle and prepulse plus startle stimuli
Demographic data on the 340 participants with valid ERP data are presented in table 1. The sample contained more women participants (n=205, 60%) than men (n=135, 40%). Lifetime DSM-III-R alcohol dependence was found in 33% (n=44) of the men and 22% (n=45) of the women. Of those participants with lifetime alcohol dependence 28% (n=25) had three dependence symptoms in the last month. A first degree family history of alcohol dependence was found in 40% (n=135) of the population. Nine percent of the females (n=18) and 17% (n=23) of the males were diagnosed with conduct disorder or conduct/ASPD. Thirty two percent of the females (n=65) and twenty-four percent of the males (n=32) were found to have a lifetime diagnosis of affective and/or anxiety disorder. Thirty-eight percent of the men (n=51) and 30 percent of the women (n=61) had a diagnosis of another drug disorder (nicotine, cannabis, hallucinogens, stimulants, sedatives, opioids). As previously described, alcohol dependence was significantly co-morbid with: other drug dependencies, ASPD/CD, anxiety and affective disorders (see Gilder et al., 2007).
Table 1.
Demographic characteristics and eye blink responses to the startle stimuli of Mexican American participants comparing alcohol dependence groups, Antisocial personality disorder/conduct disorder groups, and anxiety or affective disorder groups (n = 340)
| Demographic | Total sample (n =340) | Alcohol dependence (n = 89) | Antisocial personality disorder and/or conduct disorder (n = 41) | Anxiety or affective disorder (n = 97) | Other drug dependence (n=112) |
|---|---|---|---|---|---|
| Age (years) | 23.5 (3.69) | 24.0 (3.77) | 24.6 (5.76) | 22.9 (3.94) | 23.5 (3.17) |
| Gender (n) | |||||
| Male | 135 | 44 1 | 23 1 | 32 | 51 |
| Female | 205 | 47 | 18 | 65 | 61 |
| Years of education | 13.24 (1.84) | 13.26 (1.89) | 12.3 (1.92) 2 | 13.1 (1.97) | 12.64 (2.12) 2 |
| Income | |||||
| < $20,000 | 55 | 15 | 13 3 | 14 | 19 3 |
| > $20,000 | 285 | 74 | 28 | 83 | 93 |
| Eye blink amplitude to startle (microvolts) | 42.31 (41.30) | 41.07 (41.13) | 39.38 (41.17) | 37.69 (40.87) | 41.33 (40.96) |
| Eye blink amplitude to startle following prepulse (microvolts) | 41.29 (40.93) | 40.1 (40.47) | 36.83 (40.53) | 36.32 (40.25) | 39.66 (40.34) |
| % prepulse inhibition | 2.41 | 2.36 | 6.48 | 3.63 | 4.04 |
Values are Means (SD) unless indicated
p < 0.05 gender difference diagnosis vs. no diagnosis
p < 0.05 education difference diagnosis vs. no diagnosis
p < 0.05 income difference diagnosis vs. no diagnosis
In this study, the presentation of auditory stimuli in the form of startle and short delay pre-pulse plus startle tones produced a robust eye blink response. Mean values for the eye blink response are presented in table 1. As predicted, using a short delay prepulse, low levels of prepulse inhibition were seen using this paradigm. This startle paradigm also produced a series of waves that could be averaged from the EEG as seen in figure 1. A series of small waves (< 7 microvolts) occurred between 10 and 100 ms in all three leads. These waves were followed by a large (10-20 microvolts) negative going wave (N1) that occurred at around 120 milliseconds in the frontal areas and at 105 milliseconds in the centro-pariental areas, that was of highest amplitude in the central leads. The N1 potential was followed by a large positive going wave (15-25 milliseconds) that occurred between 200 and 300 milliseconds and was of highest amplitude in central leads. This was followed by a negative wave that peaked at 400 milliseconds that occurred earlier and at higher amplitude in frontal areas. The late 400 millisecond wave (N4S) is the subject of this investigation.
Figure 1.
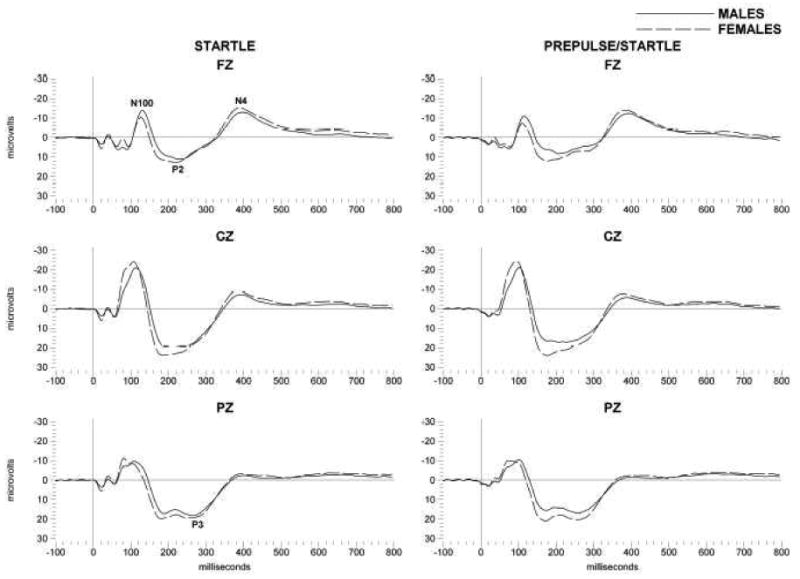
Grand averages of event-related potentials (ERPs) elicited by startle (left column) and prepulse startle (right column) stimuli in Mexican American young adults. Averages are presented for frontal (Fz) and central (Cz) and parietal (Pz). The N4 component is indicated; solid lines are for male participants and dashed lines for females. X axis represents time (milliseconds, ms) after presentation of the startle stimuli, Y axis is amplitude in microvolts.
When these N4S responses were compared in the entire population between men and women significant sex differences emerged as also seen in figure 1. Women were found to have significantly higher amplitude N4S responses to the startle (Frontal: F=11.36; df=1,339; p<0.001, Centro-parietal: F=8.5; df=1,339; p<0.004) and pre-pulse startle (Frontal: F= 6.5; df=1,339; p<0.01, Centro-parietal: F=5.2; df=1,339; p<0.02) stimuli. Women also displayed an increase in the eye blink response to startle (F=9.5; df=1,339; p<0.002) and pre-pulse startle stimuli (F=11.9; df=1,339; p<0.001).
3.2. Associations of eye blink and N4S responses to startle and prepulse plus startle stimuli in Mexican American young adults with alcohol dependence, family history of alcohol dependence, and co-morbid diagnoses
N4S amplitudes were also evaluated in the frontal and centro-parietal leads in this population (n=340), as a function of alcohol dependence. Multivariate ANOVA, that co-varied for sex, revealed that those participants with a lifetime DSM-III-R diagnosis of alcohol dependence had significantly increased amplitude N4S responses to the prepulse/startle stimuli as compared to those participants with no alcohol dependence diagnoses, in the frontal areas, as seen in figures 2 and 3 (F= 4.28; df=1,339; p<0.04). Multivariate ANOVA also revealed no significant associations between ASPD/CD, AXAF, or OTHER DRUG diagnoses, and N4S amplitudes (data not shown). There was no significant association between a family history of alcohol dependence and N4S amplitudes although there was a trend in the same direction as that found for alcohol dependence, more pre-pulse facilitation to startle stimuli in frontal areas in family history positive participants(F=1.77; df=1,339; p<0.19) (data not shown). There were no significant associations found between eye blink response to the startle or pre-pulse plus startle and alcohol dependence, family history of alcohol dependence or any of the co-morbid diagnoses.
Figure 2.
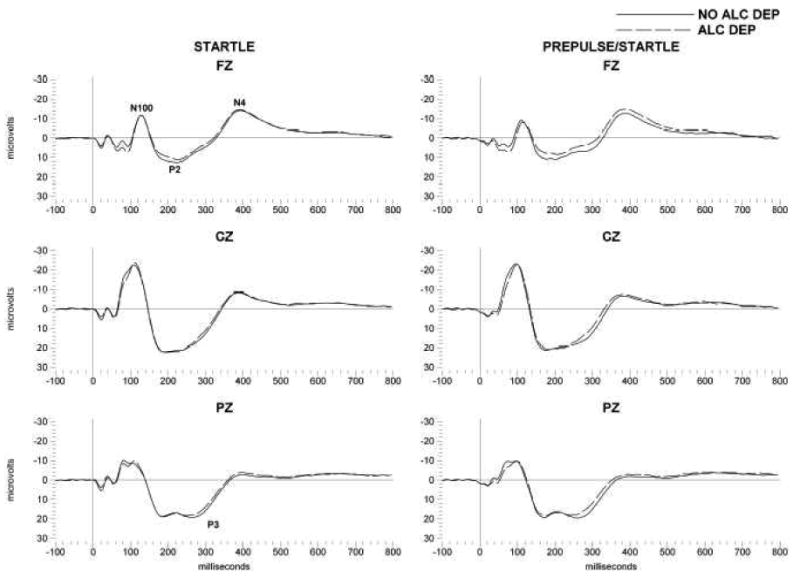
Grand averages of event-related potentials (ERPs) elicited by startle (left column) and prepulse startle (right column) stimuli in Mexican American young adults. Averages are presented for frontal (Fz) and central (Cz) and parietal (Pz). The N4 component is indicated; solid lines are for participants without a diagnosis of alcohol dependence and dashed lines for participants with a diagnosis of alcohol dependence. X axis represents time (milliseconds, ms) after presentation of the startle stimuli, Y axis is amplitude in microvolts.
Figure 3.
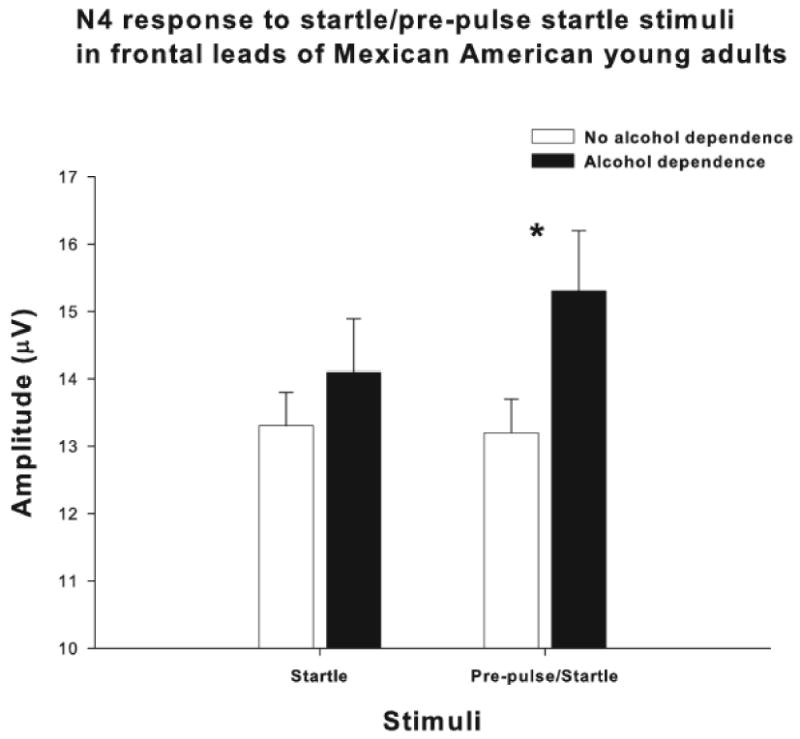
N4 component amplitudes (microvolts) to startle and prepulse startle stimuli in those participants with a lifetime diagnosis of alcohol dependence in the black bars, and in those without an alcohol dependence diagnosis in the open bars. Values are given as means ± SEM. Asterisk indicates a significant difference between groups. (*p<0.04)
4. Discussion
In the present study a startle plus short delay (<5 ms) prepulse plus startle paradigm was used to generate behavioral (eye blink) and ERP responses to the stimuli. Short delay long duration prepulses have been demonstrated to produce prepulse facilitation (PPF) in some paradigms (Hsieh et al., 2006). The present study used short delay and short duration prepulses, and this combination did not produce significant PPF or PPI in eye blink responses in this study. It has been suggested that eye blink responses may be highly sensitive to differences in a number of parameters of the startle stimuli including background noise, prepulse duration, frequency and interval. Additionally, it has been further suggested that differences in these parameters may explain failures to replicate associations between psychiatric diagnoses and eyeblink responses to the startle in some studies (see Hsieh et al., 2006). This was likely the case in the present study where eye blink responses were not found to be associated with any of the psychiatric diagnoses investigated.
Using the startle plus short delay (<5 ms) prepulse plus startle paradigm the present study did confirm previous studies that described an ERP component, in the 360-600 ms range, that could be elicited by startle/prepulse startle stimuli (Putnam and Roth, 1990). A late negative component in the 400ms range, designated the N4S was identified in the present study. It has been suggested that the neural circuits involved in blink responses to startle are different from those involved in the cortical ERP responses (Schupp et al., 1997; Ford et al., 1999). The N4S identified in the present study was found to be of the highest amplitude in the frontal areas perhaps suggesting that it may be a reflection of frontal cortical involvement in cognitive responses to startle stimuli.
The present study also extends previous studies by uncovering a series of relationships between the amplitude of this N4S ERP component, sex and alcohol dependence. In this study women displayed higher eye blink responses and also higher N4S amplitude responses to both the startle and pre-pulse startle stimuli. These data confirm our previous data analyses in a subset of the present population demonstrating that women had higher P450 amplitude responses in a facial expression recognition task using affective stimuli (happy and sad faces) than men (Criado and Ehlers, 2007). Taken together these data could be interpreted as either a greater electrophysiological response to emotional stimuli in women or a less intense response to emotional stimuli in men. Greater ERP responses to emotional stimuli in women have been reported in a number of populations. For instance, higher amplitude and longer latency P450 responses were found to happy and sad faces in women as compared to men using a facial discrimination paradigm (Orozco and Ehlers, 1998). In another study using facial expressions as stimuli, the N2b component was found to be delayed in men in response to happy stimuli as compared to fearful ones (Campanella et al., 2004). Larger P450 ERP components to negative emotional images in women as compared to men have also been reported (Yuan et al., 2009). These data also confirm what has been reported previously for simple oddball tasks (see Hoffman and Polich, 1999) where women were found overall to have higher amplitude P350 and P450 ERP components than men. However, gender effects on behavioral responses to startle have been inconsistent with some studies finding no effects of sex (Ludewig et al., 2003), and others finding that women show less PPI compared with men (Aasen et al., 2005; Kumari et al., 2008). In the present study, although overall amplitudes were different between men and women, the difference between the amplitude of the startle compared to the prepulse/startle was not different between men and women in either the N4S or the eye blink data.
The present study also evaluated whether a relationship existed between the N4S startle ERP response and a diagnosis of alcohol dependence and other co-morbid disorders. Data from a number of electrophysiological and behavioral genetics studies have converged on the idea that substance dependence and antisocial behavioral disorders comprise a spectrum that may have common risk factors (Begleiter and Porjesz, 1999; Waldman and Slutske, 2000; Iacono et al., 2003; Button et al., 2005; Du et al., 2006; Patrick et al., 2006). There is also ample evidence supporting an association between low P3 amplitude and these externalizing disorders (Bauer et al., 1994; Bauer, 1997; Bauer and Hesselbrock, 1999a, 1999b, 2003; Costa et al., 2000; Iacono et al., 2002, 2003; Kamarajan et al., 2005, 2006). Our previous studies in a subset of the present population found that a personal history of ASPD/CD was associated with significant reductions in P450 amplitudes; specifically to happy faces in frontal and centro-parietal areas and only in men, not in women. In the present study, although a facilitation of the N4S by prepulse stimuli was found in those participants with a lifetime diagnosis of alcohol dependence, a lifetime diagnosis of ASPD/CD was not associated with changes in the N4S response to the startle or pre-pulse stimuli. Therefore the N4S facilitation found associated with alcohol dependence in the present study does not appear to be a generalized marker of externalizing diagnoses.
It is not known whether the N4S facilitation of startle responses following pre-pulse stimuli seen in alcohol dependent participants in the present study is an endophenotype associated with alcohol dependence or represents a marker of alcohol exposure. Behavioral responses to startle are known to be impacted by both risk for alcoholism and chronic ethanol exposure in humans and animal models (Grillon et al., 1997; Krystal et al., 1997; Grusser et al., 2002; Saladin et al., 2002; Miranda et al., 2002, 2003; Chester et al., 2004; Zimmermann et al., 2004; Loeber et al., 2007). Generally behavioral responses to startle are decreased following ethanol administration or consumption humans (Hutchison et al., 1997, 2003; Grillon et al., 2000; Saladin et al., 2002; Zimmermann et al., 2004; Moberg and Curtin, 2009) and in rodents (Pohorecky et al., 1976; Rassnick et al., 1992; Sandbak et al., 2000; Slawecki et al., 2006).
However, during the early phases of ethanol withdrawal, ASR is increased in human alcoholics (Krystal et al., 1997) and in rats previously treated with alcohol and then withdrawn (Pohorecky et al., 1976; Rassnick et al., 1992; Macey et al., 1996; Vandergriff et al., 2000; Chester et al., 2004). It has been suggested the enhanced acoustic startle response (ASR) during the early phases of withdrawal is an index of increased anxiety (Gulinello et al., 2003; Harris and Gewirtz, 2004). As such, assessment of the startle response may provide an index of persistent anxiety-like behavior following ethanol exposure. No significant associations were found between the presence of anxiety or affective disorders and the eye blink or N4S response to the startle in the present study. However, it is possible that the facilitation of the N4S response to pre-pulse startle stimuli could represent a marker of subclinical anxiety from alcohol exposure in the alcohol dependent participants.
Both startle and PPI has been demonstrated to be impaired in children with a parental history of alcoholism (Grillon et al., 1997, 2000; Miranda et al., 2002; Zimmermann et al., 2004). Decreases in the eye blink responses to startle or pre-pulse startle were not found to be associated with a personal or family history of alcohol dependence in the present study. However, the present study used a different set of eliciting stimuli than previous investigations in that a short delay between prepulse and the startle stimulus was employed. However, in the present study, a trend was found for an association between facilitation of the N4S response to pre-pulse startle stimuli and a family history of alcohol dependence. Thus it is not clear at this time whether the facilitation of startle responses following pre-pulse stimuli seen in alcohol dependent participants in the present study is an endophenotype associated with alcohol dependence or represents a marker of alcohol exposure or a gene-environment interaction.
Although the relationship between late positive complexes (P300) and alcohol dependence has been extensively studied, fewer studies have explored late negativities in the 350-600 msec range. In one study a lexical decision task was employed to study 87 alcohol dependent subjects and 57 community controls. In that study alcohol dependent participants were found to display less attenuation of the N400 response to primed words when compared to unprimed words (Roopesh et al., 2010). Additionally, similar findings were observed for a group of high risk offspring of alcoholics as compared to low risk children where the same finding of a lack of attenuation for primed words was observed (Roopesh et al., 2009). These data and additional data demonstrating significant heritability of the N400 component (Almasy et al., 1999, 2001) suggests that the N400 recorded by Roopesh and colleagues may be an endophenotype associated with alcohol dependence. The N4S generated in the present study is not comparable to the classic N400 typically used in linguistic tasks and as used by Roopesh and colleagues (2009, 2010) and is closer to a passive auditory oddball paradigm. However, it should be noted that N400s have been generated to signed words, drawings, photos, videos, objects and environmental sounds (see Kutas and Federmeier, 2011 for review). In that context, the N4S recorded in the present study may index a process whereby an individual is determining whether the startle stimulus is something to “react to” as a “meaningful” environmental noise or whether it should be ignored. Typically, N400s to linguistic stimuli are found to be maximal at centro-parietal sites (Kutas and Van Petten, 1988). The N4S observed in the present study was found to be maximal in frontal areas, and it may have more commonality with what has been described as an “anterior negativity” (Kutas and Van Petten, 1994) that is more “automatically” generated.
Although the cognitive concomitants of the N4S recorded in the present study are currently unknown, the fact that it indexes the presence of alcohol dependence may still be an important finding. Impairments in frontal lobe function and associated behaviors such as impulsivity and executive functioning have been an important theoretical constructs in the understanding of alcoholism (see Pfefferbaum et al., 1997; Begleiter and Porjesz, 1999; Crews and Boettinger, 2009; Campanella et al., 2009; Field et al., 2010). Additional studies will be necessary to determine if the facilitation of the N4S ERP potential by prepulses indexes any of those anatomical or behavioral variables.
In summary, these data suggest that the N4S response to startle/pre-pulse startle stimuli in Mexican American young adults differs depending on sex and the presence of an alcohol dependence diagnosis. However, the results of this study should be interpreted in the context of several limitations. First, the findings may not generalize to the general American population of mixed heritage or all Mexican Americans, or all Hispanic young adult Americans. Over half of the participants in the present were women and thus findings many not generalize to previous studies that have focused on samples of entirely male participants. Second, the study was limited to young adults between the ages of 18 and 30 years, and the sample size may limit the interpretation of the relationship between the N4S and the variance in severity or chronicity of alcohol dependence. Further studies employing a longitudinal design will be required to test the relationship of N4S amplitude, sex, alcohol dependence and co-morbid disorders. The lack of participants with psychotic disorders and bipolar disease limits the ability to test whether N4S facilitation is also found in those patient populations. Despite these limitations, this report represents an important step in an ongoing investigation to determine risk and protective factors associated with development of substance use disorders in this select Mexican American population.
Acknowledgments
Supported in part by National Institutes of Health, National Institute on Alcoholism and Alcohol Abuse (NIAAA) grants AA006420 and AA010201 and the National Center for Minority Health and Health Disparities (NCMHD). JRC is recipient of the Dallas and Mary Clark Fellowship in Human Neurophysiology at the Brain Research and Treatment Center, Scripps Clinic, La Jolla, CA. The computer programs were written by Dr. James Havstad. The authors thank, Phil Lau, Derek Wills, and Susan Lopez for assistance in data collection and analyses, and Shirley Sanchez for assistance in editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasen I, Kolli L, Kumari V. Sex effects in prepulse inhibition and facilitation of the acoustic startle response: implications for pharmacological and treatment studies. Journal of Psychopharmacology. 2005;19:39–45. doi: 10.1177/0269881105048890. [DOI] [PubMed] [Google Scholar]
- Almasy L, Porjesz B, Blangero J, Chorlian DB, O'Connor SJ, Kuperman S, Rohrbaugh J, Bauer LO, Reich T, Polich J, Begleiter H. Heritability of event-related brain potentials in families with a history of alcoholism. American Journal of Medical Genetics. 1999;88:383–390. [PubMed] [Google Scholar]
- Almasy L, Porjesz B, Blangero J, Goate A, Edenberg HJ, Chorlian DB, Kuperman S, O'Connor SJ, Rohrbaugh J, Bauer LO, Foroud T, Rice JP, Reich T, Begleiter H. Genetics of event-related brain potentials in response to a semantic priming paradigm in families with a history of alcoholism. American Journal of Human Genetics. 2001;68:128–135. doi: 10.1086/316936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug and Alcohol Dependence. 1997;44:1–10. doi: 10.1016/s0376-8716(96)01311-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biological Psychiatry. 1999a;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: effects on P300 during the stroop test. Neuropsychopharmacology. 1999b;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. CSD/BEM localization of P300 sources in adolescents “at-risk”: evidence of frontal cortex dysfunction in conduct disorder. Biological Psychiatry. 2001;50:600–608. doi: 10.1016/s0006-3223(01)01066-6. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on P300 amplitude and topography in male adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM, O'Connor S, Roberts L. P300 differences between nonalcoholic young men at average and above average risk for alcoholism: effects of distraction and task modality. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:263–277. doi: 10.1016/0278-5846(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism: Clinical and Experimental Research. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophrenia Research. 2001a;49:171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001b;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Button TM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behavior Genetics. 2005;35:115–120. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- Campanella S, Rossignol M, Mejias S, Joassin F, Maurage P, Debatisse D, Bruyer R, Crommelinck M, Guerit JM. Human gender differences in an emotional visual oddball task: an event-related potentials study. Neuroscience Letters. 2004;367:14–18. doi: 10.1016/j.neulet.2004.05.097. [DOI] [PubMed] [Google Scholar]
- Campanella S, Petit G, Maurage P, Kornreich C, Verbanck P, Noel X. Chronic alcoholism: insights from neurophysiology. Neurophysiologie Clinique. 2009;39:191–207. doi: 10.1016/j.neucli.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Hughes M, Budd TW, Cooper G, Fulham WR, Karayanidis F, Hanlon MC, Stojanov W, Johnston P, Case V, Schall U. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci. 2007;26:2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcoholism: Clinical and Experimental Research. 2004;28:677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O'Connor S, Hesselbrock V, Rohrbaugh J, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry and Behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Electrophysiological responses to affective stimuli in Mexican Americans: relationship to alcohol dependence and personality traits. Pharmacology, Biochemistry and Behavior. 2007;88:148–157. doi: 10.1016/j.pbb.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley M, McManis M, Lang PJ. Probing affective pictures: attended startle and tone probes. Psychophysiology. 1998;35:344–347. doi: 10.1017/s0048577298970536. [DOI] [PubMed] [Google Scholar]
- Du J, Li J, Wang Y, Jiang Q, Livesley WJ, Jang KL, Wang K, Wang W. Event-related potentials in adolescents with combined ADHD and CD disorder: a single stimulus paradigm. Brain and Cognition. 2006;60:70–75. doi: 10.1016/j.bandc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. Association of EEG alpha variants and alpha power with alcohol dependence in Mexican American young adults. Alcohol. 2007;41:13–20. doi: 10.1016/j.alcohol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Research. 2001;105:67–78. doi: 10.1016/s0165-1781(01)00313-4. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eye blink modification: a review. Biological Psychology. 1998;47:1–43. doi: 10.1016/s0301-0511(97)00020-3. [DOI] [PubMed] [Google Scholar]
- Filion DL, Kelly KA, Hazlett EA. Behavioral analogies of short lead interval startle inhibition. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. 1st. Cambridge University Press; Cambridge, UK: 1999. pp. 269–283. [Google Scholar]
- Ford JM, Pfefferbaum A. Event-related potentials and eyeblink responses in automatic and controlled processing: effects of age. Electroencephalography and Clinical Neurophysiology. 1991;78:361–377. doi: 10.1016/0013-4694(91)90098-o. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roth WT, Menon V, Pfefferbaum A. Failures of automatic and strategic processing in schizophrenia: comparisons of event-related brain potential and startle blink modification. Schizophrenia Research. 1999;37:149–163. doi: 10.1016/s0920-9964(98)00148-0. [DOI] [PubMed] [Google Scholar]
- Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biological Psychiatry. 1994;35:96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2001;Chapter 8:8.7.1–8.7.15. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Lau P, Gross A, Ehlers CL. A co-morbidity of alcohol dependence with other psychiatric disorders in young adult Mexican Americans. Journal of Addictive Diseases. 2007;26:31–40. doi: 10.1300/J069v26n04_05. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Startle modulation in children at risk for anxiety disorders and/or alcoholism. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:925–932. doi: 10.1097/00004583-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Grillon C, Sinha R, Ameli R, O'Malley SS. Effects of alcohol on baseline startle and prepulse inhibition in young men at risk for alcoholism and/or anxiety disorders. Journal of Studies on Alcohol. 2000;61:46–54. doi: 10.15288/jsa.2000.61.46. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Heinz A, Raabe A, Wessa M, Podschus J, Flor H. Stimulus-induced craving and startle potentiation in abstinent alcoholics and controls. Eur Psychiatry. 2002;17:188–193. doi: 10.1016/s0924-9338(02)00666-1. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. European Journal of Neuroscience. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hirano C, Russell AT, Ornitz EM, Liu M. Habituation of P300 and reflex motor (startle blink) responses to repetitive startling stimuli in children. Int J Psychophysiol. 1996;22:97–109. doi: 10.1016/0167-8760(96)00018-9. [DOI] [PubMed] [Google Scholar]
- Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. International Journal of Psychophysiology. 1999;31:163–174. doi: 10.1016/s0167-8760(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Swerdlow NR, Braff DL. Effects of background and prepulse characteristics on prepulse inhibition and facilitation: implications for neuropsychiatric research. Biological Psychiatry. 2006;59:555–559. doi: 10.1016/j.biopsych.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Wooden A, Blumenthal T, Ito T. Startle magnitude and prepulse inhibition: effects of alcohol and attention. Psychopharmacology (Berl) 2003;167:235–241. doi: 10.1007/s00213-002-1332-7. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Rohsenow D, Monti P, Palfai T, Swift R. Prepulse inhibition of the startle reflex: preliminary study of the effects of a low dose of alcohol in humans. Alcoholism: Clinical and Experimental Research. 1997;21:1312–1319. [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clinical Neurophysiology. 2005;116:1049–1061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biological Psychiatry. 2006;59:625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain Research. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, III, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacology (Berl) 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Papadopoulos A, Bojang F, Poon L, Halari R, Cleare AJ. A comparison of prepulse inhibition in pre- and postmenopausal women and age-matched men. Neuropsychopharmacology. 2008;33:2610–2618. doi: 10.1038/sj.npp.1301670. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Zachariah E, Galea A, Aasen I, Ettinger U, Mitterschiffthaler MT, Sharma T. Structural brain correlates of prepulse inhibition of the acoustic startle response in healthy humans. Neuroimage. 2005;26:1052–1058. doi: 10.1016/j.neuroimage.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty Years and Counting: Finding Meaning in the N400 Component of the Event-Related Brain Potential (ERP) Annual Review of Psychology. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Van Petten C. Event-related brain potential studies in language. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in Psychophysiology. Vol. 3. JAI Press, Inc; Greenwich, CT: 1988. pp. 139–187. [Google Scholar]
- Kutas M, Van Petten C. Psycholinguistics electrified: event-related brain potential investigations. In: Gernsbacher MA, editor. Handbook of Psycholinguistics. Vol. 1. Academic Press; San Diego, CA: 1994. pp. 83–143. [Google Scholar]
- Loeber S, Croissant B, Nakovics H, Zimmer A, Georgi A, Klein S, Diener C, Heinz A, Mann K, Flor H. The startle reflex in alcohol-dependent patients: changes after cognitive-behavioral therapy and predictive validity for drinking behavior. A pilot study. Psychotherapy and Psychosomatics. 2007;76:385–390. doi: 10.1159/000107567. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer MA, Vollenweider FX. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biological Psychology. 2003;63:311–323. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A. Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection. Neuroreport. 1997;8:3029–3037. doi: 10.1097/00001756-199709290-00007. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Buchanan TW, Lovallo WR. Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2002;26:441–448. [PubMed] [Google Scholar]
- Miranda R, Jr, Meyerson LA, Myers RR, Lovallo WR. Altered affective modulation of the startle reflex in alcoholics with antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 2003;27:1901–1911. doi: 10.1097/01.ALC.0000099263.71214.F9. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I, Stocker T, Kellermann T, Ermer V, Wegener HP, Eickhoff SB, Schneider F, Shah NJ. Electrophysiology meets fMRI: neural correlates of the startle reflex assessed by simultaneous EMG-fMRI data acquisition. Human Brain Mapping. 2010;31:1675–1685. doi: 10.1002/hbm.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz EM, Gehricke JG, Russell AT, Pynoos R, Siddarth P. Modulation of startle and the startle-elicited P300 by the conditions of the cued continuous performance task in school-age boys. Clinical Neurophysiology. 2001;112:2209–2223. doi: 10.1016/s1388-2457(01)00686-1. [DOI] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biological Psychiatry. 1998;44:281–289. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Cagan M, Brick J, Jaffe SL. The startle response in rats: effect of ethanol. Pharmacology, Biochemistry and Behavior. 1976;4:311–316. doi: 10.1016/0091-3057(76)90247-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Alcoholism and human electrophysiology. Alcohol Research and Health. 2003;27:153–160. [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neuropathology. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Putnam LE, Roth WT. Automatic elicitation of cognitive components by startling stimuli. Electroencephalogr Clin Neurophysiol. 1987;40:256–262. [PubMed] [Google Scholar]
- Putnam LE, Roth WT. Effects of stimulus repetition, duration, and rise time on startle blink and automatically elicited P300. Psychophysiology. 1990;27:275–297. doi: 10.1111/j.1469-8986.1990.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Roopesh BN, Rangaswamy M, Kamarajan C, Chorlian DB, Pandey AK, Porjesz B. Reduced Resource Optimization in Male Alcoholics: N400 in a Lexical Decision Paradigm. Alcoholism: Clinical and Experimental Research. 2010;34:1905–1914. doi: 10.1111/j.1530-0277.2010.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopesh BN, Rangaswamy M, Kamarajan C, Chorlian DB, Stimus A, Bauer LO, Rohrbaugh J, O'Connor SJ, Kuperman S, Schuckit M, Porjesz B. Priming deficiency in male subjects at risk for alcoholism: the N4 during a lexical decision task. Alcoholism: Clinical and Experimental Research. 2009;33:2027–2036. doi: 10.1111/j.1530-0277.2009.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Blowers GH, Doyle CM, Kopell BS. Auditory stimulus intensity effects on components of the late positive complex. Electroencephalography and Clinical Neurophysiology. 1982;54:132–146. doi: 10.1016/0013-4694(82)90155-9. [DOI] [PubMed] [Google Scholar]
- Roth WT, Dorato KH, Kopell BS. Intensity and task effects on evoked physiological responses to noise bursts. Psychophysiology. 1984;21:466–481. doi: 10.1111/j.1469-8986.1984.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Drobes DJ, Coffey SF, Libet JM. The human startle reflex and alcohol cue reactivity: effects of early versus late abstinence. Psychology of Addictive Behaviors. 2002;16:98–105. doi: 10.1037//0893-164x.16.2.98. [DOI] [PubMed] [Google Scholar]
- Sandbak T, Rimol LM, Jellestad FK, Murison R. Relating acoustic startle reactivity and plasticity to alcohol consumption in male Wistar rats. Physiology and Behavior. 2000;68:723–733. doi: 10.1016/s0031-9384(99)00239-5. [DOI] [PubMed] [Google Scholar]
- Schall U, Catts SV, Karayanidis F, Ward PB. Auditory event-related potential indices of fronto-temporal information processing in schizophrenia syndromes: valid outcome prediction of clozapine therapy in a three-year follow-up. International Journal of Neuropsychopharmcology. 1999;2:83–93. doi: 10.1017/S1461145799001418. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer N, Lang PJ. Probe P3 and blinks: two measures of affective startle modulation. Psychophysiology. 1997;34:1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J, Gilder DA. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behavioural Brain Research. 2006;170:41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Sadeghpour M, De TJ, Ornitz EM. Prestimulation-induced modulation of the P300 component of event related potentials accompanying startle in children. Electroencephalography and Clinical Neurophysiology. 1994;90:201–213. doi: 10.1016/0013-4694(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Neurophysiology and neuropharmacology of short lead interval startle modification. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. 1st. Cambridge University Press; Cambridge, UK: 1999. pp. 114–133. [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Archives of General Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Braff DL, Geyer MA. The neural substrates of sensoimotor gating of the startle reflex: a review of recent findings and their implications. Journal of Psychopharmacology. 1992;6:176–190. doi: 10.1177/026988119200600210. [DOI] [PubMed] [Google Scholar]
- Vandergriff J, Kallman MJ, Rasmussen K. Moxonidine, a selective imidazoline-1 receptor agonist, suppresses the effects of ethanol withdrawal on the acoustic startle response in rats. Biological Psychiatry. 2000;47:874–879. doi: 10.1016/s0006-3223(00)00229-8. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Slutske WS. Antisocial behavior and alcoholism: a behavioral genetic perspective on comorbidity. Clinical Psychology Review. 2000;20:255–287. doi: 10.1016/s0272-7358(99)00029-x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Luo Y, Yan JH, Meng X, Yu F, Li H. Neural correlates of the females' susceptibility to negative emotions: an insight into gender-related prevalence of affective disturbances. Human Brain Mapping. 2009;30:3676–3686. doi: 10.1002/hbm.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Wittchen HU, Himmerich H, Landgraf R, Uhr M, Holsboer F. Arginine vasopressin and adrenocorticotropin secretion in response to psychosocial stress is attenuated by ethanol in sons of alcohol-dependent fathers. J Psychiatr Res. 2004;38:385–393. doi: 10.1016/j.jpsychires.2003.11.009. [DOI] [PubMed] [Google Scholar]


