Abstract
Cachexia is a syndrome of wasting and anorexia that worsens the prognosis of many chronic diseases including cancer, chronic kidney disease, chronic heart disease and chronic obstructive pulmonary disease. Properties of the orexigenic hormone ghrelin—including appetite-stimulation, weight-gain production and increased cardiac output—make it a logical treatment for cachexia. While endogenous ghrelin levels are increased in the setting of cachexia, treatment with ghrelin and other GHSR-1a agonists in animal models of cachexia and in humans with cachexia have demonstrated consistent effects of increased appetite and improved weight gain. These positive effects occur in multiple underlying diseases associated with cachexia and appear to be sustained over treatment duration of up to 12 weeks. The mechanism of action in producing these effects is likely related to stimulation of central appetite centers such as the central melanocortin system and to increased growth hormone release, though ghrelin’s effects may also relate to decreased systemic inflammation and other direct and indirect actions. Questions regarding the long-term safety of ghrelin treatment are still unanswered, as is the important question of whether successful treatment of cachexia will improve the prognosis of the underlying disease itself.
Introduction
Cachexia is a wasting syndrome that accompanies a wide array of chronic diseases including cancer, chronic kidney disease, chronic heart disease and chronic obstructive pulmonary disease (COPD)(Tisdale 1997; Evans et al. 2008). While these diseases are varied in their underlying pathophysiology, they each result in a loss of lean and fat mass that is in part fueled by an increase in resting energy expenditure. Perhaps the most striking characteristic of cachexia, however, is on-going anorexia at a time when energy stores are depleted. Thus far, no definitive treatment has been shown to be effective for use in humans with cachexia.
One agent that has gained attention as a potential treatment for cachexia syndromes is the gut hormone ghrelin (Kojima et al. 1999; Tschop et al. 2000; Nakazato et al. 2001). Given its properties of increasing appetite and increasing fat mass accumulation—that is, the opposite of processes observed during cachexia—ghrelin has been investigated in a growing number of animal models and human trials testing its efficacy in treating cachexia. As we will see in this review, ghrelin and other agonists of the growth hormone secretegogue recepetor (GHSR-1a) also exhibit additional properties that may further benefit specific underlying diseases associated with cachexia.
Physiology of Ghrelin
Ghrelin is a 28-amino acid hormone whose discovery was based on the ability for ghrelin to bind to the GHSR-1a in the hypothalamus and stimulate growth hormone release (Kojima et al. 1999). Ghrelin contains a unique n-octylation of serine residue ser-3 catalyzed by ghrelin-O-acyltransferase (GOAT) prior to its secretion (Gutierrez et al. 2008; Yang et al. 2008). In acylating ghrelin, GOAT utilizes fatty acids absorbed from the diet, including C6–C10 fatty acids, with a strong preference for C8 (Nishi et al. 2005). This acetylation is essential for ghrelin’s binding to the GHSR-1a (Kojima et al. 1999) but as described below, may not be necessary for all of its actions.
Ghrelin is released primarily from endocrine cells in the antrum of the stomach and levels of total ghrelin rise in response to time since last feed (Cummings et al. 2002). The regulation of ghrelin release is at least partly in response to the availability of energy dense fatty acids (Kirchner et al. 2009). As such, while ghrelin has traditionally been seen as a meal-initiating hormone, it may also have properties of alerting growth-and appetite-regulating centers in the organism regarding the availability of calorie-dense food sources.
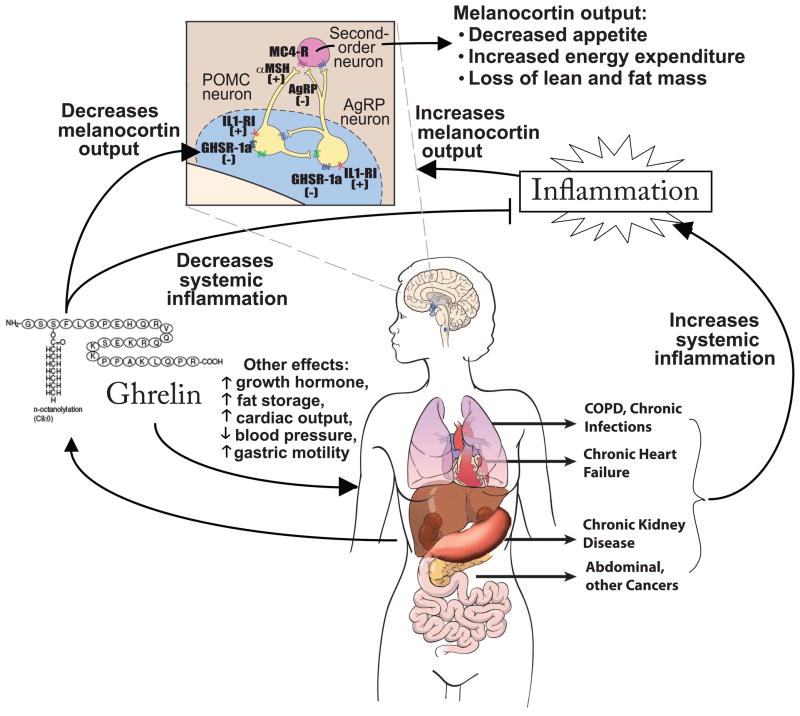
Ghrelin’s actions on energy balance are mediated in part via its effects on central appetite centers such as the central melanocortin system (Figure 1)(DeBoer and Marks 2006; DeBoer 2010). Ghrelin binds to GHSR-1a in the arcuate nucleus and ventromedial nucleus. In the case of the melanocortin system, ghrelin stimulation results in an increase in expression and release of the orexigenic peptide agouti-related peptide (AgRP) and neuropeptide-Y (Chen et al. 2004) and a decrease in expression of the anorexigenic peptide pro-opiomelanocortin (POMC), which is then cleaved to α-melanocyte-stimulating peptide (α-MSH)(Cowley et al. 2003). These effects ultimately lead to a decrease in signaling at the melanocortin-4 receptor (MC4R) and an increase in signaling at the Y-1 receptor, with the result being altered signaling at second order neurons and downstream effects of increased food-seeking behavior and decreased basal metabolic rate (Marks et al. 2001; Laviano et al. 2008).
Figure 1. A model of ghrelin’s mechanisms of action in cachexia.
Cachexia in the setting of multiple different underlying etiologies appears to have in common an increase in inflammatory cytokines. This results in—among other things—effects on the central melanocortin system of the hypothalamus, a key site of ghrelin’s appetite-stimulating actions. Inflammatory cytokines act on receptors such as IL-1 receptor I (IL1-RI) to increase activity of neurons expressing pro-opiomelanocortin (POMC), increasing release of α-melanocyte stimulating hormone (αMSH), which activates the melanocortin-4 receptor (MC4-R) on downstream neurons. Inflammatory cytokines also decrease activity of neurons expressing agouti-related peptide (AgRP), a natural antagonist of the MC4-R. The net result of these processes is a decrease in appetite and increase in degradation of lean mass. Conversely, ghrelin acts on GHSR-1a to increase expression and release of AgRP and Neuropeptide Y. In addition to counter-acting the effects of inflammatory cytokines in the melanocortin system, ghrelin treatment also decreases systemic inflammation in some settings and has other potentially-important systemic effects. Abbreviations: COPD, chronic obstructive pulmonary disease. (Adapted from DeBoer Nature Clinical Practice: Endocrinology and Metabolism 2(8):459–466 and DeBoer Nutrition 26:146–151.
However, ghrelin’s actions pertinent to cachexia are not limited to these central effects. Additional effects are as follows:
1. Inflammation
The GHS-R is expressed on lymphocytes, and administration with ghrelin or GHS-R agonists has been shown to decrease expression of inflammatory cytokines in monocytes and T-cells (Dixit et al. 2004) and decrease systemic inflammation in rodent models of inflammation (Chang et al. 2003; Granado et al. 2005), which may decrease the severity of processes related to cachexia.
2. Cardiovascular
Ghrelin has cardiovascular effects, including increased cardiac output and decreased blood pressure (Nagaya et al. 2001c; Nagaya et al. 2004), as reviewed by Isgaard and Granata in this special issue. These effects are discussed further in the section on cachexia from chronic heart failure (CHF).
3. Fat storage
Ghrelin has potent effects on fat storage, which may provide important energy reserves for the organism as the processes of cachexia continue (Tschop et al. 2000).
4. Gastric motility
Ghrelin administration accelerates the rate of gastric emptying in humans even in the presence of vagotomy (Tack et al. 2005; Binn et al. 2006), as reviewed by Jeffrey et al. in this special issue.
5. Blood sugar maintenance during fasting
Potentially pertinent to long-standing anorexia seen in cachexia, acyl ghrelin is necessary for growth-hormone mediated maintenance of fasting glucose levels during a prolonged fast (Zhao et al. 2010) as reviewed by Ukkola in this special issue.
In addition to these effects of acyl ghrelin via GHSR-1a, desacyl ghrelin—which does not bind to the GHSR-1a—also may exert other metabolic effects. Mice over-expressing desacyl ghrelin are smaller than wild-type mice (Ariyasu et al. 2005). Desacyl ghrelin appears to increase insulin sensitivity, increase fatty acid uptake in cardiomyocytes and decrease fat mass (Zhang et al. 2008; Lear et al. 2010). Similarly, treatment with desacyl ghrelin has effects including a partially-protective effect against myocardial damage in an animal model of chemically-induced cardiac injury (Li et al. 2006). Desacyl ghrelin may also have an effect on suppressing food intake (Asakawa et al. 2005), though these data have been conflicting (Ariyasu et al. 2005).
As we will see, most cachexia syndromes have been shown to have elevated levels of desacyl ghrelin at baseline and it is not known whether these elevated levels of desacyl ghrelin are causative of symptoms or a physiologic response to cachexia. Most disease states resulting in cachexia also demonstrate an elevation in acyl-ghrelin that may be expected following loss of body mass. As suggested above, the expression of GHS-R in a variety of tissues provides a wide scope of potential effects of ghrelin as a treatment for cachexia. Research has thus focused on whether use of acyl-ghrelin or other GHS-R agonists may be able to overcome the catabolic processes of cachexia.
Cancer Cachexia
Cachexia is a feature that complicates the course of multiple different malignancies. In certain kinds of cancer—particularly gastrointestinal cancers—up to 85% of patients experience cachexia, and cachexia contributes to at least 20% of cancer deaths overall (Tisdale 2002). The weight loss experienced by patients can be severe, including loss of up to 75% of muscle mass (Fearon 1992), though even subtle amounts of weight loss and anorexia are associated with a worsened prognosis, poorer response to chemotherapy and increased morbidity (Barber et al. 1999). The loss of appetite may be a particularly important sign in that one survey of patients with terminal cancer found the presence of nausea or emesis was associated with a 68% decrease in survival and contributed to a substantial decrease in quality of life (Vigano et al. 2004).
As is true of most diseases associated with cachexia, the presence of underlying inflammation has been implicated as exhibiting important contributions in the production of cancer cachexia and may be an important target of ghrelin’s actions in this setting. Pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α can be produced by tumor cells, as well as from the host response to tumor (Deans and Wigmore 2005). Up to 50% of cancer patients exhibit evidence of elevated inflammation at diagnosis (Falconer et al. 1995) and the associated increase in cytokines is strongly implicated in producing anorexia, at least partly due to action at the central melanocortin system (Marks et al. 2001), as discussed in the previous section (Figure 1).
In the setting of malignancy, serum levels of acyl and desacyl ghrelin are already elevated 25–50% above normal among individuals bearing a variety of cancers, including those due to lung, breast, colon and prostate cancers (Shimizu et al. 2003; Garcia et al. 2005; Malendowicz et al. 2009). The cause of these higher levels may be multi-factorial, as each of these types of cancer and several others have been reported to express ghrelin (Nikolopoulos et al. 2010). Nevertheless, the elevation in ghrelin in cancer cachexia is likely also in part a physiologic response to the decrease in lean and fat mass in cancer (Shiiya et al. 2002; Soriano-Guillen et al. 2004). Indeed, levels of acyl-ghrelin are 50% higher in cancer patients with cachexia, when compared to those without cachexia and 80% higher than in non-cancer patients (Garcia et al. 2005).
On the surface, the presence of anorexia in the face of elevated acyl ghrelin levels suggests either an element of resistance to ghrelin’s orexigenic properties in the setting of cancer cachexia or overwhelming anorexic effects of other processes. Attempts at treating cancer cachexia with ghrelin have thus required supraphysiologic doses of ghrelin but have yielded almost universally positive effects on appetite, suggesting that ghrelin’s effect on appetite-stimulating centers is not saturated in the setting of cancer cachexia (DeBoer 2008).
Use of ghrelin and other GHSR-1a agonists have been tested in animal models of cancer cachexia (DeBoer 2009) and have demonstrated a 20–37% increase in food intake, 10% weight gain, and reversal of lean and fat mass losses (Hanada et al. 2003; Wang et al. 2006; DeBoer et al. 2007). These effects appear to be mediated at least in part due to effects at the level of the melanocortin system, as ghrelin treatment was accompanied by increased expression of the orexigenic neuropeptides AgRP and NPY (DeBoer et al. 2007).
Nevertheless, these animal experiments involved extreme doses of ghrelin (500–800 nmol/kg/day) several orders of magnitude above those used in trials among healthy humans (2–6 nmol/kg/day)(Wren et al. 2001), raising the potential that ghrelin would not be as effective in humans with cachexia. Early trials in human subjects with melanoma, breast and colon cancers involved administering a blinded dose of ghrelin vs. placebo, demonstrating increased appetite (Neary et al. 2004; Strasser et al. 2008)(Table 1 (DeBoer 2008)). Longer term treatment was reported by Garcia et al. using a GHSR-1a-agonist called RC-1291 (Aminorhelin; Saphire Therapeutics, Bridgewater, NJ)(Garcia et al. 2007). Subjects with multiple cancer types, predominantly lung cancer, were randomized to receive oral doses of Aminorhelin (50 mg, molecular weight not reported) or placebo for 12 weeks. Treatment yielded improvements in weight gain and lean mass vs. placebo. The investigators also measured quality-of-life and IGF-1 levels, both of which were increased with RC-1291 treatment. Testing of RC-1291 in healthy volunteers for 5–6 days had also revealed dose-dependent increases in IGF-1 and insulin-like-growth-factor binding protein 3 (IGF-BP3)(Garcia and Polvino 2009).
Table 1.
Summary of animal and human trials of GHSR-1a agonists for the treatment of cachexia
| Publication | Underlying Disease/Model | GHS-1a Agent | Dose, Route | Duration of treatment | Effects |
|---|---|---|---|---|---|
| CANCER | |||||
| Human trials | |||||
| Neary (2004) | Melanoma, breast, colon cancers | ghrelin | 450 pmol/kg as 90 min. IV infusion | 1 dose (crossover placebo controlled) | Food intake (single meal): ghrelin 31% more than saline** |
| Garcia (2007) | Multiple cancer types | GHSR-1a agonist RC-1291 (Anamorelin) | 50 mg/day PO | 12 weeks |
Total mass: RC-1291 +0.6%; placebo: −1.45%* Lean body mass: RC-1291 +1.75%; placebo: +0.4% NS |
| Strasser (2008) | Multiple cancer types | ghrelin | 2 ug/kg and 8 ug/kg (2.4 nmol/kg) as IV infusion | 2 doses (one of each dose strength 1 wk apart) | Food intake (one day): ghrelin +56% over placebo NS |
| Lundholm (2010) | Gastro-intestinal cancer | ghrelin | 0.7 ug/kg/d (0.24 nmols/kg/d) or 13 ug/kg/d (4.4 nmols/kg/d) as daily SC injection | 8 weeks |
Fat mass change: high dose ghrelin −1%; low dose ghrelin −2.3%* Lean mass change: high dose ghrelin +6.7%; low dose ghrelin +1.8% NS |
| Adachi (2010) | Stomach cancer (after gastrectomy) | ghrelin | 3 ug/kg IV | 10 days |
Body weight change: ghrelin −1.4%; placebo −3.7%* Fat mass change: ghrelin −7.6%; placebo −8.8 NS Lean mass change: ghrelin −2.9%; placebo −7.8% NS |
| CHRONIC KIDNEY DISEASE | |||||
| Human trials | |||||
| Wynne (2005) | Multiple causes of chronic kidney disease, on peritoneal dialysis | ghrelin | 3.6 nmol/kg as a single SQ dose | 1 dose (crossover placebo controlled) | Food intake (one meal): ghrelin 57% more than placebo** |
| Ashby (2009) | ghrelin | 12 ug/kg/d (4 nmols/kd/d) SC | 8 days | Food intake (one week): ghrelin 19% more than saline* | |
| HEART FAILURE/COPD | |||||
| Human trials | |||||
| Nagaya (2004) | Congestive heart failure | ghrelin | 4 ug/kg/d (1.35 nmols/kg/day) as twice daily IV infusions (no placebo control) | 3 weeks |
Weight: ghrelin +1.6% vs. baseline NS; control no change Food intake: +8% vs. baseline*; control no change Lean body mass: ghrelin +2%* vs. baseline*; control no change LV ejection fraction: ghrelin +15% vs. baseline*; control no change |
| Nagaya (2005) | COPD | ghrelin | 4 ug/kg/d (1.35 nmols/kg/day) as twice daily IV infusions (no control) | 3 weeks |
Weight: ghrelin +2% vs. baseline* Food intake: +9% vs. baseline* Lean body mass: ghrelin +1.8%* vs. baseline* |
| Kodama | Recurrent lung infection | ghrelin | 4 ug/kg/d 1.2 nmols/kg(1.35 nmols/kg/day) as twice daily IV infusions (no control) | 3 weeks |
Weight: ghrelin +6% vs. baseline* Food intake: +25% vs. baseline* |
| Gertner (2009) | COPD | GHSR-1a agonist SUN11031 | 10 or 20 ug/kg twice daily SC | 3 weeks | Weight: SUN11031 20 ug/kg: +4%; placebo +1% * |
Significance: NS=not significant (p>0.05);
p<0.05;
p<0.01;
p<0.001.
Abreviations: IV, intravenous; PO, oral; SC, subcutaneous; LV, left ventricular; COPD, chronic obstructive pulmonary disease.
Recently the results of another long-term trial of ghrelin treatment have been reported among 31 subjects with progressive, unresponsive gastrointestinal cancers (Lundholm et al. 2010). These subjects were randomized to receive low-dose (0.7 ug/kg/d) or high dose (17 ug/kg/day) ghrelin for 8 weeks, to further test whether the effects of ghrelin could be sustained over time. This group administered ghrelin as a once-daily subcutaneous (SC) injection to be administered 30 minutes before the main meal of the day. Subjects receiving high-dose ghrelin reported increased appetite (as measured via visual-analogue scale), though their total food intake was not different from the group receiving low-dose ghrelin. The group receiving high-dose ghrelin had a decrease in loss of fat mass and trended toward increased lean mass and increased energy balance. Interestingly, subjects in both groups exhibited a non-significant 9–28% decrease in IGF-1 levels over the course of treatment. This study thus emphasizes that ghrelin’s effects on body mass may be mediated without increased food intake or increases in IGF-1. Finally, treatment with ghrelin following removal of gastric cancer decreased the loss of body weight, though this may have been partly due to replacement of normal endogenous ghrelin secretion (Adachi et al. 2010).
While moderate-length treatment courses of ghrelin have had beneficial effects, there exists potential for tolerance to ghrelin’s effects or that counter-regulating processes would overwhelm beneficial effects. Of additional concern regarding the use of ghrelin in cancer patients is the potential for stimulating tumor growth. This remains an important possibility, given ghrelin’s ability to stimulate processes related to cancer progression (as reviewed by Chopin et al. in this special issue) and to stimulate growth hormone release. Although many tumor cells express the IGF-1 receptor on their membranes (Maki 2010), neither animal models nor human studies—albeit over short time periods—have reported increases in tumor volume or disease severity during treatment with ghrelin. Studies have been mixed regarding the effect of ghrelin or GHSR-1a-agonist treatment on levels of growth hormone itself, with some reporting an increase in growth hormone (Garcia et al. 2007) and some reporting no difference vs. controls (DeBoer et al. 2007; Lundholm et al. 2010). Finally, while ghrelin has been reported to have anti-inflammatory properties in other settings, decreases in inflammation have not been reported in the setting of cancer cachexia.
In summary, although cancer cachexia involves elevated levels of acyl ghrelin, treatment with supraphysiologic doses of ghrelin nevertheless results in improvement in appetite. While long-term studies have not demonstrated increases in food intake, there have been demonstrable increases in weight stabilization and fat mass, suggesting metabolic effects that may improve an organism’s energy reserve. Nevertheless, questions regarding safety still need to be carefully addressed, and dosing regimens and long-term efficacy will need to be optimized prior to clinical application. At the time of this writing, further trials are on-going (www.clinicaltrials.gov accessed 11/18/2010) regarding the efficacy of ghrelin as a treatment for cancer cachexia, raising interest that treatment with ghrelin or GHSR-1a agonists may be an option for patients with cancer cachexia in the future.
Renal Cachexia
Similar to what is seen in cancer cachexia, in the setting of chronic kidney disease cachexia involves a loss of lean body mass, increased energy expenditure and decreased appetite. This constellation of symptoms affects up to 60% of patients on hemodialysis (Kalantar-Zadeh and Balakrishnan 2006). Given a prominent decrease in serum protein levels in this setting, this syndrome is also referred to as protein energy wasting (Muscaritoli et al. 2009; Naufel et al. 2010). Inflammation—which is present in CKD at least in part due to uremic toxins, decreased tissue perfusion and impaired renal clearance of cytokines (Muscaritoli et al. 2009)—appears to play a role, in that decreased hunger (as assessed by visual analogue scales) correlates with markers of inflammation (Zabel et al. 2009). Other potential causative factors include metabolic acidosis (Muscaritoli et al. 2009) and oxidative stress due to uremia and dialysis itself (Locatelli et al. 2003). Muscle wasting in CKD is facilitated by the ubiquitome protease system (Mitch and Goldberg 1996) and is complicated by abnormalities in mitochondrial function, including a decrease in oxidative enzymes and their activity (Adey et al. 2000).
As seen in other cachexia states, levels of ghrelin are elevated in renal cachexia. A more striking finding in this state, however, is the sharp difference in desacyl ghrelin vs. acyl ghrelin. Desacyl ghrelin levels are elevated by 2.2–2.8 fold vs. controls among CKD patients on hemodialysis and peritoneal dialysis (Yoshimoto et al. 2002; Buscher et al. 2010; Naufel et al. 2010) and are higher in hemodialysis patients with anorexia than without (Muscaritoli et al. 2007). While acyl ghrelin levels do not differ overall between CKD patients and controls (Perez-Fontan et al. 2005; Buscher et al. 2010; Naufel et al. 2010), CKD patients with cachexia have higher levels of acyl ghrelin than patients without cachexia (Bossola et al. 2009).
As in other forms of cachexia, the higher levels of acyl ghrelin in renal cachexia may be a physiologic response to a starved nutritional state and present the possibility that treatment with additional doses would be saturated. Animal models of treatment of ghrelin or other GHSR-1a agonists over a moderate time frame (14 days) demonstrated an increase in overall food intake and increased total mass and lean body mass (DeBoer et al. 2008). However, whereas adipogenesis has been a major effect of ghrelin treatment in non-diseased animals (Tschop et al. 2000), in renal cachexia, treatment with ghrelin and other GHS-R agonists did not change the loss of fat mass. While the main mechanism behind these changes is uncertain, the clinical improvements were accompanied by altered expression of neuropeptides and enzymes in the melanocortin system, a decrease in systemic inflammatory cytokines, a decrease in the ubiquitome-protease system and a normalization of mitochondrial enzyme activity, and it is likely that a combination of these effects contributes to the changes in body mass (DeBoer et al. 2008; Barazzoni et al. 2010).
Among human subjects with end stage renal disease (ESRD), a single subcutaneous (SC) dose of ghrelin (vs. placebo) in a blinded, cross-over trial resulted in an initial increase in food intake that abated over the following 24 hours (Wynne et al. 2005), raising the question of whether sustained treatment would continue to increase appetite (Table 1). The same group subsequently performed a one-week randomized, cross-over trial of daily injections of ghrelin vs. placebo, demonstrating sustained increase in food intake (Ashby et al. 2009).
Questions remain regarding longer-term use of ghrelin in CKD, including whether treatment with native acyl ghrelin would lead to further accumulations of desacyl ghrelin in the presence of poor renal clearance, and whether this would eventually affect efficacy. Treatment using GHSR-1a agonists that cannot be converted to the desacyl state would avoid this issue. Administration of ghrelin in the setting of renal failure has been shown to decrease blood pressure (discussed further below), which is a potentially beneficial effect in chronic kidney disease (Wynne et al. 2005; Ashby et al. 2009). Finally, a common outcome of renal cachexia in childhood is poor linear growth, which is treated with injections of human growth hormone. An uncertain but important consideration is whether improved nutrition parameters with ghrelin could improve adult height outcomes among children with CKD.
Chronic Heart Failure
Chronic heart failure (CHF) as a result of myocardial infarction and dilated cardiomyopathy is a relatively common cause of cachexia, as 5.7 million people in the United States have CHF and up to 15% of these will develop cachexia (Anker et al. 1997a; American_Heart_Association 2005). Individuals with CHF who develop unintended weight loss, decreased lean and fat mass and a decrease in appetite exhibit tripling of mortality rate over an 18-month period, from 17% to 50% (Anker et al. 1997a; Anker et al. 1997b).
Cachexia due to CHF is related to systemic inflammation, and patients with CHF cachexia exhibit over a two-fold elevation in TNF-α and IL-6 levels compared to those with non-cachexic CHF (Levine et al. 1990; Torre-Amione et al. 1996). These elevated cytokines are independent risk factors for mortality (Rauchhaus et al. 2000; Deswal et al. 2001) and are postulated to arise from the failing myocardium, response to endotoxin released through edematous bowel wall or from hypoxic tissue (von Haehling et al. 2009). Again, these inflammatory cytokines are thought to act at the level of the melanocortin system, as genetic and pharmacologic blockade of this system improves body mass parameters (Scarlett et al. 2010).
Total ghrelin levels are 41% higher in individuals with CHF compared to controls (Nagaya et al. 2001b) and are 50% higher in those with cachectic CHF vs. non-cachectics (Xin et al. 2009). Differences in levels of acyl ghrelin have not been reported. As in other disease states underlying cachexia, administration of ghrelin and ghrelin agonists in animal models of CHF has been shown to improve accrual of lean mass, fat mass and body weight several fold vs. placebo over 3–4 week treatment periods (Nagaya et al. 2001c; Xu et al. 2005; Akashi et al. 2009).
However, particularly appealing as an intervention for CHF cachexia are ghrelin’s additional effects on cardiovascular function (reviewed by Isgaard and Granata in this special issue). While the cause of these effects may be multi-faceted, the GHSR-1a is expressed in the myocardium, raising the potential for direct as well as indirect effects (von Haehling et al. 2009). In animal studies, administration with ghrelin has been shown to increase cardiac output (Nagaya et al. 2001c), decrease cardiac sympathetic tone (Schwenke et al. 2008), decrease in atrial natriuretic peptide (Xu et al. 2005) and decrease cardiomyocyte apoptosis (Xu et al. 2005), with ultimate improvements in mortality rates (Schwenke et al. 2008). Nevertheless, these effects have not been universally demonstrated as other investigators have not found changes in left ventricular (LV) ejection fraction or other markers of cardiac function despite 2-fold increases over placebo in weight and in lean body mass (Akashi et al. 2009).
Systemic cardiovascular effects have also been reported in human studies of ghrelin administration. An unblinded trial of twice-daily IV ghrelin for 3 weeks to subjects with CHF demonstrated increased LV ejection fraction, decreased end-diastolic function and increased peak workload, as well as decreased B-type natriuetic peptide (a marker of LV wall stress)(Nagaya et al. 2004) (Table 1). Other researchers noted blinded doses of ghrelin (vs. placebo) to result in decreased mean arterial pressure and increased cardiac output (without change in heart rate) in subjects with CHF (Nagaya et al. 2001a) and decreased blood pressure in ESRD patients (Wynne et al. 2005; Ashby et al. 2009). It remains unclear whether these are direct actions or secondary to other effects of ghrelin. Ghrelin also exerts effects on body mass in cardiac cachexia in that a three-week course of ghrelin treatment resulted in significant increases in lean mass and muscle strength, gains which were not noted in untreated patients (Nagaya et al. 2004).
Pulmonary cachexia
Chronic respiratory diseases also can result in a cachexia syndrome, including chronic obstructive pulmonary disease and recurrent pneumonias. Each of these results in increased inflammatory cytokines, and as in other syndromes, the presence of cachexia is linked to increased mortality (Andreas et al. 2005). Levels of total ghrelin are elevated in COPD (Itoh et al. 2004); nevertheless, treatment with ghrelin as a twice-daily IV infustion resulted in increased food intake as well as increases in body weight and lean mass (Nagaya et al. 2005). When ghrelin was administered in an unblinded fashion to subjects with recurrent lung infections and unintended weight loss (2 ug/kg IV twice daily for three weeks), this resulted in increased food intake and increased body weight. Moreover, subjects had a decrease in neutrophils and cytokines in their sputum and improved exercise tolerance (Kodama et al. 2008) (Table 1).
Recently, a synthetic GHS-R analogue was shown to increase appetite and increase weight gain over placebo when given as twice-daily SC injections to subjects with COPD (von Haehling et al. 2010). The subcutaneous delivery suggests clear potential for home administration as a cachexia treatment.
Cachexia or anorexia from other underlying diseases
Treatment with ghrelin has also been show to be of benefit in other animal models of cachexia or anorexia, including radiation injury (Shah et al. 2009), chemotherapy (Liu et al. 2006; Garcia et al. 2008) and burn injury (Balasubramaniam et al. 2006). Interestingly, in the case of cisplatin-induced anorexia, ghrelin secretion is blunted (Yakabi et al. 2010), underscoring potential differences in etiology of anorexia among various conditions and—thus far—the ability of ghrelin to stimulate appetite and weight gain despite these differences in etiology (Garcia et al. 2008). Human studies on ghrelin’s use in these additional causes of cachexia have not yet been reported. Efficacy has also been demonstrated in the anorexia of aging, including increased food intake in an animal model (Toshinai et al. 2007) and increased lean mass accrual in a 2-year trial of an oral GHS-R agonist in healthy elderly adults (Nass et al. 2008). Again, the etiology of age-associated anorexia is likely to differ from cachexia of chronic disease, but appears to benefit from treatment with ghrelin.
Safety
While the trials reported here have not had significant side effects associated with ghrelin treatment, potential continues for pharmacologic effects of ghrelin that might limit its application in cachectic patients. Tolerability has not seemed to be a significant effect, though one blinded trial revealed a trend toward increased undesirable gastrointestinal effects among subjects receiving ghrelin (Strasser et al. 2008). These gastrointestinal effects included abdominal pain, dry mouth and an increase in bowel activity, which combined for 11 complaints during ghrelin treatment vs. 4 complaints during saline treatment (Strasser et al. 2008).
As mentioned in the Cancer Cachexia section, theoretical concerns persist regarding the possible effects of ghrelin on processes related to cancer (as reviewed by Chopin et al. in this special issue) and on downstream results of increased levels of GH and IGF-1 in malignancies. IGF-1 signaling plays a role in anchorage-dependent cell growth, which might be important in tumor cell survival (Brodt et al. 2000; Maki 2010). Thus far, these concerns have not borne out in animal models or human application, though longer-term treatment will be necessary.
Additionally, the prospect of increased levels of growth hormone raises potential for increased insulin resistance, and ghrelin administration in healthy individuals has been shown to suppress insulin action and worsen glucose tolerance (Tong et al. 2010). This may prove problematic in some cachexia-associated conditions, given that inflammation and use of steroids are also associated with insulin resistance. The only human trial reporting measures of insulin resistance showed non-significant increases following treatment with GHS-R agonist vs. placebo (Garcia et al. 2007). Nevertheless, while prior trials have not demonstrated significant problems, induction of glucose intolerance could preclude use of ghrelin in a sub-set of patients with cachexia. Clearly, longer-term trials will be necessary to answer these concerns.
Conclusion
Ghrelin has received significant attention for the treatment of cachexia. This is particularly important because no effective treatments have been shown to have such consistent effects in animal models and human trials. Moreover, the additional systemic effects in improved cardiovascular function particularly raise the potential for application in cachectic states involving heart failure. Questions persist including whether ghrelin’s effects will be sustained past the 8–12 week time points that represent the extent of current applications. Further mechanistic studies will be important to determine any synergies that ghrelin treatment may have with additional approaches to treating cachexia. These mechanistic approaches include investigation of anti-inflammatory effects (Chang et al. 2003; Dixit et al. 2004; Granado et al. 2005), beneficial cardiovascular effects (Nagaya et al. 2001c; Nagaya et al. 2004), and effects on the ubiquitome-proteosome system to help prevent losses of lean mass (Mitch and Goldberg 1996; DeBoer et al. 2008), in addition to ghrelin’s effects on appetite and fat storage. Finally, some optimism persists that successful treatment of cachexia with ghrelin may improve the prognosis of underlying causative diseases, which would add immense value to such treatment and remains a topic requiring further research.
Acknowledgments
Funding: 5K08HD060739-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, Nakajima K, Fujiwara Y, Hosoda H, Kangawa K, Mori M, Doki Y. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology. 2010;138:1312–1320. doi: 10.1053/j.gastro.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. 2000;278:E219–225. doi: 10.1152/ajpendo.2000.278.2.E219. [DOI] [PubMed] [Google Scholar]
- Akashi YJ, Palus S, Datta R, Halem H, Taylor JE, Thoene-Reineke C, Dong J, Thum T, Culler MD, Anker SD, Springer J. No effects of human ghrelin on cardiac function despite profound effects on body composition in a rat model of heart failure. Int J Cardiol. 2009;137:267–275. doi: 10.1016/j.ijcard.2008.06.094. [DOI] [PubMed] [Google Scholar]
- American_Heart_Association. Heart Disease and Stroke Statistics. American Heart Association Update; Dallas, TX: 2005. [Google Scholar]
- Andreas S, Anker SD, Scanlon PD, Somers VK. Neurohumoral activation as a link to systemic manifestations of chronic lung disease. Chest. 2005;128:3618–3624. doi: 10.1378/chest.128.5.3618. [DOI] [PubMed] [Google Scholar]
- Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997a;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- Anker SD, Swan JW, Volterrani M, Chua TP, Clark AL, Poole-Wilson PA, Coats AJ. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997b;18:259–269. doi: 10.1093/oxfordjournals.eurheartj.a015229. [DOI] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Iwakura H, Hosoda H, Akamizu T, Arai Y, Kangawa K, Nakao K. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology. 2005;146:355–364. doi: 10.1210/en.2004-0629. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby DR, Ford HE, Wynne KJ, Wren AM, Murphy KG, Busbridge M, Brown EA, Taube DH, Ghatei MA, Tam FW, Bloom SR, Choi P. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009;76:199–206. doi: 10.1038/ki.2009.114. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam A, Wood S, Joshi R, Su C, Friend LA, Sheriff S, James JH. Ghrelin stimulates food intake and growth hormone release in rats with thermal injury: synthesis of ghrelin. Peptides. 2006;27:1624–1631. doi: 10.1016/j.peptides.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Zhu X, Deboer M, Datta R, Culler MD, Zanetti M, Guarnieri G, Marks DL. Combined effects of ghrelin and higher food intake enhance skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rats with chronic kidney disease. Kidney Int. 2010;77:23–28. doi: 10.1038/ki.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber MD, Ross JA, Fearon KC. Changes in nutritional, functional, and inflammatory markers in advanced pancreatic cancer. Nutr Cancer. 1999;35:106–110. doi: 10.1207/S15327914NC352_2. [DOI] [PubMed] [Google Scholar]
- Binn M, Albert C, Gougeon A, Maerki H, Coulie B, Lemoyne M, Rabasa Lhoret R, Tomasetto C, Poitras P. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27:1603–1606. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bossola M, Scribano D, Colacicco L, Tavazzi B, Giungi S, Zuppi C, Luciani G, Tazza L. Anorexia and plasma levels of free tryptophan, branched chain amino acids, and ghrelin in hemodialysis patients. J Ren Nutr. 2009;19:248–255. doi: 10.1053/j.jrn.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Brodt P, Samani A, Navab R. Inhibition of the type I insulin-like growth factor receptor expression and signaling: novel strategies for antimetastatic therapy. Biochem Pharmacol. 2000;60:1101–1107. doi: 10.1016/s0006-2952(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Buscher AK, Buscher R, Hauffa BP, Hoyer PF. Alterations in appetite-regulating hormones influence protein-energy wasting in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2010;25:2295–2301. doi: 10.1007/s00467-010-1588-9. [DOI] [PubMed] [Google Scholar]
- Chang L, Zhao J, Yang J, Zhang Z, Du J, Tang C. Therapeutic effects of ghrelin on endotoxic shock in rats. Eur J Pharmacol. 2003;473:171–176. doi: 10.1016/s0014-2999(03)01972-1. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–269. doi: 10.1097/01.mco.0000165004.93707.88. [DOI] [PubMed] [Google Scholar]
- DeBoer MD. Emergence of ghrelin as a treatment for cachexia syndromes. Nutrition. 2008;24:806–814. doi: 10.1016/j.nut.2008.06.013. [DOI] [PubMed] [Google Scholar]
- DeBoer MD. Animal models of anorexia and cachexia. Expert Opin Drug Discov. 2009;4:1145–1155. doi: 10.1517/17460440903300842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer MD. Update on melanocortin interventions for cachexia: progress toward clinical application. Nutrition. 2010;26:146–151. doi: 10.1016/j.nut.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer MD, Marks DL. Therapy insight: Use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab. 2006;2:459–466. doi: 10.1038/ncpendmet0221. [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Zhu X, Levasseur PR, Inui A, Hu Z, Han G, Mitch WE, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149:827–835. doi: 10.1210/en.2007-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148:3004–3012. doi: 10.1210/en.2007-0016. [DOI] [PubMed] [Google Scholar]
- Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–2082. doi: 10.1002/1097-0142(19950415)75:8<2077::aid-cncr2820750808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Fearon KC. The Sir David Cuthbertson Medal Lecture 1991. The mechanisms and treatment of weight loss in cancer. Proc Nutr Soc. 1992;51:251–265. doi: 10.1079/pns19920036. [DOI] [PubMed] [Google Scholar]
- Garcia J, Boccia R, Graham C, Kumor K, Polvino W. A Phase II, randomized, placebo-controlled, double blind study of the efficacy and safety of RC-1291 for the treatment of cancer-cachexia. Journal of Clinical Oncology Supp; Abstract 2007 American Society of Clinical Oncology (ASCO) Meeting; Chicago, IL. 2007. [Google Scholar]
- Garcia JM, Cata JP, Dougherty PM, Smith RG. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149:455–460. doi: 10.1210/en.2007-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, Smith RG, Cunningham GR, Marcelli M. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–2926. doi: 10.1210/jc.2004-1788. [DOI] [PubMed] [Google Scholar]
- Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res. 2009;19:267–273. doi: 10.1016/j.ghir.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288:E486–492. doi: 10.1152/ajpendo.00196.2004. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, Osuye K, Kangawa K, Matsukura S, Nakazato M. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. 2003;301:275–279. doi: 10.1016/s0006-291x(02)03028-0. [DOI] [PubMed] [Google Scholar]
- Itoh T, Nagaya N, Yoshikawa M, Fukuoka A, Takenaka H, Shimizu Y, Haruta Y, Oya H, Yamagishi M, Hosoda H, Kangawa K, Kimura H. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:879–882. doi: 10.1164/rccm.200310-1404OC. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Balakrishnan VS. The kidney disease wasting: inflammation, oxidative stress, and diet-gene interaction. Hemodial Int. 2006;10:315–325. doi: 10.1111/j.1542-4758.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschop MH. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther. 2008;21:774–779. doi: 10.1016/j.pupt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Laviano A, Inui A, Marks DL, Meguid MM, Pichard C, Rossi Fanelli F, Seelaender M. Neural control of the anorexia-cachexia syndrome. Am J Physiol Endocrinol Metab. 2008;295:E1000–1008. doi: 10.1152/ajpendo.90252.2008. [DOI] [PubMed] [Google Scholar]
- Lear PV, Iglesias MJ, Feijoo-Bandin S, Rodriguez-Penas D, Mosquera-Leal A, Garcia-Rua V, Gualillo O, Ghe C, Arnoletti E, Muccioli G, Dieguez C, Gonzalez-Juanatey JR, Lago F. Des-acyl ghrelin has specific binding sites and different metabolic effects from ghrelin in cardiomyocytes. Endocrinology. 2010;151:3286–3298. doi: 10.1210/en.2009-1205. [DOI] [PubMed] [Google Scholar]
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang LK, Pang YZ, Pan CS, Qi YF, Chen L, Wang X, Tang CS, Zhang J. Cardioprotective effects of ghrelin and des-octanoyl ghrelin on myocardial injury induced by isoproterenol in rats. Acta Pharmacol Sin. 2006;27:527–535. doi: 10.1111/j.1745-7254.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- Liu YL, Malik NM, Sanger GJ, Andrews PL. Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother Pharmacol. 2006;58:326–333. doi: 10.1007/s00280-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- Lundholm K, Gunnebo L, Korner U, Iresjo BM, Engstrom C, Hyltander A, Smedh U, Bosaeus I. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer. 2010;116:2044–2052. doi: 10.1002/cncr.24917. [DOI] [PubMed] [Google Scholar]
- Maki RG. Small is beautiful: insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28:4985–4995. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malendowicz W, Ziolkowska A, Szyszka M, Kwias Z. Elevated blood active ghrelin and unaltered total ghrelin and obestatin concentrations in prostate carcinoma. Urol Int. 2009;83:471–475. doi: 10.1159/000251190. [DOI] [PubMed] [Google Scholar]
- Marks DL, Ling N, Cone RD. Role of the Central Melanocortin System in Cachexia. Cancer Res. 2001;61:1432–1438. [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- Muscaritoli M, Molfino A, Bollea MR, Rossi Fanelli F. Malnutrition and wasting in renal disease. Curr Opin Clin Nutr Metab Care. 2009;12:378–383. doi: 10.1097/MCO.0b013e32832c7ae1. [DOI] [PubMed] [Google Scholar]
- Muscaritoli M, Molfino A, Chiappini MG, Laviano A, Ammann T, Spinsanti P, Melchiorri D, Inui A, Alegiani F, Rossi Fanelli F. Anorexia in hemodialysis patients: the possible role of des-acyl ghrelin. Am J Nephrol. 2007;27:360–365. doi: 10.1159/000103798. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, Kangawa K. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, Kojima M, Nakanishi N, Mori H, Kangawa K. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab. 2001a;86:5854–5859. doi: 10.1210/jcem.86.12.8115. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, Hosoda H, Shimizu W, Yamagishi M, Oya H, Koh H, Yutani C, Kangawa K. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001b;104:2034–2038. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, Hosoda H, Hirota Y, Ishida H, Mori H, Kangawa K. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001c;104:1430–1435. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE, Jr, Clasey JL, Heymsfield SB, Bach MA, Vance ML, Thorner MO. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–611. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naufel MF, Bordon M, de Aquino TM, Ribeiro EB, de Abreu Carvalhaes JT. Plasma levels of acylated and total ghrelin in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2010;25:2477–2482. doi: 10.1007/s00467-010-1628-5. [DOI] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin: a potential therapeutic target for cancer. Regul Pept. 2010;163:7–17. doi: 10.1016/j.regpep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146:2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- Perez-Fontan M, Cordido F, Rodriguez-Carmona A, Garcia-Naveiro R, Isidro ML, Villaverde P, Garcia-Buela J. Acute plasma ghrelin and leptin responses to oral feeding or intraperitoneal hypertonic glucose-based dialysate in patients with chronic renal failure. Kidney Int. 2005;68:2877–2885. doi: 10.1111/j.1523-1755.2005.00761.x. [DOI] [PubMed] [Google Scholar]
- Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Bowe DD, Zhu X, Batra AK, Grant WF, Marks DL. Genetic and pharmacologic blockade of central melanocortin signaling attenuates cardiac cachexia in rodent models of heart failure. J Endocrinol. 2010;206:121–130. doi: 10.1677/JOE-09-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenke DO, Tokudome T, Kishimoto I, Horio T, Shirai M, Cragg PA, Kangawa K. Early ghrelin treatment after myocardial infarction prevents an increase in cardiac sympathetic tone and reduces mortality. Endocrinology. 2008;149:5172–5176. doi: 10.1210/en.2008-0472. [DOI] [PubMed] [Google Scholar]
- Shah KG, Wu R, Jacob A, Blau SA, Ji Y, Dong W, Marini CP, Ravikumar TS, Coppa GF, Wang P. Human ghrelin ameliorates organ injury and improves survival after radiation injury combined with severe sepsis. Mol Med. 2009;15:407–414. doi: 10.2119/molmed.2009.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–778. [PubMed] [Google Scholar]
- Soriano-Guillen L, Barrios V, Campos-Barros A, Argente J. Ghrelin levels in obesity and anorexia nervosa: effect of weight reduction or recuperation. J Pediatr. 2004;144:36–42. doi: 10.1016/j.jpeds.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschop M, Kaufmann K, Holst B, Brandle M, von Moos R, Demmer R, Cerny T. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98:300–308. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89:1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Shimbara T, Yamaguchi H, Date Y, Kangawa K, Nakazato M. Ghrelin stimulates growth hormone secretion and food intake in aged rats. Mech Ageing Dev. 2007;128:182–186. doi: 10.1016/j.mad.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Vigano A, Donaldson N, Higginson IJ, Bruera E, Mahmud S, Suarez-Almazor M. Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer. 2004;101:1090–1098. doi: 10.1002/cncr.20472. [DOI] [PubMed] [Google Scholar]
- von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–252. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- von Haehling S, Stepney R, Anker SD. Advances in understanding and treating cardiac cachexia: highlights from the 5th Cachexia Conference. Int J Cardiol. 2010;144:347–349. doi: 10.1016/j.ijcard.2010.05.042. [DOI] [PubMed] [Google Scholar]
- Wang W, Andersson M, Iresjo BM, Lonnroth C, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol. 2006;28:1393–1400. [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. [accessed 11/18/2010];J Clin Endocrinol Metab. 2001 86:5992. doi: 10.1210/jcem.86.12.8111. www.clinicaltrials.gov. [DOI] [PubMed]
- Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, Brown EA, Bloom SR, Choi P. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111–2118. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- Xin X, Ren AJ, Zheng X, Qin YW, Zhao XX, Yuan WJ, Guo ZF. Disturbance of circulating ghrelin and obestatin in chronic heart failure patients especially in those with cachexia. Peptides. 2009;30:2281–2285. doi: 10.1016/j.peptides.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, Yu XX, Guo S, Chen MC, Chen C. GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H1643–1651. doi: 10.1152/ajpheart.01042.2004. [DOI] [PubMed] [Google Scholar]
- Yakabi K, Sadakane C, Noguchi M, Ohno S, Ro S, Chinen K, Aoyama T, Sakurada T, Takabayashi H, Hattori T. Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010;151:3773–3782. doi: 10.1210/en.2010-0061. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, Kangawa K, Nakao K. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol. 2002;13:2748–2752. doi: 10.1097/01.asn.0000032420.12455.74. [DOI] [PubMed] [Google Scholar]
- Zabel R, Ash S, King N, Bauer J. The relationship between subjective appetite sensations, markers of inflammation and appetite in dialysis patients. J Hum Nutr Diet. 2009;22:343–350. doi: 10.1111/j.1365-277X.2009.00968.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chai B, Li JY, Wang H, Mulholland MW. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology. 2008;149:4710–4716. doi: 10.1210/en.2008-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]