Abstract
Rhodopsin is a prototypical G protein-coupled receptor (GPCR) – a member of the superfamily that shares a similar structural architecture consisting of seven-transmembrane helices and propagates various signals across biological membranes. Rhodopsin is embedded in the lipid bilayer of specialized disk membranes in the outer segments of retinal rod photoreceptor cells where it transmits a light-stimulated signal. Photoactivated rhodopsin then activates a visual signaling cascade through its cognate G protein, transducin or Gt, that results in a neuronal response in the brain. Interestingly, the lipid composition of ROS membranes not only differs from that of the photoreceptor plasma membrane but is critical for visual transduction. Specifically, lipids can modulate structural changes in rhodopsin that occur after photoactivation and influence binding of transducin. Thus, altering the lipid organization of ROS membranes may result in visual dysfunction and blindness.
Keywords: G protein-coupled receptor(s), rhodopsin, transducin (Gt), photoreceptor, rod outer segment, membranes, oligomerization, signal transduction, phospholipids, cholesterol, phospholipase A2
1. Introduction
Biological membranes define the boundaries of cells and intracellular organelles and protect them from various harmful substances while providing specific environments for different biological processes essential for life (1). They are composed of lipids, largely phospholipids that form a thin bilayer and embed proteins that are important for the regulation of cellular function and organization in tissues (1, 2). Membranes spontaneously self-assemble with their lipid, hydrophobic tails sequestered inside the bilayer and their hydrophilic heads exposed to the aqueous milieu. The ratio between unsaturated and saturated fatty acids in the bilayer must be well balanced so that the membrane can maintain the fluidity needed for rapid responses to environmental factors. The lipid composition of a membrane affects the structural and functional properties of integral membrane proteins and membrane associated proteins as well (2). In this review we emphasize the importance of specific lipids for the function and stability of the light receptor, rhodopsin, a prominent member of G protein-coupled receptor superfamily.
2. Rhodopsin and Gt dependence on membrane integrity
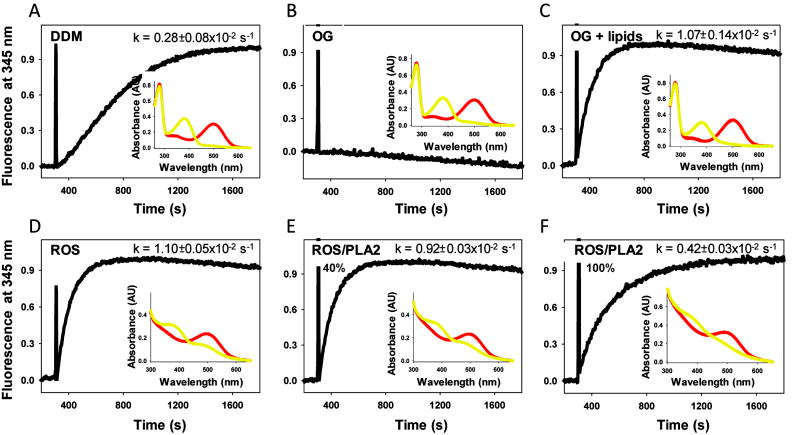
Rhodopsin, a visual photopigment, localizes in disk membranes of rod cells (3, 4) where it is embedded in a lipid bilayer surrounded by ~65-70 phospholipids per one protein molecule (5, 6). After light illumination structural changes in rhodopsin cause coupling to the photoreceptor-specific G protein, Gt or transducin (7), and initiation of the visual signaling cascade (8-10). But does the lipid membrane environment play any role in this activation process? To answer this question, we disrupted ROS membranes by using two detergents, dodecyl-β-D-maltoside (DDM) and n-octyl-glucopyranoside (OG), and then tested the solubilized rhodopsin in a Gt activation assay (Fig. 1A, B). We found that Gt was activated several times more slowly by rhodopsin extracted in DDM than by rhodopsin in native disks (Fig. 1D), whereas no Gt activation was observed when ROS membranes were solubilized with OG (Fig. 1B). However, when lipids isolated from ROS membranes were added back to the ROS membranes in OG, rates of activation were similar to those obtained for native membranes (Fig. 1C). A similar effect of DDM on ROS had previously been reported (11). Interestingly, no changes in the spectral properties of rhodopsin in DDM and OG were observed. Three events, alone or in combination, could account for this result. First, membrane solubilization with detergent may cause dissociation of rhodopsin complexes and thus affect its activation properties. Indeed, our earlier studies suggest that rhodopsin’s oligomeric state might be crucial for efficient Gt activation (7, 11, 12). Nonetheless, illumination of only one rhodopsin molecule suffices to produce a neurological response in the brain (13). In this case, the second rhodopsin/photoactivated rhodopsin or opsin would serve as a platform that supports the Gt-rhodopsin complex. Second, a specific lipid environment surrounding rhodopsin may be disorganized due to detergent treatment and formation of mixed micelles composed of lipids, detergent and proteins. Because different detergents sequester varying amounts and populations of lipids, the remaining rhodopsin-associated lipids most likely would affect rhodopsin’s functional properties. Third, detergent can affect hydrophobic anchors of Gt (myristoyl and farnesyl groups), and thereby compromise the proper formation of the complex between rhodopsin and Gt. To test the importance of membrane integrity on rhodopsin’s properties, we treated ROS membranes with phospholipase A2 (PLA2) that cleaves one acyl chain from the sn-2 position of phospholipids. Then we used the digested membranes in the Gt activation assay (Fig. 1E and F). When the efficiency of phospholipid digestion in membranes used for the assay was ~40 %, only slightly slower rates of Gt activation (~16 %) as compared to native ROS membranes were observed (Fig. 1E). However, when we used ROS membranes in which phospholipids were nearly 100 % digested, a far greater decrease (~60 %) in rates of Gt activation occurred (Fig. 1F). Whereas treatment of ROS membranes with phospholipase A2 did not change the spectral properties of rhodopsin, its functional properties were affected. This straightforward experiment clearly indicates that even mild changes in membrane architecture and composition can affect the function of membrane proteins and assigns a significant role to lipids in producing this effect.
Figure 1.
Ability of different preparations of photoactivated rhodopsin to activate Gt (transducin). Increased intrinsic fluorescence of the Gtα subunit caused by interaction with photoactivated rhodopsin: (A) solubilized in dodecyl-β-D-maltoside (DDM); (B) solubilized in n-octyl-glucopyranoside (OG); (C) solubilized in OG and complemented with lipids isolated from ROS; (D) in ROS membranes; (E) in ROS membranes treated with phospholipase A2 (PLA2) at ~ 40 % digestion efficiency; (F) in ROS membranes treated with phospholipase A2 at ~ 100 % digestion efficiency. Rhodopsin (25 nM) was mixed with Gt (250 nM) and samples were illuminatedfor 30 s with a fiber light through band pass filter 480-520 nm. After 300 s of recording, 5 μM GTPγS was added. Reactions were carried out in a continuously stirred cuvette at 20°C in buffer composed of 20 mM BTP, 120 mM NaCl, 2 mM MgCl2 plus either 2 mM DDM or 30 mM OG. No detergent was present in the buffer when Gt was activated by ROS membranes. Relative activation rates were calculated from 3 independent experiments. Intrinsic fluorescence of Gtα was measured with a LS55 luminescence spectrophotometer (Perkin Elmer, Life Science), by using excitation and emission wavelengths of 300 nm and 345 nm, respectively (125, 126). Insets, Absorption spectra of rhodopsin solubilized in DDM (A), or in OG (B), or in OG and complemented with lipids isolated from ROS (C), rhodopsin in ROS membranes (D) and rhodopsin in ROS membranes treated with phospholipase A2 with ~ 40 % digestion efficiency (E), and rhodopsin in ROS membranes treated with phospholipase A2 with ~ 100 % digestion efficiency (F). Red lines represent spectra of ground state rhodopsin. Yellow lines represent spectra of photoactivated rhodopsin illuminated for 30 s.
3. Rhodopsin oligomerization
Based on classic fluid models for cell membranes and early work on rhodopsin’s biophysical properties, this photopigment was viewed for many years as a monomeric protein freely mobile in a liquid-like environment (6, 14-16). But more recent studies on membrane proteins from the GPCR family strongly suggest their propensity for oligomerization (17-23). Although dimerization of family C GPCRs, such as the metabotropic mGlu and glutaminergic GABAB receptors, was commonly accepted (24, 25), oligomerization of receptors from rhodopsin-like family A GPCRs has long been debated. Development of high resolution imaging techniques such as atomic force microscopy (AFM) allowed direct visualization of rhodopsin in its native disk membrane environment (26-29). Spectacular AFM images revealed rhodopsin as a dimer organized in rows of densely packed oligomers about 25 nm long embedded in a ROS disk membrane lipid bilayer (Fig. 2). Thus, the simplified view of rhodopsin as a single molecule freely diffusing in a lipid bilayer must be revised in the light of more advanced physiological analyses (30) as opposed to previous measurements (15, 31). Indeed, another powerful technique, near-field scanning optical microscopy or NSOM (32) was used to observe clusters of several β2-adrenergic receptor molecules, another family A GPCR, on the surface of neonatal and embryonic cardiac myocytes isolated from mice. This result also strongly suggests that higher organization of rhodopsin-like GPCRs is required for their signaling function as well as their intracellular transport. Moreover, it is consistent with the growing number of in vivo investigative reports (e.g. see (21)) and not just those involving experimentally transformed cell lines (20).
Figure 2.
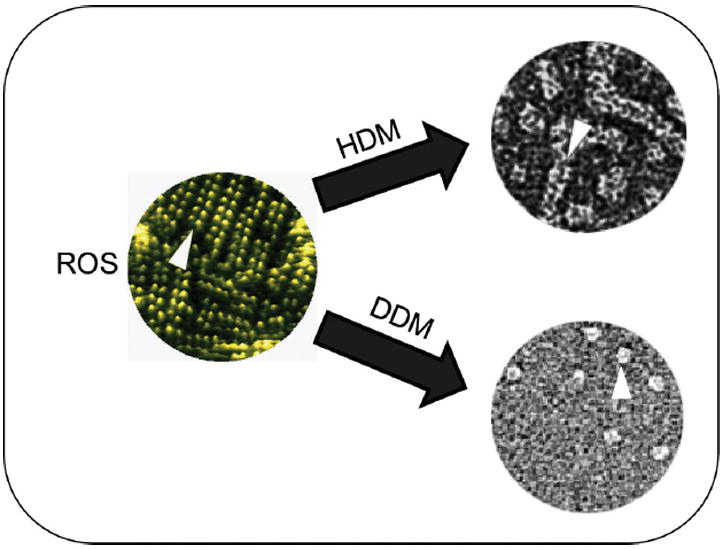
Rhodopsin oligomerization. The propensity of different preparations of rhodopsin to oligomerize is illustrated. Atomic force microscopic (AFM) image of native disk membranes is colored green (left) and organization of rhodopsin molecules in dimers, packed in rows is shown by the arrowhead. Transmission electron microscopic (TEM) images of negatively stained ROS membranes solubilized in either hexyl-β-D-maltoside (HDM) or dodecyl-β-D-maltoside (DDM) are shown on the right. Arrowheads highlight whole rows of rhodopsin dimers extracted by HDM but just single rhodopsin dimers extracted by DDM.
Rhodopsin organization can be altered by solubilization of membranes thus, depending on detergent properties, rhodopsin can be found in different oligomeric states in different detergent solutions or the same detergent at different concentrations (5, 12). For example, with the mild non-ionic detergent hexyl-β-D-maltoside (HDM), entire rows of rhodopsin dimers could be isolated as documented by transmission electron microscopy (TEM) whereas another detergent, dodecyl-β-D-maltoside (DDM), disrupted those oligomers into dimers (Fig. 2). Receptor monomers were only occasionally seen. Interestingly, analysis of total phospholipids extracted together with rhodopsin solubilized by HDM and DDM revealed that more phospholipids were sequestered along with rhodopsin in HDM than in DDM. This suggested the importance of phospholipids in stabilizing rhodopsin’s quaternary structure. Consequently, addition of lipids to rhodopsin solubilized with DDM promoted rhodopsin’s self-association and resulted in its faster migration through a gel filtration column due to oligomer formation (unpublished). After extraction by mild detergents, frog rhodopsin was visualized by blue native BN-gel electrophoresis only as oligomers consisting mostly of dimers and trimers (33), possibly because of its incorporation into detergent-resistant micro-domains. Such micro-domains with distinct lipid compositions have been identified in ROS membranes and most likely play an important role in visual transduction (34).
Implications of the membrane environment for rhodopsin higher organization have been explored by different laboratories. Rhodopsin self-association during reconstitution into lipid vesicles has been tested using fluorescence resonance energy transfer (FRET) (35, 36). Detergent-solubilized, purified and labeled with site-specific fluorophores rhodopsin, self-associated when reconstituted into asolectin liposomes, indicating that lipids play an important role in rhodopsin organization and can promote its self-assembly. Different lipid/protein ratios were tested to ensure that the observed results did not reflect protein crowding. Moreover, light-activated receptor assembly is highly dependent on membrane thickness and is promoted by its reduction. This finding was investigated in more molecular detail by using mesoscopic simulation techniques, where monomeric rhodopsin was incorporated into lipid bilayers composed of phosphatidylcholine (PC) with different acyl chain lengths (ranging from C12 to C20) in different saturation states (37, 38). Interestingly, the number of contact rhodopsin-rhodopsin interfaces was greater in (C12:0)PC and (C16:1)PC than in (C20:0) and (C20:1)PC, suggesting the possibility of non-specific protein-protein interactions in more fluid membranes. However, in thicker bilayers rhodopsin mobility decreased up to 30-fold and only three contact zones were clearly visible in formed aggregates, signifying more specific dimerization interfaces. Dimerization interfaces between helices H I/H-II/H-8, H-IV/H-V and H-VI/H-VII have been reported for rhodopsin and other GPCRs (19, 39-43).
Therefore, rhodopsin oligomerization is most likely affected by the membrane lipid environment wherein the lipid bilayer acts as a structural matrix that helps to arrange membrane proteins. Reduction of free diffusion in three dimensions to two dimensional biological membranes produces stable complexes even in cases of low affinity interactions. Specifically in ROS membranes, enrichment in phospholipids with long polyunsaturated acyl chains influences this receptor’s rigidity and higher order organization. This is not to say that such clustering must be “permanent”. Indeed, such islands of receptors can be formed transiently, but they should remain long enough for signal transduction or specifically, interactions with G proteins to take place (44, 45).
4. Lipid composition of ROS membranes
Starting in the 1970s, several laboratories have studied the lipid composition of ROS membranes (46-53) organized as schematically illustrated in Fig. 3A and B. Similar findings revealed that glycerophospholipids are the most abundant constituent comprising more than 85 mol %, or nearly 90 mol % of total lipid in ROS. Moreover, ROS contain ~10 mol % of neutral lipids and ~1-2 mol % of sphingolipids (Table 1).
Figure 3.

Schematic organization of the retinal rod photoreceptor cell. A, Rod photoreceptor cell with labeled cell segments and major cellular elements. B, Membrane organization of a single disk showing location of rhodopsin dimers. C, Schematic representation of different states of rhodopsin after light activation.
TABLE 1.
Lipid composition of rod outer segment (ROS) membranesa.
| Phospholipids (~85-90 % of total lipids) | Fatty Acid | ||
|---|---|---|---|
| Type | Amount | Type | References |
| PC | ~45 mol % | 16:0, 18:0, DHA | (55, 58, 109, 122, 123) |
| PE | ~42 mol % | DHA, 18:0 | (55, 58, 109, 123) |
| PS | ~13 mol % | DHA, 18:0 | (55, 58, 109, 123) |
| PI | Less than 1 % | 18:0, 24:4, DHA | (55) |
| PA | Less than 1 % | DHA, 18:0 | (55) |
| Other Lipids | |||
| Cholesterol | ~8-10 mol % of total lipids | Not applicable | (55, 109, 124) |
| Sphingolipids | Less than 1 % of total lipids | 18:0, 18:1, 22:4 | (55, 109) |
| Free fatty acids | ~2-3 % of total lipids | 18:0, 18:1, DHA, 16:0, 20:4, 22:4 | (55, 57, 59) |
Abbreviations: PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; PA, phosphatidic acid; DHA, docosahexaenoic acid.
The most abundant phospholipids are PC and phosphatidylethanolamine (PE) in nearly equal amounts of ~45 mol % and 42 mol %, respectively, whereas phosphatidylserine accounts for ~13 mol %. Other phospholipids such as phosphatidylinositol (PI) and phosphatidic acid (PA) were identified as well but they constitute less than 1 mol % of total lipids in ROS membranes. Interestingly, the distribution of phospholipids in disk membranes is not symmetrical. The inner (lumenal) membrane is highly enriched in PC, while the outer (cytoplasmic) membrane leaflet contains more PE and PS (each ~75 % of total ROS PE and PS) (52, 54). Importantly, this asymmetric phospholipid distribution relates to ROS membrane function and stability because it exists in a dynamic equilibrium such that redistribution between two leaflets of ROS membranes may occur during activation of the visual cascade (55). The first enzyme involved in this process is the photoreceptor disk P4-ATPase, Atp8a2, a recently identified phosphatidylserine flipase (56). ROS membranes contain relatively low amounts of neutral lipids, namely cholesterol and neutral glycerides such as diacylglycerol, which account only for ~ 10 % of their total lipids (57). Among these cholesterol is the most abundant species that makes up nearly 90 % of neutral lipids and ~8-10 mol % of total lipids in ROS membranes. Low amounts of sphingolipids have been detected in ROS membranes as well but these represent only 1-2 mol % of total lipids (58, 59). Vertebrate ROS membranes also retain small amounts (2-3 %) of free fatty acids that include 18:0, 18:1, 22:6 (DHA), 16:0, 20:4 and 22:5 (57).
Another striking feature of ROS lipid composition is an unusually high content of long-chain polyunsaturated fatty acids (LC-PUFAs) containing 18-24 carbons and 3-7 double bonds. PE and PS are especially enriched in these PUFAs while PC contains more saturated fatty acids. In mammalian ROS, ~80 mol % of total PUFAs consist of docosahexaenoic acid (DHA) 22:6, which significantly contributes to visual function (60). Moreover, ROS lipids also contain very long-chain unsaturated fatty acids (VLC-PUFAs) with more than 24 carbons (24-36) and 4, 5 and 6 double bonds. VLC-PUFAs account for ~13 % of total PUFAs in ROS and they are mostly found in PC. These fatty acids are very tightly associated with rhodopsin, and thus they might be important for its stability (61). On the other hand, VLC-PUFAs might be involved in the formation of specific micro-domains for cholesterol binding and therefore influence the stability and activity of membrane proteins. Recent studies on fatty acid elongase (ELOVL4) suggest that VLC-PUFAs probably play a unique role in the retina and may be important for its maintenance and function (62, 63). Specific mutations in the ELOVL4 gene can result in lack of retinal VLC-PUFAs biosynthesis and lead to photoreceptor cell death in Stargardt-like macular dystrophy (64).
5. Lipid-rhodopsin interaction sites
A large number of studies suggest the importance of lipid-rhodopsin interactions for activating visual processes. Lipid composition, especially specific headgroups and acyl chains influence rhodopsin stability, formation of the Meta ll active state (see different states of rhodopsin in Fig 3C) and binding of Gt. So what is the nature of the lipid-receptor association? Are there defined regions on rhodopsin’s surface that allow binding of specific lipids, which then alter rhodopsin’s properties? Can the interaction between rhodopsin and lipids be considered as a receptor-ligand interaction by analogy to other receptors from the GPCR family? In fact, lipid-like ligands have been established for other GPCRs, e.g. 2-arachidonylglycerol and arachidonoylethanolamide are endogenous ligands for the cannabinoid receptor (65). Redistribution of specific lipids within the membrane bilayer after light stimulation has been shown by EPR spin labeling experiments as well (83). Studies of rhodopsin suggest that specific lipid-binding regions exist on its surface. Several well defined phospholipids associated with protein helices near the cytoplasmic ends of H-VI and H-VII have been found in the crystal structure of dark state bovine rhodopsin (66). While the identity of these lipids is unknown because no density was observed for their head groups (possibly dipalmitoyl-sn-glycero-3-phosphatidylethanolamine),, lipid acyl chains did form contacts with specific residues such as Ile256, Ala260, and Ile263 in H-VI and with Thr297, Tyr301, Val304, Ile305, and Met309 in H-VII (Fig. 4A). Helical rearrangements in rhodopsin are followed by activation of this protein, so lipids crystallized together with ground state rhodopsin might be responsible for its stability in the non-active conformation. Moreover, a density into which a cholesterol molecule could fit has been found in 2D crystals of rhodopsin Meta I near the extracellular (lumenal) region of H-VI and H-VII of one rhodopsin molecule and the intracellular (cytoplasmic) side of H-IV of the adjacent molecule (Fig. 4B) (67). A specific binding site for cholesterol between transmembrane helices H-II, H-III and, similar to rhodopsin Meta I, the cytoplasmic end of H-IV including a highly conserved Trp4.50 (residue 161 in rhodopsin), have been found in the crystal structure of another family A GPCR, the β2 adrenergic receptor (β2AR-T4) co-crystallized in complex with the inverse agonist, timolol (68). The same area referred to as the cholesterol binding motif in the β2 adrenergic receptor has also been found in the structure of A2A adenosine receptor; however instead of cholesterol, two stearic acid molecules were bound to this region (69). Moreover, multiple members of family A GPCRs have high sequence similarity among 4 residues participating in the tightest lipid binding. Thus, it seems that a lipid interaction motif is conserved among members of family A GPCRs.
Figure 4.

Residues of rhodopsin interacting with specific lipids. (A) Aliphatic lipid chain of dipalmitoyl-sn-3-glycero-phosphatidylethanolamine (PE) interacting with cytoplasmic parts of H-VI and H-VII. (B) Cholesterol molecule interacting with two rhodopsin molecules in 2D crystals (antiparallel arrangement of rhodopsin molecules). Cholesterol interacts with the cytoplasmic side of H-IV and the extracellular sides of H-VI and H-VII. (C) Residues of rhodopsin preferentially interacting with DHA (a, a’), saturated fatty acids (b, b’), and cholesterol (c, c’). Primed panels are rotated 180°. The color scheme fo r helices of rhodopsin is: H-I - blue, H-II - cyan, H-III - green, H-IV - lime, H-V - yellow, H-VI - orange, H-VII - light red, H-8 - red. In A and B panels the lipids are shown with their hydrogen atoms; no lipids in panel C. In all panels the gray shadowing denotes rhodopsin residues interacting with lipids.
Existence of specific sites on the surface of rhodopsin for interactions with polyunsaturated and saturated lipids, and cholesterol has been reported from 100-ns molecular dynamic simulations (70). These studies indicate that tight coupling between rhodopsin and polyunsaturated DHA occurs frequently in a DHA-rich environment and that this association takes place in small well-defined regions, especially at the extracellular ends of H-VI, H-VII, and H-I (residues 36, 39, 45, 48, 50, 252, 255-256, 259, 266, 269-270, 273-274, 277-278, 286, 290, 300, 304, 307-308, 314). Some residues that tend to associate with DHA have been observed within intracellular ends of H-III and H-IV and the extracellular part of H-II as well (residues 92, 95-96, 99, 129, 130, 133, 136-137, 142-143, 146, 148, 152, 155-156, 159) (Fig. 4Ca,a’). However, cholesterol and saturated fatty acids bound less specifically to rhodopsin. Several groups of residues that preferentially interact with saturated fatty acids have been found within: i) the extracellular ends of H-III and H-IV: residues 108, 111-112, 115, 172; ii) the intracellular end of H-V: residues 217, 220-221, 224-225, 228; iii) extracellular ends of H-V and H-VI: residues 205, 208-209, 213, 273; as well as iv) middle part of H-VI and intracellular end H-VII: residues 256, 300-301, 304-305, 308-309 (Fig. 4Cb,b’). Residues interacting with cholesterol have been found within three clusters: i) H-I and H-8: residues 53, 56-57, 60, 320-321; ii) H-VI and H-VII: residues 252, 255-256, 259-260, 305, 308-309; and iii) HIII and H-IV: residues 108, 111-112, 115, 172 (Fig. 4Cc,c’). Specific interaction sites for cholesterol in the bundle of H-I/H-II/H-VII/H-8 were reported in later studies of rhodopsin done with microsecond molecular dynamic simulations, suggesting a regulatory role of cholesterol in a structural rearrangement needed for the function of all GPCRs (71). Other studies of the interaction between rhodopsin and lipids carried out by saturation transfer difference (STD) spectroscopy in combination with magic angle spinning (MAS) and nuclear magnetic resonance (NMR) (1H STD MAS NMR) revealed that polyunsaturated and monounsaturated lipids associate specifically with different sites on the rhodopsin surface (72). This technique selectively saturates membrane protein resonance without saturating lipids so magnetization redistributes within the protein via spin diffusion. Magnetization is then transferred from the protein to the first layer of lipids surrounding the protein via 1H-1H dipolar contacts and this requires short distances between protein and lipids equivalent to physical contact between molecules. All rhodopsin photointermediates transfer magnetization preferentially to lipids containing polyunsaturated DHA. Thus, rhodopsin function is influenced by the interaction with specific lipids in the lipid bilayer of disk membranes.
6. Effect of lipids on G-protein binding to rhodopsin
Lipids in disk membranes of ROS not only serve as structural support for rhodopsin but also play a critical role in the activity of this receptor (7). Thus, the lipid composition of the ROS membranes, specifically its asymmetric allocation among disk membrane leaflets, is critical for activating the visual signaling cascade. Both the head groups and acyl chains of phospholipids have a significant effect on Meta II formation (73-79) and G protein anchoring (73, 80-83). Whereas more PC is located in the inner (lumenal) disk membrane leaflets, PE and negatively charged PS are enriched in the outer (cytoplasmic) part of the disk. PS is organized in small clusters that help attract G protein to the membrane (84). Even more PS has been found in outer (cytoplasmic) membrane leaflets after rhodopsin photoactivation indicating that phospholipid redistribution may occur in response to light stimuli and most likely stabilizes the active rhodopsin conformation, Meta II (85). Indeed, redistribution of PS was enhanced by the presence of C-terminal Gt peptide that stabilizes the Meta II conformation (86). This indicates a specific role of PS in forming the complex between activated receptor and Gt, most likely by influencing the rhodopsin structure and simultaneously providing a platform for Gt anchoring to the membrane (Fig. 5). Rapid lipid flip-flop depends upon the length and unsaturation of lipid chains, increasing in more fluid membranes rich in long chain polyunsaturated lipids (87). As for fatty acids, ROS membranes are highly enriched in DHA polyunsaturated fatty acid and PS contains more DHA than PE and PC. This seems to agree with the movement propensities of these phospholipids. Very recently an enzyme involved in this flip-flop movement of phospholipids across the membrane was identified: Atp8a2 that belongs to the relatively new subfamily of P-type ATPases. Purified Atp8a2 exhibited ATPase activity stimulated by PS and to some degree by PE but not by PC. When Atp8a2 was reconstituted into liposomes containing fluorescently labeled PS, PE and PC, a flip of PS from the inner (lumenal) leaflet to the outer (cytoplasmic) leaflet of the liposome membrane was observed (56).
Figure 5.
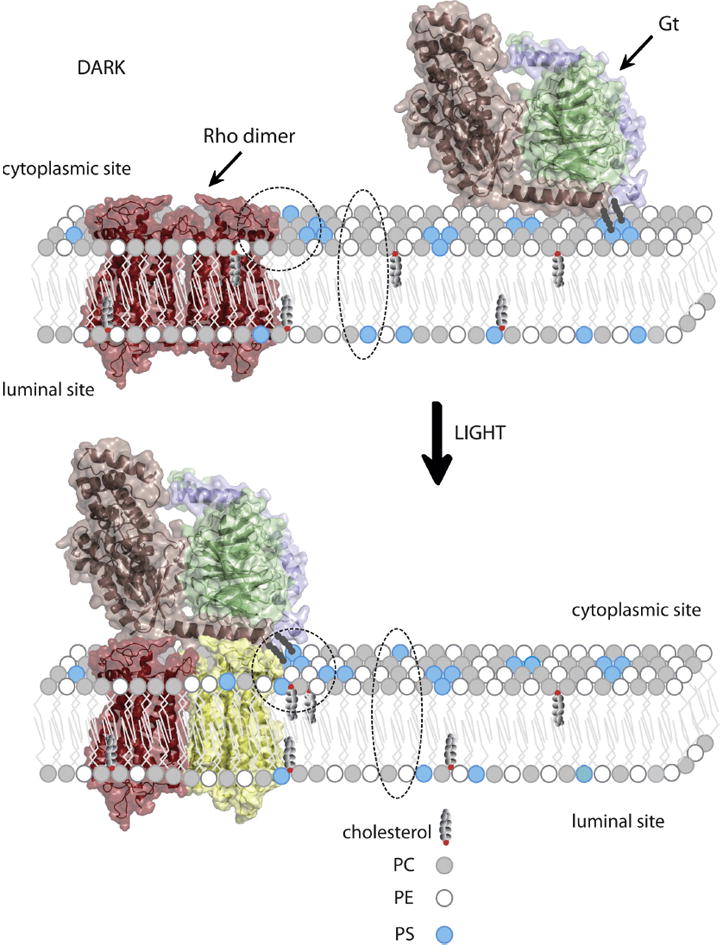
Reorganization of specific lipids in a disk membrane upon light illumination allows appropriate binding of Gt to photoexcited rhodopsin. Model of rhodopsin dimer (26, 39) is colored red in the dark state. Activated rhodopsin molecule is colored yellow. Gtα, Gtβ and Gtγ within heterotrimeric G protein are colored light pink, green and violet, respectively. Gt is attached to the membrane through myristoyl and farnesyl groups. Headgroups of phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylserine (PS) are depicted as small circles colored grey, white and blue, respectively. Movement of PS from the inner (lumenal) to the outer (cytoplasmic) leaflet of the lipid bilayer, as well as reorganization of PS, PE and cholesterol in close proximity to the activated rhodopsin molecule, is highlighted with a broken ellipsoid and circle, respectively. This lipid reorganization plays an important role in formation of the rhodopsin-Gt complex, thereby activating the visual signaling cascade.
Movement of PE across disk membrane leaflets following light activation has been suggested as well (85). Using plasmon-waveguide resonance (PWR) spectroscopy, investigators observed that PE favors the formation of Meta II and enhances Gt binding affinity to photoexcited rhodopsin (88-90). In synthetic bilayers composed of a 25:75 mol % DOPC/DOPE mixture, Gt affinity to activated rhodopsin increased from 64 nM to 0.7 nM, whereas in membranes composed entirely of DOPC, Gt affinity was much lower at 18 nM. Thus, PE most likely affects membrane fluidity, providing more freedom for rhodopsin’s structural changes to occur after light illumination, whereas PC inhibits this conversion (88). Free PE serves yet another distinct role in the retinoid cycle by acting as an acceptor for free all-trans-retinal released from rhodopsin’s chromophore binding pocket after Meta II decay. Formed N-retinal-PE then interacts with ABCA4 (91) and presumably is flipped from the inner (lumenal) leaflet to the outer cytoplasmic leaflet of the disk using ATP hydrolysis as the energy source (92, 93). Therefore, PE helps to clear released chromophore from photoreceptor cells for conversion back to 11-cis-retinal by the retinoid (visual) cycle (92, 93).
The significance of a specific lipid environment on visual signaling is emphasized by studies of rat ROS membranes (94) when DHA (22:6) was replaced by docosapentaenoic acid (DPA; 22:5 acyl chain). Here, the deficiency of DHA reduced not only rhodopsin activation, but also formation of the rhodopsin-Gt complex in addition to decreasing phosphodiesterase (PDE) activity. Similar observations were reported in rats raised on a n-3 fatty acid-deficient diet (60). Both Gt activation and PDE activity were diminished by lowering DHA levels. Thus, the composition of the lipid matrix is critical, not only for rhodopsin’s function, but also for the entire process of signal transduction.
We tested the effect of phospholipids such as asolectin, DOPC, DOPE and DOPS on the binding of Gt to DDM-solubilized rhodopsin following light exposure (11). More efficient formation of the rhodopsin-Gt complex was achieved in the presence of these lipids. DOPS had the greatest effect in this regard, which is not surprising because negatively charged PS derivatives reportedly are far more critical for Gt membrane anchoring than uncharged PC. Phospholipids also appear to be required for rapid Gt activation. Although Gt could be activated by Rho* solubilized in detergent, its activation was several times faster in the presence of either synthetic phospholipids or a mixture of native lipids isolated from ROS membranes. Both DOPS and native lipids resulted in Gt activation rates similar to those observed when Gt was activated by photoactivated rhodopsin in native disk membranes. Thus, these phospholipids most likely play a critical role in stabilizing the quaternary structure of rhodopsin for better membrane anchoring and Gt docking that guarantee rapid signal transduction and immediate responses to light (7, 12, 95).
7. Cholesterol in the function and organization of rhodopsin and Gt activation
Cholesterol is an essential membrane component affecting various functions such as cellular fusion, permeability, enzymatic activity and the operation of various receptors (2). The plasma membrane is highly enriched in cholesterol as compared to ROS membranes in the retina (96). The average cholesterol content in ROS disk membranes is ~8-10 mol % whereas plasma membranes of ROS contain ~30 mol %. Disk membranes in rod cells are formed by evagination of the plasma membrane at the base of outer segments and after separation they move to the apical portion of the ROS within a few days. Old disk membranes at ROS apices are finally shed and phagocytosed by the retinal pigmented epithelium (RPE) (97, 98). The amount of cholesterol substantially decreases as outer disk membranes mature, which has significant implications for activating the visual signaling cascade. Thus, it has been shown that formation of Meta II and binding of Gt to photoactivated rhodopsin is increased in apical disks of rod cells (99), where levels of cholesterol are lower and the amount of phospholipid containing polyunsaturated fatty acids is increased. Rhodopsin accounts for over 90 % of proteins in disk membranes but it is also present in the plasma membrane of ROS, where it constitutes about 40 % of membrane proteins. Can this plasma membrane rhodopsin activate the visual signaling cascade? A study that compared the activity of PDE resulting from rhodopsin excitation in disk and plasma membranes showed that while disk rhodopsin could fully activate the cascade, activation by plasma membrane rhodopsin was very poor. But when the plasma membrane cholesterol was oxidized to cholestenone, activation of plasma membrane rhodopsin greatly improved (100). This result clearly indicates that a high membrane content of cholesterol inhibits rhodopsin activation. A shift in transition between Meta I and Meta II back toward Meta I was also observed in a reconstituted egg PC bilayer system containing 30 % of cholesterol (101). Because cholesterol increases membrane rigidity, it most likely inhibits expansion of the rhodopsin structure in the membrane during conversion from Meta I to Meta II. Consequently, rhodopsin cannot reach its fully activated state. But cholesterol also helps to stabilize rhodopsin. Therefore, an overall balance of cholesterol must be attained in ROS membranes to ensure rhodopsin’s stability without inhibiting its function.
Cholesterol exhibits asymmetric distribution within disk membrane between inner (lumenal) and outer (cytoplasmic) bilayer leaflet as well. It can readily exchange between lipid bilayers and is sensitive to phospholipid head groups and fatty acid composition, favoring a saturated fatty acid environment rich in PC but not PE (102). In addition to lipid asymmetry between the bilayers of disk membranes, enrichment of cholesterol in micro-domains called lipid rafts or detergent resistant membranes (DRM) has been reported (103-105). These lipid rafts tend to recruit fatty-acyl modified signaling proteins and probably affect their function. For example, in bovine ROS Gtα partitions to DRMs as a consequence of rhodopsin photoexcitation, where it can activate other signaling proteins such as PDE. RGS9-1 is a phototransduction regulatory protein that most likely plays a crucial role in terminating Gtα activity (106, 107). Detailed lipid and protein analyses of DRMs indicate that they are rich in cholesterol, saturated fatty acids, free fatty acids and contain a specific PC fraction devoid of polyunsaturated fatty acids (PUFAs). DRMs hold little PE and PS, and some ceramide-containing PUFAs but no saturated fatty acids. DHA is diminished in PS recruited to DRMs but present in the PE fraction. One of major proteins found in ROS DRMs is caveolin-1. Gtα and PDE localize to DRMs in a light-dependent manner (108, 109). DRMs isolated by sucrose gradient centrifugation are not rich in rhodopsin but DRM-associated rhodopsin preferentially binds heterotrimeric Gt after light illumination as compared to rhodopsin in bulk lipids. This finding seems to contradict results indicating that enrichment of membranes in cholesterol and saturated fatty acids inhibits the Meta I → Meta II transition and formation of the rhodopsin-Gt complex. Nevertheless the observation was confirmed by a second group using fluorescence recovery after photobleaching (FRAP) (34) to study the role of lipid micro-domains in regulating membrane protein function in living rod photoreceptors from transgenic Xenopus laevis. Treatment of disk membranes with cholesterol-depleting agents such as methyl-β-cyclodextrin or fillipin III accelerated diffusion of activated GTP-Gtα as well as heterotrimeric Gt complexed with activated rhodopsin but had no effect on diffusion of either inactive GDP-Gtαβγ or ground state rhodopsin. Thus, heterogenic lipid domains must exist in a bulk lipid bilayer and both rhodopsin and Gt are distributed into these domains in a manner dependent on their functional state (106). The complex between rhodopsin and Gt formed as a result of light illumination is either recruited to specific regions or it facilitates lipid reorganization and assembly of micro-domains around itself. This could be the mechanism separating an activated pool of Gt from non-active Gt. Moreover, other studies noted that the inhibitory effect of cholesterol on formation of the rhodopsin-Gt complex is minimized by phospholipids containing DHA which, as mentioned before, is one of the major fatty acids in PS and PE (60, 109). Therefore, a precise ratio between specific lipids surrounding rhodopsin which is balanced according to lighting conditions seems to be crucial for rapid and efficient function of the visual signaling cascade.
8. Role of lipid modification in rhodopsin and Gt structures
Both rhodopsin and Gt possess hydrophobic posttranslational modifications that anchor them to ROS membranes and that are important for their stability and function. Rhodopsin is palmitoylated at two adjacent residues, Cys322 and Cys323, in its C-terminus next to H-8 at the cytoplasmic surface. Interestingly, this palmitoylation is highly conserved among the GPCR superfamily and in rhodopsin this region is involved in binding of Gt following light activation. As shown by studies with Palm-/--deficient mice, absence of palmitoylation causes reduction in rates of Gt activation as well as the forces required to unfold the C-terminal end of rhodopsin, suggesting that stability and/or rigidity of rhodopsin structure was affected (110). Faster phosphorylation rates of depalmitoylated rhodopsin have been observed as well (111). Together, these observations strongly suggest the importance of rhodopsin palmitoylation for proper signal transduction. Moreover, further studies on Palm-/--deficient mice revealed that palmitoylation is critical for stability and correct function of unliganded opsin in rod photoreceptor cells and this hydrophobic modification helps to protect the protein against light induced retinal degeneration (112). The heterotimeric G protein, Gt contains two different hydrophobic modifications in its structure, a myristoyl and a farnesy groupl. The myristoyl group is attached to the N-terminal Gly residue of Gtα and the farnesyl group is attached to the C-terminal Cys71 of Gtγ. Unfortunately both modifications are missing in the crystal structure of Gt but the N-terminus of Gtα and the C-terminus of Gtγ are in close proximity to each other, suggesting that this is a membrane-binding motif. In fact, as determined by electron crystallography, Gt binds to the membrane with a very small contact area (113) that most likely is important for fast signal transduction. Moreover, this result also suggests that most of the Gt’s surface is involved in coupling to photoactivated rhodopsin.
In addition to lipid anchors, Gt also exhibits electrostatic interactions with negatively charged membrane surfaces and thus binds more strongly to negatively charged lipids than to neutral membranes (84). Using a quantitative computational approach and calculations of electrostatic free energy, investigators reported that in fact electrostatic repulsion constrains the orientation of membrane-associated Gt. Considering both contacting membranes by lipid anchors and electrostatic interactions with membrane phospholipids, the most preferred orientation with minimum free energy was found to be a “tilted orientation” whereby the N-terminal helix of Gtα is tilted ~30° to the membrane surface (114). Different results emanating from future studies may suggest that Gt is able to achieve a number of orientations that ensure an immediate response to light with transduction of the signal.
9. Abnormal lipid composition of the retina and defective vision
An abnormal membrane lipid composition could affect visual signaling. For example, a decreased level of DHA (22:6,n-3) in the retina was found to be associated with a diet low in n-3 fatty acids. Withholding DHA and its precursor, alpha-linolenic (18:3,n-3) acid from the diet produced this effect. Therefore, the lipid composition of biological membranes can reflect the amount and composition of dietary lipids. Rats maintained on diets deprived of n-3 fatty acids were less sensitive to light and evidenced both a significantly reduced capacity for photon absorption by rhodopsin and a greatly decreased rate of rhodopsin regeneration (115). One explanation for this observation could be that rhodopsin’s structural organization was disorganized and its function compromised due to altered lipid composition of the lipid bilayer. Reduced rhodopsin activation, inhibited formation of the complex between activated rhodopsin and Gt as well as reduced PDE activation have all been demonstrated in transgenic rats with n-3 fatty acid deficient ROS (94).
Reduced levels of long chain polyunsaturated fatty acids in the blood have been found in humans and animals with inherited retinal dystrophies. Both lipid and fatty acid composition were altered in retina of the Swedish Briard dog that exhibits slowly progressive retinal dystrophy inherited in an autosomal recessive manner. These animals have relatively more PE and PI, but less PC in their retinas than control animals. Analysis of fatty acids in the retina of affected dogs also documented lower levels of n-3 unsaturated fatty acids such as 22:5,n-3 and 22:6,n-3 together with higher levels of n-6 fatty acids such as 18:2,n-6, 20:4,n-6, and 22:5,n-6 (116). There was a direct relationship between the rate of retinal degeneration and the levels of DHA in the ROS. Lower amounts of DHA were found in transgenic rats with either P23H or S334ter rhodopsin – mutations leading to retinal degeneration (117). However, the abundance of major phospholipids in the ROS membranes of these animals was not affected by their disease. Thus, reduction of DHA levels in ROS membranes due to environmental stress or genetic mutations could be considered a neuroprotective mechanism that controls the number of photons captured by rhodopsin and the efficiency of visual transduction where excessive activation can lead to cell death (118).
Perturbations in cholesterol metabolism associated with photoreceptor degeneration have been reported as well (119). In Royal College of Surgeons (RCS) rats, an animal model of retinal degeneration progressing to blindness, it was found that the distribution of cholesterol in rod disk membranes differs significantly from normal animals in which cholesterol was distributed evenly from the base to the apex of ROS. The level of cholesterol in ROS membranes was increased, even though it was decreased in rod cell plasma membranes, most likely resulting from the abnormal phospholipid composition of these rats’ disk membranes. PE/PC ratios were 0.6 in the disks and 0.2 in the ROS plasma membranes of RCS rats compared to ratios of 0.9 and 0.4 in normal animals. This result is consistent with the observation that cholesterol partitions out of membranes enriched in PE.
A spectrum of visual defects has been reported for the Smith-Lemli-Optiz (SLO) syndrome in humans characterized by high levels of the cholesterol precursor, 7-dehydroxycholesterol, and low levels of cholesterol (120). This defect is caused by an array of mutations with consequences manifested in other tissues besides the eye.
Deficient synthesis of very long polyunsaturated C32-C36 acyl fatty acids (VLC-PUFAs) has been found in autosomal-dominant Stargardt-like macular dystrophy (62). Patients exhibiting this rare disease also carry several mutations in the gene encoding ELOVL4, an enzyme involved in elongation of VLC-PUFAs (59, 121). Therefore, decreased levels of VLC-PUFAS in Stargardt-like syndrome relate to abnormal functioning of ELOVL4 elongase. But the role of VLC-PUFAs is not well understood. Phospholipids containing these fatty acids tightly associate with rhodopsin and possibly stabilize its structure. They also may be involved in clearing retinal compounds from disk membranes after photoexcitation as suggested by results obtained in transgenic, heterozygous ELOVL4 knock-in mice. These mice carrying Stargardt-like associated mutations in their EOVL4 gene exhibited a deficiency in C32-C36 acyl chains and accumulation of lipofusions in RPE cells following degeneration of rod and cone photoreceptor cells. Surprisingly, homozygous ELOVL4 knockout mice exhibited normal prenatal retinal development but died several hours after birth due to increased skin permeability (64). Thus, VLC-PUFAs may not be required for retinal development but rather for retinal maintenance and function.
10. Conclusions
Rhodopsin remains an ideal model for biophysical and structural studies because it is the only GPCR purified in its native state. But how does a light signal captured by this receptor transfer through the plasma membrane and affect a G protein located 40 Å or more away, initiating a cellular response? How do changes in the structure and/or dynamics of rhodopsin lead to Gt binding? To answer these questions, a structure that captures this process is needed. Although structures of variously activated individual components of the rhodopsin-Gt complex have been solved at different resolutions, not much is known about the mechanism by which photoactivated rhodopsin binds to its cognate G protein and catalyzes nucleotide exchange. Certainly this system is not limited to just two proteins. Rather additional small molecules play key roles in the functional complex that includes chromophore, nucleotides, lipids, and integral-structural water molecules. As summarized in this review, neutral and phospholipids are predictably critical to this process and they do modulate rhodopsin conformational changes. But they also affect properties of the cognate G protein and are essential for proper formation of the complex between photoactivated rhodopsin and Gt. How we sort out these interactions at a high resolution structural level has remained a technical and intellectual challenge for years. The rhodopsin-Gt system also continues to represent one of the most productive models for investigating membrane biology.
Acknowledgments
We thank Dr. Leslie T. Webster, Jr. (Case Western Reserve University) and Susan Farr (Case Western Reserve University) for valuable comments on the manuscript. This work was supported in part by NIH grants RO1-EY008061, and R01 GM079191.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muller DJ, Wu N, Palczewski K. Vertebrate membrane proteins: structure, function, and insights from biophysical approaches. Pharmacol Rev. 2008;60:43–78. doi: 10.1124/pr.107.07111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 3.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SO. Structure and activation of the visual pigment rhodopsin. Annu Rev Biophys. 2010;39:309–328. doi: 10.1146/annurev-biophys-101209-104901. [DOI] [PubMed] [Google Scholar]
- 5.Aveldano MI. Phospholipid solubilization during detergent extraction of rhodopsin from photoreceptor disk membranes. Arch Biochem Biophys. 1995;324:331–343. doi: 10.1006/abbi.1995.0046. [DOI] [PubMed] [Google Scholar]
- 6.Calvert PD, Govardovskii VI, Krasnoperova N, Anderson RE, Lem J, Makino CL. Membrane protein diffusion sets the speed of rod phototransduction. Nature. 2001;411:90–94. doi: 10.1038/35075083. [DOI] [PubMed] [Google Scholar]
- 7.Jastrzebska B, Tsybovsky Y, Palczewski K. Complexes between photoactivated rhodopsin and transducin: progress and questions. Biochem J. 2010;428:1–10. doi: 10.1042/BJ20100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 9.Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo DG, Xue T, Yau KW. How vision begins: an odyssey. Proc Natl Acad Sci U S A. 2008;105:9855–9862. doi: 10.1073/pnas.0708405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jastrzebska B, Goc A, Golczak M, Palczewski K. Phospholipids are needed for the proper formation, stability, and function of the photoactivated rhodopsin-transducin complex. Biochemistry. 2009;48:5159–5170. doi: 10.1021/bi900284x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jastrzebska B, Fotiadis D, Jang GF, Stenkamp RE, Engel A, Palczewski K. Functional and structural characterization of rhodopsin oligomers. J Biol Chem. 2006;281:11917–11922. doi: 10.1074/jbc.M600422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- 14.Liebman PA, Weiner HL, Drzymala RE. Lateral diffusion of visual pigment in rod disk membranes. Methods Enzymol. 1982;81:660–668. doi: 10.1016/s0076-6879(82)81091-4. [DOI] [PubMed] [Google Scholar]
- 15.Poo M, Cone RA. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974;247:438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- 16.Chabre M, Cone R, Saibil H. Biophysics: is rhodopsin dimeric in native retinal rods? Nature. 2003;426:30–31. doi: 10.1038/426030b. discussion 31. [DOI] [PubMed] [Google Scholar]
- 17.Lee SP, O’Dowd BF, George SR. Homo- and hetero-oligomerization of G protein-coupled receptors. Life Sci. 2003;74:173–180. doi: 10.1016/j.lfs.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Park PS, Lodowski DT, Palczewski K. Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes. Annu Rev Pharmacol Toxicol. 2008;48:107–141. doi: 10.1146/annurev.pharmtox.48.113006.094630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filizola M. Increasingly accurate dynamic molecular models of G-protein coupled receptor oligomers: Panacea or Pandora’s box for novel drug discovery? Life Sci. 2009;86:590–597. doi: 10.1016/j.lfs.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palczewski K. Oligomeric forms of G protein-coupled receptors (GPCRs) Trends Biochem Sci. 2010 doi: 10.1016/j.tibs.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivero-Muller A, Chou YY, Ji I, Lajic S, Hanyaloglu AC, Jonas K, Rahman N, Ji TH, Huhtaniemi I. Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci U S A. 2010;107:2319–2324. doi: 10.1073/pnas.0906695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay D, Kellett E, McVey M, Rees S, Milligan G. Homo- and hetero-oligomeric interactions between G-protein-coupled receptors in living cells monitored by two variants of bioluminescence resonance energy transfer (BRET): hetero-oligomers between receptor subtypes form more efficiently than between less closely related sequences. Biochem J. 2002;365:429–440. doi: 10.1042/BJ20020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angers S, Salahpour A, Bouvier M. Biochemical and biophysical demonstration of GPCR oligomerization in mammalian cells. Life Sci. 2001;68:2243–2250. doi: 10.1016/s0024-3205(01)01012-8. [DOI] [PubMed] [Google Scholar]
- 24.Moepps B, Fagni L. Mont Sainte-Odile: a sanctuary for GPCRs. Confidence on signal transduction of G-protein-couple receptors. EMBO Rep. 2003;4:237–243. doi: 10.1038/sj.embor.embor777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovira X, Pin JP, Giraldo J. The asymmetric/symmetric activation of GPCR dimers as a possible mechanistic rationale for multiple signalling pathways. Trends Pharmacol Sci. 2010;31:15–21. doi: 10.1016/j.tips.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 2004;564:281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Fotiadis D, Maeda T, Maeda A, Modzelewska A, Filipek S, Saperstein DA, Engel A, Palczewski K. Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J Biol Chem. 2004;279:48189–48196. doi: 10.1074/jbc.M408362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Govardovskii VI, Korenyak DA, Shukolyukov SA, Zueva LV. Lateral diffusion of rhodopsin in photoreceptor membrane: a reappraisal. Mol Vis. 2009;15:1717–1729. [PMC free article] [PubMed] [Google Scholar]
- 31.Cone RA. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972;236:39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- 32.Ianoul A, Grant DD, Rouleau Y, Bani-Yaghoub M, Johnston LJ, Pezacki JP. Imaging nanometer domains of beta-adrenergic receptor complexes on the surface of cardiac myocytes. Nat Chem Biol. 2005;1:196–202. doi: 10.1038/nchembio726. [DOI] [PubMed] [Google Scholar]
- 33.Shukolyukov SA. Aggregation of frog rhodopsin to oligomers and their dissociation to monomer: application of BN- and SDS-PAGE. Biochemistry (Mosc) 2009;74:599–604. doi: 10.1134/s0006297909060029. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Zhang X, Zhang L, He F, Zhang G, Jamrich M, Wensel TG. Activation-dependent hindrance of photoreceptor G protein diffusion by lipid microdomains. J Biol Chem. 2008;283:30015–30024. doi: 10.1074/jbc.M803953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kota P, Reeves PJ, Rajbhandary UL, Khorana HG. Opsin is present as dimers in COS1 cells: identification of amino acids at the dimeric interface. Proc Natl Acad Sci U S A. 2006;103:3054–3059. doi: 10.1073/pnas.0510982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansoor SE, Palczewski K, Farrens DL. Rhodopsin self-associates in asolectin liposomes. Proc Natl Acad Sci U S A. 2006;103:3060–3065. doi: 10.1073/pnas.0511010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Periole X, Huber T, Marrink SJ, Sakmar TP. G protein-coupled receptors self-assemble in dynamics simulations of model bilayers. J Am Chem Soc. 2007;129:10126–10132. doi: 10.1021/ja0706246. [DOI] [PubMed] [Google Scholar]
- 38.Dell’Orco D, Schmidt H. Mesoscopic Monte Carlo simulations of stochastic encounters between photoactivated rhodopsin and transducin in disc membranes. J Phys Chem B. 2008;112:4419–4426. doi: 10.1021/jp709963f. [DOI] [PubMed] [Google Scholar]
- 39.Filipek S, Krzysko KA, Fotiadis D, Liang Y, Saperstein DA, Engel A, Palczewski K. A concept for G protein activation by G protein-coupled receptor dimers: the transducin/rhodopsin interface. Photochem Photobiol Sci. 2004;3:628–638. doi: 10.1039/b315661c. [DOI] [PubMed] [Google Scholar]
- 40.Mobarec JC, Sanchez R, Filizola M. Modern Homology Modeling of G-Protein Coupled Receptors: Which Structural Template to Use? J Med Chem. 2009 doi: 10.1021/jm9005252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filizola M, Weinstein H. The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J. 2005;272:2926–2938. doi: 10.1111/j.1742-4658.2005.04730.x. [DOI] [PubMed] [Google Scholar]
- 42.Casciari D, Seeber M, Fanelli F. Quaternary structure predictions of transmembrane proteins starting from the monomer: a docking-based approach. BMC Bioinformatics. 2006;7:340. doi: 10.1186/1471-2105-7-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Javitch JA. The ants go marching two by two: oligomeric structure of G-protein-coupled receptors. Mol Pharmacol. 2004;66:1077–1082. doi: 10.1124/mol.104.006320. [DOI] [PubMed] [Google Scholar]
- 44.Lambert NA. GPCR dimers fall apart. Sci Signal. 2010;3:pe12. doi: 10.1126/scisignal.3115pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert NA, Johnston CA, Cappell SD, Kuravi S, Kimple AJ, Willard FS, Siderovski DP. Regulators of G-protein signaling accelerate GPCR signaling kinetics and govern sensitivity solely by accelerating GTPase activity. Proc Natl Acad Sci U S A. 2010;107:7066–7071. doi: 10.1073/pnas.0912934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson RE, Maude MB. Phospholipids of bovine outer segments. Biochemistry. 1970;9:3624–3628. doi: 10.1021/bi00820a019. [DOI] [PubMed] [Google Scholar]
- 47.Anderson RE, Sperling L. Lipids of ocular tissues VII Positional distribution of the fatty acids in the phospholipids of bovine retina rod outer segments. Arch Biochem Biophys. 1971;144:673–677. doi: 10.1016/0003-9861(71)90374-2. [DOI] [PubMed] [Google Scholar]
- 48.Anderson RE, Benolken RM, Kelleher PA, Maude MB, Wiegand RD. Chemistry of photoreceptor membrane preparations from squid retinas. Biochim Biophys Acta. 1978;510:316–326. doi: 10.1016/0005-2736(78)90032-9. [DOI] [PubMed] [Google Scholar]
- 49.Dudley PA, Landis DJ, Anderson RE. Further studies on the chemistry of photoreceptor membranes of rats fed an essential fatty acid deficient diet. Exp Eye Res. 1975;21:523–530. doi: 10.1016/0014-4835(75)90034-2. [DOI] [PubMed] [Google Scholar]
- 50.Anderson RE, Benolken RM, Dudley PA, Landis DJ, Wheeler TG. Proceedings: Polyunsaturated fatty acids of photoreceptor membranes. Exp Eye Res. 1974;18:205–213. doi: 10.1016/0014-4835(74)90149-3. [DOI] [PubMed] [Google Scholar]
- 51.Anderson RE, Risk M. Lipids of ocular tissues. IX. The phospholipids of frog photoreceptor membranes. Vision Res. 1974;14:129–131. doi: 10.1016/0042-6989(74)90127-8. [DOI] [PubMed] [Google Scholar]
- 52.Miljanich GP, Sklar LA, White DL, Dratz EA. Disaturated and dipolyunsaturated phospholipids in the bovine retinal rod outer segment disk membrane. Biochim Biophys Acta. 1979;552:294–306. doi: 10.1016/0005-2736(79)90284-0. [DOI] [PubMed] [Google Scholar]
- 53.Organisciak DT, Noell WK. The rod outer segment phospholipid/opsin ratio of rats maintained in darkness or cyclic light. Invest Ophthalmol Vis Sci. 1977;16:188–190. [PubMed] [Google Scholar]
- 54.Tsui FC, Sundberg SA, Hubbell WL. Distribution of charge on photoreceptor disc membranes and implications for charged lipid asymmetry. Biophys J. 1990;57:85–97. doi: 10.1016/S0006-3495(90)82509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giusto NM, Pasquare SJ, Salvador GA, Castagnet PI, Roque ME, Ilincheta de Boschero MG. Lipid metabolism in vertebrate retinal rod outer segments. Prog Lipid Res. 2000;39:315–391. doi: 10.1016/s0163-7827(00)00009-6. [DOI] [PubMed] [Google Scholar]
- 56.Coleman JA, Kwok MC, Molday RS. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284:32670–32679. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aveldano de Caldironi MI, Giusto NM, Bazan NG. Polyunsaturated fatty acids of the retina. Prog Lipid Res. 1981;20:49–57. doi: 10.1016/0163-7827(81)90013-8. [DOI] [PubMed] [Google Scholar]
- 58.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 59.Brush RS, Tran JT, Henry KR, McClellan ME, Elliott MH, Mandal MN. Retinal sphingolipids and their very-long-chain fatty acid-containing species. Invest Ophthalmol Vis Sci. 2010;51:4422–4431. doi: 10.1167/iovs.09-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell DC, Niu SL, Litman BJ. Enhancement of G protein-coupled signaling by DHA phospholipids. Lipids. 2003;38:437–443. doi: 10.1007/s11745-003-1081-1. [DOI] [PubMed] [Google Scholar]
- 61.Aveldano MI. Phospholipid species containing long and very long polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry. 1988;27:1229–1239. doi: 10.1021/bi00404a024. [DOI] [PubMed] [Google Scholar]
- 62.Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res. 2010;51:1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agbaga MP, Brush RS, Mandal MN, Elliott MH, Al-Ubaidi MR, Anderson RE. Role of elovl4 protein in the biosynthesis of docosahexaenoic Acid. Adv Exp Med Biol. 2010;664:233–242. doi: 10.1007/978-1-4419-1399-9_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res. 2010 Oct;49(4):476–92. doi: 10.1016/j.plipres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hurst DP, Grossfield A, Lynch DL, Feller S, Romo TD, Gawrisch K, Pitman MC, Reggio PH. A lipid pathway for ligand binding is necessary for a cannabinoid G protein-coupled receptor. J Biol Chem. 2010;285:17954–17964. doi: 10.1074/jbc.M109.041590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 67.Ruprecht JJ, Mielke T, Vogel R, Villa C, Schertler GF. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 2004;23:3609–3620. doi: 10.1038/sj.emboj.7600374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc Natl Acad Sci U S A. 2006;103:4888–4893. doi: 10.1073/pnas.0508352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khelashvili G, Grossfield A, Feller SE, Pitman MC, Weinstein H. Structural and dynamic effects of cholesterol at preferred sites of interaction with rhodopsin identified from microsecond length molecular dynamics simulations. Proteins. 2009;76:403–417. doi: 10.1002/prot.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soubias O, Teague WE, Gawrisch K. Evidence for specificity in lipid-rhodopsin interactions. J Biol Chem. 2006;281:33233–33241. doi: 10.1074/jbc.M603059200. [DOI] [PubMed] [Google Scholar]
- 73.Brown MF. Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids. 1994;73:159–180. doi: 10.1016/0009-3084(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 74.Wiedmann TS, Pates RD, Beach JM, Salmon A, Brown MF. Lipid-protein interactions mediate the photochemical function of rhodopsin. Biochemistry. 1988;27:6469–6474. doi: 10.1021/bi00417a041. [DOI] [PubMed] [Google Scholar]
- 75.Gibson SK, Parkes JH, Liebman PA. Phosphorylation stabilizes the active conformation of rhodopsin. Biochemistry. 1998;37:13910. doi: 10.1021/bi985049k. [DOI] [PubMed] [Google Scholar]
- 76.Gibson NJ, Brown MF. Lipid headgroup and acyl chain composition modulate the MI-MII equilibrium of rhodopsin in recombinant membranes. Biochemistry. 1993;32:2438–2454. doi: 10.1021/bi00060a040. [DOI] [PubMed] [Google Scholar]
- 77.Gibson NJ, Brown MF. Membrane lipid influences on the energetics of the metarhodopsin I and metarhodopsin II conformational states of rhodopsin probed by flash photolysis. Photochem Photobiol. 1991;54:985–992. doi: 10.1111/j.1751-1097.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 78.Gibson NJ, Brown MF. Influence of pH on the MI-MII equilibrium of rhodopsin in recombinant membranes. Biochem Biophys Res Commun. 1990;169:1028–1034. doi: 10.1016/0006-291x(90)91997-7. [DOI] [PubMed] [Google Scholar]
- 79.Isele J, Sakmar TP, Siebert F. Rhodopsin activation affects the environment of specific neighboring phospholipids: an FTIR spectroscopic study. Biophys J. 2000;79:3063–3071. doi: 10.1016/S0006-3495(00)76541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Botelho AV, Martinez GV, Brown MF. Electrostatic properties of membrane lipids coupled to metarhodopsin II formation in visual transduction. J Am Chem Soc. 2002;124:7690–7701. doi: 10.1021/ja0200488. [DOI] [PubMed] [Google Scholar]
- 81.Kisselev O, Ermolaeva M, Gautam N. Efficient interaction with a receptor requires a specific type of prenyl group on the G protein gamma subunit. J Biol Chem. 1995;270:25356–25358. doi: 10.1074/jbc.270.43.25356. [DOI] [PubMed] [Google Scholar]
- 82.Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Lipid modification at the N terminus of photoreceptor G-protein alpha-subunit. Nature. 1992;359:749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- 83.Fukada Y, Takao T, Ohguro H, Yoshizawa T, Akino T, Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990;346:658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- 84.Hessel E, Heck M, Muller P, Herrmann A, Hofmann KP. Signal transduction in the visual cascade involves specific lipid-protein interactions. J Biol Chem. 2003;278:22853–22860. doi: 10.1074/jbc.M302747200. [DOI] [PubMed] [Google Scholar]
- 85.Hessel E, Herrmann A, Muller P, Schnetkamp PP, Hofmann KP. The transbilayer distribution of phospholipids in disc membranes is a dynamic equilibrium evidence for rapid flip and flop movement. Eur J Biochem. 2000;267:1473–1483. doi: 10.1046/j.1432-1327.2000.01147.x. [DOI] [PubMed] [Google Scholar]
- 86.Hessel E, Muller P, Herrmann A, Hofmann KP. Light-induced reorganization of phospholipids in rod disc membranes. J Biol Chem. 2001;276:2538–2543. doi: 10.1074/jbc.M009061200. [DOI] [PubMed] [Google Scholar]
- 87.Armstrong VT, Brzustowicz MR, Wassall SR, Jenski LJ, Stillwell W. Rapid flip-flop in polyunsaturated (docosahexaenoate) phospholipid membranes. Arch Biochem Biophys. 2003;414:74–82. doi: 10.1016/s0003-9861(03)00159-0. [DOI] [PubMed] [Google Scholar]
- 88.Alves ID, Salgado GF, Salamon Z, Brown MF, Tollin G, Hruby VJ. Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy. Biophys J. 2005;88:198–210. doi: 10.1529/biophysj.104.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hruby VJ, Tollin G. Plasmon-waveguide resonance (PWR) spectroscopy for directly viewing rates of GPCR/G-protein interactions and quantifying affinities. Curr Opin Pharmacol. 2007;7:507–514. doi: 10.1016/j.coph.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salamon Z, Wang Y, Soulages JL, Brown MF, Tollin G. Surface plasmon resonance spectroscopy studies of membrane proteins: transducin binding and activation by rhodopsin monitored in thin membrane films. Biophys J. 1996;71:283–294. doi: 10.1016/S0006-3495(96)79224-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- 92.Tsybovsky Y, Molday RS, Palczewski K. The ATP-Binding Cassette Transporter ABCA4: Structural and Functional Properties and Role in Retinal Disease. Adv Exp Med Biol. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molday RS, Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog Lipid Res. 2010;49:476–492. doi: 10.1016/j.plipres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niu SL, Mitchell DC, Lim SY, Wen ZM, Kim HY, Salem N, Jr, Litman BJ. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem. 2004;279:31098–31104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- 95.Jastrzebska B, Maeda T, Zhu L, Fotiadis D, Filipek S, Engel A, Stenkamp RE, Palczewski K. Functional characterization of rhodopsin monomers and dimers in detergents. J Biol Chem. 2004;279:54663–54675. doi: 10.1074/jbc.M408691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Albert AD, Boesze-Battaglia K. The role of cholesterol in rod outer segment membranes. Prog Lipid Res. 2005;44:99–124. doi: 10.1016/j.plipres.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Young RW. The renewal of rod and cone outer segments in the rhesus monkey. J Cell Biol. 1971;49:303–318. doi: 10.1083/jcb.49.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young JE, Albert AD. Transducin binding in bovine rod outer segment disk membranes of different age/spatial location. Exp Eye Res. 2000;70:809–812. doi: 10.1006/exer.2000.0821. [DOI] [PubMed] [Google Scholar]
- 100.Boesze-Battaglia K, Albert AD. Cholesterol modulation of photoreceptor function in bovine retinal rod outer segments. J Biol Chem. 1990;265:20727–20730. [PubMed] [Google Scholar]
- 101.Mitchell DC, Straume M, Miller JL, Litman BJ. Modulation of metarhodopsin formation by cholesterol-induced ordering of bilayer lipids. Biochemistry. 1990;29:9143–9149. doi: 10.1021/bi00491a007. [DOI] [PubMed] [Google Scholar]
- 102.House K, Badgett D, Albert AD. Cholesterol movement between bovine rod outer segment disk membranes and phospholipid vesicles. Exp Eye Res. 1989;49:561–572. doi: 10.1016/s0014-4835(89)80055-7. [DOI] [PubMed] [Google Scholar]
- 103.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 104.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 105.Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 106.Elliott MH, Fliesler SJ, Ghalayini AJ. Cholesterol-dependent association of caveolin-1 with the transducin alpha subunit in bovine photoreceptor rod outer segments: disruption by cyclodextrin and guanosine 5’-O-(3-thiotriphosphate) Biochemistry. 2003;42:7892–7903. doi: 10.1021/bi027162n. [DOI] [PubMed] [Google Scholar]
- 107.Elliott MH, Nash ZA, Takemori N, Fliesler SJ, McClellan ME, Naash MI. Differential distribution of proteins and lipids in detergent-resistant and detergent-soluble domains in rod outer segment plasma membranes and disks. J Neurochem. 2008;104:336–352. doi: 10.1111/j.1471-4159.2007.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seno K, Kishimoto M, Abe M, Higuchi Y, Mieda M, Owada Y, Yoshiyama W, Liu H, Hayashi F. Light- and guanosine 5’-3-O-(thio)triphosphate-sensitive localization of a G protein and its effector on detergent-resistant membrane rafts in rod photoreceptor outer segments. J Biol Chem. 2001;276:20813–20816. doi: 10.1074/jbc.C100032200. [DOI] [PubMed] [Google Scholar]
- 109.Martin RE, Elliott MH, Brush RS, Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Invest Ophthalmol Vis Sci. 2005;46:1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- 110.Park PS, Sapra KT, Jastrzebska B, Maeda T, Maeda A, Pulawski W, Kono M, Lem J, Crouch RK, Filipek S, Muller DJ, Palczewski K. Modulation of molecular interactions and function by rhodopsin palmitylation. Biochemistry. 2009;48:4294–4304. doi: 10.1021/bi900417b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Z, Wen XH, Ablonczy Z, Crouch RK, Makino CL, Lem J. Enhanced shutoff of phototransduction in transgenic mice expressing palmitoylation-deficient rhodopsin. J Biol Chem. 2005;280:24293–24300. doi: 10.1074/jbc.M502588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maeda A, Okano K, Park PS, Lem J, Crouch RK, Maeda T, Palczewski K. Palmitoylation stabilizes unliganded rod opsin. Proc Natl Acad Sci U S A. 2010;107:8428–8433. doi: 10.1073/pnas.1000640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Z, Melia TJ, He F, Yuan C, McGough A, Schmid MF, Wensel TG. How a G protein binds a membrane. J Biol Chem. 2004;279:33937–33945. doi: 10.1074/jbc.M403404200. [DOI] [PubMed] [Google Scholar]
- 114.Kosloff M, Alexov E, Arshavsky VY, Honig B. Electrostatic and lipid anchor contributions to the interaction of transducin with membranes: mechanistic implications for activation and translocation. J Biol Chem. 2008;283:31197–31207. doi: 10.1074/jbc.M803799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bush RA, Malnoe A, Reme CE, Williams TP. Dietary deficiency of N-3 fatty acids alters rhodopsin content and function in the rat retina. Invest Ophthalmol Vis Sci. 1994;35:91–100. [PubMed] [Google Scholar]
- 116.Anderson RE, Maude MB, Narfstrom K, Nilsson SE. Lipids of plasma, retina, and retinal pigment epithelium in Swedish briard dogs with a slowly progressive retinal dystrophy. Exp Eye Res. 1997;64:181–187. doi: 10.1006/exer.1996.0195. [DOI] [PubMed] [Google Scholar]
- 117.Martin RE, Fliesler SJ, Brush RS, Richards MJ, Hopkins SA, Anderson RE. Lipid differences in rod outer segment membranes of rats with P23H and S334ter opsin mutations. Mol Vis. 2005;11:338–346. [PubMed] [Google Scholar]
- 118.Anderson RE, Penn JS. Environmental light and heredity are associated with adaptive changes in retinal DHA levels that affect retinal function. Lipids. 2004;39:1121–1124. doi: 10.1007/s11745-004-1338-8. [DOI] [PubMed] [Google Scholar]
- 119.Boesze-Battaglia K, Organisciak DT, Albert AD. RCS rat retinal rod outer segment membranes exhibit different cholesterol distributions than those of normal rats. Exp Eye Res. 1994;58:293–300. doi: 10.1006/exer.1994.1020. [DOI] [PubMed] [Google Scholar]
- 120.Boesze-Battaglia K, Damek-Poprawa M, Mitchell DC, Greeley L, Brush RS, Anderson RE, Richards MJ, Fliesler SJ. Alteration of retinal rod outer segment membrane fluidity in a rat model of Smith-Lemli-Opitz syndrome. J Lipid Res. 2008;49:1488–1499. doi: 10.1194/jlr.M800031-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, Li Y, Metzker ML, Allikmets R, Zack DJ, Kakuk LE, Lagali PS, Wong PW, MacDonald IM, Sieving PA, Figueroa DJ, Austin CP, Gould RJ, Ayyagari R, Petrukhin K. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet. 2001;27:89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]
- 122.Aveldano MI, Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J Biol Chem. 1987;262:1180–1186. [PubMed] [Google Scholar]
- 123.Boesze-Battaglia K, Albert AD. Phospholipid distribution among bovine rod outer segment plasma membrane and disk membranes. Exp Eye Res. 1992;54:821–823. doi: 10.1016/0014-4835(92)90040-y. [DOI] [PubMed] [Google Scholar]
- 124.Boesze-Battaglia K, Fliesler SJ, Albert AD. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J Biol Chem. 1990;265:18867–18870. [PMC free article] [PubMed] [Google Scholar]
- 125.Heck M, Hofmann KP. Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J Biol Chem. 2001;276:10000–10009. doi: 10.1074/jbc.M009475200. [DOI] [PubMed] [Google Scholar]
- 126.Farrens DL, Khorana HG. Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J Biol Chem. 1995;270:5073–5076. doi: 10.1074/jbc.270.10.5073. [DOI] [PubMed] [Google Scholar]