Abstract
Attention can be readily measured in experimental animal models. Animal models of attention have been used to better understand the neural systems involved in attention, how attention is impaired, and how therapeutic treatments can ameliorate attentional deficits. This review focuses on the ways in which animal models are used to better understand the neuronal mechanism of attention and how to develop new therapeutic treatments for attentional impairment. Several behavioral test methods have been developed for experimental animal studies of attention, including a 5-choice serial reaction time task (5-CSRTT), a signal detection task (SDT), and a novel object recognition (NOR) test. These tasks can be used together with genetic, lesion, pharmacological and behavioral models of attentional impairment to test the efficacy of novel therapeutic treatments. The most prominent genetic model is the spontaneously hypertensive rat (SHR). Well-characterized lesion models include frontal cortical or hippocamapal lesions. Pharmacological models include challenge with the NMDA glutamate antagonist dizocilpine (MK-801), the nicotinic cholinergic antagonist mecamylamine and the muscarinic cholinergic antagonist scopolamine. Behavioral models include distracting stimuli and attenuated target stimuli. Important validation of these behavioral tests and models of attentional impairments for developing effective treatments for attentional dysfunction is the fact that stimulant treatments effective for attention deficit hyperactivity disorder (ADHD), such as methylphenidate (Ritalin®), are effective in the experimental animal models. Newer lines of treatment including nicotinic agonists, α4β2 nicotinic receptor desensitizers, and histamine H3 antagonists, have also been found to be effective in improving attention in these animal models. Good carryover has also been seen for the attentional improvement of nicotine in experimental animal models and in human populations. Animal models of attention can be effectively used for the development of new treatments of attentional impairment in ADHD and other syndromes in which have attentional impairments occur, such as Alzheimer’s disease and schizophrenia.
Keywords: Sustained attention, Cognition, Animal models, Treatment, Signal detection
Introduction
Attention stands at the forefront of cognition. To optimize learning and remembering an environmental stimulus or event, one must first attend to it. Attentional impairments are seen in a variety of conditions, obviously including attention deficit hyperactivity disorder (ADHD), but also schizophrenia and Alzheimer’s disease, which have pronounced attentional impairments as a component of the syndrome. Cognitive enhancement to reverse the deficits in these syndromes refers to improvement of learning, memory and attention.
This article reviews the principal rodent models of attention and how they are used to discover and characterize novel treatments for attentional impairment. It follows upon previous reviews in this area, which provide important coverage of the neurobiology of attention, the value and drawbacks of various animal models and the effects of specific classes of drugs on attention (Bushnell, 1998; Bushnell et al., 2000; Chudasama and Robbins, 2004). This review is intended to provide insight into animal models for developing potential therapeutic treatments for attentional deficits. Some treatments may be useful only in counteracting a specific mechanism causing attentional impairment while others may have a more general improvement in a variety of attention deficit models and even in the unimpaired subject. Thus, it is important to have a variety of models to test specificity vs. generality of effect. This variety can help both with developing new effective therapeutic treatments and with improving understanding of the neurobehavioral mechanisms of attention.
Varieties of Attention and Methods for Assessing them in Animals
Attention is a hypothetical construct that is conceived to underlie processing information about the environment and its relation to the individual. It is not measured directly, but is inferred from behavior in controlled environments. Over the past century there has been considerable research concerning attention using a wide variety of paradigms and models. There is an enormous literature concerning attention with a variety of conceptualizations and ways to subdivide it. In this review we have kept to much more practical issues of how different animal models shed light on the neural systems involved in attention and how they point to promising avenues for therapeutic drug development.
To this end, we consider just two of the many possible “varieties” of attention (James, 1890, reprinted in 1950), which can be categorized as “selective” and “sustained”. Selective attention is thought to be engaged when an animal faces multiple stimuli and chooses among them; this choice is defined by the animals’ observed behavior. Novelty plays an important role in determining the subsequent behavior of the animal and hence its attentional selection. Sustained attention, by contrast, is thought to be engaged when an animal’s behavior is controlled by a single stimulus that occurs unpredictably in time or space. For example, a light flash or auditory chirp may signal the availability of food and guide the animal’s choice of responses required to obtain that food.
There are several commonly used methods of assessing selective and sustained attention in rodents (Bushnell and Strupp, 2009). Sustained attention is frequently assessed using the 5-choice serial reaction time test (5-CSRTT) (Carli et al., 1983) and an operant signal detection task (SDT) (Bushnell, 1999; Bushnell et al., 1994; McGaughy and Sarter, 1995a; Rezvani and Levin, 2003b). Selective attention can be assessed using a novel object recognition test (Grayson et al., 2007) or a sand-digging task (Birrell and Brown, 2000). They each have particular advantages and drawbacks.
Tests of sustained attention employ operant techniques in which animals are trained to perform the task and are then given daily multiple-trial test sessions in which a critical stimulus is presented unpredictably in time and/or space. Thus, the animal must sustain attention to that stimulus to earn food or water, by responding correctly to the presence or absence of the stimulus. The 5-choice serial reaction time test (5-CSRTT) utilizes stimuli that vary spatially and can also be varied in terms of intensity and temporal unpredictability. The 5-CSRTT tests attentiveness to multiple locations over time and thus requires both sustained and selective attention. Whereas they are not independent in this task, the two components can be manipulated separately to preferentially “load” one function or the other. For example, sustained attention can be stressed by increasing the temporal unpredictability of the stimulus, and selective attention can be emphasized by increasing the number of locations at which the stimulus may occur. The task has the advantages of a rich literature base and multiple measures of performance that reflect attention and response speed. As described below, there are many studies, which have assessed effects of drugs acting on a variety of neurotransmitter receptors. The principal drawbacks of the task are the extensive training needed to attain a stable baseline of attentional performance, and the fact that the timing of trials is under the control of the subject, rather than of the experimenter.
The SDT described here (Fig. 1) was devised by Bushnell (Bushnell et al., 1994) to assess effects of solvents on sustained attention; McGaughy and Sarter (McGaughy and Sarter, 1995a) provided parametric and pharmacological manipulations in an early characterization of behavior in the task. The SDT uses a signal that varies only in time and intensity, and has the advantages of providing separate measures of hits, misses, false alarms and correct rejections as well as measures of response latency and response omissions. Stimulus modality, intensity and duration can be varied to provide parametric tests of attentiveness and sensory threshold. Rats and humans behave similarly in this task (Fig. 2), with rats (Bushnell et al., 2003) responding similarly to changes in several parameters known to affect sustained attention in humans (Parasuraman, 1984). There have been a moderate number of studies using this task. As reviewed below, drugs acting on a variety of neurotransmitter systems and other chemical exposures have been assessed using the SDT. As with the 5-CSRTT task, its principal drawback is the considerable training required to achieve the stable behavioral baseline necessary for assessment of attention.
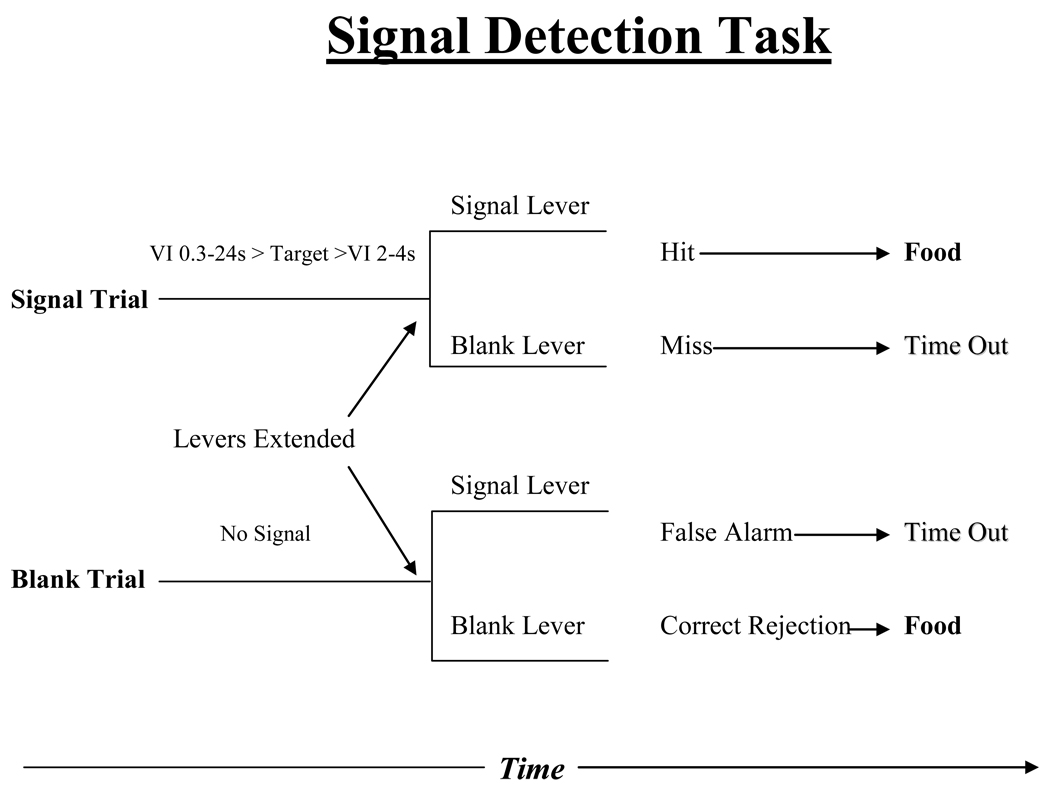
Figure 1.
Diagram of operant signal detection attention task. This task comprised two types of trials, signal and blank, which differed only in that a signal was presented in each signal trial and omitted in blank trials. In each trial, the rat pressed either of two retractable levers to report that a signal had or had not occurred in that trial. Four possible outcomes result: hit, miss, false alarm and correct rejection. Hits and correct rejections were followed by delivery of food; misses and false alarms by a 2 sec “time out” period without food. An increase in hit and or correct rejection indicates improvement in sustained attention and a decrease in these parameters indicates impairment in attentional performance. VI stands for variable intervals for pre- and post-signal during the signal trial (Bushnell, 1998; Bushnell et al., 2003; Bushnell et al., 1997b; Rezvani et al., 2002; Rezvani et al., 2011; Rezvani et al., 2009a; Rezvani et al., 2009b; Rezvani et al., 2008).
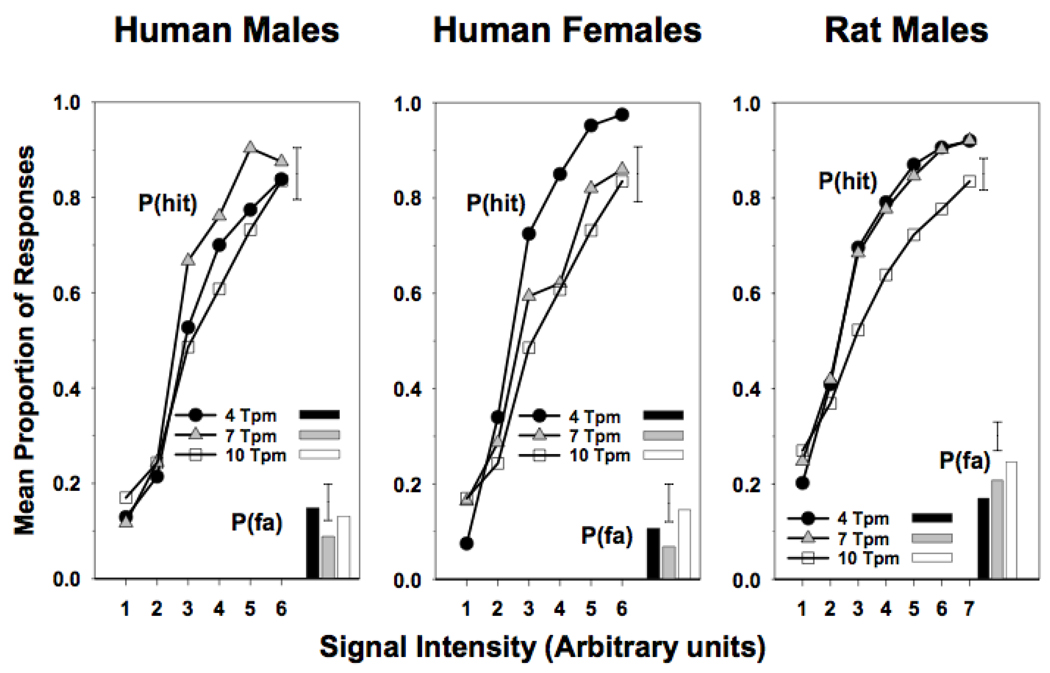
Figure 2.
Attentional performance of rats and humans on signal detection Tasks (SDTs) on proportions of hits relative to the total signal trial responses [P(hit]) and false alarms relative to the total blank trial responses [P(fa)] as a function of the signal intensity (Bushnell et al., 2003). The effect of the rate of trial presentation (trials per minute or TPM) was assessed. Both rats and humans showed a specific TPM for optimal attentional accuracy.
Selective attention may be assessed with the novel object recognition (NOR) test and the sanddigging (S-D) test (Birrell and Brown, 2000). These tasks rely on unconditioned behaviors of rodents that are elicited by presentation of novel stimuli. The principal advantage of the NOR test is the rapid testing sequence. There is no training necessary other than the initial exposure session. This test has been used frequently in the pharmaceutical industry for rapid assessment of candidate nootropic drugs, although much of this information is not in the open literature due to the confidential nature of the drugs tested. Nevertheless, results are available for enough studies to evaluate the method. The main drawback of the NOR test is that its specificity for assessing attention is more limited than in the operant methods previously described. Other factors such as changes in novelty preference, sensory function, or memory must be independently evaluated to determine whether changes in performance can be ascribed to altered attention.
There are other tasks, which have not been as extensively developed, but could be useful in future research. For example, the S-D test has been used to assess selective attention in terms of set-shifting (Birrell and Brown, 2000). Based on the Wisconsin card-sorting test (Milner, 1963), this method evaluates an animal’s attention to a stimulus dimension and the facility with which this attending can be shifted to another dimension. For example, the critical cue attended to could shift from odor to texture of the stimulus object. This method has the advantage of being procedurally similar to tests of attention used in clinical studies. However, the degree to which set shifting as measured in the S-D task models selective attention is debatable. As the field progresses new tests of animal attention continue to be developed which are more efficient and have better validity for predicting efficacy of treatments for attentional impairments in humans.
Animal Models of Attentional Impairment
A wide variety of animal models of attentional deficits have been described (Davids et al., 2003). These include genetic models, lesion models, pharmacological models and behavioral models. Each model has particular advantages and disadvantages (Davids et al., 2003). Each has a place in the effort to better understand the neurobehavioral bases of attentional function and dysfunction and development of more efficacious therapeutic treatments. The models of attentional impairment are described in this section and the efficacy of drug treatments in reversing the attentional impairments in the following section on therapeutic treatments.
Genetic Models
The most extensively used genetic model is the spontaneously hypertensive rat (SHR) (Berger and Sagvolden, 1998; Sagvolden, 2000; Sagvolden et al., 1992). Originally developed and widely used for hypertension research, SHR rats develop hypertension in later adulthood. Before they develop hypertension, these rats show symptoms of ADHD, including attentional impairment as measured by the set shifting task and a lateralized reaction time task (Jentsch, 2005; Kantak et al., 2008). SHR are not impaired in spatial learning and memory as such, but appear to have more difficulty in relating motivational state to choice behavior, that is they did not associate a conditioned cue to their response as well as controls (Clements et al., 2007; Clements and Wainwright, 2007) and controlling impulsivity (Adriani et al., 2003). Reductions in nicotinic receptor number may be in part responsible for the cognitive impairments of SHR rats (Gattu et al., 1997a; Gattu et al., 1997b). Nicotinic receptors in SHR rats were hypo-responsive to up-regulation with chronic nicotine administration (Hohnadel et al., 2005).
Lesion Models
Loss of telencephalic cholinergic innervation has been postulated to play a significant role in cognitive function associated with aging and dementia. Manipulations of corticopetal cholinergic projections from the basal forebrain have revealed bidirectional control of sustained attention in rats. Partial loss of these cortical cholinergic inputs induced by infusion of 192 IgG-saporin into the basal forebrain of rats decreased the relative number of hits while the relative number of correct rejections (and false alarms) remained unaffected (McGaughy et al., 1996), suggesting that cholinergic input to the cortex facilitates responses to environmental targets. Because behaviorally-stimulated ACh release from the basal forebrain is augmented by activation of glutamatergic pathways, cortical cholinergic activity is enhanced by NMDA activity in the basal forebrain. This interaction provides a way to manipulate task-dependent cholinergic activity in the cortex. In an elegant application of this system, Turchi and Sarter (Turchi and Sarter, 2001) showed that intrabasalis infusions of NMDA increased the false alarm rate without affecting hits, whereas infusions of the competitive NMDA antagonist DL-2-amino-5-phosphonovaleric acid (APV) increased the hit rate without affecting false alarms. A similar dissociation was obtained when effects of intrabasalis infusions of 192 IgG-saporin were compared to effects of infusions of iobotenic acid, which reduced the GABA-mediated inhibition of the cholinergic neurons in the nucleus basalis (Burk and Sarter, 2001). Thus, increasing the cholinergic input to the cortex appears to increase the animal’s responses for signals (shifting the animal’s bias toward reporting hits) and decreasing the cholinergic input to the cortex appears to have the opposite effect. Nevertheless, none of these manipulations actually increased the accuracy of signal detection, but rather impaired it by creating a bias to respond either “signal” or “blank”, depending upon the direction of change in cholinergic tone.
Thus, sustained attention appears to be optimal in intact animals whose corticopetal cholinergic inputs are appropriately balanced. Attentional impairments associated with aging or neurodegeneration may reflect a loss of balance in the cholinergic innervation of the cortex. Changes in bias in such individuals may be useful for identifying the nature of the imbalance. However, pharmacological treatment of such imbalances is not likely to be simple. For example, systemic administration of ABT-418, a nicotinic acetylcholine receptor agonist, increased the relative number of hits in control rats but not in animals whose detection of signals was impaired by intrabasalis infusions of 192 IgG-saporin (McGaughy et al., 1999).
Pharmacological Models
A wide variety of transmitter receptor antagonists have been shown to reversibly impair choice accuracy on attentional tasks. Extensive work with a variety of systemically-administered drugs in the 5-CSRTT was summarized by Robbins (Robbins, 2002). In brief, this review suggests the following conclusions. The ascending adrenergic systems appear to maintain arousal, modulate “response vigor”, and reduce the influence of distracting events, although inconsistent effects of manipulations of these systems do not afford firm conclusions. Attenuation of striatal dopaminergic systems primarily slows responding, with little effect on accuracy; however, dopaminergic lesions restricted to the prefrontal cortex impair accuracy without response latency effects. Loss of cortical acetylcholine exerts profound effects on response accuracy, as has also been observed in the SDT, consistent with the lesion work described above, and pharmacological manipulations to follow. Finally, reduced serotonergic tone is generally associated with increases in premature responding, which has been interpreted as impulsiveness.
A subsequent review (Chudasama and Robbins, 2004) related these experimental findings to potential pharmacological therapies for schizophrenia, as screened with the 5-CSRTT, including treatment with dopamine D1 and D2 agonists, adrenergic α1 agonists, 5HT1A agonists and 5HT2A antagonists, nicotine, and physostigmine. In particular, evidence from a variety of sources points to abnormalities in the function of α7 nicotinic receptors as a key mechanism in the cognitive impairments of schizophrenia, and new α7 nicotinic agonists have shown promise for treatment of the attentional impairment of schizophrenia (Martin et al., 2004).
As discussed above, acetylcholine has been shown to play key roles in sustained attention, in both the 5-CSRTT and the SDT. Regarding the latter test, both the muscarinic cholinergic antagonist scopolamine (Bushnell et al., 1997b; McGaughy and Sarter, 1995b; Rezvani et al., 2009b) and the nicotinic cholinergic antagonist mecamylamine (Rezvani et al., 2002; Rezvani et al., 2009b) substantially impair accuracy. Changes in adrenergic and cholinergic tone appear to affect sustained attention in a parallel manner. Thus, reduced tone (induced by clonidine, mecamylamine or scopolamine) lowered signal detection accuracy by reducing hits and increasing false alarms, whereas elevated tone (induced by idazoxan, nicotine or pilocarpine) reduced hits without affecting false alarms Fig. 3). The effects of cholinergic antagonists scopolamine and mecamylamine are generally consistent with the results of manipulations of corticopetal cholinergic projections from the nucleus basalis described above) with regard to hit accuracy but not with regard to false alarms. As is often the case with cognitive function, there appears to be a level of activation of cholinergic and adrenergic systems that optimizes attention, while increases or decreases both impair function. It should be noted that clonidine, acting on postsynaptic adrenergic receptors, can have a net stimulatory effect on noradrenergic systems and has been found in some studies to improve attention in ADHD (Sallee, 2010).
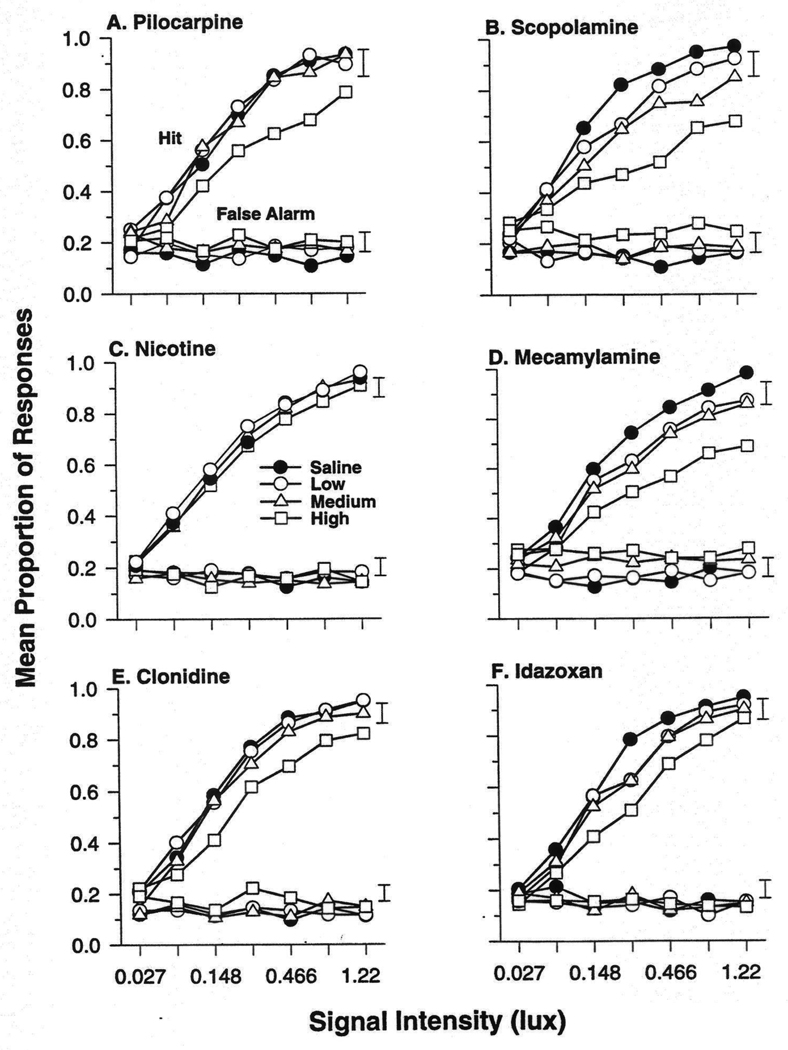
Figure 3.
Effects of cholinergic and alpha2-adrenergic drugs on proportions of hits relative to the total signal trial responses P(hit) and false alarms relative to the total blank trial responses [P(fa)] as a function of the signal intensity. In each panel, the upper curves show values of P(hit) and lower curves P(fa). The values on the abscissa indicate the intensities of the 300-msec signals as increments in intensity above a background illumination of 1.20 lux. In the key, Saline refers to vehicle; Low, Medium and High refer to the three doses of each drug administered. Doses were as follows: Pilocarpine: 1.0, 1.8, and 3.0 mg/kg; Scopolamine: 0.030, 0.056, 0.10 mg/kg; Nicotine: 0.083, 0.25, and 0.75 mg/kg; Mecamylamine: 1.8, 3.0, and 5.6 mg/kg; Clonidine: 0.003, 0.010, 0.030 mg/kg; and Idazoxan: 1.0, 3.0, and 10.0 mg/kg. Values are means; the error bars indicate SEM above and below the means.
Sustained attention in the SDT also depends on glutamatergic and GABA-ergic pathways. For example, dizocilpine (MK-801), an NMDA glutamate antagonist, substantially impairs signal detection behavior (Rezvani et al., 2009b; Rezvani et al., 2008) (Fig. 4). Chlordiazepoxide, a benzodiazepine agonist that potentiates the effects of GABA at GABA-ergic receptors, impairs performance of the SDT in young and old rats (McGaughy and Sarter, 1995b), but may do so by affecting visual thresholds rather than impairing attention (Bushnell et al., 1997a). Alcohol and many organic solvents, which among other things potentiate chloride flux through GABA-mediated ion channels and inhibit activity at glutamatergic ion channels, impair sustained attention in this task (Bushnell, 1997; Bushnell et al., 2007; Rezvani and Levin, 2003a). Antipsychotic drugs such as clozapine, risperidone and haloperidol, which block a variety of receptors including dopamine, serotonin, norepinephrine, histamine and cholinergic receptors, also impair sustained attention in rats (Rezvani and Levin, 2004b). The deficits in attention caused by these drugs serve as good pharmacological models of impaired attentional performance against which to evaluate potential therapeutic effects of novel attention improving drugs.
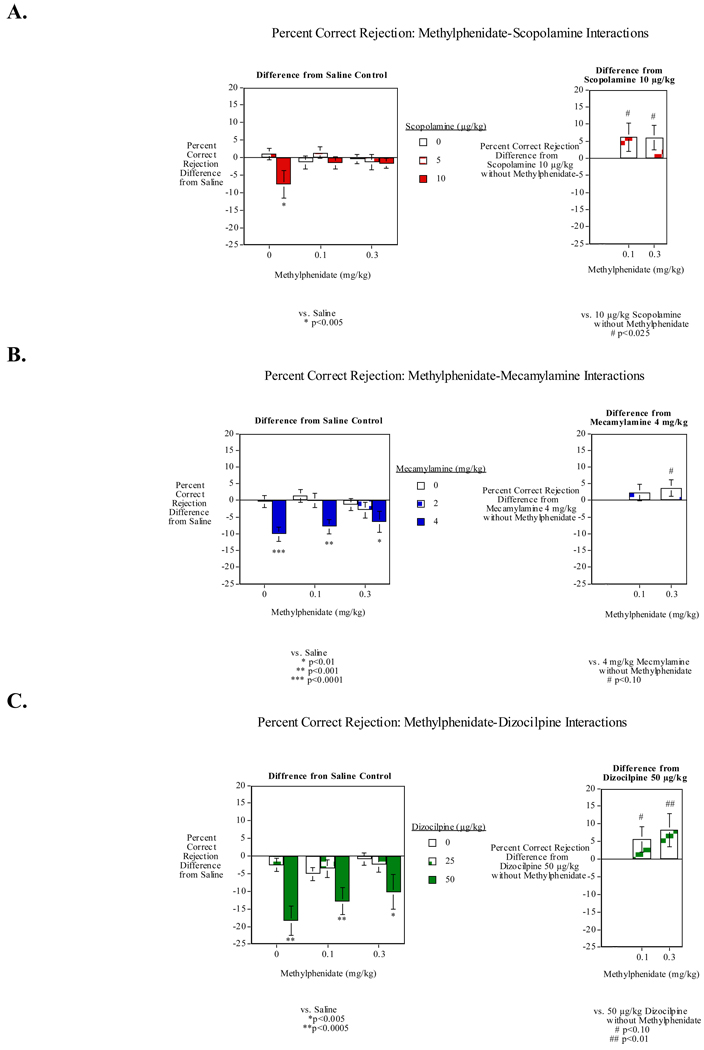
Figure 4.
- The muscarinic cholinergic antagonist scopolamine;
- The nicotinic antagonist mecamylamine; and
- The NMDA glutamate antagonist dizocilpine: percent correct rejection (mean±sem)
Behavioral Models
The primary behavioral models of attentional impairment involve attenuating the target stimuli, varying their predictability, and imposing distracting stimuli, all of which have been used to challenge sustained attention. Target stimuli have been attenuated both by reducing their intensity and duration. Manipulating the duration of the stimulus is more frequently done, because automated test systems typically used for this work do not enable software control of the voltage to the stimulus lamp or loudspeaker. Nevertheless, varying the intensity of the stimulus provides a means to detect the influence of sensory threshold shifts as well as deficits in attention (Bushnell et al., 1997a). Reducing the duration of the stimulus is thought to increase the “attentional load” of the task (Muir et al., 1993), by reducing time that the stimulus is present and thus the probability of its detection.
Distracting stimuli can be effective in impairing sustained attention (Johnson and Burk, 2006; McGaughy and Sarter, 1995a; Newman and McGaughy, 2008), although the timing of the presentation of the distractors is critical, and animals adapt to their occurrence (Himmelheber et al., 2000). Finally, the rate at which target stimuli are presented, regardless of their predictability, affects the accuracy of detection, in both rats and humans (Bushnell, 1999; Bushnell et al., 2003).
Therapeutic Medications
Stimulants
Drugs that have proven to be therapeutically useful in the treatment of attentional deficits in humans are quite useful for validating the animal models of attentional impairment. This is true both for the behavioral tasks and the source of impairment, whether it is in genetics, transmitter receptor blockade, lesion or behavioral challenge. Predictive validity of the animal model would be shown by a therapeutically useful drug reversing an impairment in the model. In the operant SDT, methylphenidate (Ritalin®) has been shown to reverse the impairments caused by the NMDA glutamate antagonist dizocilpine, the muscarinic acetylcholine antagonist scopolamine, and the nicotinic acetylcholine antagonist mecamylamine (Rezvani et al., 2009b) (Fig. 4). This reversal of pharmacologically-induced attentional impairment with a proven treatment for ADHD serves as a good validation of this model of ADHD. Methylphenidate also eliminated impairments of attention and memory in SHR rats (Kantak et al., 2008; Paule et al., 2000). Methamphetamine (0.3 mg/kg) has been shown to shorten the choice reaction time in rats; however it also increases the number of lever presses (Mishima et al., 2002). Also, methylphenidate has been shown to improve performance in a multi-trial attention task in juvenile rats (Zhu et al., 2010). Activating noradrenergic α1 receptors enhanced the attentional processing, while blocking these receptors impaired attention (Mishima et al., 2002).
Cholinergic systems have been shown to be important for several aspects of cognitive function including attention, learning and memory (Levin et al., 2006). Both nicotinic and muscarinic acetylcholine receptors have been found to be involved. In particular cholinergic projections to the hippocampus and frontal cortex play key roles in cognitive function (Bartus et al., 1987).
Neuronal nicotinic systems have been shown in both laboratory animals and humans to play major roles in cognitive functions such as learning and memory and attention (Grilly, 2000; Levin et al., 2006; Mirza and Bright, 2001; Mirza and Stolerman, 1998; Muir et al., 1995; Rezvani et al., 2002; Rezvani and Levin, 2003b; Stolerman et al., 2000). Because of the efficacy of nicotine in improving attention, a variety of nicotinic analogs have been developed as possible treatments for cognitive impairment. Nicotinic analog treatments have been shown to improve attention. Terry et al. (Terry et al., 2002) found that the nicotinic agonist SIB-1553A significantly improves performance of rats on 5-CSRTT, but only when accuracy was reduced pharmacologically by injecting the rats with dizocilpine, an NMDA-sensitive glutamate receptor antagonist or behaviorally with a distracting stimulus.
Nicotine also has been shown to improve accuracy of intact rats in the operant SDT (Rezvani et al., 2002; Rezvani et al., 2006; Rezvani and Levin, 2004a; Turchi et al., 1996). Nicotine also effectively reversed impairment induced by dizocilpine (Rezvani et al., 2008; Rezvani and Levin, 2003b) and alcohol (Rezvani and Levin, 2003a).
The involvement of α7 nicotinic receptors in attentional processes remains unclear. The α7 nicotinic agonist ARR17779 was not found to significantly affect attentional performance in the 5-CSRTT (Grottick and Higgins, 2000; Hahn et al., 2003), however, the α7 nicotinic agonist R3487/MEM3454 significantly improved attentional function as measured by the operant SDT (Rezvani et al., 2009a). Furthermore, the α7 nAChR antagonist methyllycaconitine (MLA) given systemically did not significantly attenuate the effects of nicotine on attention (Blondel et al., 2000).
The α4β2 nAChRs appear to be critically involved in cognitive function. The α4β2 nAChR agonist SIB 1765F has been shown to significantly improve accuracy in the 5-CSRTT (Grottick and Higgins, 2000). Recently, it has been demonstrated that similar to α4β2 nAChR agonists, sazetidine-A, a novel nicotinic partial agonist that desensitizes these receptors (Xiao et al., 2006; Xiao et al., 2008), reverses dizocilpine and scopolamine-induced impairment in the SDT (Rezvani et al., 2011). This finding indicates an important role for α4β2 nAChRs in sustained attention.
Other Compounds
Other drugs with more complex actions have been shown to reverse attentional impairments. The antipsychotic drug clozapine reversed the attentional impairment caused by dizocilpine (Rezvani et al., 2008). Interestingly, it also blocks nicotine-induced attenuation of dizocilpine-induced attentional impairment. This effect is also seen with the 5HT2 antagonist ketanserin, which indicates that the 5HT2 antagonist effects of clozapine are key in the clozapine-induced blockade of nicotine effects on attention (Rezvani and Levin, 2004b). Clozapine also has been shown to reverse the attentional impairment caused by chronic PCP as measured by the novel object recognition task (Grayson et al., 2007).
Adenosine receptor antagonist treatment: It has been demonstrated that pre-training administration of caffeine (1–10 mg/kg, i.p.), the selective adenosine receptor antagonists DPCPX (8-cyclopenthyl-1,3-dipropylxanthine; Adenosine 1 antagonist, 5 mg/kg, i.p.) and ZM241385 (Adenosine 2A antagonist, 1.0 mg/kg, i.p.), or the comination of ineffective doses of DPCPX (3 mg/kg) and ZM241385 (0.5 mg/kg), improved the performance of spontaneous hypertensive rats in the object-recognition task. These findings suggest that adenosinergic antagonists might represent potentially interesting drugs for the treatment of ADHD (Aspide et al., 2000). Caffeine has been found to improve learning in a rat model of ADHD the spontaneously hypertensive rat (Prediger et al., 2005), however, clinical studies have not yet shown provide clinically effective attenuation of ADHD symptoms with caffeine (Lara, 2010).
The histamine H3 receptor has been suggested as an attractive target for the treatment of cognitive disorders (Esbenshade et al., 2008). Preclinical data suggest that potent and selective H3 antagonists may offer a novel therapeutic approach for the treatment of cognitive dysfunction in ADHD, Alzheimer’s Disease and schizophrenia. A number of H3 receptor antagonists including thioperamide, ABT-239, GT-2331, and ciproxifan have been shown to be efficacious in five-trial inhibitory avoidance in spontaneously hypertensive pups (for review see (Esbenshade et al., 2008)). The H3 antagonist ciproxifan was shown to significantly reduce the accuracy impairment in the 5-CSRTT when signals of short duration were used (Ligneau et al., 1998). H3 receptors in the brain have been shown to promote the release of acetylcholine as well as monoaminergic transmitters dopamine, norepinephrine and histamine (Esbenshade et al., 2008). Thus, blockade of these receptors by H3 antagonist may improve attention by affecting a variety neurotransmitter systems. However, the efficacy of H3 antagonist treatment for reduction of attentional impairment in the clinic have been mixed with further study needed to determine what place H3 antagonists may have in the spectrum of therapeutic agents for attentional impairment (Brioni et al., 2011).
Discussion
Animal models of attention are being actively used in the development of treatment for attention deficits. Their utility for this purpose will be determined by their effectiveness in identifying novel drugs which later prove to be effective for reversing attentional impairments in people. The demonstration of the efficacy of drugs such as methylphenidate, which is known to be therapeutically useful in treating attentional deficits in humans, provides important validation of the behavioral tasks and models of attentional impairment used in the experimental animal literature. Deficit models provide valid tools with which to test the effectiveness of new treatments for combating attentional impairment.
Dose-effect functions need to be carefully assessed in testing drug effects on attentional function in animal models because all drugs which improve attention have an inverted U-shaped dose-effect function in which either lower or higher than optimal doses are less effective. In fact, for many drugs there is an inverted J-shaped dose-effect function in which higher doses can impair attention. The combination of this characteristic non-monotonic dose-effect function with the fact that there is often considerable individual variability in drug sensitivity can complicate detection of beneficial responses to attention-improving drugs. With clinical medicine, the standard practice is to adjust doses upward or downward to achieve the best possible therapeutic effect with minimal side effects. This approach would be good to use in the development of drug treatments for attentional improvement especially given the typical inverted U-shaped dose-effect curve of attention-enhancing drugs and inevitable individual differences in response.
Care needs to be taken however, in the interpretation of the results of the models for predicting effects in humans. Some of this is due to limitations of the animal models in precisely replicating the complexities of the human syndromes of attentional impairment. But some of the problems in extrapolation from animal models to humans arise from uncertainties of the human conditions. A recent review (Nestler and Hyman, 2010) has discussed in detail how difficulties arise in developing animal models of psychopathology because of nebulous clinical categorizations of the different types of psychopathology. Clinical diagnosticians do their best to cope with the reality that often human disease in general and psychopathology in particular is a complex process with multiple etiologies and multiple means of expression. It is not just the boundaries of diagnostic conditions that are fuzzy, that is, who is included vs. excluded in the condition; the fuzziness pervades the entire set of people in each category. This arises from the fact that each category of psychopathology is a syndrome with a variety of symptoms and impairments, in which each has its spectrum of severity. More fundamental to the vagueness of clinical categorization is the fact that similar functional impairments can arise from quite different physiological mechanisms, such as seen with aging-related dementia resulting from Alzheimer’s disease and multiple small infarcts. This diversity is key to developing effective therapeutic treatments. The heterogeneity within each of the human syndromes of attentional impairment limits even the best animal model for completely capturing the human condition. In addition, human conditions of attentional impairment are very often accompanied by co-morbidities, psychosis predominantly in schizophrenia, but also often in Alzheimer’s disease, memory impairment in schizophrenia and increased rates of drug abuse (primarily tobacco smoking) in schizophrenia and ADHD. Also, concurrent drug treatment for other aspects of the disease or life stage in schizophrenia and Alzheimer’s disease and finally, impaired behavioral control in ADHD, schizophrenia and Alzheimer’s disease make extrapolation from animal models to human conditions challenging. Despite these potential impediments, animal models of attentional impairment and therapeutic treatment have proven to be quite useful in modeling different aspects of human conditions and devising new treatments. Animal models are especially useful in understanding the variety of neurobehavioral mechanisms of impairment and how treatments can help counteract or work around these disordered mechanisms.
There are several ways by which experimental animal models of attentional function can be improved for development of new drug therapies. One way would be incorporating individual differences in dose-effect functions in the evaluation of efficacy for improving attention. This could be easily accomplished by using the “best dose” test method proposed by Bartus (Bartus, 2000) and pioneered in drug development research by Buccafusco and Terry (Buccafusco and Terry Jr, 2001). With this method there are two phases of testing. The first phase of testing is to determine the most effective dose for improving attention for each subject (best dose). The second phase of testing evaluates the effect of the best dose vs. control treatment. The use of the best dose assessment more accurately reflects the clinical practice of medicine in which the physician titrates the dose to achieve the most effective clinical outcome. This is particularly important with treatments of cognitive dysfunction, which often show non-monotonic dose effect functions.
The impact of polypharmacy should also be considered. Drug treatments to improve attentional function in schizophrenia certainly need to be tested in conjunction with antipsychotic medications since the use of attention-improving drugs would be given in conjunction with antipsychotic drugs in this population. In fact, we found that co-administration of the antipsychotic drug clozapine significantly attenuated the attentional improvement caused by nicotine (Rezvani et al., 2008). Polypharmacy should also be considered in modeling use of attention-improving drugs for Alzheimer’s disease. In this elderly population, there is most often a variety of other drugs being taken. Those which may interact with attention-improving therapies would be antihypertensive β-adrenergic blockers, antidepressants, benzodiazepines and antipsychotic drugs given for behavioral control in Alzheimer’s disease patients.
To mimic human conditions, when assessing the possible efficacy of drugs for treating attentional impairment, it is important to test their efficacy in reversing attentional deficits, not just improvements in normal baseline performance. Also, the nature of the impairment should be considered. Some impairments, which are temporary, such as acute blockade of transmitter receptors, may be useful for initial studies, but for further analysis it is important to model chronic impairments in attention such as are seen in the diseases for which the drugs are being developed. There are many neural system accommodations that take place in the chronic deficit situation that would not be present with the acute deficit. Along with chronic deficits, it is critical to test chronic efficacy of the drug being tested to determine possible tolerance development or induction of a persisting effect. Animal models of attention are valuable tools in developing novel attentional improving drugs and will become more valuable as the models continue to improve.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: This manuscript has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Aspide R, Fresiello A, de Filippis G, Carnevale UA, Sadile AG. Non-selective attention in a rat model of hyperactivity and attention deficit: subchronic methylphenydate and nitric oxide synthesis inhibitor treatment. Neurosci Biobehav Rev. 2000;24:59–71. doi: 10.1016/s0149-7634(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Flicker C. Cholinergic psychopharmacology: An integration of human and animal research on memory. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. New York: Raven Press; 1987. pp. 219–232. [Google Scholar]
- Berger DF, Sagvolden T. Sex differences in operant discrimination behaviour in an animal model of attention-deficit hyperactivity disorder. Behavioural Brain Research. 1998;94:73–82. doi: 10.1016/s0166-4328(97)00171-x. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel A, Sanger D, Moser P. Characterisation of the effects of nicotine in the five-choice serial reaction task in rats: Antagonist studies. Psychopharmacology. 2000;149:293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Esbenshade TA, Garrison TR, Bitner SR, Cowart MD. Discovery of histamine H3 antagonists for the treatment of cognitive disorders and Alzheimer's disease. J Pharmacol Exp Ther. 2011;336:38–46. doi: 10.1124/jpet.110.166876. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV., Jr . Nicotine and cognition in young and aged non-human primates. In: Levin E, editor. Nicotine and the Nervous System. New York: CRC Press; 2001. [Google Scholar]
- Burk JA, Sarter M. Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. Neuroscience. 2001;105:899–909. doi: 10.1016/s0306-4522(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Bushnell P. Behavioral approaches to the assessment of attention in animals. Psychopharmacology. 1998;138:231–259. doi: 10.1007/s002130050668. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ. Concentration-time relationships for the effects of inhaled trichloroethylene on signal detection behavior in rats. Fundam Appl Toxicol. 1997;36:30–38. doi: 10.1006/faat.1997.2287. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ. Detection of visual signals by rats:Effects of signal intensity, event rate and task type. Behavioural Processes. 1999;46:141–150. doi: 10.1016/s0376-6357(99)00030-3. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Benignus VA, Case MW. Signal detection behavior in humans and rats: A comparison with matched tasks. Behavioural Processes. 2003;64:121–129. doi: 10.1016/s0376-6357(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Kelly KL, Crofton KM. Effects of toluene inhalation on detection of auditory signals in rats. Neurotoxicol Teratol. 1994;16:149–160. doi: 10.1016/0892-0362(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Levin ED, Marrocco RT, Sarter MF, Strupp BJ, Warburton DM. Attention as a target of intoxication: insights and methods from studies of drug abuse. Neurotoxicol Teratol. 2000;22:487–502. doi: 10.1016/s0892-0362(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Padnos BK. Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology (Berl) 1997a;134:230–241. doi: 10.1007/s002130050446. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Padnos BK. Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology. 1997b;134:230–241. doi: 10.1007/s002130050446. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Samsam TE, Benignus VA, Krantz QT, Kenyon EM. A dosimetric analysis of the acute behavioral effects of inhaled toluene in rats. Toxicol Sci. 2007;99:181–189. doi: 10.1093/toxsci/kfm146. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Strupp BJ. Assessing Attention in Rodents. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton: CRC Press; 2009. pp. 119–144. [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology. 2004;174:86–98. doi: 10.1007/s00213-004-1805-y. [DOI] [PubMed] [Google Scholar]
- Clements KM, Saunders AJ, Robertson BA, Wainwright PE. Spontaneously hypertensive, Wistar Kyoto and Sprague-Dawley rats differ in their use of place and response strategies in the water radial arm maze. Neurobiol Learn Mem. 2007;87:285–294. doi: 10.1016/j.nlm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Clements KM, Wainwright PE. Spontaneously hypertensive, Wistar Kyoto and Sprague-Dawley rats differ in performance on a win-stay task and a conditioned cue preference task in the water radial arm maze. Behavioural Brain Research. 2007;183:169–177. doi: 10.1016/j.bbr.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Research Reviews. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol. 2008;154:1166–1181. doi: 10.1038/bjp.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattu M, Pauly JR, Boss KL, Summers JB, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: role of central nicotinic receptors. I. Brain Res. 1997a;771:89–103. doi: 10.1016/s0006-8993(97)00793-2. [DOI] [PubMed] [Google Scholar]
- Gattu M, Terry AV, Jr, Pauly JR, Buccafusco JJ. Cognitive impairment in spontaneously hypertensive rats: role of central nicotinic receptors. Part II. Brain Res. 1997b;771:104–114. doi: 10.1016/s0006-8993(97)00794-4. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behavioural Brain Research. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Grilly DM. A verification of psychostimulant-induced improvement in sustained attention in rats: effects of d-amphetamine, nicotine, and pemoline. Exp Clin Psychopharmacol. 2000;8:14–21. doi: 10.1037//1064-1297.8.1.14. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behavioural Brain Research. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Hohnadel EJ, Hernandez CM, Gearhart DA, Terry AV., Jr Effect of repeated nicotine exposure on high-affinity nicotinic acetylcholine receptor density in spontaneously hypertensive rats. Neurosci Lett. 2005;382:158–163. doi: 10.1016/j.neulet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. New York: Dover Publications Inc; 1890. reprinted 1950. [Google Scholar]
- Jentsch JD. Impaired visuospatial divided attention in the spontaneously hypertensive rat. Behavioural Brain Research. 2005;157:323–330. doi: 10.1016/j.bbr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Burk JA. Effects of gonadectomy and androgen supplementation on attention in male rats. Neurobiol Learn Mem. 2006;85:219–227. doi: 10.1016/j.nlm.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, Deschepper CF, Dwoskin LP. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis. 2010;20 Suppl 1:S239–S248. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. J Pharmacol Exp Ther. 1998;287:658–666. [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology. 1999;144:175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995a;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Effects of chlordiazepoxide and scopolamine, but not aging, on the detection and identification of conditional visual stimuli. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 1995b;50:B90–B96. doi: 10.1093/gerona/50a.2.b90. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Archives of Neurology. 1963;9:90–99. [Google Scholar]
- Mirza NR, Bright JL. Nicotine-induced enhancements in the five-choice serial reaction time task in rats are strain-dependent. Psychopharmacology. 2001;154:8–12. doi: 10.1007/s002130000605. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology. 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Mishima K, Fujii M, Aoo N, Yoshikawa T, Fukue Y, Honda Y, Egashira N, Iwasaki K, Shoyama Y, Fujiwara M. The Pharmacological characterization of attentional processes using a two-lever choice reaction time task in rats. Biological & Pharmaceutical Bulletin. 2002;25:1570–1576. doi: 10.1248/bpb.25.1570. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. Reversal of visual attentional dysfunction following lesions of the cholinergic basal forebrain by physostigmine and nicotine but not by the 5-HT3 receptor antagonist, ondansetron. Psychopharmacology. 1995;118:82–92. doi: 10.1007/BF02245253. [DOI] [PubMed] [Google Scholar]
- Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ. Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res. 1993;57:123–131. doi: 10.1016/0166-4328(93)90128-d. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nature Neuroscience. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Cholinergic deafferentation of prefrontal cortex increases sensitivity to cross-modal distractors during a sustained attention task. J Neurosci. 2008;28:2642–2650. doi: 10.1523/JNEUROSCI.5112-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R. The psychobiology of sustained attention. In: Warm J, editor. Sustained attention in human performance. New York: Wiley; 1984. pp. 61–101. [Google Scholar]
- Paule MG, Rowland AS, Ferguson SA, Chelonis JJ, Tannock R, Swanson JM, Castellanos FX. Attention deficit/hyperactivity disorder: characteristics, interventions and models. Neurotoxicol Teratol. 2000;22:631–651. doi: 10.1016/s0892-0362(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Pamplona FA, Fernandes D, Takahashi RN. Caffeine improves spatial learning deficits in an animal model of attention deficit hyperactivity disorder (ADHD) -- the spontaneously hypertensive rat (SHR) International Journal of Neuropsychopharmacology. 2005;8:583–594. doi: 10.1017/S1461145705005341. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Bushnell PJ, Levin ED. Nicotine and mecamylamine effects on choice accuracy in an operant signal detection task. Psychopharmacology. 2002;164:369–375. doi: 10.1007/s00213-002-1221-0. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Caldwell DP, Levin ED. Chronic nicotine interactions with clozapine and risperidone and attentional function in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30:190–197. doi: 10.1016/j.pnpbp.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Sexton H, Xiao X, Brown MA, Paige MA, McDowell BE, Kellar KJ, Levin ED. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent reverses dizocilpine and scopolamine-induced attentional impairments in rats. Psychopharmacology. 2011 doi: 10.1007/s00213-010-2161-8. in press. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Brucato FH, Callahan PM, Lowe DA, Levin ED. Effect of R3487/MEM3454, a novel nicotinic alpha7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009a;33:269–275. doi: 10.1016/j.pnpbp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Cauley MM, Levin ED. Attenuation of pharmacologically-induced attentiona impairment by methylphenidate in rats. Pharmacology, Biochemistry and Behavior. 2009b;92:141–146. doi: 10.1016/j.pbb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Dawson E, Levin ED. Nicotine and clozapine effects on attentional performance impaired by the NMDA antagonist dizocilpine in female rats. International Journal of Neuropsychopharmacology. 2008;11:63–70. doi: 10.1017/S1461145706007528. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacology, Biochemistry and Behavior. 2003a;76:75–83. doi: 10.1016/s0091-3057(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotinic-glutamatergic interactions and attentional performance on an operant visual signal detection task in female rats. Eur J Pharmacol. 2003b;465:83–90. doi: 10.1016/s0014-2999(03)01439-0. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-antipsychotic drug interactions and attentional performance in female rats. Eur J Pharmacol. 2004a;486:175–182. doi: 10.1016/j.ejphar.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-antipsychotic drug interactions and attentional performance in female rats. Eur J Pharmacol. 2004b;486:175–182. doi: 10.1016/j.ejphar.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behavioral & Neural Biology. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgraduate Medicine. 2010;122:78–87. doi: 10.3810/pgm.2010.09.2204. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–154. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- Terry AVJ, Risbrough VB, Buccafusco JJ, Menzaghi F. Effects of (+/−)-4-[[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride (SIB-1553A), a selective ligand for nicotinic acetylcholine receptors, in tests of visual attention and distractibility in rats and monkeys. J Pharmacol Exp Ther. 2002;301:284–292. doi: 10.1124/jpet.301.1.284. [DOI] [PubMed] [Google Scholar]
- Turchi J, Holley LA, Sarter M. Effects of benzodiazepine receptor inverse agonists and nicotine on behavioral vigilance in senescent rats. J Gerontol A Biol Sci Med Sci. 1996;51:B225–B231. doi: 10.1093/gerona/51a.3.b225. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Bidirectional modulation of basal forebrain N-methyl-D-aspartate receptor function differentially affects visual attention but not visual discrimination performance. Neuroscience. 2001;104:407–417. doi: 10.1016/s0306-4522(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Molecular Pharmacology. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Yasuda RP, Sahibzada N, Horton L, DiPietro JR, Iwueze AF, Paige MA, McDowell B, Brown ML, Wolfe BB, Kellar KJ. Pharmacological properties of sazetidine-A, a selective ligand of α4β2 nicotinic acetylcholine receptors. Society for Neuroscience 38th Annual Meeting; Washington, DC. 2008. [Google Scholar]
- Zhu N, Weedon J, Dow-Edwards DL. The multifaceted effects of oral administration of methylphenidate in juvenile rats: Anxiety, activity, and attention. European Neuropsychopharmacology. 2010;20:236–244. doi: 10.1016/j.euroneuro.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]