Abstract
Background
Children’s respiratory health has been linked to many factors, including air pollution. The impacts of urban land-use on health are not fully understood, although these relationships are of key importance given the growing populations living in urban environments.
Objectives
We investigated whether the degree of urban land-use near a family’s residence is associated with severity of respiratory symptoms like wheeze among infants.
Methods
Wheeze occurrence was recorded for the first year of life for 680 infants in Connecticut for 1996–1998 from a cohort at risk for asthma development. Land-use categories were obtained from the National Land Cover Database. The fraction of urban land-use near each subject’s home was related to severity of wheeze symptoms using ordered logistic regression, adjusting for individual-level data including smoking in the household, race, gender, and socio-economic status. Nitrogen dioxide (NO2) exposure was estimated using integrated traffic exposure modeling. Different levels of urban land-use intensity were included in separate models to explore intensity-response relationships. A buffer distance was selected based on the log-likelihood value of models with buffers of 100–2,000m by 10m increments.
Results
A 10% increase in urban land-use within the selected 1,540m buffer of each infant’s residence was associated with 1.09-fold increased risk of wheeze severity (95% confidence interval, 1.02–1.16). Results were robust to alternate buffer sizes. When NO2, representing traffic pollution, was added to the model, results for urban land-use were no longer statistically significant, but had similar central estimates. Higher urban intensity showed higher risk of prevalence and severity of wheeze symptoms.
Conclusions
Urban land-use was associated with severity of wheeze symptoms in infants. Findings indicate that health effect estimates for urbanicity incorporate some effects of traffic-related emissions, but also involve other factors. These may include differences in housing characteristics or baseline healthcare status.
Keywords: Wheeze Symptom, Land-Use, Infants’ health, Traffic, Urbanicity
1. Introduction
Prevalence of childhood adverse respiratory symptoms, such as asthma or wheeze, has increased in recent decades (Akinbami et al., 2009). For instance, prevalence of schoolchildren’s wheeze increased 255% in Hong Kong in the early 1990s (Leung et al., 1997). In the U.S., more than 10 million children have been diagnosed with asthma (Bloom et al., 2009). Numerous studies investigated factors associated with this health response. For example, young male children had higher risk of wheeze and asthma than young females, although the biological cause is unknown (Almqvist et al., 2008). Others found associations between asthma and family history, which could be explained by genetics or similar environmental exposures within families (Burke et al., 2003). Housing characteristics such as parental smoking status or house mold was reported as potential contributors to adverse respiratory symptoms and conditions (Prescott, 2008; Salo et al., 2004). An association between respiratory symptoms and exposure to air pollution is another explanation. Nitrogen dioxide (NO2) was found to exacerbate asthma severity (Chauhan et al., 2003). An association was observed between sulfur dioxide (SO2) and peak expiratory flow and incidence of upper respiratory symptoms (Timonen and Pekkanen, 1997). Ozone was associated with children’s respiratory symptoms and rescue medication use (Gent et al., 2003). A literature review reported that six studies found associations between children’s respiratory symptoms and traffic exposure (Boothe and Shendell, 2008).
A common method of estimating air pollution exposure in urban areas is to aggregate values from nearby outdoor air monitors or use values from the monitor closest to the residence. An advantage of this approach is that investigators can use existing monitoring sites, often established by regulatory agencies such as U.S. Environmental Protection Agency (EPA). This approach assumes homogenous exposure levels within a certain distance or district; however, pollutant levels can be heterogeneous in a given area (Bell et al., in press). Personal monitors can be used to estimate individual exposure levels, although this approach is often impractical with children and generally cost-prohibitive (Frumkin, 2005).
Estimating personal exposure levels using land-use regression modeling is attractive with respect to cost, availability of satellite images, and emergence of geographic information systems (GIS) (Elliott and Wartenberg, 2004). Many studies of urban environmental effects on respiratory symptoms applied land-use regression models to estimate traffic-related exposure levels based on land type and traffic volume (Briggs et al., 1997; Ross et al., 2005; Su et al., 2008). Developed land-use could be used as an indicator of traffic and other urban-related air pollution and to provide estimates of exposure for places and time periods without ambient outdoor air monitors. A comparison of land-use regression model results and traffic emissions data found reasonable agreement (Rosenlund et al., 2008). Land-use regression and ambient monitor approaches differ as land-use methods estimate exposure from a particular type of land-use associated with several sources whereas monitor approaches measure levels of a particular pollutant. The relative benefits of land-use or ambient monitoring approaches depend on the resolution of input data available. Given the strong contribution of traffic to air pollution in urban centers, the degree of urbanicity may be linked to air pollution levels. This is one pathway through which urbanization could affect health (Vlahov and Galea, 2002). The use of an exposure metric based on urbanicity thereby incorporates traffic-related emissions, but also includes other urban factors.
Urbanization may also affect health through other environmental exposures that may be more prevalent in urban environments (e.g., industrial emissions, urban heat island effect, and noise), stress and social factors. The fraction of urban land-use in a person’s neighborhood provides an intriguing proxy measure of health risks that could be used to represent multiple facets of the urban environment. Given that more than 50% of the world’s population lives in urban environments, a better understanding of how development affects health is needed (United Nations, 2001).
Within an urban environment, subpopulations may differ by race and socioeconomic status (SES), which can result in public health inequalities (Marmot, 2005). While SES and/or race appear to play a role in health, these alone do not explain differential health risks (Lara et al., 2006). A potential explanation is that environmental conditions can disproportionately affect subsets of the population. Previous research demonstrated that lower SES people suffer more adverse health responses from ambient environmental exposures (Bell et al., 2007).
We explored the effect of urban land-use on severity of respiratory symptoms for a cohort of infants at high risk for asthma development in Connecticut, United States. We also evaluated traffic pollution, assessed through estimated NO2 levels, to explore its association with respiratory symptoms and whether urban land-use functioned as a proxy for traffic. Furthermore, we investigated whether different levels of urban intensity affect risk of symptoms. As sensitivity analysis, we examined whether infants with lower SES are more affected by urban land-use than infants with higher SES.
Our study region is of particular interest given that childhood asthma is relatively common in Connecticut, which could be related to urbanicity, inter-state highways, and large cities with dense traffic (Perrin et al., 1989). While there are urban areas in the state, most of Connecticut consists of forests, permitting a range of urban land-use levels among subjects.
2. Materials and Methods
This study combined data from a Connecticut asthma cohort, land-use data obtained from Multi-Resolution Land Characteristics Consortium, and NO2 at each subject’s residence estimated from an integrated traffic exposure model.
2.1. Cohort Subjects
Infants’ data were obtained from the Yale Childhood Asthma Study, a longitudinal asthma cohort study conducted by the Yale Center for Perinatal, Pediatric and Environmental Epidemiology (Gent et al., 2002; Leaderer et al., 2002). All cohort subjects were born in Connecticut or Massachusetts between September 1996 and December 1998, and have at least one sibling less than 11 years of age at the time of enrollment who was diagnosed with asthma by a physician. Further description of this cohort can be found elsewhere (Belanger et al., 2003; Leaderer et al., 2002). The study was approved by the Human Investigations Committee of Yale University, New Haven, Connecticut, and each mother agreed to participate prior to her child’s enrollment.
Within four months of birth, a trained interviewer visited the home to obtain household characteristics from the mother, including residential address, infants’ gender, infants’ race, mother’s educational attainment, maternal and paternal asthma histories, family income, smoking in the home, and presence of persistent mold. Interviewers asked whether infants exhibited wheeze symptoms, and, if so, how many days the infant had symptoms in each month. After the interview, the mother was provided with a calendar and asked to prospectively record infants’ daily wheeze symptoms histories. These detailed data were collected every 3 months by phone interview until the infant reached an age of 12 months.
Diagnosis of infants’ asthma is particularly unreliable (Rabe et al., 2004; Stempel, 2002). However, persistence of wheeze symptoms can be an indicator of asthma development (Castro-Rodríguez et al., 2000; Martinez et al., 1995). Therefore, in this study, total wheeze days summed over the 12-month period was used as an indicator of adverse respiratory symptoms. Two outcome variables were defined: 1) a categorical variable for the number of wheeze days in a year (none: 0 days, mild: less than 30 days, severe: 30 or more days); and 2) dichotomous variables for the presence or absence of wheeze symptoms within a year (yes/no). Previous analysis of this cohort used the same outcome categories (Belanger et al., 2003).
The initial cohort consisted of 1002 infants. This study used the subset of subjects living in Connecticut (833 residents), because of the availability of land-use data. Twenty-seven subjects (3.2% of the 833 Connecticut subjects) had missing residential address, or/and basic household characteristics, and therefore were omitted from analysis. Of the remaining 806 subjects, 45 (5.6%) were omitted because they had less than seven months of data on wheeze symptoms, leaving 761 subjects. Among these, subjects were omitted if they moved during the one-year follow up with an unknown move date (2.8%) or moved before age 10 months (7.9%), leaving 680 subjects for analysis. Study subjects are primarily from Fairfield (29.4%), Hartford (30.9%), and New Haven Counties (33.1%).
2.2. Land-use Data
National Land Cover Database 2001 was used to obtain land-use variables. This U.S. database contains sixteen classes of land-use within Connecticut, which were derived from multiple satellite images covering multiple seasons from Landsat-5 and Landsat-7 satellites and other ancillary data to yield a resolution of 30×30m land-use data for 2001 to 2006. Applying this database for our study period (1996–1999) is appropriate because: 1) land-use classification of this database does not depend on a single time period, 2) land-use is unlikely to change dramatically over the span of a few years, and 3) the timeframe for this database is the closest available to our study period as the previous National Land Cover Database was constructed in 1992. Sixteen land-use classes were produced, which we aggregated into five categories: urban, grass/farm, rock/sand/clay, forest, and water. To explore whether exposure to different levels of urban intensity affected the association, urban land-use was classified into three levels based on National Land Cover Database 2001 definitions: high intensity (impervious surfaces accounted for 80% or more of the pixel), medium intensity (impervious surfaces accounted for 50% or more but less than 80% of the pixel), and low intensity (impervious surfaces accounted for 20% or more but less than 50% of the pixel). In this work, the definition of “urban” land-use was defined as single-family housing units, apartment complexes, row houses or commercial/industrial areas based on the National Land Cover Database. Specifically, low and medium intensity urban areas corresponded to single housing units with a mixture of constructed materials and vegetation. High intensity urban areas corresponded to apartment complexes or industrial areas where people reside or work in high numbers. Further information on the National Land Cover Database 2001 can be found elsewhere (Homer et al., 2004). Each category contains a heterogeneous mixture of the urban landscape and environmental conditions; however, these broad categories can help illuminate the overall impact of urbanicity.
Residential addresses at enrollment were projected as a point layer using a geocoding function in ESRI®’s GIS software, Arcview® (ESRI, Inc., Redlands, CA) (Mitchell, 2005). A circular buffer was created for each residence to estimate exposure levels of urban land-use for each study subject. For each urban land-use intensity, the fraction of pixels within a circular buffer around each subject’s residence was used as a predictor of wheeze symptoms. The buffer distance (i.e. radius of the circle) was selected based on a maximum likelihood estimate of the buffer radius (described in Section 2.4), and sensitivity analysis was conducted with varying buffer sizes.
2.3. Estimated Exposure to Traffic-related Air Pollution
Several studies found associations between adverse respiratory symptoms for children and traffic exhaust, which is a source of NO2, carbon monoxide (CO) and particulate matter (Pershagen et al., 1995; Schwartz, 2004). Only limited information was available for ambient pollution in our study region and time period. The U.S. EPA’s air monitoring in Connecticut for the study period consists of only five NO2 outdoor monitors, prohibiting effective use of proximity or interpolation approaches to estimate exposure. The largest distance between a study subject and the closest NO2 monitor was 64.9km.
We estimated exposure to traffic-related outdoor NO2 for each subject using a GIS integrated traffic exposure model developed in our earlier research (Holford et al., 2010). In this work, exposure from traffic on a highway, a mobile line source, was assumed to be proportional to a line integral of the dispersion function and annual average daily traffic. Thus the approach incorporated information on distance between residence and highways, length of highway segments, and annual averaged daily traffic volume of each highway segment within 2km of a residence. A regression model was applied to estimate the dispersion of traffic-related NO2 pollution. The outcome was observed NO2 levels, measured outside subjects’ homes using Palmes tubes in a previously conducted cohort study (Belanger et al., 2003; Leaderer et al., 2002; Palmes et al., 1976). Each monitor was attached to a tree or outdoor clothes line at least 5 feet from the ground and as close to the home as possible, and left for 10–14 days. Traffic model estimates showed reasonable agreement with measured values (correlation between observed and predicted NO2 levels was 0.71). Using this model, we estimated outdoor NO2 levels for each study subject’s residence in the current study (Supplemental Figure S1). Additional details on this integrated exposure model are published elsewhere (Holford et al., 2010).
2.4. Statistical Analysis
As noted above, exposure to urban land-use was measured using the proportion of pixels of a given classification within a specified distance from a residence. We selected the buffer size as the value that maximized the log-likelihood for a model estimating the association between fraction of urban land-use and wheeze symptom response adjusted by infants’ gender, race (Caucasian/African American/Hispanic/Other), parental asthma history (history of asthma for either parent), indicator of family income (family income less than $20,000/year, equal to or more than $20,000/year), smoking in the home (yes/no), presence of persistent mold (yes/no/missing), and maternal educational attainment (less than 12 years, equal to or more than 12 years) for buffer distances of 100m to 2000m at 10m intervals. The categories for family income were designed in relation to the poverty threshold for a family of four during the study period, $16,000/year, defined by the U.S. Census (US Census Bureau, 2000). This cohort study collected income data as a categorical variable using $10,000 cut-points, so $20,000 was the available value closest to the Census definition. The resulting selected buffer distance was used for subsequent analyses to preserve nesting of hypotheses required for likelihood ratio testing.
Applying the selected buffer size, three ordered logistic regression models (proportional odds model), described below, were fitted using three levels of the prevalence and severity of wheeze symptoms (none, less than 30 days/year, 30 or more days/year) as the outcome. Models estimated the odds ratio (OR) of increased severity of wheeze symptoms and urban land-use, or estimated NO2 levels, with adjustment for covariates described above. This model structure assumes that the OR for a given increment in exposure (i.e. urban land-use) comparing presence versus absence of wheeze is equivalent to the OR comparing 30 or more days of wheeze per year to less than 30 days of wheeze per year. Similar models were used in previous publications using this data (Belanger et al., 2003; Holford et al., 2010). A proportional odds model was selected for main analysis rather than the alternative ordered logistic regression models (continuation ratio model) because of its directional independence and ability to provide good fit to the data, as discussed elsewhere (Scott et al., 1997). Choice of symptom stratification points (0 and 30 days/year) is consistent with earlier publications based on this cohort (Belanger et al., 2003).
The first model explored the association between urban land-use and the prevalence and severity of wheeze symptoms. For each study subject, exposure to urban land-use was characterized as the number of pixels of urban land-use within the buffer of the subjects’ residence, which represents the fraction of urban land-use area. The second model explored the association between NO2 exposure, estimated by the integrated traffic exposure model, and severity of wheeze symptoms. Finally, variables for both urban land-use and estimated NO2 levels were included simultaneously, which enables us to examine whether urban land-use served as a proxy for traffic pollution. To explore whether other land-use categories were associated with prevalence and severity of wheeze symptoms, we also fitted similar models, replacing urban land-use with other land-use categories. Furthermore, logistic regression was performed using dichotomized wheeze symptoms (yes/no) as an outcome to investigate whether results were similar to those from ordered logistic regression. As an alternative analysis, we used three levels of urban intensity as the exposures in separate models. The ORs of each urban intensity level were compared to explore intensity-response relationships.
As sensitivity analysis, we studied whether family income status modified the association between urban land-use and severity of wheeze symptoms by fitting a model with interaction terms between urban land-use and income level (less than $20,000, $20,000 or more/year). A similar interaction model was considered for income status and maternal educational attainment to evaluate possible effect modification.
3. Results
Table 1 provides summary statistics for the study subjects. Over 40 % of infants had at least one day with wheeze symptoms within 12 months after birth. About 10% of infants experienced severe wheeze symptoms (30 or more days/year). At least one parent had a history of asthma for 45.7% of infants. Most subjects were Caucasian (62.7%).
Table 1.
Summary statistics of cohort study subjects
| Number of subjects (%) | Percentage of urban land-use within 1,540m buffer of residence (standard deviation) | |
|---|---|---|
| Presence and Severity of Wheeze | ||
| Yes (severe): ≥30 days of wheeze symptoms within the first 12 months of life | 72 (10.6%) | 54.4 (32.2) |
| Yes: 1 to 29 days of wheeze symptoms within the first 12 months of life | 224 (32.9%) | 49.1 (28.9) |
| No: No wheeze symptoms within the first 12 months of life | 384 (56.5%) | 42.2 (28.2) |
| Infants’ Race | ||
| Caucasian | 426 (62.7%) | 32.4 (24.4) |
| African American | 92 (13.5%) | 63.2 (19.8) |
| Hispanic | 134 (19.7%) | 74.8 (18.8) |
| Other | 28 (4.1%) | 53.9 (29.5) |
| Infants’ Gender | ||
| Female | 352 (51.8%) | 46.6 (29.1) |
| Male | 328 (48.2%) | 45.0 (29.3) |
| Parental History of Asthma | ||
| Yes: one or more parent with asthma | 311 (45.7%) | 47.8 (29.9) |
| No: no parent with asthma history | 369 (54.3%) | 44.1 (28.5) |
| Family Income | ||
| < $20,000/year | 136 (20.0%) | 70.2 (19.8) |
| ≥ $20,000/year | 544 (80.0%) | 39.7 (27.9) |
| Mother’s Educational Attainment | ||
| Less than high school diploma | 75 (11.0%) | 70.7 (22.2) |
| High school or more | 605 (89.0%) | 42.7 (28.5) |
| Smoking in the home | ||
| Yes | 278 (40.9%) | 53.5 (27.8) |
| No | 402 (59.1%) | 40.5 (28.9) |
| House Mold Status | ||
| None | 518 (76.2%) | 46.6 (29.2) |
| Mold data missing | 20 (2.9%) | 68.7 (24.9) |
| Mold | 142 (20.9%) | 39.7 (27.7) |
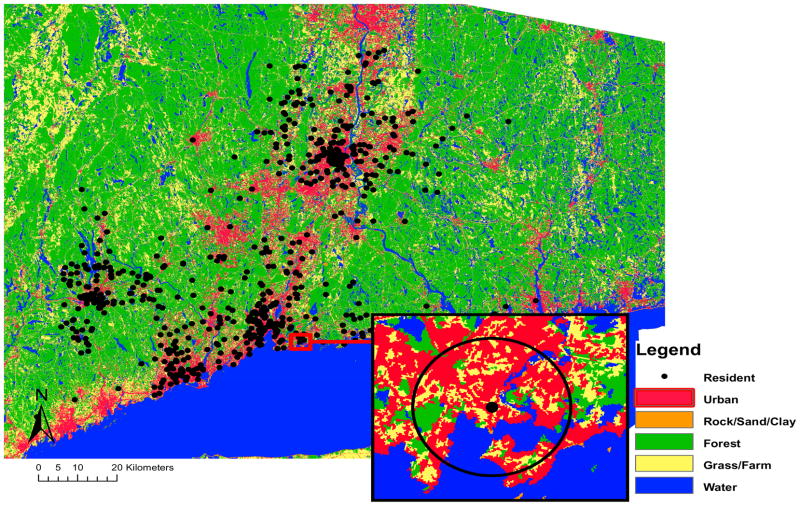
Based on a model of urban land-use and severity and prevalence of wheeze symptoms adjusted for the covariates described previously, a buffer size of 1,540m was employed for the remaining analysis. Figure 1 shows the land-use categories and approximate location of residences of study subjects’ in Connecticut. The figure also provides an example of land-use pixels within a 1,540m buffer around a residence. The percent of urban land-use within 1,540m from a subject’s residence averaged 45.8% (standard deviation 29.2%, range 0.1% to 97.4%). Supplemental Table S1 provides summary statistics for land-use categories and estimated NO2 levels.
Figure 1.
Map of land-use categories with 30m×30m resolution and approximate residential locations of study subjects, and a buffer distance of 1,540m from the resident.
Table 2 presents correlations among land-use categories within buffers and estimated NO2 levels, representing traffic-related outdoor air pollution. Higher fraction of urban land-use was correlated with higher estimated NO2 levels (0.63).
Table 2.
Correlation of land-use around residences of study subjects and estimated NO2 levels
| Land-use categories | Traffic pollution estimated NO2 | |||||
|---|---|---|---|---|---|---|
| Forest | Grass/Farm | Rock/Sand/Clay | Water | |||
| Land-use | Urban | −0.92 | −0.53 | −0.07 | −0.32 | 0.63 |
| Forest | 0.46 | −0.06 | 0.06 | −0.58 | ||
| Grass/Farm | 0.03 | 0.00 | −0.35 | |||
| Rock/Sand/Clay | 0.33 | −0.09 | ||||
| Water | −0.11 | |||||
Note: Land-use is based on a 1,540m buffer. NO2 levels are estimated and designed to be a marker for traffic-related air pollutants more broadly.
Table 3 shows the relationship between study subjects’ covariates and severity of wheeze symptoms based on the ordered logistic model without land-use variables adjusted by other covariates (infants’ gender, race, parental asthma history, family income, smoking in the home, presence of persistent mold, and maternal educational attainment). Male infants were 1.68 (95% CI: 1.24, 2.28) times more likely to have severe wheeze symptoms than females. Infants with parental history of asthma were 1.49 (95% CI: 1.10, 2.02) times more likely to have symptoms than those without such a parental history. Infants of mothers with lower educational attainment were 1.88 (95% CI: 1.11, 3.19) times more likely to show severe wheeze symptoms. Presence of persistent mold was associated with adverse respiratory symptoms with OR of 1.77 (95% CI: 1.23, 2.54), which corresponds to results from our previous study (Belanger et al., 2003). Statistically significant differences in severity of wheeze symptoms were not observed by infants’ race, smoking in the home, or family’s income. Some of the variables in Table 3 may be indicators for or have associations with other factors. As an example, housing type is correlated with income (r2=0.65). The variable for whether smoking was present in the home is associated with housing type; those living in apartments were 3.37 times more likely to have smoking in the home than those living in single-family or duplex homes.
Table 3.
Odds ratio of severity of wheeze symptoms for subject characteristics for a model without land-use variables
| Parameter | Number of subjects (%) | OR | 95% Confidence interval |
|---|---|---|---|
| Infants’ Race | |||
| Caucasian (reference) | 426 (62.7%) | 1.00 | |
| Hispanic | 134 (19.7%) | 1.57 | (1.00,2.47) |
| African American | 92 (13.5%) | 1.61 | (1.00,2.60) |
| Other | 28 (4.1%) | 1.66 | (0.79,3.52) |
| Parental History of Asthma | |||
| None (reference) | 369 (54.3%) | 1.00 | |
| Parent with asthma | 311 (45.7%) | 1.49 | (1.10,2.02) |
| Family Income | |||
| ≥$20,000/year (reference) | 544 (80.0%) | 1.00 | |
| <$20,000/year | 136 (20.0%) | 0.82 | (0.51,1.30) |
| Mother’s Education | |||
| High school or more (reference) | 605 (89.0%) | 1.00 | |
| < high school | 75 (11.0%) | 1.88 | (1.11,3.19) |
| Gender | |||
| Female (reference) | 352 (51.8%) | 1.00 | |
| Male | 328 (48.2%) | 1.68 | (1.24,2.28) |
| Smoking Status | |||
| non-Smoking (reference) | 402 (59.1%) | 1.00 | |
| Smoking Status | 278 (40.9%) | 1.04 | (0.76,1.44) |
| Mold | |||
| Non-Mold (reference) | 518 (76.2%) | 1.00 | |
| Mold Info Missing | 20 (2.9%) | 2.31 | (0.98,5.47) |
| Mold | 142 (20.9%) | 1.77 | (1.23,2.54) |
Note: Results are based on the proportional odds model with health outcome defined as the severity of wheeze symptoms (none, mild, severe in the first year of life). Models do not include a variable for land-use. Models adjust for infants’ gender, race, parental asthma history, family income, smoking in the home, presence of persistent mold, and maternal educational attainment.
Table 4 shows results of ordered logistic models relating urban land-use as a continuous variable to severity of wheeze symptoms adjusted by subjects’ covariates. Urban land-use was associated with increased severity of wheeze symptoms. A 10% increment of urban area within 1,540m of a subject’s residence, approximately 0.75 km2, was associated with an OR of 1.09 (95% CI: 1.02, 1.16) for severity of wheeze symptoms. Another alternative ordered logistic regression (continuation ratio model) provided similar results (OR=1.08 (95% CI: 1.02, 1.15)). We performed sensitivity analysis on buffer size, finding associations at all buffer sizes considered up to 2,000m, with statistically significant associations at buffers of about 540m or larger (Supplemental Figure 2). The fraction of urban land-use within the buffer monotonically decreases from 100m (62.3%) to 2,000m (44.0%).
Table 4.
Odds ratio of wheeze symptoms for multiple model structures
| Estimated NO2 (Odds Ratio and 95 % Confidence Interval) | Urban land-use (Odds Ratio and 95 % Confidence Interval) | |
|---|---|---|
| Model 1 Univariate Model-Urban land-use | 1.09 (1.02, 1.16) | |
| Model 2 Univariate Model- Estimated NO2 | 1.22 (0.98, 1.51) | |
| Model 3 Multivariate Model- Urban land-use and NO2 | 1.10 (0.86, 1.40) | 1.07 (0.99, 1.16) |
Note: NO2 results are per IQR increment of estimated traffic NO2 (9.21ppb). Urban land-use results are per 10% increment within the selected buffer, 1,540m, which is about 185 acres. Results are based on the proportional odds model with health outcome defined as the severity of wheeze symptoms (none, mild, severe in the first year of life). Models adjust for infants’ gender, race, parental asthma history, family income, smoking in the home, presence of persistent mold, and maternal educational attainment.
Sensitivity analysis using logistic regression with dichotomized wheeze symptoms (yes/no) supports results of ordered logistic regression; the OR for risk of wheeze per 10% increment of urban land-use was 1.09 (95% CI: 1.02, 1.16). We estimated the relationship between traffic-related outdoor NO2 exposure and severity of wheeze symptoms in a separate model without the urban land-use variable. An interquartile range (IQR) increase in estimated NO2 exposure (9.21 ppb) was associated with an OR of 1.22 (95% CI: 0.98, 1.51) for severity of wheeze symptoms. When variables for estimated NO2 and urban land-use were included simultaneously, associations remain but are attenuated and neither is statistically significant. The OR for severity of wheeze symptoms associated with a 10% increase in exposure was 1.07 (95% CI: 0.99, 1.16) for urban land-use, and 1.10 (95% CI: 0.86, 1.40) for estimated NO2. The correlation between the fraction of urban land-use within the buffer and estimated traffic NO2 level was 0.63 (Table 2).
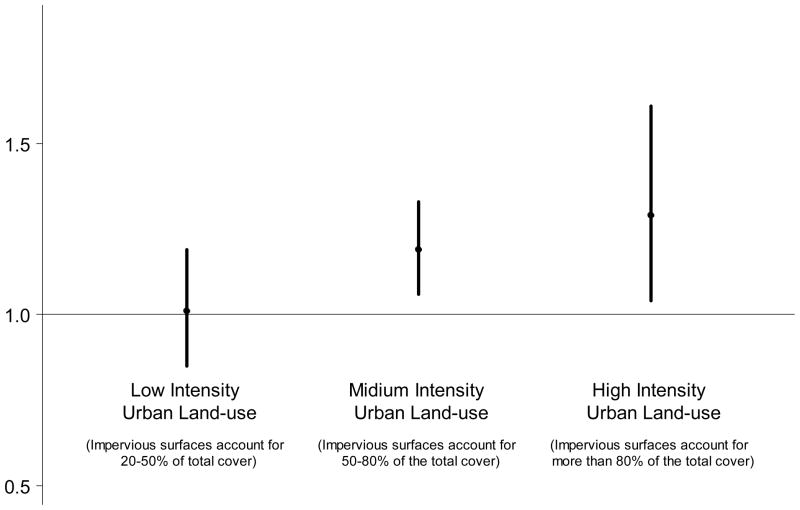
To explore the relationship between urban intensity and wheeze symptoms, separate analysis was conducted with the three levels of urban intensity for land-use (low, medium, high intensity) (Figure 2). We observed a clear ordered relationship between urban intensity and risk of wheeze response: infants living in a more urban setting were at higher risk of wheeze symptoms.
Figure 2.
Odds ratio of severity of wheeze symptoms for various levels of urban land-use intensity
Note: Results are odds ratio per 10% increment of urban land-use within buffer (about 185 acres). The point represents the central estimate and the vertical line represents the 95% confidence interval. Results are based on the proportional odds model with health outcome defined as the severity of wheeze symptoms (none, mild, severe in the first year of life). Models adjust for infants’ gender, race, parental asthma history, family income, smoking in the home, presence of persistent mold, and maternal educational attainment.
Effect estimates for other land-use categories did not show any statistically significant associations (results are not shown). The OR for a 10% increment of grass/farm land-use was 0.88 (95% CI: 0.63, 1.22) and forest was 0.94 (95% CI: 0.87, 1.01). Table 5 summarizes results of a model with interaction terms between urban land-use and family SES. Results imply that the association between urban land-use and severity of wheeze symptoms differed by family SES, although the significance level is marginal.
Table 5.
Odds ratio of wheeze severity for 10% increase in developed land-use within buffer, by socio-economic status (SES)
| Lower SES (income-based) (Odds Ratio and 95 % Confidence Interval) | Higher SES (income-based) (Odds Ratio and 95 % Confidence Interval) | |
|---|---|---|
| Interaction term with urban land-use and income-based SES | 1.07 (0.99, 1.14) | 0.92 (0.81, 1.05) |
| Interaction term with urban land-use and education-based SES | 1.26 (1.01, 1.57) | 1.07 (1.00, 1.15) |
Note: Income-based SES has lower SES at annual income less than $20,000, and higher SES at ≥ $20,000. Education-based SES has lower SES at maternal educational attainment less than high school diploma, and higher SES at least high school diploma. Results are based on the proportional odds model with health outcome defined as the severity of wheeze symptoms (none, mild, severe in the first year of life). Models adjust for infants’ gender, race, parental asthma history, family income, smoking in the home, presence of persistent mold, and maternal educational attainment.
4. Discussion
The study population is a particularly sensitive group – infants with at least one sibling with physician-diagnosed asthma. In fact, we found that infants with at least one parent with asthma history had higher severity of wheeze symptoms (Table 3). This could be explained by genetics or exposures that are common to family members (Bisgaard et al., 2009). While some variables could be independent risk factors for developing wheeze symptoms (e.g., infants with parental history of asthma, presence of persistent mold), we found that living in a more urban area was associated with higher risk of wheeze symptoms, after adjustment for other variables.
There are multiple mechanisms through which urban land-use could affect wheeze symptoms, such as air pollution, noise, and stress (Klinnert et al., 2003; Ryan et al., 2005). Our original hypothesis was that the fraction of urban land-use around residence would be associated with severity of infants’ respiratory symptoms, with the concept that urban land-use captures multiple aspects of urban burdens, including traffic and other pollutants, noise and other factors relating to urbanicity. An association has been identified between factors reflecting urbanicity and adverse respiratory symptoms (Wright, 2007). While our study examined urbanicity in general, we were able to address some of these factors.
Traffic-related outdoor air pollution is anticipated to be higher in urban centers because of high traffic volume and concentrated roadways. Several studies used developed land-use as a marker of traffic emissions (Clougherty et al., 2008; Henderson et al., 2007). Many used shorter distances than we used in this work (e.g., 500m compared to our selected buffer of 1,540m); however, the nature of the buffer differs as many such studies consider only the presence or absence of a specified land-use within a certain distance of the residence, whereas we considered the degree of land-use (e.g., fraction of area) within a specified distance (English et al., 1999; Zhou and Levy, 2007).
We selected buffer size based on the value that maximized the log-likelihood and performed sensitivity analysis with other sizes. Results were essentially unchanged (OR 1.02 to 1.09) when we modified the buffer size from 100m to 2,000m, although estimates were not statistically significant for the first a few hundred meters. This might be explained by two reasons. First, traffic-related outdoor NO2 level could affect infants’ health further than the distance many studies considered. A recent study reported that NO2 levels could be explained in part by traffic emissions and land-use within 6,000m from a residence (Skene et al., 2010). Another possible explanation is that urbanicity may capture factors relevant to human health other than traffic pollutants. Our analysis suggested the fraction of urban land-use within the 1,540m buffer around residences is somewhat correlated with estimated traffic-related outdoor NO2 levels (0.63). This correlation was similar (0.51 to 0.64) across a range of buffer sizes of 100–2,000m. When adjusted for estimated NO2 levels, the association between urban land-use and severity of wheeze lost statistical significance although the central estimate was similar (OR of 1.07 with NO2 adjustment, 1.09 without NO2 adjustment). The NO2 levels were based on a GIS traffic model that incorporated data on highway patterns and traffic flow, and did not explicitly use land-use categories as inputs; however, urbanicity and traffic are linked, as evidenced by the correlation between NO2 estimates and the urban land-use category.
Thus, findings indicate that the observed association between urban land-use and infants’ respiratory symptoms incorporates some effects of traffic-related outdoor air pollution, although these results cannot be explained solely by traffic. Highway and non-highway vehicles account for over 90% of emissions for CO, about 36% for volatile organic compounds (VOCs), and over 70% for nitrogen oxides. Area point sources (e.g., small commercial and industrial firms) contribute about 40% of VOC emissions, whereas stationary point sources (e.g., utilities, industry) contribute about 13% of nitrogen oxide emissions (CT Dept. of Environmental Protection, 2005). Another interesting finding is the clear trend between the different levels of urban intensity and severity of wheeze symptoms. High intensity urban areas, pixels with 80% or more impervious surfaces, are more likely to contain industrial centers and be surrounded by heavy traffic, while low intensity urban areas, pixels with 20% or more and less than 50% impervious surfaces, are more likely to be residential, less polluted areas.
Our results imply that the association between urban land-use and severity of wheeze was higher for infants of lower SES families. One potential explanation is housing characteristics. Families with higher income may be more likely to afford home air conditioning (AC). Earlier work showed lower effect estimates for air pollution for communities or individuals with central AC, which may relate to changes in the penetration of ambient air pollutants indoors or filtration (Bell et al., 2009; Medina-Ramon et al., 2006; Waring and Siegel, 2008). The correlation between county’s median income and AC prevalence in Connecticut was 0.65 (US Census Bureau, 2000; US Department of Commerce, 1997). Another explanation could be that susceptibility to environmental stressors from urban environments may be related to baseline healthcare status or healthcare, which could be related to SES (O’Neill et al., 2007).
A key strength of this study is the cohort design; we were able to adjust for infants’ race, parental asthma history, mother’s educational attainment, smoking in the home, presence of persistent mold, and family income on an individual level, and base exposure on residential location. We were able to estimate a marker for traffic-related outdoor air pollutants, NO2, using a GIS integrated traffic exposure model, for the study time period and area where monitoring data were unavailable. Although actual measurements would be preferable, such information is often prohibited by cost or data availability. The methods used in this study to estimate NO2 based on a GIS model and to obtain land-use based on satellite imagery could be applied to other studies, and in fact related approaches have been used (Jerrett et al., 2005).
Limitations of this study include the potential misclassification of land-use categories and sub-scale heterogeneity given land-use resolution of 30×30m. However, we anticipate that any such misclassification would be non-differential and drive effect estimates towards the null. Additional refinements to land-use categorization methods and satellite imagery, perhaps with higher spatial resolution, would be beneficial. While we addressed traffic-related outdoor air pollution, this issue warrants further attention. Our ability to separate effects of traffic and other urban characteristics is limited because of their moderate correlation. Also, our method using NO2 as an indicator of overall traffic-related outdoor air pollution may not fully capture the effect of some pollutants or other sources on adverse respiratory symptoms. Traffic contributes to a variety of pollutants including NO2, PM2.5, and CO, and previous research indicated that other air pollutants, such as PM2.5, are associated with respiratory outcomes (Dominici et al., 2006). Other sources such as industry also contribute to NO2 (Holgate, 1999). Although our method has the spatial advantage of individual-level exposure, the NO2 traffic approach has a temporal limitation due to the use of annual averages, which were the only available form of traffic data. Additional research in this area is warranted. Future research could investigate actual observed air pollutants, rather than estimated values, and other environmental stressors in relation to urban land-use and respiratory symptoms. Additionally, although the NO2 estimation model performs well, it could be improved with consideration of seasonal traffic volume and non-highway roads for which data were unavailable, and wind direction which was not incorporated in the exposure model (Holford et al., 2010).
In conclusion, we found an association between urban land-use and severity of wheeze symptoms for infants, with higher effect estimates with higher degree of urbanicity. Our cohort subjects are at high risk of adverse respiratory outcomes, and further work could investigate whether results apply to a more general population. Urban land-use represents several aspects of urban environment. Urbanicity may affect health not only through traffic-related outdoor air pollution, but also through other pathways, and its effect may be modified by SES. Further research is needed to investigate the various characteristics of an urban environment that affect respiratory health, given the environmental justice implications.
Supplementary Material
Acknowledgments
Grant support: Funding for this project was provided by the National Institute of Environmental Health Sciences (R01ES07456, ES05410, and ES07456).
The authors thank Dr. Janneane Gent, Yale Center for Perinatal, Pediatric and Environmental Epidemiology.
Abbreviations
- CO
carbon monoxide
- GIS
geographic information systems
- IQR
interquartile range
- NO2
nitrogen dioxide
- OR
odds ratio
- PM2.5
particulate matter with aerodynamic diameter equal to or less than 2.5 microns
- SES
socio-economic status
- SO2
sulfur dioxide
- U.S. EPA
U.S. Environmental Protection Agency
Footnotes
Disclaimers/Conflict of Interest: The authors declare no conflict of interest.
Human Subjects: The study was approved by the Human Investigations Committee of Yale University, New Haven, Connecticut, and the mothers of the children agreed to participate prior to their child’s enrollment.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of Childhood Asthma in the United States, 1980–2007. Pediatrics. 2009;123:S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- Belanger K, Beckett W, Triche E, Bracken MB, Holford T, Ren P, McSharry J-e, Gold DR, Platts-Mills TAE, Leaderer BP. Symptoms of wheeze and persistent cough in the first year of life: associations with indoor allergens, air contaminants, and maternal history of asthma. Am J Epidemiol. 2003;158:195–202. doi: 10.1093/aje/kwg148. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD. Community-Level Spatial Heterogeneity of Chemical Constituent Levels of Fine Particulates and Implications for Epidemiological Research. Journal of Exposure Science and Environmental Epidemiology. doi: 10.1038/jes.2010.24. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Dominici F. Adverse health effects of particulate air pollution: modification by air conditioning. Epidemiology. 2009;20:682–686. doi: 10.1097/EDE.0b013e3181aba749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Bonnelykke K, Sleiman PMA, Brasholt M, Chawes B, Kreiner-Moller E, Stage M, Kim C, Tavendale R, Baty F, Pipper CB, Palmer CNA, Hakonarsson H. Chromosome 17q21 Gene Variants Are Associated with Asthma and Exacerbations but Not Atopy in Early Childhood. Am J Respir Crit Care Med. 2009;179:179–185. doi: 10.1164/rccm.200809-1436OC. [DOI] [PubMed] [Google Scholar]
- Bloom B, Cohen RA, Freeman G. Summary health statistics for US children: National Health Interview Survey, 2008. Vital Health Stat. 2009;10:1–81. [PubMed] [Google Scholar]
- Boothe VL, Shendell DG. Potential health effects associated with residential proximity to freeways and primary roads: review of scientific literature, 1999–2006. Journal of Environmental Health. 2008;70:33–41. [PubMed] [Google Scholar]
- Briggs DJ, Collins S, Elliott P, Fischer P, Kingham S, Lebret E, Pryl K, VanReeuwijk H, Smallbone K, VanderVeen A. Mapping urban air pollution using GIS: a regression-based approach. Int J Geogr Inf Sci. 1997;11:699–718. [Google Scholar]
- Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. American Journal of Preventive Medicine. 2003;24:160–169. doi: 10.1016/s0749-3797(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- Chauhan AJ, Inskip HM, Linaker CH, Smith S, Schreiber J, Johnston SL, Holgate ST. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361:1939–1944. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Wright RJ, Baxter LK, Levy JI. Land use regression modeling of intra-urban residential variability in multiple traffic-related air pollutants. Environmental Health: A Global Access Science Source. 2008;7:17–17. doi: 10.1186/1476-069X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CT Dept. of Environmental Protection, B. o. A. M. [Accessed 15 December 2010];2002 Periodic Ozone and Carbon Monoxide Emissions Inventory. 2005 Available : http://www.ct.gov/dep/lib/dep/air/regulations/proposed_and_reports/att_g_2002_periodic_inventory.pdf.
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA: The Journal of the American Medical Association. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P, Wartenberg D. Spatial epidemiology: current approaches and future challenges. Environ Health Perspect. 2004;112:998–1006. doi: 10.1289/ehp.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English P, Neutra R, Scalf R, Sullivan M, Waller L, Zhu L. Examining associations between childhood asthma and traffic flow using a geographic information system. Environmental Health Perspectives. 1999;107:761–7. doi: 10.1289/ehp.99107761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumkin H. Environmental Health: from global to local. Jossey-Bass; San Francisco, CA: 2005. [Google Scholar]
- Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, Leaderer BP. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect. 2002;110:A781–786. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- Henderson SB, Beckerman B, Jerrett M, Brauer M. Application of Land Use Regression to Estimate Long-Term Concentrations of Traffic-Related Nitrogen Oxides and Fine Particulate Matter. Environ Sci Technol. 2007;41:2422–2428. doi: 10.1021/es0606780. [DOI] [PubMed] [Google Scholar]
- Holford TR, Ebisu K, McKay LA, Gent JF, Triche EW, Bracken MB, Leaderer BP. Integrated exposure modeling: a model using GIS and GLM. Stat Med. 2010;29:116–129. doi: 10.1002/sim.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST. Air pollution and health. Academic Press; San Diego, CA: 1999. [Google Scholar]
- Homer C, Huang C, Yang L, Wylie B, Coan M. Development of a 2001 national landcover database for the United States. Photogrammetric Engineering and Remote Sensing. 2004;70:829–840. [Google Scholar]
- Jerrett M, Arain A, Kanaroglou P, Beckerman B, Potoglou D, Sahsuvaroglu T, Morrison J, Giovis C. A review and evaluation of intraurban air pollution exposure models. J Expo Anal Environ Epidemiol. 2005;15:185–204. doi: 10.1038/sj.jea.7500388. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Price MR, Liu AH, Robinson JL. Morbidity patterns among low-income wheezing infants. Pediatrics. 2003;112:49–57. doi: 10.1542/peds.112.1.49. [DOI] [PubMed] [Google Scholar]
- Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117:43–53. doi: 10.1542/peds.2004-1714. [DOI] [PubMed] [Google Scholar]
- Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, McSharry JeJ-e, Platts-Mills TAE, Chapman MD, Bracken MB. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung R, Wong G, Lau J, Ho A, Chan JK, Choy D, Douglass C, Lai CK. Prevalence of asthma and allergy in Hong Kong schoolchildren: an ISAAC study. Eur Respir J. 1997;10:354–360. doi: 10.1183/09031936.97.10020354. [DOI] [PubMed] [Google Scholar]
- Marmot M. Social determinants of health inequalities. Lancet. 2005;365:1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- Medina-Ramon M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006;163:579–88. doi: 10.1093/aje/kwj078. [DOI] [PubMed] [Google Scholar]
- Mitchell A. The ESRI Guide to GIS Analysis. Vol. 2. ESRI Press; Redlands, CA: 2005. [Google Scholar]
- O’Neill MS, McMichael AJ, Schwartz J, Wartenberg D. Poverty, environment, and health: the role of environmental epidemiology and environmental epidemiologists. Epidemiology. 2007;18:664–668. doi: 10.1097/EDE.0b013e3181570ab9. [DOI] [PubMed] [Google Scholar]
- Palmes ED, Gunnison AF, DiMattio J, Tomczyk C. Personal sampler for nitrogen dioxide. Am Ind Hyg Assoc J. 1976;37:570–7. doi: 10.1080/0002889768507522. [DOI] [PubMed] [Google Scholar]
- Perrin JM, Homer CJ, Berwick DM, Woolf AD, Freeman JL, Wennberg JE. Variations in rates of hospitalization of children in three urban communities. N Engl J Med. 1989;320:1183–1187. doi: 10.1056/NEJM198905043201805. [DOI] [PubMed] [Google Scholar]
- Pershagen G, Rylander E, Norberg S, Eriksson M, Nordvall SL. Air pollution involving nitrogen dioxide exposure and wheezing bronchitis in children. Int J Epidemiol. 1995;24:1147–1153. doi: 10.1093/ije/24.6.1147. [DOI] [PubMed] [Google Scholar]
- Prescott SL. Effects of early cigarette smoke exposure on early immune development and respiratory disease. Paediatr Respir Rev. 2008;9:3–10. doi: 10.1016/j.prrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Adachi M, Lai CKW, Soriano JB, Vermeire PA, Weiss KB, Weiss ST. Worldwide severity and control of asthma in children and adults: The global Asthma Insights and Reality surveys. J Allergy Clin Immunol. 2004;114:40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Rosenlund M, Forastiere F, Stafoggia M, Porta D, Perucci M, Ranzi A, Nussio F, Perucci CA. Comparison of regression models with land-use and emissions data to predict the spatial distribution of traffic-related air pollution in Rome. J Expo Sci Environ Epidemiol. 2008;18:192–199. doi: 10.1038/sj.jes.7500571. [DOI] [PubMed] [Google Scholar]
- Ross Z, English PB, Scalf R, Gunier R, Smorodinsky S, Wall S, Jerrett M. Nitrogen dioxide prediction in Southern California using land use regression modeling: potential for environmental health analyses. J Expos Sci Environ Epidemiol. 2005;16:106–114. doi: 10.1038/sj.jea.7500442. [DOI] [PubMed] [Google Scholar]
- Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, Wilson K, Villareal M, Burkle J, Lockey J. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Salo PM, Xia J, Johnson CA, Li Y, Avol EL, Gong J, London SJ. Indoor allergens, asthma, and asthma-related symptoms among adolescents in Wuhan, China. Ann Epidemiol. 2004;14:543–50. doi: 10.1016/j.annepidem.2003.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and children’s health. Pediatrics. 2004;113:1037–43. [PubMed] [Google Scholar]
- Scott SC, Goldberg MS, Mayo NE. Statistical assessment of ordinal outcomes in comparative studies. J Clin Epidemiol. 1997;50:45–55. doi: 10.1016/s0895-4356(96)00312-5. [DOI] [PubMed] [Google Scholar]
- Skene KJ, Gent JF, McKay LA, Belanger K, Leaderer BP, Holford TR. Modeling effects of traffic and landscape characteristics on ambient nitrogen dioxide levels in Connecticut. Atmos Environ. 2010;44:5156–5164. doi: 10.1016/j.atmosenv.2010.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel DA. The myth of mild asthma. Ann Allergy Asthma Immunol. 2002;89:340–343. doi: 10.1016/S1081-1206(10)62030-3. [DOI] [PubMed] [Google Scholar]
- Su JG, Brauer M, Ainslie B, Steyn D, Larson T, Buzzelli M. An innovative land use regression model incorporating meteorology for exposure analysis. Sci Total Environ. 2008;390:520–529. doi: 10.1016/j.scitotenv.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Timonen KL, Pekkanen J. Air pollution and respiratory health among children with asthmatic or cough symptoms. Am J Respir Crit Care Med. 1997;156:546–552. doi: 10.1164/ajrccm.156.2.9608044. [DOI] [PubMed] [Google Scholar]
- United Nations. World Population Prospects: The 2000 Revision. United Nations; New York, NY: 2001. [Google Scholar]
- US Census Bureau. Census 2000, Summary File 1. US Census Bureau; Washington, DC: 2000. [Google Scholar]
- US Department of Commerce. American Housing Survey. US Department of Commerce, US Department of Housing and Urban Development; Washington D.C: 1997. [Google Scholar]
- Vlahov D, Galea S. Urbanization, urbanicity, and health. J Urban Health. 2002;79:S1–S12. doi: 10.1093/jurban/79.suppl_1.S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring MS, Siegel JA. Particle loading rates for HVAC filters, heat exchangers, and ducts. Indoor Air. 2008;18:209–24. doi: 10.1111/j.1600-0668.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology. 2007;21:8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89. doi: 10.1186/1471-2458-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.