Abstract
Retromer deficiency has been implicated in sporadic AD and animals deficient in retromer components exhibit pronounced neurodegeneration. Because retromer performs retrograde transport from the endosome to the Golgi apparatus and neuronal Aβ is found in late endosomal compartments, we speculated that retromer malfunction might enhance amyloidogenic APP processing by promoting interactions between APP and secretase enzymes in late endosomes. We have evaluated changes in amyloid precursor protein (APP) processing and trafficking as a result of disrupted retromer activity by knockdown of Vps35, a vacuolar sorting protein that is an essential component of the retromer complex. We found that knocking down retromer activity produced no change in the quantity or cellular distribution of total cellular APP and had no affect on internalization of cell-surface APP. Retromer deficiency did, however, increase the ratio of secreted Aβ42:Aβ40 in HEK-293 cells over-expressing APP695, due primarily to a decrease in Aβ40 secretion. Recent studies suggest that the retromer-trafficked protein, Wntless, is secreted at the synapse in exosome vesicles and that these same vesicles contain Aβ. We therefore hypothesized that retromer deficiency may be associated with altered exosomal secretion of APP and/or secretase fragments. In exosomal vesicles secreted from HEK-293 cells, we detected holo-APP, Presenilin and APP C-terminal fragments. Levels of total APP C-terminal fragments were significantly increased in exosomes secreted by retromer deficient cells. These data suggest that reduced retromer activity can mimic the effects of familial AD Presenilin mutations on APP processing and promote export of amyloidogenic APP derivatives.
Keywords: Retromer, Vps35, APP, Aβ, exosome, endosome
Introduction
Deficits in retromer transport are associated with sporadic AD and pronounced neurodegeneration in AD animal models (Small et al., 2005). The human retromer is a multimeric complex consisting of vacuolar sorting protein (Vps)26, Vps35, Vps29, and sorting nexin (SNX)1 and SNX2 that mediates retrograde transport of proteins from the endosome to the trans-Golgi network (TGN) (Bonifacino and Hurley, 2008). In mice, reduction of the retromer protein Vps26 is associated with modest increases in brain soluble APP and Aβ peptide (Muhammad et al., 2008). In flies, over expression of human APP and beta-site APP-cleaving enzyme (BACE), in conjunction with knockout of the fly’s Vps35 homolog, results in a modest increase of total Aβ and pronounced neurodegeneration (Muhammad et al., 2008). These data support an association between retromer deficiency and neurodegeneration and suggest parallel increases in Aβ generation.
The role of retromer in APP metabolism is unknown. Because neuronal intracellular Aβ is found in endosomes (Jacobsen et al., 2001), deficient recycling of internalized APP from the endosome is an attractive hypothesis to explain the effects of retromer malfunction. In yeast, retromer binds and facilitates retrograde transport of Vps10p from the endosome to the Golgi apparatus (Jacobsen et al., 2001). In mammals, there are five Vps10 domain proteins, one of which, the sortilin-related receptor (SorLA), is genetically associated with sporadic AD (Rogaeva et al., 2007). SorLA localizes in late endosomal and Golgi compartments (Offe et al., 2006) and promotes APP retention in the Golgi apparatus (Andersen et al., 2005; Rogaeva et al., 2007). Deficiency of SorLA is associated with increased production of Aβ (Offe et al., 2006; Rogaeva et al., 2007). In light of the role of Vps10p and retromer in yeast, these observations suggest that retromer may be required for retrograde transport of SorLA and APP (Small and Gandy, 2006). It is currently unclear, however, whether shuttling of SorLA between the endosome and trans-Golgi network (TGN) is mediated by retromer, Golgi-localizing, γ-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins, or both (Jacobsen et al., 2002; Nielsen et al., 2007). Further, the role of retromer in APP processing may be either direct (retrieving APP from endosomes) or indirect (retrieving SorLA from endosomes and consequently retaining APP in the secretory pathway). In either case, disruption of retromer is predicted to cause accumulation of APP or APP derivatives in late endosomal compartments.
Currently, there is no direct evidence supporting the retention of either APP or its derivatives in a metabolically active compartment when retromer transport is disrupted. Retromer-associated changes in the secretion of Aβ species may result from changes in Golgi processing of APP or from trafficking of either internalized APP or enzymes involved in APP processing. Recent evidence suggests that retromer malfunction may affect the endosomal transport of BACE and lead to increased secretion of Aβ (Okada et al., 2010). The simplest prediction of the effect of retromer deficiency on APP processing is the retention of APP derivatives in an amyloidogenic endosomal compartment. Evidence suggests that the late endosomes are the principle site of Aβ accumulation in cortical neurons in AD brain and in cultured neurons (Hu et al., 2009; Troncoso et al., 1998). Immuno-electron microscopy studies have demonstrated Aβ in association with the internal vesicles of late endosomal multivesicular bodies (Takahashi et al., 2002). Moreover, both APP and Aβ have been detected in exosomal vesicles that are secreted from late endosomal compartments when the MVB fuses with the plasma membrane (Rajendran et al., 2006). If retromer transport is required for normal trafficking of APP and/or secretase enzymes through the endosome, then retromer disruption would predictably cause changes in APP localization and metabolism within the cell.
Late endosomal organelles can fuse with the plasma membrane and discharge select proteins that accumulate in the internal vesicles of the multivesicular body (MVB). The Wntless protein, which is necessary for secretion of Wnt proteins and is recycled by retromer (Belenkaya et al., 2008), has been found in secreted exosomal vesicles at the neuromuscular junction (Korkut et al., 2009), indicating that retromer recycled proteins may include regulated exosomal cargo proteins. These observations suggest the hypothesis that retromer malfunction may lead to altered exosomal secretion of proteins associated with the internal vesicles of the MVB, including, potentially, APP and its derivatives. Previous studies have demonstrated the presence of APP, Aβ, and Presenilin-1 (PS-1) in exosomal vesicles secreted by cells in culture (Vella et al., 2008). However, no studies have evaluated exosomal cargo following retromer disruption.
In this study, we have evaluated APP processing in cells in which retromer activity has been altered by shRNA-mediated knockdown of Vps35 expression. We report that Vps35 knockdown leads to an increase in the amount of APP C-terminal fragments (CTFs) in secreted exosome vesicles, while PS-1and holo-APP secretion is unchanged. We also observe a decrease in secretion of Aβ40 that is associated with an increased ratio of Aβ42 to Aβ40 (Aβ42:Aβ40) in the media. We observe no change in total cellular APP and APP CTF. These findings are consistent with the hypothesis that retromer deficiency causes increased amyloidogenic APP fragments in the endosomal secretory pathway.
Materials and methods
Cell culture and viral transduction
Wild type HEK-293 cells (293WT) were maintained in DMEM containing 10% FBS and antibiotics. HEK-293 cells over expressing hAPP-695 (293695) were maintained in DMEM containing 10% FBS, antibiotics and 400 μg/ml G418 (Gibco). Lentivirus particles were generated using the Trans-Lentiviral GIPZ Packaging System (Open Biosystems) for transduction of cells with empty vector or vectors expressing shRNA (5’ – TGCTGTTGACAGTGAGCGCGCTTAGTAATGTTCTGGATTATAGTGAAGCCACAGATG TATAATCCAGAACATTACTAAGCATGCCTACTGCCTCGGA – 3’) directed against vps35 derived mRNA. After infection, polyclonal cell lines were maintained in puromycin for selection of transduced cells.
Exosome preparation
Exosomes were isolated (using a modified version of the methods described by Mathias et al. (Mathias et al., 2009) and Thery et al. (Thery et al., 2001)) from 293695 cells with or without shRNA knockdown of Vps35. Cells were plated at 40% confluence in T-150 flasks and grown to confluence in 20 ml medium over 3 days. Medium was collected and sequentially centrifuged at 3,000 × g (to remove cells), 10,000 × g (to remove large membrane fragments) and 150,000 × g (to isolate exosome particles). Pellets from the high-speed centrifugation were resuspended in PBS with a dissolved protease inhibitor cocktail tablet (Roche) for protein analysis. Enrichment (>100-fold) of exosomal proteins was verified by western blotting using anti-Alg-2 interacting protein (ALIX) antibody (kindly provided by Dr. Arthur Polans, University of Wisconsin). The absence of exosome protein in identically processed unconditioned medium was verified by Western blotting using anti-ALIX antibody.
Microscopy
Cells were grown on poly-L-lysine-coated chamber slides (BD Biosciences) and fixed in 4% paraformaldehyde in PBS for 15 min. Cells were rinsed three times in PBS before permeabilization in PBS with 0.1% TX-100 for 3 min. Cells were rinsed again and blocked for 60 min in PBS containing 5% donkey serum and 5% BSA (blocking solution). Primary antibodies [anti-Vps35 (Imgenex, IMG-3575), anti-CI-M6P (abcam, ab2733), anti-LAMP1 (Affinity Bioreagents, PA1-654), anti-APP C-terminus (Sigma, A8717), anti-APP N-terminus (Millipore, MAB348), anti-Golgi 58K (Sigma, G2404), anti-EEA1 (Calbiochem, 324610)] diluted in blocking solution were incubated with cells overnight at 4ºC. Cells were washed with PBS and treated with fluorescence-labeled secondary antibodies (Invitrogen). Coverslips were mounted and cells were visualized using a Leica SP5 scanning laser confocal microscope.
ELISA
ELISAs for the detection of Aβ40 and Aβ42 were performed according to manufacturer’s instructions (Invitrogen). Confluent cultures of 293695 cells were cultured in fresh medium for 24 hours before medium collection for ELISA. Total Aβ (pg) was determined in each media sample by comparison to a standard curve of synthetic Aβ. These values were then adjusted for differences in cellular protein in the culture of origin and presented as relative levels.
Western blot analysis
Cellular lysates were prepared in RIPA buffer (Pierce) supplemented with a protease inhibitor cocktail tablet (Roche). Cellular lysates and exosomal samples (described previously) were combined 1:1 with LDS sample loading buffer (Invitrogen) containing 2% 2-mercaptoethanol. Proteins were separated on Bis-Tris gels (Invitrogen) and transferred to Immobilon-P membranes (Millipore). Protein detection was carried out as described previously (Sullivan et al., 2009). Essentially, membranes were blocked for 1 hour at room temperature in Superblock (Pierce) containing 0.1% Tween-20. Blocked membranes were incubated with primary antibodies [anti-Vps35 (Imgenex, IMG-3575), anti-ALIX (provided courtesy of Dr. Arthur Polans), anti-APP C-terminal domain (Sigma, A8717), anti-APP βCTF (Millipore, AB1510), anti-TSG101 (abcam, ab83) or anti-β-actin (Sigma, A1978)]. Membranes were washed with TBST and treated for an additional hour with horse radish peroxidase-linked secondary antibodies (Vector Labs). Membranes were washed again with TBST before detection of secondary antibodies using a chemiluminescent substrate (Pierce). Densitometry of chemiluminescence was carried out after phosphoimaging with the use of AlphaEase FluorChem image analysis software (Alpha Innotech).
Real time RT-PCR
Real Time RT-PCR was conducted with total RNA harvested using the RNeasy kit (Qiagen). Total RNA was converted to cDNA by RT-PCR using the Superscript III First Strand Synthesis System (Invitrogen). Resulting cDNA was combined with primers in SYBR Green master-mix (Applied Biosystems) and used for Real Time RT-PCR using the Applied Biosystems 7300 Real Time PCR System (Applied Biosystems). Primers for the detection of Vps35 mRNA (5’-GGAGTCGCCATGCCTACAAC-3’ and 5’-GTTCGTAGAGATCTGCCACTTTCC-3’) were designed using Primer Express software (Applied Biosystems). Previously described primers [5’-AGGAATTGACGGAAGGGCAC-3’ and 5’-GGACATCTAAGGGCATCACA-3’ (Williams et al., 2003)] were used to detect and normalize against 18 S rRNA. Values are expressed as relative levels of mRNA expression ± SD.
Generation of APP CTF constructs
V5-tagged APP CTF constructs coding for either a 99 amino acid segment of the C-terminus (C99), an 83 amino acid segment (C83) and a control construct consisting of the intracellular and transmembrane domains of C99 plus the ectodomain amino acids in reverse order (IC99) were constructed de novo (Retrogen). The DNA sequences were used as templates for PCR and were cloned into pcDNA3.1 V5/HIS Topo. Correct orientation and frame were confirmed by sequencing and expression of the appropriate sized APP CTF epitopes (not shown).
Statistical analysis
Data are presented as mean values + SD from a representative experiment, except where indicated that the mean + SD represents the average from 3 independent experiments. Where two samples are compared, data were analyzed using a 2-tailed unpaired Student’s t test. Significant differences were reported when P<0.05. Where multiple comparisons are made, data were analyzed by ANOVA. Relative comparisons were included in Bonferroni post-hoc analysis and statistically significant differences reported when P<0.05. Asterisks are used to indicate statistical significance where appropriate.
Results
Vps35 knockdown leads to retromer dysfunction
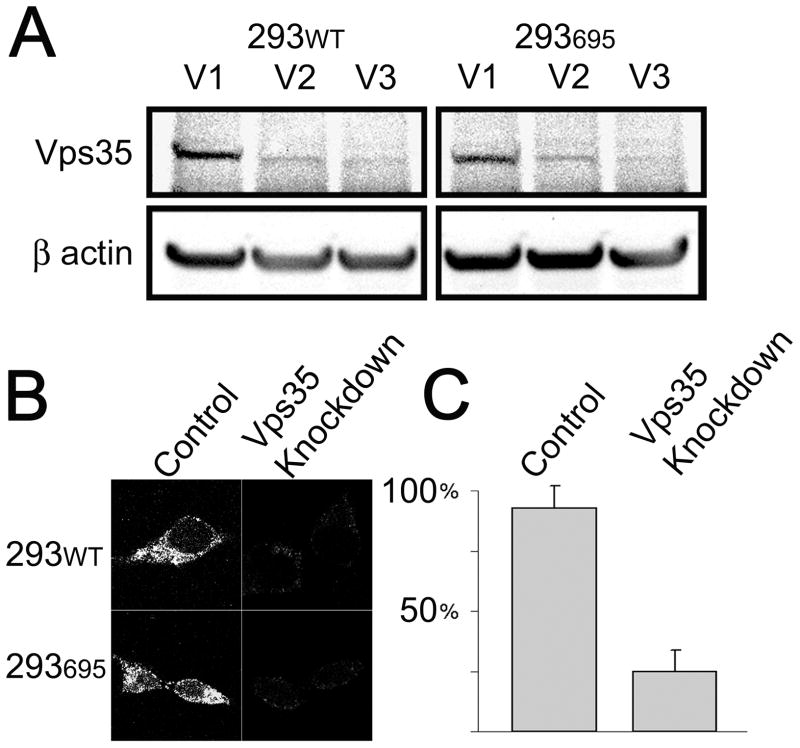
In order to test the effects of retromer dysfunction on APP metabolism, stable cell lines were generated by transducing HEK-293 cells (293WT) and HEK-293 cells over expressing hAPP-695 (293695) with lentiviral vectors expressing either a nonspecific shRNA (V1) or one of two shRNAs (V2 and V3) targeting Vps35 mRNA. After several rounds of selection (puromycin), cell lysates were harvested and analyzed for Vps35 protein and mRNA to confirm knockdown. Western blot analysis indicated that Vps35 levels were reduced after transduction with V2 and V3 (Figure 1A). The reduction in protein was determined to be ~80% in V3 lines by densitometry analysis. Transduced cell lines were also examined by immunofluorescence microscopy for Vps35 expression, revealing reduced levels of Vps35 in knockdown cells (Figure 1B). Real Time RT-PCR analysis demonstrated that Vps35 mRNA levels were reduced by 75% (Figure 1C). These findings indicate successful knockdown of Vps35 by viral transduction in both 293WT and 293695 cells. V3 transduced lines were selected for subsequent experiments.
Figure 1.
Vps35 expression is reduced in stably transduced cell lines. Stably transduced cell lines were created using lentiviral delivery of a vector coding for either nonspecific sequence control (V1) or Vps35 specific shRNA (V2 and V3) in HEK 293WT and 293695 cells. (A) Western blotting was carried out to verify knockdown of Vps35 in cultures raised from cells transduced with V1, V2 or V3. Cell lines with stabile expression of V1 or V3 shRNA were used in all subsequent experiments. (B) Vps35 expression was examined by immunofluorescence microscopy in 293WT and 293695 stabile shRNA expressing cell lines. (C) Vps35 message levels were examined by Real Time RT-PCR in 293WT stabile shRNA expressing cell lines.
One function of the retromer complex is to recycle CI-M6PR from endosomal compartments back to the Golgi (Arighi et al., 2004). Previous studies have shown that in the absence of the Vps26 retromer component, CI-M6PR immunofluorescence expands from a punctate, perinuclear distribution to a more diffuse distribution (Arighi et al., 2004), indicating loss of the retromer-dependent recycling of CI-M6PR and its subsequent restriction to endosomal compartments. These patterns of CI-M6PR staining were also observed in the Vps35 knockdown cells (Figure 2), indicating retromer dysfunction as a result of Vps35 knockdown. Taken together, these data indicate that the stable cell lines generated for this study have high levels of Vps35 knockdown and have disrupted retromer function.
Figure 2.
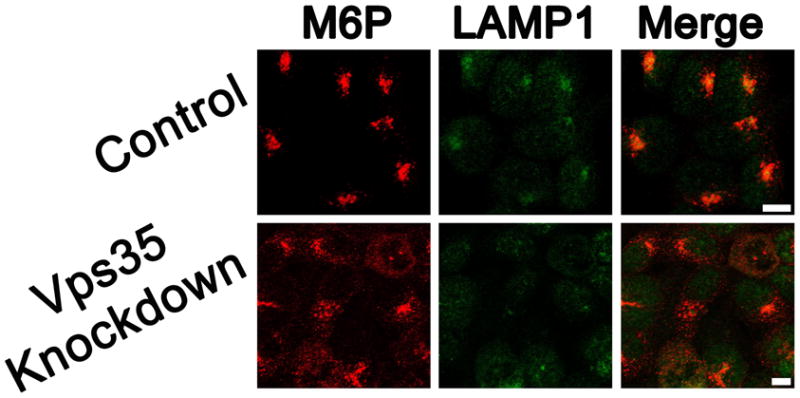
Vps35 knockdown disrupts CI-M6PR cellular localization. Cellular localization of CI-M6PR in relation to lysozomal LAMP1 was compared in 293WT control and Vps35 knockdown cells by immunofluorescence microscopy (scale bars represent 10 μm).
Retromer dysfunction does not alter total APP or cellular distribution
Retromer disruption leads to rapid lysosomal degradation and/or altered cellular localization of cargo proteins that are normally recycled from the endosome. To assess for changes in APP levels in cells with retromer dysfunction, Western blotting was conducted using lysates from transduced 293WT cell lines as well as 293695 cell lines. No changes in protein levels of either full length APP or APP CTF were observed in any of the Vps35 knockdown cell extracts (Figure 3). In previous studies where proteins have been observed to be recycled in a retromer-dependent process, retromer dysfunction has been shown to prevent the return of recycled proteins to the Golgi apparatus (Canuel et al., 2008). To assess changes in APP cellular localization between control and knockdown cell lines, 293695 cells were analyzed by immunofluorescence confocal microscopy. Cellular APP was detected using anti-APP antibodies (Sigma) that recognize the C-terminus of the protein. No changes in APP distribution or colocalization with an antibody recognizing the Golgi 58K protein (Sigma) were observed (Figure 4).
Figure 3.
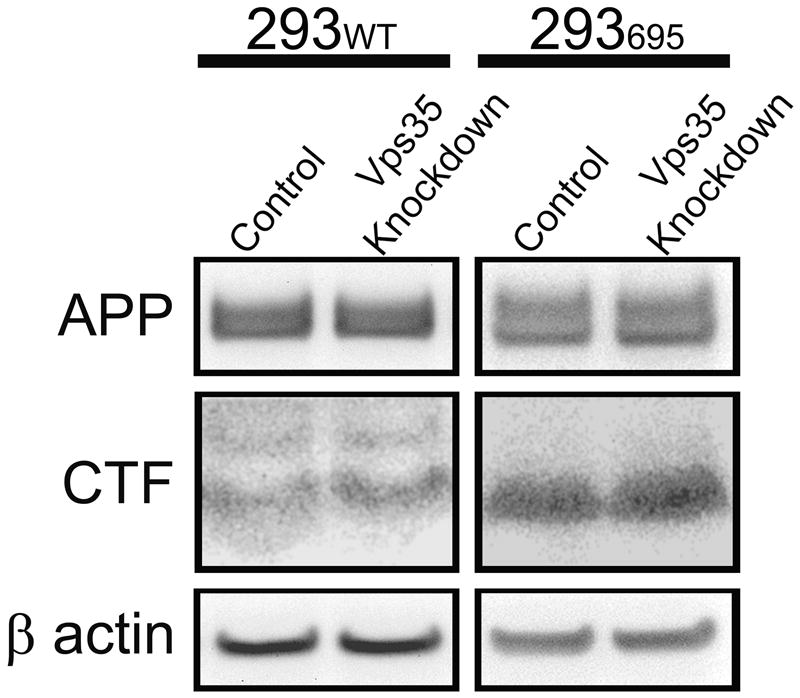
Loss of Vps35 does not alter cellular levels of APP. Cell lysates from control and Vps35 knockdown cells were probed by Western blot with an antibody that recognizes β actin or the C-terminus of APP. Bands corresponding to full length APP, CTF and β actin are shown.
Figure 4.
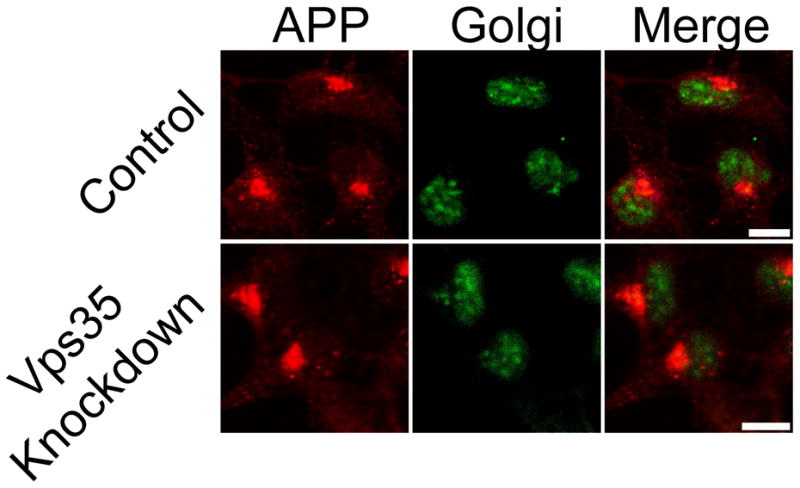
Vps35 knockdown does not alter intracellular APP localization. APP (C-terminus) and Golgi 58K (a Golgi marker) were fluorescently labeled in transduced cell lines by immunostaining before observation by confocal microscopy. Representative images are shown (scale bars represent 10 μm)..
To determine the cellular localization of newly synthesized APP CTF in control and Vps35 knockdown cells, we created V5-tagged human APP C99 or C83 constructs along with a control construct, IC99, in which the C99 ectodomain amino acids were inverted. These APP CTF constructs were transiently expressed in 293WT cells with or without functional retromer trafficking. Cellular distribution of these constructs in relation to LAMP1 was then analyzed by immunofluorescence confocal microscopy. C99 was found to localize near the cell surface (Figure 5). We did not observe any increase in peripheral membrane labeling or increased co-localization of C99, C83 or IC99 with early endosomal proteins, EEA1 and Rab5 (not shown). As with the cell-surface labeling, this approach has limitations, notably the question of whether the newly synthesized APP CTF is transported to the plasma membrane and then internalized to the endosome where retromer transport may exert an effect on distribution. With this limitation in mind, we conclude that retromer disruption did not affect the transport of newly synthesized APP CTFs.
Figure 5.
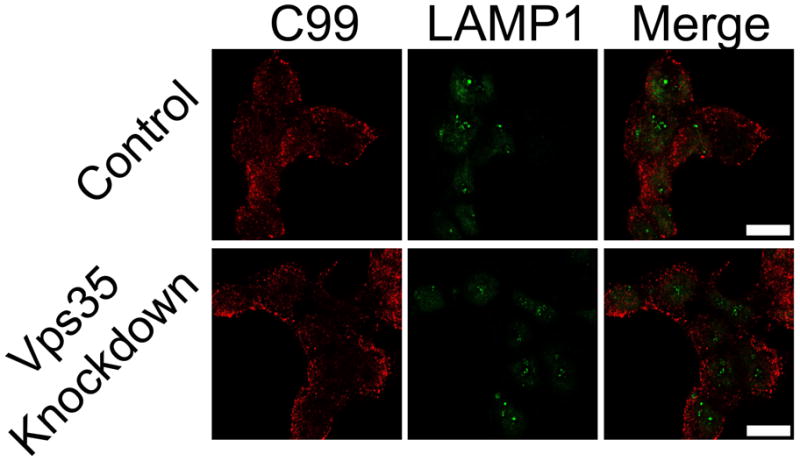
Cellular distribution of APP CTF in relation to lysosomal compartments is unchanged in the absence of Vps35. Control and Vps35 knockdown 293WT cells were transiently transfected with plasmids expressing a peptide corresponding to the final 99 amino acids in the C-terminus of human APP with a V5 tag. Cells were then labeled by immunofluorescence staining for V5 and LAMP1. Confocal images are shown with individual panels for V5 (red) and LAMP1 (green) as well as merged images (scale bars represent 10 μm).
Retromer-deficiency does not affect the internalization of APP or its cellular distribution
We next examined APP following internalization from the cell surface. Surface APP molecules were labeled with anti-APP ectodomain (N-terminal) antibodies (Millipore) at 4°C and then allowed to internalize for 30 minutes. At 30 minutes, we found no changes in the co-localization of internalized APP and the early endosomal marker EEA1 (Figure 6). There are two limitations to this approach. First, the antibody may dissociate from the APP in early (acidic) endosomal compartments, and, second, only a limited portion of internalized APP may be affected by retromer deficiency. With these caveats, we conclude that retromer knockdown did not alter the endosomal trafficking of internalized APP molecules. Overall, these findings suggest that knockdown of Vps35 does not dramatically alter the steady-state expression, endosomal trafficking, or intracellular distribution of total APP in HEK-293 cells.
Figure 6.
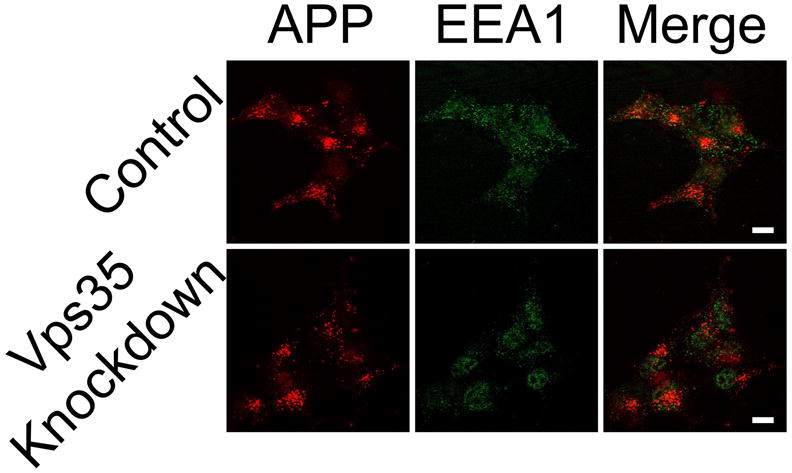
Internalization of cell surface APP is unchanged in the absence of Vps35. Cell surface APP was labeled by the addition of anti-APP antibody (recognizing the N-terminus) at 4ºC. After 30 minutes of chase (incubation using standard culture conditions at 37ºC,) internalized antibody and endosomal protein EEA1 were detected by fluorescent immunostaining. Representative confocal images are shown (scale bars represent 10 μm).
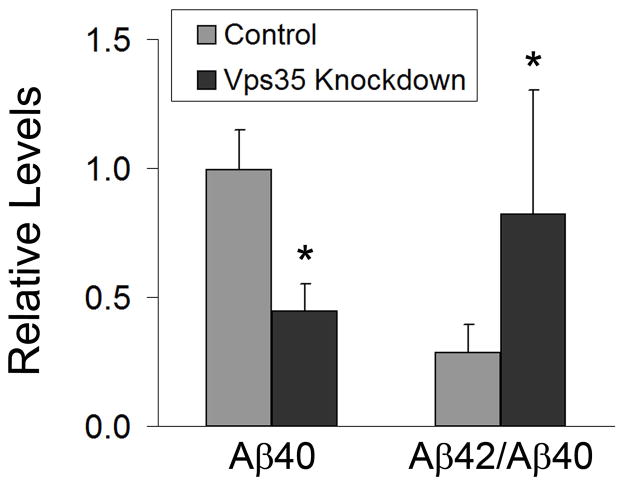
Retromer deficiency decreases Aβ40 secretion
To assess Aβ secretion, we measured Aβ40 and Aβ42 by ELISA in the tissue culture medium of 293695 cells with and without Vps35 knockdown (Figure 7). Levels of Aβ40 were significantly reduced, while levels of Aβ42 remained unchanged, resulting in an increased Aβ42:Aβ40 ratio. These findings suggest that retromer dysfunction may promote amyloidosis by lowering the secretion of Aβ40 and elevating the ratio of Aβ42:Aβ40.
Figure 7.
Aβ40 secretion is decreased and the Aβ42:Aβ40 ratio is increased after Vps35 knockdown. ELISA analysis was carried out on media from control and Vps35 knockdown 293695 cultures to detect levels of secreted Aβ40 and Aβ42. Aβ levels were detected and normalized against cellular protein levels. The ratio of Aβ42 to Aβ40 was also determined for each cell line. Relative values from a representative experiment are shown. The difference in Aβ40 levels between control and Vps35 knockdown cells is significant (P<0.05). The difference in the ratio of Aβ42 to Aβ40 between control and Vps35 knockdown is also significant (P<0.05).
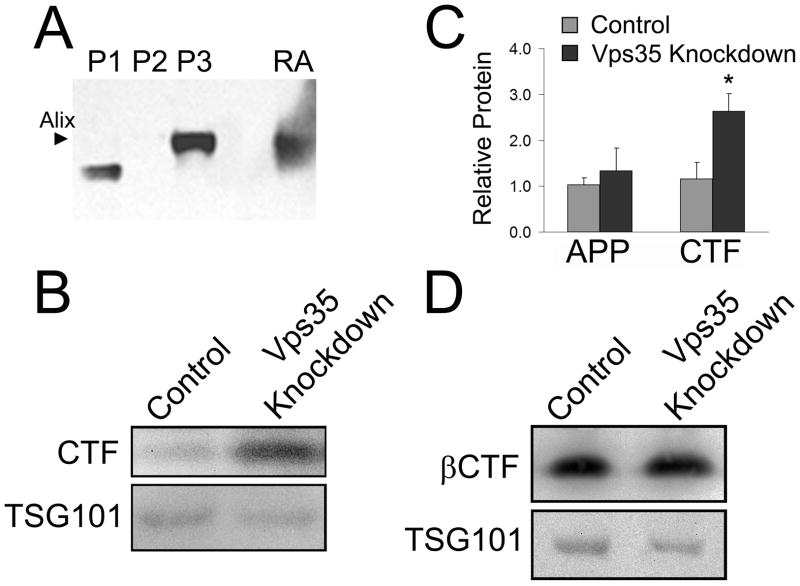
Retromer dysfunction leads to an increase in exosomal APP CTFs
Exosomal vesicles are formed in late endosomal compartments and are released when the multivesicular endosome (MVE) fuses with the plasma membrane (Stahl and Barbieri, 2002). Thus, exosomes represent another potential route of cellular secretion of amyloidogenic APP fragments (Vingtdeux et al., 2007). In theory, retromer dysfunction could result in the accumulation of protein, including APP and its derivatives, in late endosomal compartments, enhancing their exosomal secretion. To determine whether retromer disruption affects exosomal APP secretion, exosomes were separated by centrifugation from medium collected from 293695 cell cultures with and without Vps35 knockdown. Figure 8A demonstrates the isolation of Alg2 Interacting Protein 1 (Alix) rich exosomal vesicles in the high speed pellet separated by this method. APP and APP CTF were detected in lysates from the isolated exosomes by Western blot (Figure 8B) and quantified by densitometry (Figure 8C). To account for variation in cell numbers and exosome recovery, APP signal was normalized to Tsg 101, a late endosome protein that is secreted in exosomal vesicles (Aharon et al., 2008). As demonstrated in Figures 8B and 8D, recovery of Tsg 101 did not vary significantly between independent exosome preparations. Total APP CTF levels increased significantly relative to Tsg 101 in exosomes derived from retromer-deficient cells, while increases in APP were not statistically significant (Figure 8B, 8C). Exosome fractions were also probed for APP βCTF. As shown in Figure 8D, APP βCTF is present in exosomes and was unchanged by Vps35 knockdown. These data indicate that retromer dysfunction promotes the release of total APP CTF, but not holo-APP, in exosomes.
Figure 8.
Total APP CTF levels, but not βCTF levels, are elevated in exosomes secreted from Vps35 knockdown cells. Culture media components were separated by sequential centrifugation at varying speeds to isolate exosomes. (A) The purity of the exosomal isolates in the high speed pellet were confirmed by Western blot for the exosomal protein, Alix (P1 – low speed pellet, P2 – intermediate speed pellet, P3 – high speed pellet, RA – recombinant Alix). Exosomes isolated from 293695 control and Vps35 knockdown cultures were resuspended in lysis buffer and analyzed by Western blot for APP, CTF, βCTF and TSG101. (B) Western blot comparing total CTF expression relative to TSG101 in exosomes from control and Vps35 knockdown cultures. (C) Levels of APP and total CTF were determined by densitometry analysis of Western blots and levels of APP and CTF were normalized against TSG101 levels. An average of normalized densitometry values from three independent experiments are shown as relative values. The increase in CTF in Vps35 knockdown cells is significant (P<0.05). (D) Western blot comparing βCTF levels in exosomes from control and Vps35 knockdown cultures.
Discussion
We have investigated the effects of retromer disruption on APP processing and trafficking in HEK-293 cells. In this study, we have disrupted retromer trafficking by knocking down the cargo-binding Vps35 subunit and we find modest, but significant changes in APP metabolism. We examined both total full-length APP and total APP CTFs in 293WT and 293695 cell lines and identified no changes in the steady-state levels of these APP species when Vps35 is knocked down by ~80% (Figure 3). In contrast, the localization of CI-M6PR, which recycles between endosomal and Golgi membranes, is altered by Vps35 knockdown (Figure 2). Thus, our results suggest that Vps35 knockdown causes retromer dysfunction that is comparable to those reported in other cells (Arighi et al., 2004). We conclude that any effects on APP metabolism are likely to involve a limited pool of APP molecules in the endosomal compartment. Indeed, very recent data implicates a pool of APP characterized by phosphorylation at S655 of the APP ICD (Vieira et al., 2010).
Our data suggest that either retromer-mediated transport is not the major mechanism of retrograde APP transport or that retromer disruption is associated with only a subtle change in APP transport. In response to retromer disruption, we observed no change in the levels or cellular distribution of APP species in 293WT cells, in cells expressing a ten-fold excess of APP (293695) or in SH-SY5Y cells (not shown). Our internalization experiment indicates that internalized APP is internalized identically by cells with and without functional retromer-mediated trafficking, and that the intracellular distribution of internalized APP is similar (Figure 6) in relation to EEA1-containing endosomal compartments in these cell lines. While more robust retromer-mediated transport of APP may occur in specialized cells or under selected conditions, our data suggest that separate transport mechanisms probably exist for retrograde trafficking of APP.
Nevertheless, Vps35 knockdown does produce a modest, but significant change in APP metabolism. We have detected decreased secretion of Aβ40 which results in an increase in the Aβ42:Aβ40 ratio (Figure 7). This finding is consistent with a previous study of SNX6 knockdown, which resulted in enhanced retromer transport and accelerated endosomal transit of BACE1 to a perinuclear location colocalizing with the Golgi apparatus (Okada et al., 2010). This effect is associated with accumulation of total cellular APP βCTF (and not αCTF) and increased Aβ40 secretion. The authors speculate that SNX6 may negatively modulate retrograde transport from the endosome to Golgi-like membranes. By accelerating retrograde transport, SNX6 knockdown leads to increased Aβ40 secretion, presumably from the Golgi apparatus. These data are consistent with our finding that disruption of retrograde transport reduces Aβ40 secretion (Figure 7). Whether endosomal BACE1 trafficking is altered in our cells is unknown and currently under investigation.
Two previous studies have reported increased Aβ secretion in cells in which retromer components were knocked down. One study knocked down Vps35 in Hela cells and found a 37% increase in Aβ40 (Small et al., 2005), derived from endogenous APP. A second study knocked down Vps26 in HEK293 cells over expressing APPSw and found increased secretion of Aβ40 (89%) and Aβ42 (102%) (He et al., 2005). These differences may reflect different cell types or lineages, altered expression levels, changes in retromer subunit functions, or differences in APP species. APP isoforms may be differentially modified (e.g. phosphorylated), and hence differentially transported within the endosome (Vieira et al., 2010). Differences arising between knockdown of individual retromer subunits are particularly intriguing, as one would expect that retromer malfunction would be the net result of knockdown of each component. Both Vps26 and Vps35 are structural components of a self assembling membrane coat structure (Hierro et al., 2007), while Vps35 alone is thought to contain cargo recognition information. Yet Vps35, but not Vps26, has been associated genetically with late-onset AD (Small et al., 2005). These data may point to a non-trafficking function of Vps35 in relation to APP metabolism.
Importantly, the exact site of Aβ production is unknown in all of the studies cited and in no case is it possible to mechanistically link retromer malfunction to altered Aβ40 generation. In these studies, the ELISA data might reflect Aβ that is generated from newly synthesized APP and secreted from the Golgi apparatus. If retrograde transport regulates the amount of secretase present in the Golgi apparatus, as suggested by Okada and colleagues (Okada et al., 2010), then attempts to infer changes in retromer-dependent APP trafficking from measurements of secreted Aβ40 are problematic. To date, no evidence of APP redistribution resulting from retromer disruption has been published, and further analysis of retromer dependent secretase transport is warranted.
Another difference between this and previously reported studies is the degree of reduction in retromer protein. In our experimental paradigm, Vps35 is decreased approximately 2 fold more than in previous studies. A complete (~80%) blockade of retromer function could, in principle, produce a qualitatively different effect than partial (~35%) reduction (Small et al., 2005). This is an important distinction since available data suggest that Vps35 protein may be reduced, but not absent, in AD brain (Muhammad et al., 2008). In addition, available data from human brain do not distinguish levels of Vps35 in individual cell types, so that Vps35 might be mildly depleted in many cells or severely depleted in a specific cell population, such as neurons. Neuropathological studies of Vps35 expression in sporadic AD would be useful in addressing this question.
Retromer deficiency has also been associated with neurotoxicity in mouse brain, but the mechanisms remain unclear. One study examining the brains of retromer-deficient mice reported a modest increase of Aβ40 and Aβ42 (~20%), and the soluble APP N-terminal BACE-cleaved (APPS-β) fragment (~15%), with no changes in holo APP or APP βCTF (Muhammad et al., 2008). Measurements in brain reflect mechanisms of both production and clearance of Aβ, and also include both secreted and intracellular Aβ. In addition, since these measurements were made on brain tissue from 3–6 month old mice, they reflect the accumulation of Aβ over an interval during which the cells were exposed to accumulating neurotoxicity, as reflected by the pronounced neurodegeneration. In contrast, our measurements are of only secreted Aβ from healthy cells over only 16h, and thus may represent only the initial stages of amyloidogenesis.
We hypothesized that retromer deficiency might lead to accumulation of APP derivatives in exosomal compartments and subsequent ejection from the cell in exosomal vesicles. Our data support this hypothesis and also suggest that retromer deficiency may increase alpha-cleavage of holo-APP, at least in relation to the limited pool of APP that is destined for exosomal secretion (Figure 8). Specifically, we have detected an increase in total APP CTF, but not in βCTF. We interpret this finding with caution, since the majority of cell-surface APP is subject to constitutive alpha-cleavage by extracellular ADAM family proteases. Although the APP ectodomain is luminal in secreted exosomes, we cannot be certain that holo-APP is not processed after secretion from the cell. While it is technically possible that exosomal holo-APP is differentially processed after secretion from retromer-deficient cells, our finding that total APP CTF is increased relative to holo-APP suggests to us that more APP CTF is exported from retromer-deficient cells. The increased exosomal export of APP CTF with Vps35 deficiency could account for the reduction in the secretion of its γ-secretase-cleaved product (Aβ). Therefore, a pool of Aβ precursor proteins may be escaping the cell before being processed to Aβ. Since exosomal vesicles also contain PS-1 (Sharples et al., 2008), APP CTF that is secreted in exosomes could, theoretically be cleaved extracellularly to produce Aβ.
Disruption of the retromer may increase exosomal APP CTFs by impeding the interaction between APP CTFs and the γ-secretase complex. Consistent with this notion, increased APP CTFs in the exosome have been reported in cells in which γ-secretase activity has been blocked (Sharples et al., 2008). In the absence of γ-secretase inhibition, increased exosomal APP CTFs could result from redistribution of either the substrate or the enzyme (or both), thereby altering the accessibility of APP CTF to the γ-secretase complex. In the absence of γ-secretase inhibition, increased APP CTF production would be predicted to cause increased Aβ generation. Our observation that Vps35 knockdown results in increased exosomal APP CTF and decreased Aβ40 suggests that retromer disruption may generate a secretase-resistant pool of APP CTF in the late endosome. Very recent data from studies of Down syndrome fibroblasts suggests that APP CTFβ may be associated with endosomal toxicity, although the mechanism remains unclear (Jiang et al., 2009). Assessment of endosomal toxicity in our retromer deficient endosomes is an important goal for future studies.
Whether a small increase in the Aβ42:Aβ40 ratio could contribute to neuropathology when the effects are aggregated over decades is difficult to estimate. The increase observed in 293695 cells in which Vps35 is significantly knocked down is consistent with what has been reported in the same cell lines over expressing PS-1 with familial AD (FAD) mutations (Borchelt et al., 1996; Wolfe, 2007). In mice over expressing FAD-PS-1, neuropathology appears within a few months, with or without co-expression of human APP (Garcia-Alloza et al., 2006). Also, in the Vps35-deficient fly, an increase of Aβ40 of only ~30% was associated with pronounced neurodegeneration over a period of weeks (Muhammad et al., 2008). These observations suggest that even small changes in Aβ generation may lead to significant neuropathology over extended periods of time, depending on clearance mechanisms. Moreover, several endosomal trafficking genes have been associated with late-onset AD, including SorL1, Vps26a (Small et al., 2005) and SorCS1 (Reitz et al., 2011). In each case, both the genetic association and the effect of the gene on phenotype are relatively weak. It is possible that the full phenotype of late-onset AD requires combinations of perturbations in trafficking genes and that modest effects of individual genes are, in isolation, misleading. Systems designed to evaluate ‘multiple hit’ scenarios in cells or systems biology approaches may be necessary to identify key genetic combinations.
In summary, our results confirm that disruption of retromer-mediated trafficking alters APP processing. Retromer disruption is associated with increased APP CTF secretion in exosomal vesicles and changes in Aβ production. While the magnitude of this effect is small, its direction is decidedly amyloidogenic [increased Aβ42:Aβ40 ratio]. We conclude that variation in retromer activity may contribute to the risk for developing sporadic Alzheimer’s disease by modulating APP trafficking and metabolism in the late endosome.
Footnotes
This work was supported by a VA Merit Award (PJM), a VISN1 VA Career Development Award (CPS), and an NIA BU Alzheimer’s Disease Center Pilot Project Award (PJM). Correspondence:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharon A, et al. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100:878–85. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- Andersen OM, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–6. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arighi CN, et al. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–31. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–36. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–13. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Canuel M, et al. AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem Biophys Res Commun. 2008;366:724–30. doi: 10.1016/j.bbrc.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, et al. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–24. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- He X, et al. GGA proteins mediate the recycling pathway of memapsin 2 (BACE) J Biol Chem. 2005;280:11696–703. doi: 10.1074/jbc.M411296200. [DOI] [PubMed] [Google Scholar]
- Hierro A, et al. Functional architecture of the retromer cargo-recognition complex. Nature. 2007;449:1063–7. doi: 10.1038/nature06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, et al. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci U S A. 2009;106:20324–9. doi: 10.1073/pnas.0911281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L, et al. Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem. 2001;276:22788–96. doi: 10.1074/jbc.M100857200. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, et al. The sorLA cytoplasmic domain interacts with GGA1 and -2 and defines minimum requirements for GGA binding. FEBS Lett. 2002;511:155–8. doi: 10.1016/s0014-5793(01)03299-9. [DOI] [PubMed] [Google Scholar]
- Jiang Y, et al. Alzheimer's-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci U S A. 2009;107:1630–5. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkut C, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RA, et al. Isolation of extracellular membranous vesicles for proteomic analysis. Methods Mol Biol. 2009;528:227–42. doi: 10.1007/978-1-60327-310-7_16. [DOI] [PubMed] [Google Scholar]
- Muhammad A, et al. Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci U S A. 2008;105:7327–32. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, et al. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA. Mol Cell Biol. 2007;27:6842–51. doi: 10.1128/MCB.00815-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offe K, et al. The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci. 2006;26:1596–603. doi: 10.1523/JNEUROSCI.4946-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, et al. Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. 2010;24:2783–94. doi: 10.1096/fj.09-146357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, et al. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–7. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, et al. SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer's disease risk. Ann Neurol. 2011;69:47–64. doi: 10.1002/ana.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples RA, et al. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008;22:1469–78. doi: 10.1096/fj.07-9357com. [DOI] [PubMed] [Google Scholar]
- Small SA, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, et al. Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann Neurol. 2005;58:909–19. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- Stahl PD, Barbieri MA. Multivesicular bodies and multivesicular endosomes: the “ins and outs” of endosomal traffic. Sci STKE. 2002;2002:pe32. doi: 10.1126/stke.2002.141.pe32. [DOI] [PubMed] [Google Scholar]
- Sullivan CP, et al. Secretory phospholipase A2, group IIA is a novel serum amyloid a target gene; Activation of smooth muscle cell expression by an interleukin-1 receptor-independent mechanism. J Biol Chem. 2009 doi: 10.1074/jbc.M109.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, et al. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–79. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, et al. Neuropathology of preclinical and clinical late-onset Alzheimer's disease. Ann Neurol. 1998;43:673–6. doi: 10.1002/ana.410430519. [DOI] [PubMed] [Google Scholar]
- Vella LJ, et al. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J. 2008;37:323–32. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- Vieira SI, et al. Retrieval of the Alzheimer's amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol Neurodegener. 2010;5:40. doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, et al. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem. 2007;282:18197–205. doi: 10.1074/jbc.M609475200. [DOI] [PubMed] [Google Scholar]
- Williams NS, et al. Identification and validation of genes involved in the pathogenesis of colorectal cancer using cDNA microarrays and RNA interference. Clin Cancer Res. 2003;9:931–46. [PubMed] [Google Scholar]
- Wolfe MS. When loss is gain: reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:136–40. doi: 10.1038/sj.embor.7400896. [DOI] [PMC free article] [PubMed] [Google Scholar]