Abstract
Periventricular leukomalacia (PVL) is the predominant pathology in premature infants, characterized by prominent cerebral white matter injury, and commonly caused by hypoxia–ischemia and inflammation. Activated microglia trigger white matter damage and play a major role in the development of PVL. Erythropoietin (EPO) and its derivative carbamylated erythropoietin (CEPO) have been shown to be neuroprotective in several brain disease models. Here we investigated whether EPO and CEPO could provide protection in mouse models of PVL induced by hypoxia–ischemia or hypoxia–ischemia-inflammation. We administered EPO or CEPO to mice with PVL, and found that both EPO and CEPO treatments decreased microglia activation, oligodendrocyte damage and myelin depletion. We also noted improved performance in neurological function assays. Inhibited disease progression in PVL mice by EPO or CEPO treatment was associated with decreased poly-(ADP-ribose) polymerase-1 (PARP-1) activity. PARP-1 activity was increased dramatically in activated microglia in untreated mice with PVL. Furthermore, we demonstrated that the neuroprotective properties of EPO and CEPO were diminished after PARP-1 gene depletion. The therapeutic doses of EPO and CEPO used in this study did not interfere with normal oligodendrocyte maturation and myelination. Together, our data demonstrate that EPO and CEPO are neuroprotective in cerebral white matter injury via a novel microglial PARP-1 dependent mechanism, and hold promise as a future treatment for PVL and other hypoxic–ischemic/inflammatory white matter diseases.
Keywords: Erythropoietin, Carbamylated erythropoietin, White matter injury, Oligodendrocyte, Microglia, PARP-1, Hypoxia–ischemia, Lipopolysaccharide, Periventricular leukomalacia
Introduction
Periventricular leukomalacia (PVL) is the most notable neuropathological lesion correlating with cerebral white matter impairment in premature infants (Khwaja and Volpe, 2008; Deng, 2010). Approximately 63,000 infants are born with a very low birth weight (≤ 1500 g) in the United States every year (Martin et al., 2008). Advances in medical care allow more infants to survive prematurity but the incidence of subsequent encephalopathy in long-term survivors has increased. Encephalopathy underlies the occurrence of cognitive deficits and cerebral palsy in premature infants with which PVL is predominantly correlated (Dammann et al., 2002; Platt et al., 2007; Volpe, 2009). The pathogenesis of PVL involves cerebral ischemia and inflammation, with the latter often due to maternal intrauterine or fetal systemic infections (Dammann et al., 2002; Goncalves et al., 2002). PVL comprises diffuse lesions in the cerebral white matter characterized by loss of premyelinating oligodendrocytes, astrogliosis and microglial infiltration (Volpe, 2003, 2005). The peak incidence of PVL occurs at 24–32 weeks of gestation, when rapid brain development occurs in humans. In PVL, damaged oligodendrocyte progenitors lack the capacity for differentiation and maturation, causing hypomyelination in the developing brain (Volpe, 2001; Segovia et al., 2008). Microglia become abundant during the third trimester, and play a crucial role in responding to ischemia and inflammation. Dramatic microglial activation occurs in PVL, is associated with oligodendrocyte injury and myelin disturbances, and contributes to neuronal excitotoxicity (Rivest, 2003; Billiards et al., 2006).
Experimental rodent PVL models have been developed to better our understanding of the mechanisms involved in this disorder. Brain development studies in neonatal humans and rodents provided evidence for the time course of oligodendrocyte maturation. In humans, oligodendrocyte differentiation and maturation starts in the third trimester, whereas in rodents it begins postnatally (Follett et al., 2004). This insight enables us to specifically elicit PVL-like lesions using in vivo mouse models. The most common methods for PVL include eliciting cerebral hypoxia–ischemia and administration of bacterial lipopolysaccharide (LPS) (Deng et al., 2008).
EPO, a 34 kDa pleiotropic cytokine, was first recognized for its hematopoietic properties. EPO and its surface receptor (EPOR) were detected in multiple tissues including kidney, liver, lung and brain (Marti et al., 1996; Sasaki, 2003). Recently, EPO was identified as a neurotrophic factor in several central nervous system disease models, independent of its erythropoietic properties (Brines et al., 2000; Buemi et al., 2003; Csete et al., 2004). CEPO, a modified version of EPO, was developed by carbamylating lysine residues of EPO. It is a nonerythropoietic EPO alternative that retains EPO's cytoprotective properties and avoids its erythropoietic side effects (Leist et al., 2004; Montero et al., 2007; Wang et al., 2007) and may prove to be a suitable treatment for PVL and other central nervous system diseases.
In the present study, we hypothesized that EPO and CEPO have similar protective effects in experimental models of PVL. We investigated whether EPO and CEPO could provide protection against PVL and improve behavioral function after cerebral white matter injury, and examined and compared potential mechanisms underlying these effects of the two agents. Our results demonstrate that EPO and CEPO use the poly-(ADP-ribose) polymerase-1 (PARP-1) pathway to provide neuroprotection and functional recovery in two mouse models of PVL.
Materials and methods
Mouse models of PVL
All procedures were performed in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals and were approved by the Animal Use and Care Committee of the University of California, Davis. Mice (C57BL/6, Jackson Laboratory, Bar Harbor, ME) were maintained in a temperature-controlled animal care facility under a 12-h light/dark cycle with a constant supply of food and water. We wanted to develop mouse models of PVL consistent with cerebral white matter injury in the human infant. Myelin basic protein (MBP) expression in the neonatal mouse brain is minimal prior to postnatal day 6 (P6). From P7 through adulthood, MBP expression progressively increases, indicating that the majority of cells in the white matter are immature oligodendrocytes at P6. Thus, we induced insults in P6 mouse pups using ischemia induced by unilateral carotid ligation (UCL) followedwith hypoxia (H/I), which resulted in selective injury to the periventricular white matter. Two mouse models of PVL were established using P6 mice. In one model, mice underwent UCL and hypoxia (hypoxia–ischemia model, H/I). In the other model, mice underwent UCL, hypoxia and received intraperitoneal (i.p.) injection of LPS (hypoxia–ischemia-LPS model, H/I/L). For both models, mice were anesthetized under ice (indirect cooling) and then underwent UCL followed by a 1-h recovery interval during which the pups were housed with the dam and kept on a thermal blanket to maintain body temperature at 33–34 °C. Next, the pups were placed in an airproof isolation chamber with 6.0% O2 for 35 min. In the H/I/L model, LPS (0111:B4, Sigma), a potent activator of inflammation and microglial activation, was given at a dose of 0.1 mg/kg body weight immediately after hypoxia–ischemia. After this procedure, pups were housed with the dam for intervals of 24, 48, 72 or 96 h, as required for further experiments.
EPO and CEPO treatments
In adult rodent ischemia models, EPO treatment provides neuroprotection and promotes functional recovery (Brines et al., 2000; Buemi et al., 2003). Thus, we tested whether EPO or CEPO treatment could afford protection in our postnatal PVL models. Three experimental groups were designed to evaluate EPO and CEPO effects in cerebral white matter injury. After completion of PVL procedures, the animals were randomly assigned to one of the three groups (n = 8 mice/group) and received i.p. injections of 10 µl phosphate-buffered saline (PBS), or EPO (40 µg/kg or 5000 IU/kg; Invitrogen, Camarillo, CA), or CEPO (40 µg/kg or 5000 IU/kg; a generous gift from Warren Pharmaceuticals, Ossining, NY and H. Lundbeck A/S, Denmark). The dosing regimen was based on previously published studies regarding EPO effects on brain injury (Brines et al., 2000; Villa et al., 2003; Wang et al., 2004; Wei et al., 2006).
Tissue preparation
For immunohistochemistry, animals were deeply anesthetized and transcardially perfused with 0.1 M PBS (pH 7.4), followed by 4% paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed for 4 h in the same fixative, followed by 24-h incubation in 30% sucrose (made in PBS) at 4 °C, and then embedded in Tissue-Tek® O.C.T.™ freezing media (Sakura Finetek, Torrance, CA). Coronal sections were cut at 14 µm thickness using a cryostat, and every 4th section was collected.
For total RNA isolation, the corpus callosum tissue was removed from gross coronal sections at approximately Bregma 0 mm and −2 mm. Sagittal cuts were then made through the cingulum, medial to each lateral ventricle, followed by a cut above and below the corpus callosum to remove the majority of cortex and hippocampus. The tissue was immediately submerged in liquid nitrogen and stored at −80 °C until use.
Reverse transcriptase real-time polymerase chain reaction (RT-PCR)
In order to investigate the roles of EPO and EPOR in cerebral white matter injury, we first examined the adaptive response of endogenous EPO and EPOR gene expression levels after H/I using real-time PCR. Total RNA was extracted from manually homogenized corpus callosum tissue with the RNeasy® Lipid Tissue Mini Kit (Qiagen, Valencia, CA). Messenger RNA, in 1 µg total RNA, was reverse transcribed with the Taqman® reverse transcription reagents (Applied Biosystems™, Carlsbad, CA). Obtained cDNA was quantified by real time PCR performed on the LightCycler® System (Roche Diagnostics), using the QuantiTect SYBR® Green PCR Kit and QuantiTect Primer Assays, which includes proprietary sequences (Qiagen, Valencia, CA), for EPO, EPOR and GAPDH analysis. Results of the targeted mRNA were normalized against the internal control, GAPDH.
Immunohistochemistry and histological analysis
Sections were permeabilized with 0.1% Triton X-100 and 5% goat serumin PBS for 1 h at room temperature. Goat serum was used to block nonspecific antibody binding. Sections were rinsed in PBS and incubated with primary antibodies overnight at 4 °C. The following primary antibodies were used: mouse monoclonal MBP (1:1000; SMI-99, Sternberger Monoclonals, Baltimore, MD), rat anti-mouse GFAP (1:1000, Chemicon, Temecula, CA); mouse monoclonal O1 (1:100, Chemicon), rat anti-mouse CD68 (1:100, Serotec, Raleigh, NC); mouse monoclonal PARP-1 (1:100, Trevigen, Gaithersburg, MD) and mouse monoclonal poly-(ADP-ribose) (PAR; 1:100, Trevigen). Following primary antibody incubation, sections were rinsed 3 times with PBS, and then fluorescent-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) appropriate to the primary antibody species were applied to sections for 3 h at room temperature. Sections were coverslipped with anti-fade DAPI (4′, 6-diamidino-2-phenylindole) Fluoromount-G medium (Southern Biotechnology, Birmingham, AL) to stain nuclei. Fluorescent images were obtained on an Olympus BX61-DSU microscope (Olympus Corp., Melville, NY), with a digital camera (Hamamatsu Photonics, Shizuka, Japan) and Slidebook 4.2 software (Olympus Corp., Melville, NY).
To evaluate white matter injury, we used a previously established (Follett et al., 2004) semi-quantitative scoring scale (0–5) based on the absence of MBP: 0 = no MBP loss; 1 = some loss of processes perpendicular to capsule; 2 = moderate loss of processes; 3 = complete loss of processes with intact capsule; 4 = loss of processes with thinning or breaks in capsule; and 5 = loss of processes with complete loss of capsule (Follett et al., 2000, 2004). O1 was used as a complementary marker for evaluating white matter injury, and also scored on a 0–5 scale, with 0 being no loss, and 5 being virtually complete loss. Cell counting was performed to evaluate changes in all other immunohistochemical markers, including GFAP, CD68, and PARP-1. We counted all positive cells present within the field of view at 200× magnification in the white matter track overlying the hippocampus for six fields of each view/section and six evenly spaced coronal sections per animal. Cell counting was performed in hemispheres ipsilateral and contralateral to the ligation to take into account developmental variations between mice, and all tests were performed by an observer blinded to treatment groups or the side of carotid artery ligation. Five to eight animals per group were included in all analyses.
TUNEL assay
Cell death was assessed using the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) kit (Promega, Madison, WI). Briefly, thin sections were permeabilized with proteinase K (20 µg/ml) for 20 min. The DNA fragments of dead cells were end-labeled by incubation in fluorescent-conjugated nucleotide mix through the action of terminal deoxynucleotidyl transferase at 37 °C for 1 h. Labeled cells were identified by green fluorescence. Negative controls without the transferase were included in each assay. For quantification, we counted the number of TUNEL-positive cells in the whole white matter track in 5 sections per animal at 200× magnification.
Neurological deficits assessment
Cerebral white matter injury in premature human infants is the predominant form of brain lesion leading to cerebral palsy and is characterized by motor deficits which include spastic diplegia and hemiplegia. These impairments appear early in life and usually persist through adulthood. Mice exposed to cerebral insults display motor deficits according to the severity and location of lesions and generally undergo some level of spontaneous recovery. We tested whether treatment with EPO or CEPO could improve motor function after white matter injury. After induction of white matter injury and EPO/CEPO/vehicle treatment, the pups were raised with their dams for 2 weeks, until P21. At P21, motor strength and neurological deficits were evaluated to determine if treatment correlated with functional changes. Mortality and change of body weight were also observed and documented. Animals were scored as follows: (1) spontaneous activity in cage was observed for 5 min (score 0–3), (2) symmetry in the movement of four limbs was tested by holding a mouse in the air by the tail to examine whether the contralateral limbs extend less or more slowly than the ipsilateral side (score 0–3), (3) symmetry in the outstretching of forelimbs was examined by holding a mouse by the tail and placing the forelimbs on the edge of a wire cage to make the mouse move on forelimbs (score 0–3), and (4) climbing on the wall of a wire cage. We set the slope angle at 45° and the slope length at 0.1 m, which was challenging for P11–P21 injured PVL mice but allowed most normal mice (negative controls) to pass easily (score 0– 3, 0 for falling down right after being placed on the slope, 1 for hanging on or circling instead of climbing, 2 for climbing up slowly without sliding, 3 for running up). We performed three trials on each animal. The final score given to each animal was the summation of the four test scores. The minimum score is 0 (severe injury) and the maximum is 12 (no injury) (Garcia et al., 1995; Follett et al., 2004).
Statistical analysis
All statistical analyses were performed using SigmaStat (Systat Software, Inc, Chicago, IL). Data are presented as mean ± SEM. Comparisons between experimental groups were analyzed using one-way ANOVA followed by Tukey post hoc analysis. Statistical significance was defined at p<0.05.
Results
Endogenous EPO and EPOR messenger RNA levels transiently increased in PVL mice before demyelination
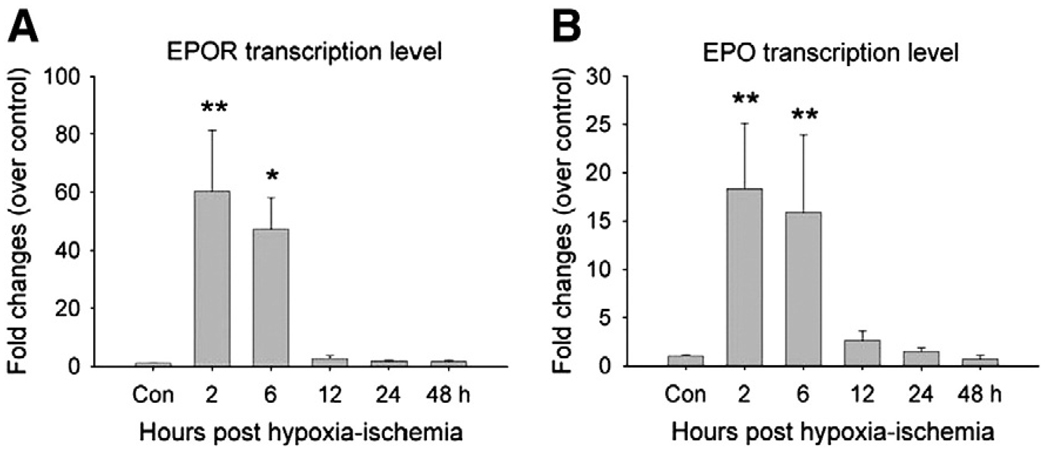
We first examined the adaptive response of endogenous EPO and EPOR gene expression levels after H/I using real-time PCR. The EPO and EPOR genes are expressed at different levels in different brain areas, so we restricted our analysis to the white matter area. We found very low levels of EPO and EPOR in the white matter area of normal controls. Next, we examined whether EPO and EPOR gene expression levels are altered after H/I. We collected white matter tissue from H/I animals 2, 6, 12, 24, or 48 h after injury. We observed peak EPOR transcription levels at 2 h after injury, which increased 60.3 ± 21.02 fold (ncontrol = 4, n2 h = 6, p<0.01). EPOR levels remained high at 6 h, increased by 47.2 ± 10.8 fold (ncontrol = 4, n6 h = 6, p<0.05), and then decreased back to normal levels by 12 h (Fig. 1A). EPO transcription levels increased 18.3 ± 6.7 fold at 2 h (ncontrol = 4, n2 h = 6, p<0.01) and 15.8 ± 8.0 fold at 6 h (ncontrol = 4, n6 h = 6, p<0.01), then decreased (Fig. 1B).
Fig. 1.
Transcription levels of EPOR and EPO transiently increase after H/I injury. White matter injury was induced by UCL and hypoxia exposure (H/I) and corpus callosum tissues were collected for real-time PCR evaluation. (A) The corpus callosum showed an increase of EPOR transcription level at 2 h and 6 h after H/I (ncontrol = 4, n2 h = 6, n6 h = 6; **, p<0.01; *, p<0.05). (B) After H/I, the transcription level of EPO in corpus callosum increased at 2 h and 6 h post-injury (ncontrol = 4, n2 h = 6, n6 h = 6; **, p<0.01).
Hypoxia–ischemia and inflammation induce selective neonatal white matter injury
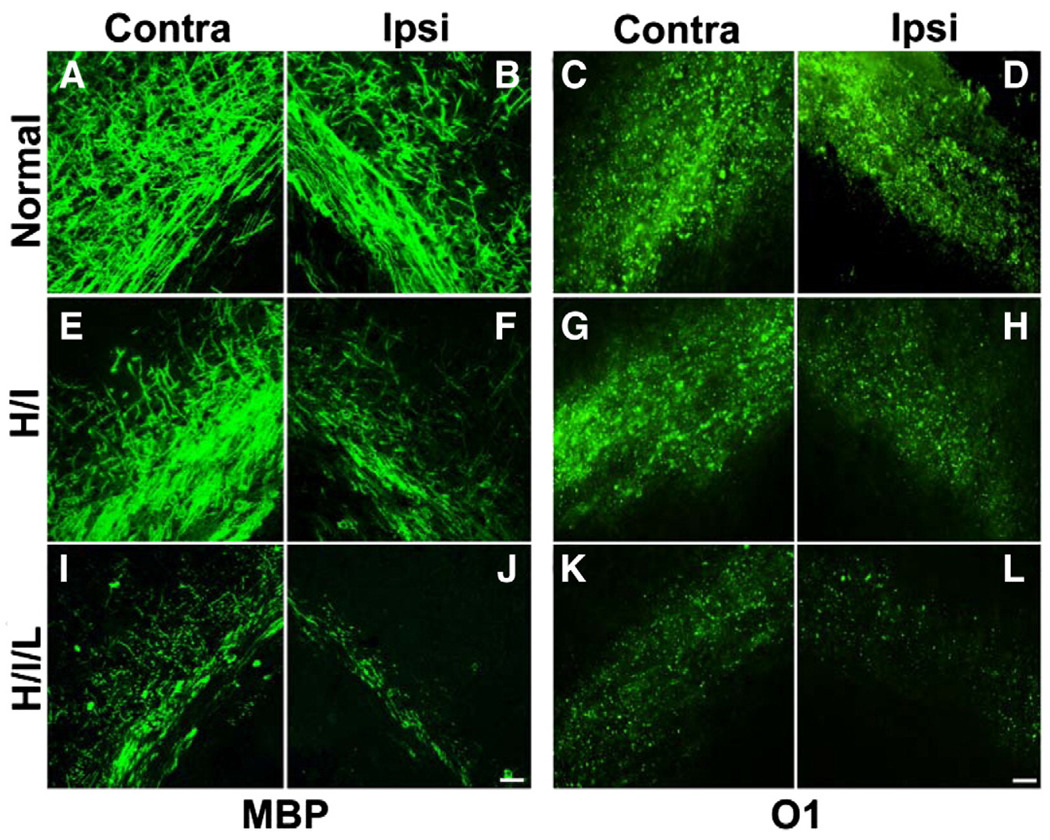
We induced insults in P6 mouse pups using UCL followed with hypoxia (H/I), which resulted in specific injury to the periventricular white matter. Immunostaining for MBP and O1 antibodies was performed to evaluate the severity of white matter injury 96 h after insult. In H/I mice, MBP and O1 expression decreased significantly in the ipsilateral white matter (Figs. 2F and H), while the contralateral side showed minimal changes (Figs. 2E and G) relative to normal mice (Figs. 2A–D). We also used another method to induce neonatal white matter injury, in which pups underwent UCL combined with hypoxia exposure and LPS injection at P6 (H/I/L). LPS elicits a strong inflammatory response in rodents, and was expected to promote microglial activation and exacerbate injury. In the H/I/L model, inflammation was more predominant than with H/I alone. In H/I/L mice, MBP and O1 expression was decreased dramatically in white matter on the ipsilateral side (Figs. 2J and L) compared with normal mice (Figs. 2B and D). MBP and O1 expression was reduced in the white matter on the contralateral side as well (Figs. 2I and K), although to a lesser extent than on the ipsilateral side. In general, H/I/L mice showed exacerbated MBP and O1 depletion compared to H/I mice. Furthermore, H/I/L mice showed bilateral lesions rather than the unilateral lesion observed in H/I mice.
Fig. 2.
Oligodendrocyte loss and myelin depletion occur after H/I or H/I/L. White matter injury was induced by UCL and hypoxia exposure (H/I) or UCL combined with hypoxia exposure and LPS administration (H/I/L). (A–B) The corpus callosum of normal mice exhibited strong MBP immunoreactivity. After H/I, MBP expression decreased substantially in the ipsilateral side with a slight reduction in the contralateral side as well (E–F). After exposure to H/I/L, MBP-immunoreactivity diminished substantially in both the ipsilateral and contralateral white matter track (I–J). Similarly, normal mice showed substantial and equal O1 immunoreactivity in the corpus callosum of both hemispheres (C–D) while mice exposed to H/I exhibited a dramatic reduction in O1 staining in the ipsilateral corpus callosum (G) but no change in O1 staining in the contralateral white matter (G). Mice exposed to H/I/L displayed a dramatic reduction in O1 immunoreactivity in both white matter tracks (K–L). Scale bar, 10 µm. Contra = contralateral, Ipsi = ipsilateral.
Time course of induced white matter injury in PVL mouse models
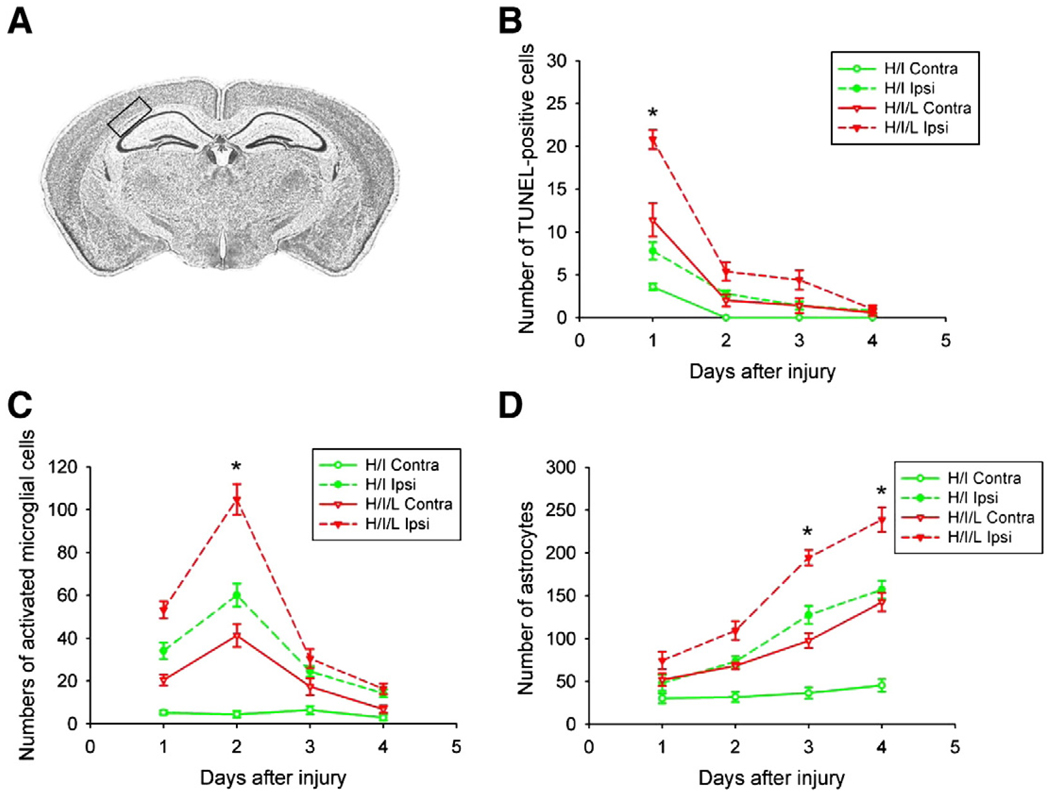
We next sought to determine the time course of PVL pathogenesis by evaluating cell death, reactive gliosis and microglial activation, which commonly occur as a result of cerebral ischemia. To elucidate the disease pathology during PVL, animals were sacrificed 24, 48, 72, or 96 h after injury. In the pre-H/I/L and sham-treated control animals, and the H/I mice under the conditions of our models, there was no apparent injury contralateral to the lesion. We used the TUNEL assay to evaluate cell death. We found the most TUNEL-positive cells present 24 h after injury (Fig. 3B). As anticipated, the ipsilateral white matter of H/I/L mice contained the most TUNEL-positive cells, significantly more than the contralateral side or either side of the H/I mice (Fig. 3B, p<0.01). To identify activated microglia, we performed immunohistochemistry with an antibody to CD68. We counted the number of CD68-positive cells present within the white matter track after H/I or H/I/L. We found increased microglial presence, which peaked 48 h after injury and was highest in the ipsilateral white matter of H/I/L mice (Fig. 3C, p<0.01). Next, we evaluated reactive gliosis by performing immunostaining with an antibody to GFAP. We observed a steady increase in the number of GFAP-positive cells for 4 days after injury with GFAP expression peaking at 4 days post-insult (Fig. 3D, p<0.05). GFAP expression was highest in the ipsilateral white matter of H/I/L mice (Fig. 3D), with noticeable astroglial hypertrophy occurring in this area.
Fig. 3.
Time course of PVL progression. We performed immunohistochemical analysis at 24, 48, 72, and 96 h after PVL induction to determine the progression of injury pathology. (A) Cresyl violet staining of a coronal brain section at the level of the hippocampus, where analyses occurred. The black box outlines the white matter area used for all immunohistochemical data analysis. (B) We counted the number of TUNEL-positive cells present in the white matter and found their presence peaked in the contralateral white matter 24 h after H/I/L (*, p<0.05). (C) We counted CD68-positive cells to determine peak microglial recruitment and found it to occur 2 days after injury, with significant increases in the contralateral white matter of H/I/L mice (*, p<0.05). (D) We used GFAP as a marker for reactive astrocytes and observed reactive gliosis peaking in the contralateral white matter 96 h after H/I/L (*, p<0.05). In the H/I and H/I/L models, the time courses of TUNEL, microglial recruitment, and astrocyte activation followed the same pattern.
EPO and CEPO attenuate cerebral white matter injury in mouse models of PVL
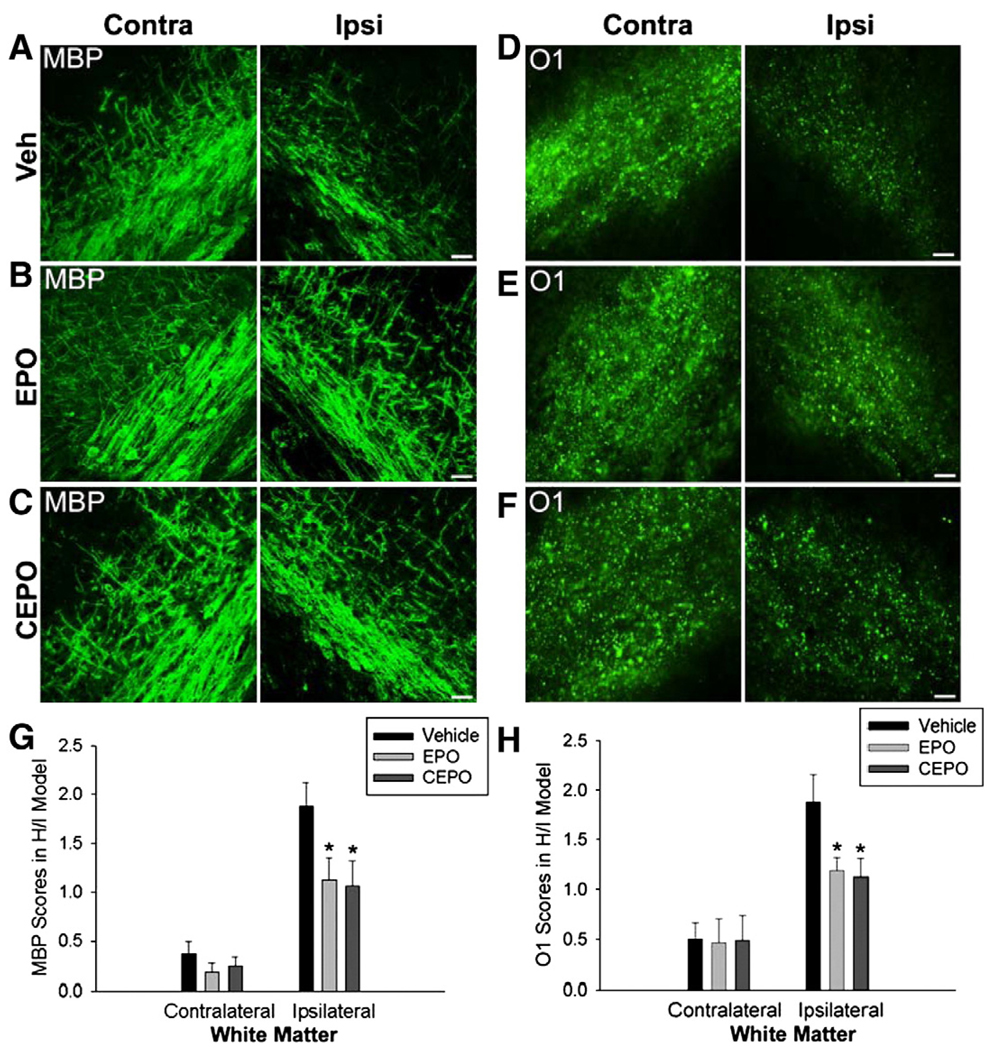
Mice received a single i.p. injection of EPO (40 µg/kg), CEPO (40 µg/kg) or vehicle (10 µl) immediately after H/I or H/I/L and were evaluated 96 h later (n = 8/group). We evaluated white matter injury severity after treatment by scoring MBP and O1 immunohistochemistry staining. Mice underwent H/I and then were administered a single dose of EPO, CEPO or vehicle and were evaluated 96 h later (n = 8/group). In the H/I model, the contralateral white matter track is not afflicted and lesions are generally less severe in the ipsilateral white matter than in H/I/L. We observed no difference in MBP staining in the contralateral white matter of mice exposed to H/I with or without EPO or CEPO treatment (Figs. 4A–C). To the contrary, we observed enhanced MBP staining in the ipsilateral white matter of H/I mice treated with EPO or CEPO compared with vehicle-treated H/I mice (Figs. 4A–C). Using the lesion severity scale, we noted a significant decrease in lesion severity based on MBP immunohistochemistry after treatment with EPO or CEPO as compared with vehicle-treated mice (Fig. 4G; p<0.05). Similarly, we saw no difference in O1 staining in the contralateral white matter but observed increases in the ipsilateral white matter track after EPO or CEPO treatment (Figs. 4D–F). Quantification of lesion scoring confirmed these observations (Fig. 4H; p<0.05).
Fig. 4.
EPO or CEPO protect against H/I cerebral white matter injury. UCL and hypoxia resulted in selective white matter injury in only the ipsilateral white matter area. Lesion severity was scored (0–5) based on the degree of MBP and O1 depletion. (A) At 96 h after lesion induction, depletion of MBP was observed in ipsilateral white matter, but not in the contralateral side. (B) Systemic treatment with EPO (40 µg/kg, i.p.) attenuated MBP loss in the ipsilateral side but had no effect on the uninjured contralateral side. (C) Intraperitoneal administration of CEPO (40 µg/kg) also attenuated MBP depletion in the ipsilateral white matter. (D) Immature oligodendrocyte marker O1 was also utilized to evaluate white matter injury at 96 h after insult. After H/I, O1 labeling was reduced in the ipsilateral white matter, but not in the contralateral side. (E) EPO treatment ameliorated loss of O1 expression in the ipsilateral cerebral white matter. (F) CEPO treatment also restored O1 expression in the ipsilateral cerebral white matter. (G) Quantitative scores from 0 to 5 were used to indicate severity of myelin depletion and oligodendrocyte loss. We found that EPO or CEPO treatment after H/I significantly improved MBP lesion severity scores (*p<0.05). (H) With either EPO or CEPO treatment, quantification confirmed a significant reduction in O1 scored lesion severity in the ipsilateral hemisphere, compared with vehicle-control mice (*p<0.05). Scale bar, 10 µm. Veh = vehicle.
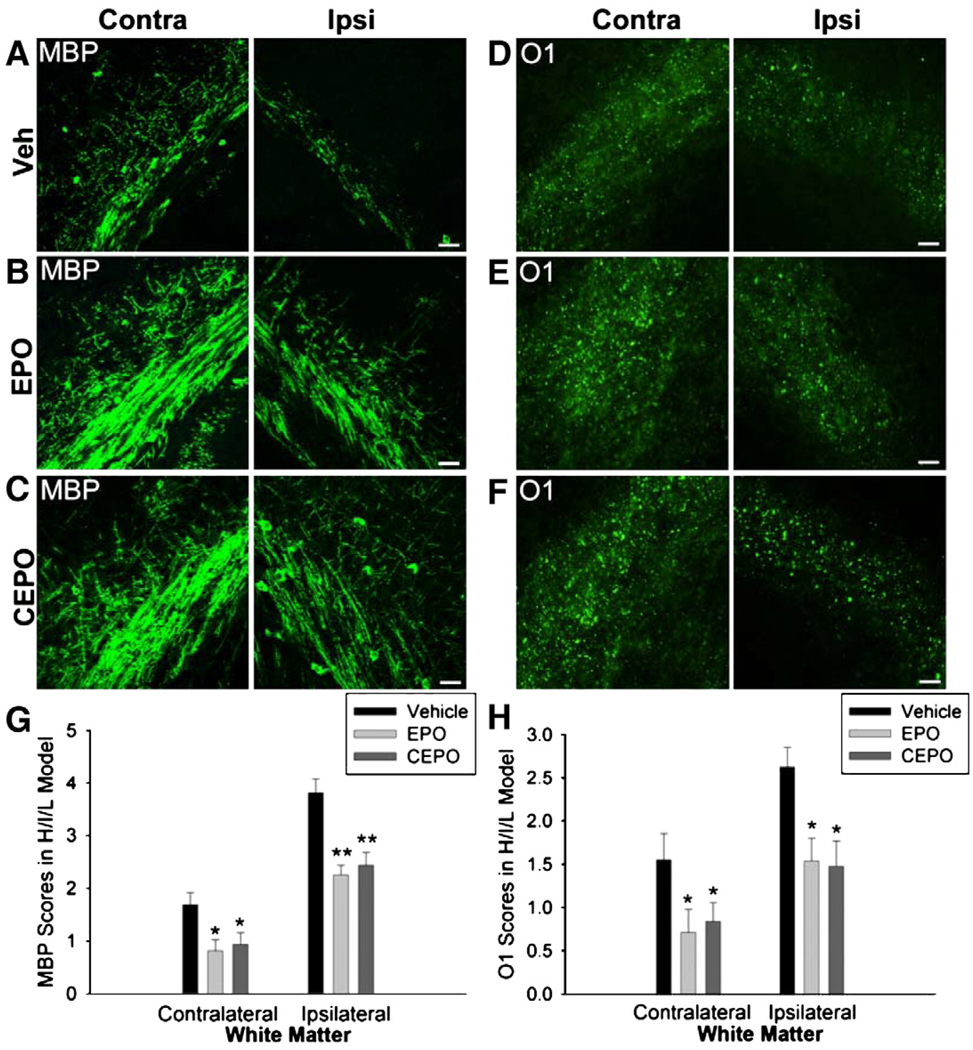
Next, we evaluated whether EPO or CEPO treatment could afford similar protection after H/I and LPS treatment. Mice exposed to H/I/L that received EPO or CEPO treatment showed a marked reduction in lesion severity as demonstrated by enhanced MBP staining compared with vehicle-treated mice (Figs. 5A–C). Quantification revealed a significant decrease in lesion severity on both the contralateral (Fig. 5G; p<0.05) and ipsilateral sides (Fig. 5G; p<0.01) after EPO or CEPO treatment compared with vehicle. In addition, EPO or CEPO treated H/I/L mice exhibited increased O1 staining in the white matter track compared with vehicle treated H/I/L mice (Figs. 5D–F). We quantified lesion severity using O1 immunohistochemistry and discovered a significant decrease in severity scores in both the ipsilateral and contralateral white matter tracks after EPO or CEPO treatment compared with controls (Fig. 5H; p<0.05). Together, these data suggest that EPO or CEPO treatment provide significant protection for neonatal PVL with or without systemic inflammation.
Fig. 5.
EPO or CEPO protect against white matter injury in H/I/L. UCL with hypoxia and LPS injection caused selective injury in both the ipsilateral and contralateral white matter. (A) At 96 h after insult, MBP depletion was observed in cerebral white matter of PVL mouse pups. (B, C) After injury, EPO or CEPO treatment attenuated MBP loss in both ipsilateral and contralateral sides. (D) After H/I/L, O1 expression was reduced in the white matter of both hemispheres. (E, F) Treatment with EPO or CEPO reduced O1 depletion. (G) Quantification revealed significant protection of MBP cells after treatment with EPO or CEPO in the contralateral white matter (*p<0.05) and ipsilateral white matter (**p<0.01). (H) Both EPO and CEPO treatments increased O1 expression in the injured white matter (*p<0.05). Scale bar, 10 µm.
EPO and CEPO improve neurobehavioral performance
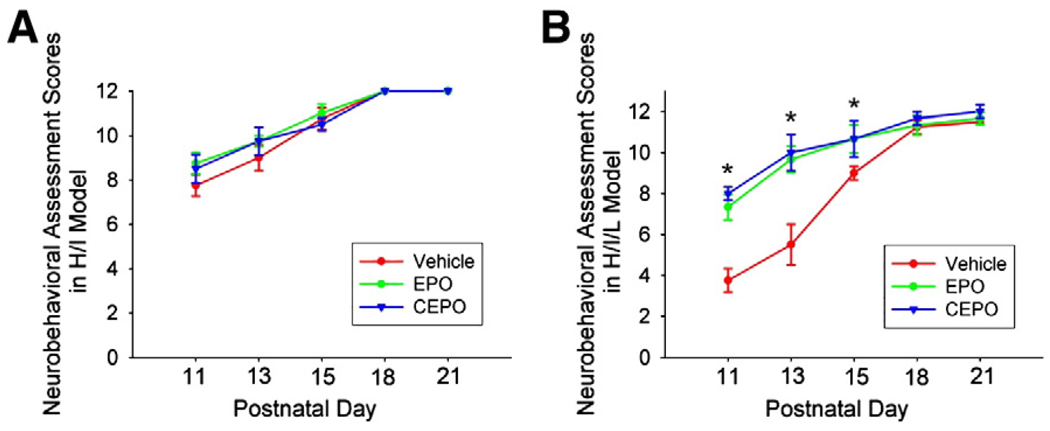
Next, we tested whether treatment with EPO or CEPO could improve motor function after white matter injury. Mice were exposed to H/I or H/I/L, and then randomly assigned to EPO or CEPO or vehicle groups (n = 5/group). We performed multiple motor deficit assessments every other day from P11 through P21. We found no significant difference in mortality rate or body weight change between the experimental groups. We evaluated muscle strength and movement coordination by observing spontaneous activity and the ability to climb a wire wall sloped at a given angle (Follett et al., 2004). Mice exposed to H/I or H/I/L show more severe motor dysfunction on the contralateral limbs and less muscle tone (Tomimatsu et al., 2002; Follett et al., 2004). We observed limb use symmetry and muscle strength and found that the contralateral limbs showed less frequent and weaker movements than ipsilateral limbs in H/I and H/I/L mice. Neurobehavioral scores were assigned based on performance in these tests. We observed no significant difference between treatment groups in mice exposed to H/I (Fig. 6A). Mice that underwent H/I/L and were treated with EPO or CEPO showed a significant improvement in neurobehavioral scores within the first 10 days after injury (Fig. 6B, p<0.05). After P16 we observed no significant difference between treatment groups, likely due to spontaneous recovery and normal brain development.
Fig. 6.
EPO and CEPO improve behavioral performance after H/I/L injury. Mice underwent H/I or H/I/L and were treated with EPO (40 µg/kg, i.p.), CEPO (40 µg/kg, i.p.), or vehicle. Animals were tested at distinct time points from P11 to P21. (A) In mice exposed to H/I, we observed no significant difference in motor functions between treatment groups. (B) After exposure to H/I/L, mice treated with EPO or CEPO showed increased neurobehavioral scores up to 10 days post-injury compared with vehicle treated mice (*, p<0.05).
EPO and CEPO prevent cell death in white matter injury
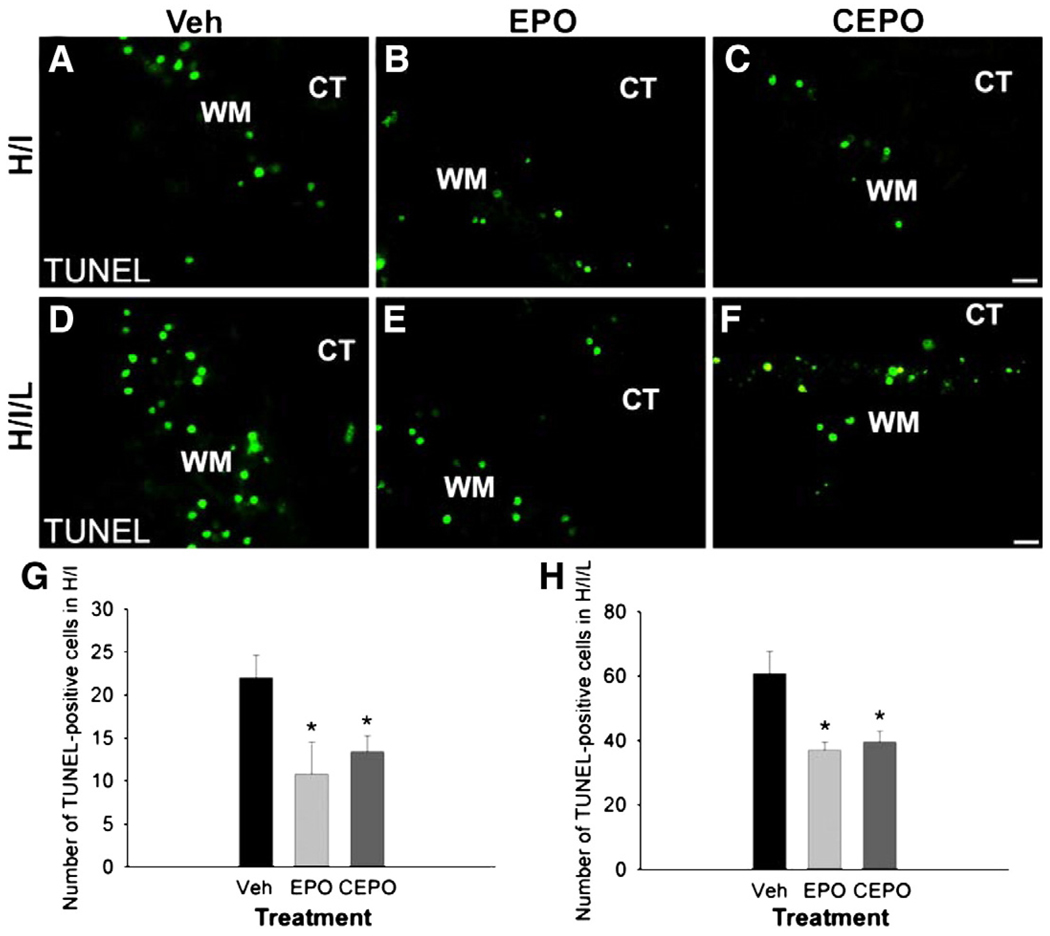
We noted diminished lesion severity after treatment with EPO or CEPO and sought to determine whether this was due to decreased cell death in the white matter track. We evaluated cell death 24 h after injury by performing the TUNEL assay, which identifies DNA strand breaks. At 24 h after H/I, we observed a decrease in TUNEL positive staining with EPO or CEPO treatment (Figs. 7A–C). We counted the number of TUNEL positive cells in the white matter track and found approximately half as many cells present in the EPO or CEPO treated groups as in the vehicle-treated H/I mice (Fig. 7G; p<0.05, n = 5 mice/group). Similarly, we noted a reduction in TUNEL immunoreactivity in H/I/L mice treated with EPO or CEPO compared to vehicle-treated mice (Figs. 7D–F). This decrease was not as dramatic as with H/I mice, however EPO or CEPO H/I/L mice had significantly fewer TUNEL positive cells than vehicle treated mice (Fig. 7H; p<0.05; n = 5 mice/group). Together, these data suggest that EPO or CEPO treatment provides protection after white matter injury by reducing cell death in the white matter track.
Fig. 7.
EPO and CPEO decrease white matter apoptosis after neonatal cerebral ischemia. Mice underwent H/I or H/I/L and received i.p. injections of EPO, CEPO or vehicle immediately after the induction of injury. We evaluated TUNEL immunoreactivity 24 h after treatment. TUNEL+ apoptotic cells were identified and counted in the white matter area. In both the H/I model (A–C) and the H/I/L model (D–F), EPO and CEPO reduced the appearance of TUNEL-positive cells. (G, H) We counted the TUNEL-positive cells and confirmed their number was significantly reduced in EPO or CEPO treatment groups compared with control groups (*, p<0.05). Scale bar, 10 µm. WM, white matter. CT, cortex.
EPO and CEPO inhibit microglial activation in inflammatory white matter injury
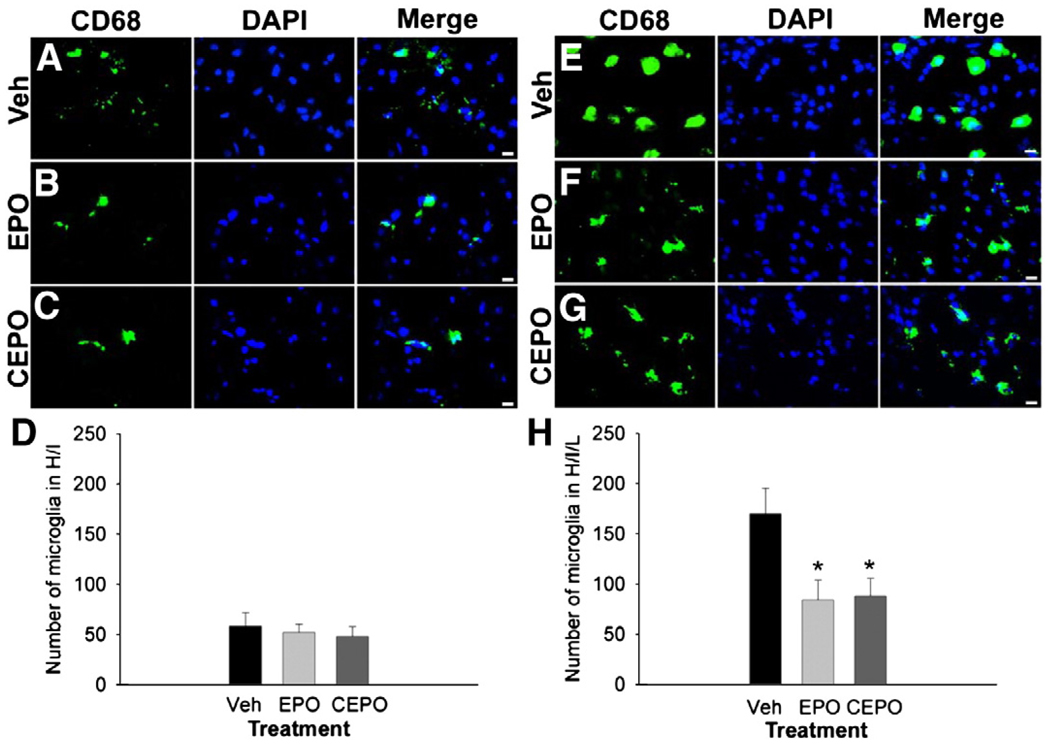
Microglia are resident macrophage lineage cells in the CNS which, after activation, function as antigen-presenting cells and promote inflammation (Olson and Miller, 2004). Microglial activation is a key component of PVL and may interrupt oligodendrocyte maturation and myelination. In our mouse models of PVL, we noted the peak time point for microglial recruitment was 48 h after insult (Fig. 3C). Thus, we evaluated whether EPO or CEPO treatment decreased microglial recruitment after H/I or H/I/L. We evaluated microglial activation at 48 h post-injury by performing immunohistochemistry for the macrophage marker CD68 and counting CD68-positive cells in the white matter. Numerous CD68-positive cells were identified 48 h after H/I and treatment with EPO or CEPO did not appear to alter their presence (Figs. 8A–C). Cell counting confirmed that EPO or CEPO treatment did not diminish the number of CD68-positive cells in the white matter after H/I (Fig. 8D, n = 5 mice/group). We observed a huge spike in the presence of CD68-positive cells in the white matter of H/I/L mice (Fig. 8E) compared with H/I alone (Fig. 8A). In contrast from H/I mice, we noted a decrease in CD68-positive cells in the white matter of H/I/L mice treated with EPO or CEPO (Figs. 8F and G). Quantification revealed that approximately twice as many CD68-positive cells were present in the white matter of H/I/L mice (Fig. 8H) as compared to H/I mice (Fig. 8D). In addition, EPO or CEPO treatment drastically reduced the number of CD68-positive cells in H/I/L mice Fig. 8H; p<0.05; n = 5 mice/group). These data suggest that EPO and CEPO may play a role in inhibiting the inflammatory response in white matter injury.
Fig. 8.
EPO and CEPO decrease microglial activation after white matter injury. We performed immunohistochemistry for the microglial marker CD68 48 h after H/I or H/I/L on mice treated with. vehicle, EPO (40 µg/kg, i.p.) or CEPO (40 µg/kg, i.p.). (A–D) H/I mice exhibited CD68+ activated microglia in the white matter. Treatment with EPO (B) or CEPO (C) did not diminish the presence of these cells compared with vehicle-treated mice (D). (E–H) In contrast, H/I/L mice showed significant microglial recruitment and CD68-immunoreactivity in the white matter (E), and treatment with EPO (F) or CEPO (G) significantly reduced the number of CD68+ cells. Quantification in the white matter track confirmed this (H, *, p<0.05). Scale bar, 10 µm.
EPO and CEPO do not reduce reactive astrogliosis after cerebral white matter injury
Astrocytes are present in the developing white matter and become activated in response to injury such as PVL. We noted that astroglial activation started to increase 72 h after H/I or H/I/L as seen by enhanced GFAP expression (Fig. 3D). EPO or CEPO treatment reduced microglial activation and cell death in our PVL models, so we next examined whether these treatments could also reduce injury-induced astrogliosis. We examined GFAP expression in the white matter after treatment with EPO or CEPO and found no obvious difference between groups after H/I or H/I/L (data not shown).
EPO and CEPO inhibit PARP-1 activity in cerebral white matter injury
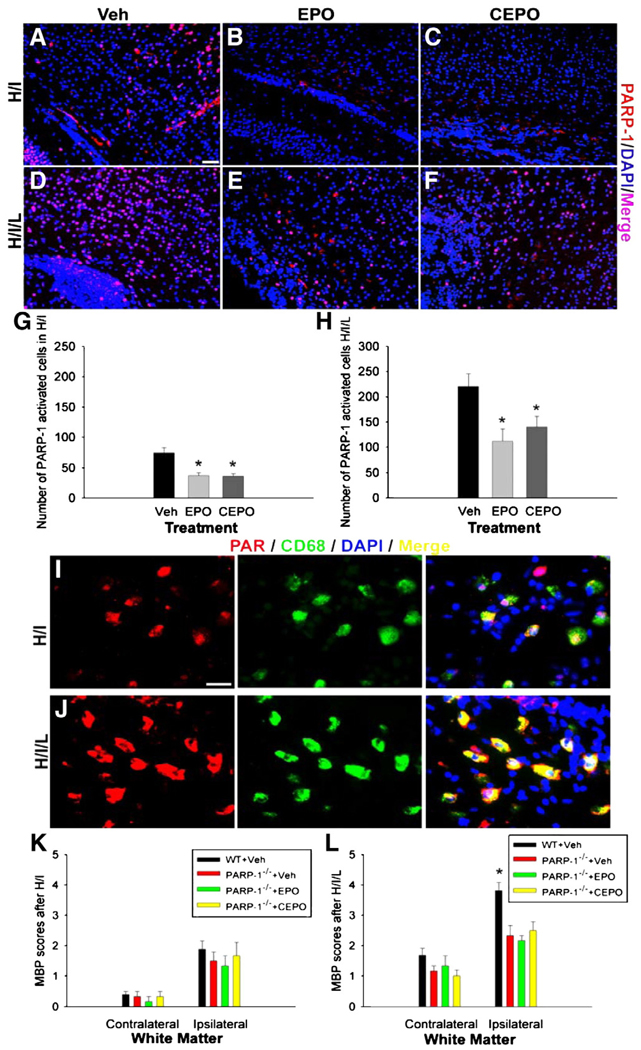
PARP-1 is activated in response to DNA damage and functions in DNA replication, transcription and repair (Virág and Szabó, 2002; Yu et al., 2003). PARP-1 also acts as a co-activator of transcriptional factors such as nuclear factor-κB (NF-κB) which regulates many aspects of the inflammatory response (Chiarugi and Moskowitz, 2003; Nakajimaet al., 2004). PARP-1 activation occurs in microglial cells in several cerebral ischemic models (Kauppinen and Swanson, 2005; Hamby et al., 2007). To study whether EPO or CEPO has an effect on PARP-1 activation, especially in activated microglia, antibodies to PARP-1 and PAR, an enzymatic product of PARP-1, were used to determine PARP-1 activity at 48 h after H/I or H/I/L. We noticed that PARP-1 immunoreactivity increased after H/I (Fig. 9A), and this appeared attenuated after treatment with EPO or CEPO (Figs. 9B and C). We counted PARP-1 immunoreactive cells in the white matter track overlying the hippocampus and found a significant decrease of PARP-1 positive cells after treatment with EPO or CEPO(Fig. 9G; p<0.05; n = 5 mice/group). When we examined PARP-1 immunoreactivity after H/I/L injury, we noted a dramatic decrease in its presence in EPO or CEPO treated mice (Figs. 9E and F) compared to vehicle-treated mice (Fig. 9D). Cell counting revealed that the number of PARP-1 immunoreactive cells was doubled in H/I/L vehicle-treated mice (Fig. 9H) compared to H/I vehicle-treated mice (Fig. 9G). In addition, we noted a significant decrease in PARP-1 positive cells in the white matter of H/I/L mice after treatment with EPO or CEPO (Fig. 9H; p<0.05; n = 5 mice/group). Next, we examined which cell type predominantly activated PARP-1 by co-labeling for PAR, as a read-out of PARP-1 activity, and cell-specific markers. We observed PAR accumulation in white matter after injury and found that most positive cells double-labeled with the microglial marker CD68 (Figs. 9I and J). These data suggest that microglial PARP-1 activity may play a role in PVL pathogenesis. Furthermore, these data demonstrate a novel mechanism for EPO and CEPO neuroprotection, in which these drugs attenuate injury by decreasing microglial PARP-1 activation. To further investigate the protective mechanisms of EPO and CEPO against PVL, we exposed neonatal PARP-1−/− mice (29S-Adprt1tm1Zqw strain, Jackson Laboratory, Bar Harbor, ME) to H/I or H/I/L and treated them with EPO or CEPO using the same protocol as aforementioned (n = 4/group). We used the same MBP scale as above to score the severity of white matter injury. After H/I insult, PARP-1−/− mice displayed similar MBP disruptions as wild-type mice and treatment with EPO or CEPO did not alter lesion severity in these mice (Fig. 9K). PARP-1−/− mice exposed to H/I/L showed a slight decrease in lesion severity by MBP scoring in the contralateral white matter compared to wild-type mice (Fig. 9L). Interestingly, we found that PARP-1−/− mice showed attenuated white matter injury in the ipsilateral side after H/I/L compared with wild-type mice, despite using the same methods as were used in the C57BL/6 strain (Fig. 9L). Contrary to our findings with C57BL/6 mice, PARP-1−/− mice exposed to H/I/L showed no further reduction in lesion severity after treatment with EPO or CEPO. Together, these data indicate that the EPO and CEPO protective effects rely on inhibition of PARP-1 activity. Although PARP-1 effects are well known to be gender-dependent in brain injury models in adult animals, we found that PARP-1 effects are gender-independent in our models using baby mice at P6.
Fig. 9.
EPO and CEPO reduce microglial PARP-1 activation in cerebral white matter injury. We performed immunohistochemistry with an antibody for PARP-1 and examined PARP-1 expression in the white matter after H/I or H/I/L. (A–C) We noticed PARP-1 immunoreactivity present in the white matter after H/I and this appeared to decrease with EPO or CEPO treatment. (D–F) We observed strong PARP-1 immunoreactivity in the white matter after H/I/L, which appeared increased compared with H/I alone (D vs. F). Mice treated with EPO or CEPO after H/I/L displayed decreased PARP-1 immunoreactivity (E, F) compared with vehicle-treated mice (D). We performed cell counting to confirm these findings. We found a significant decline in the number of PARP-1+ cells in EPO and CEPO treated mice exposed to H/I (G, *p<0.05). Similarly, the number of PARP-1+ cells significantly decreased in H/I/L mice treated with EPO or CEPO (H, *p<0.05). (I, J) Immunostaining to identify the PARP-1 enzymatic product, PAR, was performed to show PARP-1 activity. Accumulation of PAR mainly co-localized with the macrophage lineage marker CD68 in the white matter area after H/I or H/I/L. (K, L) We examined whether EPO or CEPO treatment altered white matter injury in PARP-1−/− mice by performing the same H/I or H/I/L procedure and treatment on P6. Lesion severity was assessed using MBP immunostaining and the same 0–5 scoring scale as in above experiments. (K) We found no difference in lesion severity between wild-type vehicle treated, PARP-1−/− vehicle treated, PARP-1−/− EPO treated or PARP-1−/− CEPO treated H/I mice. (L) After H/I/L, PARP-1 deficient mice showed relatively minor injuries compared with wild-type mice (*, p<0.05). However, EPO or CEPO treatment to PARP-1−/− mice exposed to H/I/L did not alter lesion severity. Scale bar in A (for A–F), 20 µm. Scale bar in I (for I–J), 10 µm.
EPO and CEPO do not influence oligodendrocyte differentiation and maturation
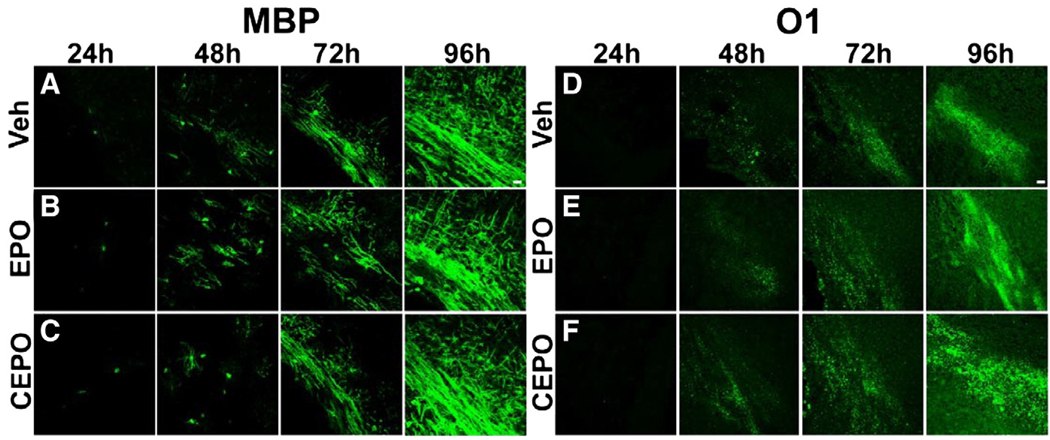
To demonstrate that EPO or CEPO affords protection against white matter injury, rather than directly influence normal oligodendrocyte differentiation and myelination, we administered EPO or CEPO to normal C57BL/6 mice at P6. Mice received i.p. injections of the same dosages used for the H/I and H/I/L studies. We evaluated MBP and O1 immunostaining at 24, 48, 72, and 96 h post-injection (n = 4 mice/treatment/time point). We observed no difference in MBP staining between groups at each time point examined (Figs. 10A–C). Similarly, we found no noticeable differences in O1 staining between vehicle, EPO or CEPO treated groups at any time point evaluated (Figs. 10D–F). Thus, EPO and CEPO do not alter normal oligodendrocyte differentiation and myelination.
Fig. 10.
EPO and CEPO do not interfere with the physiological progress of oligodendrocyte maturation and myelination. P6 mice received i.p. injections of EPO, CEPO or vehicle and were evaluated for MBP or O1 immunoreactivity 24, 48, 72 or 96 h later. We found no difference in the onset of myelination, as shown with MBP staining, between the groups at any time point (A–C). Similarly, we found no difference in O1 immunostaining between groups at any time point examined (D–F). Scale bar, 20 µm.
Discussion
PVL is the predominant pathology in premature infants and the most common cause of cerebral palsy, yet no therapy currently exists for it. Previous studies report that EPO and its nonerythropoietic derivative CEPO are neuroprotective in various experimental models of brain injury (Rees et al., 2010; Iwai et al., 2007, 2010; Gonzalez et al., 2007, 2009; McPherson and Juul, 2010). However, none of these studies investigated their efficacy against white matter injury using appropriate animal models of PVL. PVL occurs in premature infants suffering from cerebral H/I with or without prior exposure to maternal-fetal infection/inflammation, so we used hypoxia/ischemia with or without LPS injection to produce in immature mice the neuropathology similar to that seen clinically. The most common neuropathology in preterm infants is diffuse white matter damage injury, and overall our P6 model recapitulated the human pathological feature nicely. The transient increase in EPO and EPOR transcription levels implicated that both EPO and EPOR genes were induced by hypoxia–ischemia and provided rationale for pharmacological treatment of EPO and its derivatives. A single dose of EPO or CEPO (40 µg/kg, i.p.) administered after injury was significantly protective against white matter injury and improved neurobehavioral functional outcome. We further demonstrated that the protective effects of EPO and CEPO were associated with reduced activation of microglial PARP-1. The protection afforded by EPO and CEPO was diminished in PARP-1 knockout mice, indicating that EPO and CEPO provide protection in a PARP-1-dependent manner. In addition, therapeutic doses of EPO and CEPO did not affect normal myelination in the developing white matter. We have thus far conducted drug trials with several FDA-approved drugs as potential protective agents for PVL and found EPO and CEPO afforded the best neuroprotection in our mouse models of PVL. EPO and CEPO have relatively few adverse effects, and may represent therapeutic agents with translational potential for white matter injury underlying PVL.
Recently, EPO was tested as a clinically relevant neuroprotective agent, having the ability to cross the blood–brain barrier (BBB) after peripheral administration (Milano and Collomp, 2005; Grasso et al., 2007; Lykissas et al., 2007; Van der Kooij et al., 2008). CEPO was developed as a chemical nonerythropoietic derivative of EPO to alleviate concerns regarding over-stimulation of the EPOR, which can result in hematopoietic and thromboembolic complications (Mahmood et al., 2007; Montero et al., 2007; Villa et al., 2007). The results of the present study demonstrate that EPO and CEPO both protect against cerebral white matter injury in mouse models of PVL. Systemic administration of EPO or CEPO attenuates myelin depletion and oligodendrocyte loss that occurs in white matter after these insults.
Two PVL models were employed in the present study, one using hypoxia–ischemia (H/I) and the other combining hypoxic–ischemic and inflammatory insults (H/I/L). The use of these two models allowed us to explore two different pathological paradigms. In the H/I model, mice displayed unilateral (ipsilateral to the ligation) white matter lesions, while mice exposed to H/I/L showed myelin depletion in the entire cerebral white matter area, with more severe injury ipsilaterally. LPS is a potent endotoxin that binds to the innate immune receptor, Toll-like receptor 4 (TLR4), expressed on microglia. Microglia respond to TLR stimuli and once activated, initiate the inflammatory cascade (Kreutzberg, 1996; Poltorak et al., 1998; Qureshi et al., 1999; Olson and Miller, 2004). Using systemic LPS injections for the H/I/L model, we observed greater microglial activation than with H/I alone, indicating that the H/I/L model is more relevant to CNS inflammatory responses. Based on our previous unpublished studies using these PVL models, we found that inhibitors of microglial activation, such as minocycline, only rescued white matter injury in H/I/L mice, but not in H/I mice. In contrast, the results from our present study suggest that EPO and CEPO provide protection against neonatal white matter injury in both PVL models. The results suggest that EPO and CEPO not only act on the anti-inflammatory pathway, but also elicit other cytoprotective effects. Many studies have shown that EPO affords neuroprotection in experimental models of brain disease, and its derivative CEPO showed similar protective properties in ischemic and traumatic brain injury models (Mahmood et al., 2007; Montero et al., 2007). Despite numerous studies on EPO's effects on brain pathology, the underlying mechanisms remain unclear. Our studies on the neuroprotective role of EPO and CEPO in these two PVL models provide novel insight on the mechanisms involved in their CNS neuroprotection.
Previous studies reported neuroprotection and improved behavioral function in traumatic brain injury or cerebral ischemia models after EPO treatment (Milano and Collomp, 2005; Lykissas et al., 2007; Villa et al., 2007). In our study, we observed compensated motor asymmetry and improved muscle tone after treatment with EPO or CEPO. Although both EPO and CEPO provided neuroprotection and improved motor functions in neonatal white matter injury, this trend was apparent only in the early days after injury. Spontaneous recovery occurs in these PVL models and although EPO and CEPO initially promoted recovery, they did not provide additional long-term benefits. The lack of long-term improvement could be due to the treatment protocol or the severity of injury. A single injection of EPO or CEPO was administered, immediately after insult, and perhaps additional treatments are necessary to provide long-term benefits. These injuries are milder in comparison to a cerebral ischemic insult that encompasses both white and gray matter. Perhaps the effects of functional recovery are less noticeable due to the spontaneous recovery that follows this milder insult. Our H/I model and the H/I/L model only showed a transient behavioral deficit. In rodent models of PVL, behavioral recovery is generally robust. Thus, the significance of the experiments in rodents lies heavily on the observed therapeutic effect several weeks after injury. Effective translation of experimental work to human therapy for PVL requires further studies on mid-size and large animals, which may allow for observation of long-term behavioral consequences. Nevertheless, we observed acute functional recovery with EPO or CEPO treatment after H/I/L, suggesting that future research is warranted to determine its therapeutic potential.
We characterized the time course of disease development in both PVL models. We observed specific pathological events peaking at distinct time points. Cell death was apparent 24 h after insult, while microglial recruitment peaked 48 h after insult. Finally, reactive gliosis increased 72 h after insult. Determining the stage at which each event occurs allows for targeted therapeutic approaches. If these pathologies occur in the same time table in the human disease, treatment can be targeted at the specific stage in which disease onset is identified. One of the standout findings of the present study is that EPO and CEPO decrease microglial recruitment in white matter injury. Activated microglia generate free radicals and produce inflammatory cytokines, which can lead to immature oligodendrocyte injury and interruption of myelin formation (Agresti et al., 1996; Buntinx et al., 2004; Li et al., 2005; Pang et al., 2005). Therefore, regulating the immune processes which contribute to myelin depletion in PVL would be of utmost importance to alleviating this disease. Our data demonstrate that EPO and CEPO reduce microglial activation and provide clues as to how EPO and CEPO alter PVL pathogenesis.
Several studies reported that PARP-1 gene interruption or pharmacological inhibition elicits neuroprotection (Jagtap and Szabo, 2005; Lescot et al., 2010; Veto et al., 2010). We examined the effects of EPO and CEPO on PARP-1 activity in these PVL models and found decreased PARP-1 activity in the white matter after treatment. Furthermore, in mice lacking the PARP-1 gene, we observed no significant difference in myelin loss between EPO, CEPO, or vehicle groups after PVL. Thus, regulation of PARP-1 activity could be an important component of EPO- and CEPO-induced neuroprotection. PARP-1 is a DNA-repair and protein-modifying enzyme (Smith, 2001; Yu et al., 2002) but its overactivation results in nicotinamide adenine dinucleotide consumption, thereby depleting cellular energy stores and leading to cell death (Huber et al., 2004; Burkle, 2005). Many CNS insults generate DNA damage, which triggers PARP-1 activation, either promoting cell repair or cell death, depending on the extent of damage and activation level. PARP-1 overactivation can also elicit the release of apoptosis-inducing factor from the mitochondria and therefore promote cell death (Susin et al., 1999; Ye et al., 2002; Li et al., 2010). Furthermore, PARP-1 interactions with NF-κB influence microglial activation and the release of nitric oxide and cytokines (Ullrich et al., 2001b, 2001a; Kauppinen and Swanson, 2005). Therefore, inhibition of PARP-1 activity could be one of the mechanisms regulating the EPO- and CEPO-induced reduction of microglial activation and white matter damage. Our data suggest that microglial PARP-1 stimulation might be more of a component with the addition of LPS.
The present study demonstrated that exogenous EPO or CEPO treatment limits the inflammatory reaction and cell damage that occurs in neonatal white matter insults. Our data provide novel evidence to suggest that the mechanisms of EPO and CEPO neuroprotection in neonatal white matter injury are due to a combination of anti-apoptotic properties and limiting the extent of microglial PARP-1 activation. In PVL, there is concomitant microglial activation with myelin loss, and this microglial activation plays a crucial role in inducing myelin depletion in neonatal white matter injury. We demonstrated that PARP-1 over-activation secondary to cell damage is important for microglial activation, and that EPO and CEPO inhibit this PARP-1-mediated microglial activation. The underlying mechanisms of EPO and CEPO inhibition of PARP-1 are unknown. Future experiments should focus on clarifying the interactions of EPO and CEPO with the downstream events of PARP-1 signaling pathways in the mitochondria and cytosol. In addition to providing novel insight to the mechanisms of EPO and CEPO neuroprotection, we report the use of two experimental mouse models of PVL which provide a useful tool to investigate candidate drug pharmacodynamic properties.
Our data showed that there were no essential differences in pharmacological efficacy between EPO and CEPO. However, unlike EPO, CEPO has no affinity to the EPO receptor EPOR, and thus does not exhibit the potential erythropoietic side effects of the parent compound. The lack of differences in the neuroprotective effects between EPO and CEPO indicates that the protective effects are unlikely mediated through EPOR. In the present study, we demonstrated that a mechanism underlying the neuroprotective potential of EPO and CEPO is related to PARP-1 deactivation. Our results also indicated that the protective effect of EPO and CEPO was not complete. As the pathogenesis of white matter injury is likely multifaceted and involves multiple mechanisms, including oxidative stress, excitotoxicity, and inflammation, all of which may work together in a synergistic manner. Combinatorial therapy using pharmacological agents working through different mechanisms would likely lead to more complete protection.
Acknowledgments
We thank Warren Pharmaceuticals, Ossining, NY and H. Lundbeck A/S, Denmark for generously providing carbamylated erythropoietin. This study was supported by grants to W.D. from the NIH (R01 NS059043 and R01 ES015988), Roche Foundation for Anemia Research (RoFAR), National Multiple Sclerosis Society, Feldstein Medical Foundation, and Shriners Hospitals for Children.
Footnotes
The authors declare no conflicts of interest.
References
- Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumor necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur. J. Neurosci. 1996;8:1106–1116. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, Kinney HC. Development of microglia in the cerebral white matter of the human fetus and infant. J. Comp. Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc. Natl Acad. Sci. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemi M, Cavallaro E, Floccari F, Sturiale A, Aloisi C, Trimarchi M, Corica F, Frisina N. The pleiotropic effects of erythropoietin in the central nervous system. J. Neuropathol. Exp. Neurol. 2003;62:228–236. doi: 10.1093/jnen/62.3.228. [DOI] [PubMed] [Google Scholar]
- Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Ameloot M. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J. Neurosci. Res. 2004;76:834–845. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- Burkle A. Poly (ADP-ribose). The most elaborate metabolite of NAD+ FEBS J. 2005;272:4576–4589. doi: 10.1111/j.1742-4658.2005.04864.x. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Moskowitz MA. Poly (ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neuro-degenerative disorders. J. Neurochem. 2003;85:306–317. doi: 10.1046/j.1471-4159.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- Csete M, Rodriguez L, Wilcox M, Chadalavada S. Erythropoietin receptor is expressed on adult rat dopaminergic neurons and erythropoietin is neurotrophic in cultured dopaminergic neuroblasts. Neurosci. Lett. 2004;359:124–126. doi: 10.1016/j.neulet.2004.01.068. [DOI] [PubMed] [Google Scholar]
- Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- Deng W. Neurobiology of injury to the developing brain. Nat. Rev. Neurol. 2010;6(6):328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- Deng W, Pleasure J, Pleasure D. Progress in periventricular leukomalacia. Arch. Neurol. 2008;65:1291–1295. doi: 10.1001/archneur.65.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX Attenuates excitotoxic injury in developing white matter. J. Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J. Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, Ferriero DM. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev. Neurosci. 2007;29(4–5):321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev. Neurosci. 2009;31(5):403–411. doi: 10.1159/000232558. Epub 2009 Aug 11. PubMed PMID: 19672069; PubMed Central PMCID: PMC2820334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Meli F, Passalacqua M, Fodale V, Buemi M, Giambartino F, Lacopino DG, Tomasello F. The role of erythropoietin in neuroprotection: therapeutic perspectives. Drug News Perspect. 2007;20:315–320. doi: 10.1358/dnp.2007.20.5.1120219. [DOI] [PubMed] [Google Scholar]
- Hamby AM, Sun SW, Kauppinen TM, Swanson RA. Use of a poly (ADP-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia-reperfusion. Stroke. 2007;38:632–636. doi: 10.1161/01.STR.0000250742.61241.79. [DOI] [PubMed] [Google Scholar]
- Huber A, Bai P, de Murcia JM, de Murcia G. PARP-1, PARP-2 and ATM in the DNA damage response: functional synergy in mouse development. DNA Repair. 2004;3:1103–1108. doi: 10.1016/j.dnarep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38(10):2795–2803. doi: 10.1161/STROKEAHA.107.483008. Oct, Epub 2007 Aug 16. PubMed PMID: 17702962. [DOI] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41(5):1032–1037. doi: 10.1161/STROKEAHA.109.570325. May, Epub 2010 Apr 1. PubMed PMID: 20360553; PubMed Central PMCID: PMC2919308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly (ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Swanson RA. Poly (ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J. Immunol. 2005;174:2288–2296. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. (93 Edition) 2008:153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Lescot T, Fulla-Oller L, Palmier B, Po C, Beziaud T, Puybasset L, Plotkine M, Gillet B, Meric P, Marchand-Leroux C. Effect of acute poly(ADP-ribose) polymerase inhibition by 3-AB on blood-brain barrier permeability and edema formation after focal traumatic brain injury in rats. J. Neurotrauma. 2010;27:1069–1079. doi: 10.1089/neu.2009.1188. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl Acad. Sci. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Klaus JA, Zhang J, Xu Z, Kibler KK, Andrabi SA, Rao K, Yang ZJ, Dawson TM, Dawson VL, Koehler RC. Contributions of poly(ADP-ribose) polymerase-1 and −2 to nuclear translocation of apoptosis-inducing factor and injury from focal cerebral ischemia. J. Neurochem. 2010;113:1012–1022. doi: 10.1111/j.1471-4159.2010.06667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykissas MG, Korompillias AV, Vekris MD, Mitsionis GI, Sakellariou E, Beris AE. The role of erythropoietin in central and peripheral nerve injury. Clin. Neurol. Neurosurg. 2007;109:639–644. doi: 10.1016/j.clineuro.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythro-poietin. J. Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Erythropoietin gene expression in human, monkey and murine brain. Eur. J. Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Martin JA, Kung HC, Mathewsa TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- McPherson RJ, Juul SE. Erythropoietin for infants with hypoxic-ischemic encephalopathy. Curr. Opin. Pediatr. 2010;22(2):139–145. doi: 10.1097/MOP.0b013e328336eb57. Apr, Review. PubMed PMID: 20090525; PubMed Central PMCID: PMC2879270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano M, Collomp R. Erythropoietin and neuroprotection: a therapeutic perspective. J. Oncol. Pharm. Pract. 2005;11:145–149. doi: 10.1191/1078155205jp162oa. [DOI] [PubMed] [Google Scholar]
- Montero M, Poulsen FR, Noraberg J, Kirkeby A, van Beek J, Leist M, Zimmer J. Comparison of neuroprotective effects of erythropoietin (EPO) and carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal slice cultures. Exp. Neurol. 2007;204:106–117. doi: 10.1016/j.expneurol.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Nagaso H, Kakui N, Ishikawa M, Hiranuma T, Hoshiko S. Critical role of the automodification of poly (ADP-ribose) polymerase-1 in nuclear factor-kappaB-dependent gene expression in primary cultured mouse glial cells. J. Biol. Chem. 2004;279:42774–42786. doi: 10.1074/jbc.M407923200. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai ZW, Rhodes PG. Effects of tumor necrosis factor-alpha on developing optic nerve oligodendrocytes in culture. J. Neurosci. Res. 2005;80:226–234. doi: 10.1002/jnr.20450. [DOI] [PubMed] [Google Scholar]
- Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, Krageloh-Mann I. Trends in cerebral palsy among infants of very low birth weight (<1500 g) or born prematurely (<32 weeks) in16 European centres: a database study. Lancet. 2007;369:43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J. Exp. Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Hale N, De Matteo R, Cardamone L, Tolcos M, Loeliger M, Mackintosh A, Shields A, Probyn M, Greenwood D, Harding R. Erythropoietin is neuroprotective in a preterm ovine model of endotoxin-induced brain injury. J. Neuropathol. Exp. Neurol. 2010 Mar;69(3):306–319. doi: 10.1097/NEN.0b013e3181d27138. [DOI] [PubMed] [Google Scholar]
- Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav. Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Sasaki R. Pleiotropic functions of erythropoietin. Intern. Med. 2003;42:142–149. doi: 10.2169/internalmedicine.42.142. [DOI] [PubMed] [Google Scholar]
- Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann. Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. The world according to PARP. Trends Biochem. Sci. 2001;26:174–179. doi: 10.1016/s0968-0004(00)01780-1. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tomimatsu T, Fukuda H, Endoh M, Mu J, Watanabe N, Kohzuki M, Fujii E, Kanzaki T, Oshima K, Doi K, Kubo T, Murata Y. Effects of neonatal hypoxic-ischemic brain injury on skilled motor tasks and brainstem function in adult rats. Brain Res. 2002;926:108–117. doi: 10.1016/s0006-8993(01)03311-x. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Diestel A, Eyupoglu IY, Nitsch R. Regulation of microglial expression of integrins by poly (ADP-ribose) polymerase-1. Nat. Cell Biol. 2001a;3:1035–1042. doi: 10.1038/ncb1201-1035. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Diestel A, Bechmann I, Homberg M, Grune T, Hass R, Nitsch R. Turnover of oxidatively damaged nuclear proteins in BV-2 microglial cells is linked to their activation state by poly-ADP-ribose polymerase. FASEB J. 2001b;15:1460–1462. doi: 10.1096/fj.00-0540fje. [DOI] [PubMed] [Google Scholar]
- Van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res. Rev. 2008;59:22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Veto S, Acs P, Bauer J, Lassmann H, Berente Z, Setalo GJ, Borgulya G, Sumegi B, Komoly S, Gallyas FJ, Illes Z. Inhibiting poly (ADP-ribose) polymerase: potential therapy against oligodendrocyte death. Brain. 2010;133:822–834. doi: 10.1093/brain/awp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, van Beek J, Larsen AK, Gerwien J, Christensen S, Cerami A, Brines M, Leist M, Ghezzi P, Torup L. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J. Cereb. Blood Flow Metab. 2007;27:552–563. doi: 10.1038/sj.jcbfm.9600370. [DOI] [PubMed] [Google Scholar]
- Virág L, Szabó C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr. Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant — more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, Lu M, Pool C, Heavner G, Chopp M. Postischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br. J. Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Han BH, Li Y, Keogh CL, Holtzman DM, Yu SP. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J. Pharmacol. Exp. Ther. 2006;317:109–116. doi: 10.1124/jpet.105.094391. [DOI] [PubMed] [Google Scholar]
- Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G, Wu H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat. Struct. Biol. 2002;9:680–684. doi: 10.1038/nsb836. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF. Mediation of poly (ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang H, Dawson TM, Dawson VL. Poly (ADP-ribose) polymerase-1 and apoptosis inducing factor in neurotoxicity. Neurobiol. Dis. 2003;14:303–317. doi: 10.1016/j.nbd.2003.08.008. [DOI] [PubMed] [Google Scholar]