Abstract
CHFR has been implicated as a tumor suppressor in a multitude of cancers. It was originally identified as a major component of the antephase checkpoint. Recently, CHFR was reported to interact with MAD2, an important component of the spindle assembly checkpoint, where CHFR knockdown resulted in mislocalization of MAD2 and disruption of the MAD2/CDC20 interaction. To further understand how CHFR interacts with MAD2, we deleted key functional domains of CHFR, and investigated the effect on MAD2 binding and function. Here we show that deletion of the cysteine-rich domain of CHFR is required for the CHFR/MAD2 interaction as well as proper localization of MAD2 in the cell. Furthermore, the cysteine-rich domain deletion exhibits impaired ability to promote the MAD2/CDC20 interaction, leading to an increase in mitotic defects relative to wild type CHFR. These data support a critical role for CHFR in the MAD2 spindle checkpoint. Furthermore, these data establish the cysteine-rich domain of CHFR as the essential domain for the CHFR/MAD2 interaction and for promoting interaction between MAD2 and CDC20 to inhibit the anaphase-promoting complex.
Introduction
CHFR (Checkpoint with FHA and Ring finger) is an E3 ubiquitin ligase that functions as a mitotic checkpoint protein and has been implicated as a tumor suppressor in a wide array of cancer types [1-3]. Chfr-/- mice are predisposed to cancers and show increased tumor formation in response to DMBA treatment [4]. In addition, Chfr knockout (Chfr-/-) mouse embryo fibroblasts (MEFs) display mitotic defects and become aneuploid over time in culture [4]. A similar aneuploidy and mitotic defect phenotype was observed in immortalized breast epithelial cells when CHFR was knocked down by shRNA [5]. Aneuploidy is often observed as a consequence of mitotic checkpoint defects. In fact, CHFR was initially identified as an antephase checkpoint protein essential for triggering the mitotic stress checkpoint in response to nocodazole treatment [1, 3]. Subsequent studies have identified Aurora A and Kif22 as ubiquitination targets of CHFR, implicating CHFR in the spindle-assembly checkpoint occurring later in mitosis [4, 6].
Mitotic arrest deficient 2 (MAD2) has been identified as a key protein responsible for detecting proper spindle attachment to kinetochores, and triggers delay through inhibition of the anaphase-promoting complex (APC) when attachments are incomplete [7]. This inhibition occurs through binding of MAD2 to CDC20, which inhibits its activation of APC [8]. While this checkpoint has been extensively studied, full understanding of the mechanism by which MAD2 and CDC20 interact to trigger the spindle checkpoint remains elusive. Recently, MAD2 was identified as a CHFR-interacting protein by yeast-two-hybrid, and this interaction was verified using cultured cells [9]. Notably, knockdown of CHFR via siRNA resulted in mislocalization of MAD2 during mitosis, and inhibited the MAD2/CDC20 interaction [9]. These data implicate CHFR in regulation of the MAD2/CDC20 interaction, and may point to a complex role of CHFR in the spindle checkpoint, but further analyses supporting an interaction between CHFR and MAD2 have not yet been reported.
To better understand the role of CHFR in the MAD2-dependent spindle checkpoint, we deleted key CHFR domains and examined the effect on MAD2. We find that the FHA and Ring domains are not required for MAD2 binding to CHFR, while the C-terminal cysteine-rich domain is required for this interaction. Furthermore, the FHA and Ring domain deletions had no effect on MAD2-CDC20 binding, while deletion of the cysteine-rich domain inhibited this interaction. Finally, deletion of the cysteine-rich domain resulted in mislocalization of MAD2 in mitotic cells, leading to improper chromosome migration. Together, this data suggest that CHFR binding to MAD2 is important for proper cellular localization of MAD2 during mitosis and effective activation of the mitotic spindle checkpoint.
Materials and Methods
Plasmids and Antibodies
CHFR deletion constructs were created using the QuikChange site-directed mutagenesis kit (Stratagene). MAD2 antibodies were obtained from BD Biosciences (blotting) and Santa Cruz (Immunoprecipitation). CHFR antibody was a gift from the Yu lab (University of Michigan); CDC20 antibody was from BD biosciences. Anti-Flag and anti-HA antibodies were from Sigma and Covance, respectively. Fluorescent secondary antibodies were purchased from Jackson ImmunoResearch.
Cell Culture and Immunoprecipitation
HEK293T cells were obtained from ATCC and cultured in DMEM containing 10% FBS. Immortalized mouse embryonic fibroblasts were a gift from the Yu lab (University of Michigan). Transfection of HEK293T cells was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. MEF cells were transfected by electroporation (BioRad Gene Pulser XCell). Transfected cells were treated with nocodazole for 18 hours, then lysis with NETN buffer [4], spun at 4 °C and supernatant collected. For immunoprecipitation, lysate was incubated overnight with Protein A (Invitrogen), Protein G (Invitrogen) or anti-Flag beads (Sigma), and the indicated antibodies. After binding, beads were washed three times with NETN buffer and subjected to Western blot.
Immunofluorescence
After electroporation, MEFs were plated to glass coverslips and allowed to recover for 24 hours, then treated with nocodazole for 18 hours. After treatment, cells were fixed in cold methanol for 10 minutes, then stained with the indicated antibodies at 1:1000 dilution in 8% goat serum/PBS for one hour. Secondary antibodies were applied at 1:600 dilution for 30 minutes, followed by 1 minute of dapi/PBS.
Results
The Cystein-rich domain is required for CHFR-MAD2 binding
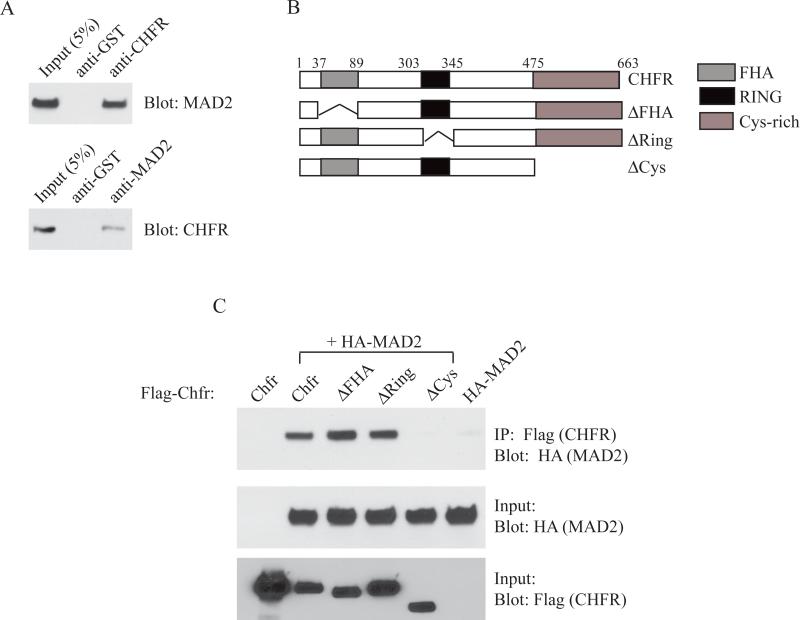
The interaction between CHFR and MAD2 was originally reported in 2008 by Privette et al [9]. We verified this interaction by immunoprecipitation using antibodies against endogenous CHFR and MAD2 in HEK293T cells (Figure 1A). To further examine this interaction we utilized three deletion constructs of CHFR. CHFR has several reported domains including the forkhead associated (FHA), the RING finger (RING) and the cysteine-rich (Cys) domains (Figure 1B) [3]. The FHA domain is thought to be a phosphothreonine binding domain, although no binding target for the CHFR FHA domain has been reported [10]. The RING domain is required for the ubiquitin ligase activity of CHFR[11], and is required for the mitotic stress checkpoint [1]. Finally, the Cys domain of CHFR has been identified as a protein-binding domain, required for the interaction of CHFR with its ubiquitination targets Aurora A and Kif22 [4, 6]. Using co-immunoprecipitation with individual deletion constructs, we investigated which domain is required for MAD2 binding. As shown in Figure 1C, only the CHFRCys mutant protein failed to pull down HA-tagged MAD2, while CHFRFHA and CHFRRing deletions had no effect on HA-MAD2 binding. This indicated that the Cys domain was likely the domain of MAD2 binding, consistent with previous findings that the Cys domain is important for CHFR protein-protein interactions.
Figure 1.
The cysteine-rich domain of CHFR is required for MAD2 binding. (A) Co-immunoprecipitation (Co-IP) of MAD2 with CHFR in HEK293T cells. (B) Schematic of CHFR functional domains and deletion constructs used. (C) HEK293T cells cotransfected with HA-Mad2 and Flag-Chfr constructs were subjected to immunoprecipitation using anti-Flag beads and blotted with anti-HA antibody. Lower two blots show input controls.
CHFRCys expression cannot rescue abnormal MAD2 localization in CHFR-/- MEFs
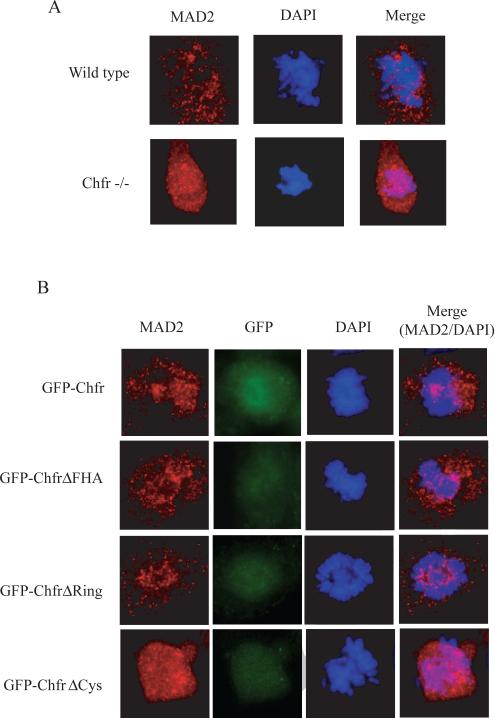
Previous work has shown that siRNA reduction of CHFR in human cell lines results in mislocalized MAD2 and disruption of the MAD2-dependent spindle-assembly checkpoint [9]. We were able to confirm this phenotype using immunofluorescence of endogenous MAD2 in immortalized Chfr-/- mouse embryonic fibroblasts (MEFs). While MAD2 in wild type MEFs displayed the characteristic punctate staining by immunofluorescence, Chfr-/- MEFs showed more diffuse MAD2 localization (Figure 2A). Transfection of GFP-tagged CHFR, as well as GFP-CHFRFHA and GFP-CHFRRing constructs into CHFR-/- MEFs restored the punctate staining, while GFP-CHFRCys was unable to restore punctate MAD2 staining in mitotic cells (Figure 2B). These data suggested that CHFR plays a role in MAD2 localization, which is dependent on binding via the cysteine-rich domain of CHFR.
Figure 2.
The cysteine-rich domain is necessary for MAD2 localization in mitosis. (A) Endogenous MAD2 and CHFR proteins stained using indirect immunofluorescence in immortalized MEFs. (B) Endogenous MAD2 stained as above, with GFP-tagged CHFR visualized to identify transfected cells. Dapi staining for mitotic DNA.
CHFRCys does not rescue segregation defects in CHFR-/- MEFs
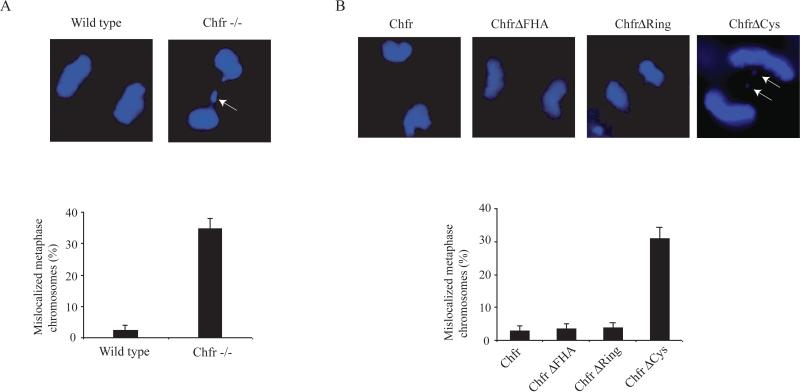
MAD2 functions in the cell as part of the spindle assembly checkpoint, to ensure proper chromosome segregation during mitosis. Considering the MAD2 localization changes we observed in transfected CHFR-/- MEFs, we wondered if the localization of MAD2 correlated to a spindle-assembly checkpoint phenotype. Thus, we investigated if the CHFRCys construct, and other deletion constructs, could rescue the chromosome segregation defects seen in CHFR-/- MEFs. Dapi staining of CHFR-/- and wild type MEFs indicates that CHFR-/- MEFs have a higher incidence of lagging chromosomes, in agreement with previous reports using primary MEF cells [9] (Figure 3A). Transfection of CHFR-/- MEFs with CHFR, CHFRFHA, and CHFRRing constructs was able to reduce the incidence of lagging chromosomes, while cells expressing CHFRCys had an incidence of mitotic defects similar to untransfected CHFR-/- cells (Figure 4B).
Figure 3.
Chromosome segregation defects persist in CHFRCys-expressing cells. (A) Representative images of wild type and CHFR-/- MEFs after chromosome segregation. Percentage of mitotic cells with lagging chromosomes is indicated in the histogram below. (B) Representative images of transfected CHFR-/- MEFs after chromosome segregation. (C) Incidence of chromosome segregation defects was quantitated for each transfected cell line (below).
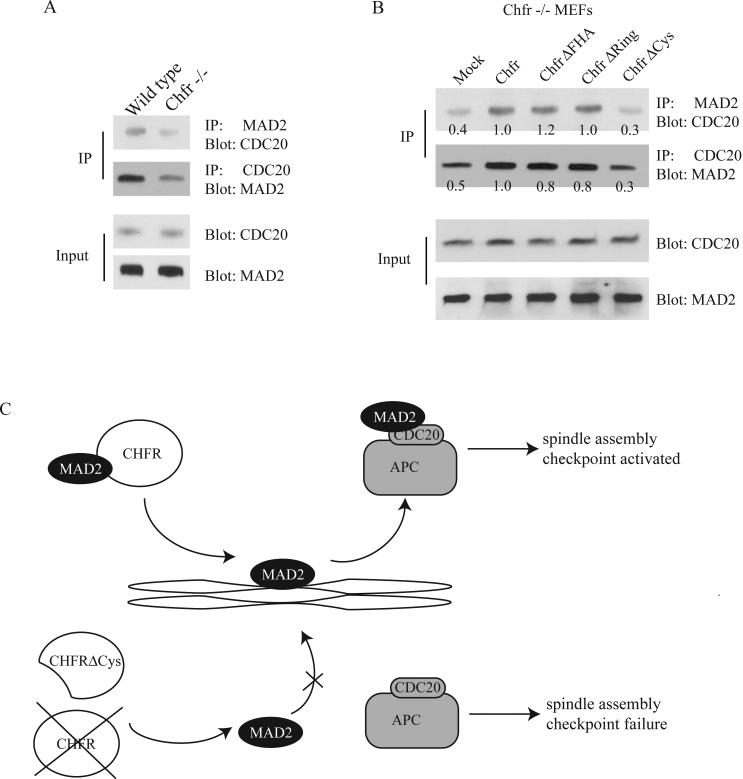
Figure 4.
The MAD2/CDC20 interaction is impaired in CHFR-/- and CHFRCys-expressing cells. (A) Immunoprecipitation of MEF lysates with either MAD2 or CDC20 antibodies was performed and blotted with the indicated antibodies. (B) Immunoprecipitation of transfected MEF lysates with either MAD2 or CDC20 antibodies was performed and blotted with the indicated antibodies. Approximate band intensities are marked below bands. Intensity was estimated using ImageJ and normalized to input. (C) Model of CHFR/MAD2 interaction and spindle checkpoint control. Above, in the presence of full length CHFR, CHFR binds to MAD2 and MAD2 is localized to the kinetochore, leading to binding to CDC20 to inhibit APC and trigger the spindle-assembly checkpoint. Below, in the presence of CHFRCys, or in the absence of CHFR protein, MAD2 fails to localize to the kinetochore and does not bind CDC20 to trigger the checkpoint.
The Cystein-rich domain of CHFR is important for MAD2-CDC20 binding
The mitotic defect seen in CHFR-/- MEFs and maintained when those cells expressed CHFRCys suggests that the cysteine-rich domain of CHFR may be critical for proper MAD2 function. To further investigate the effect of CHFRCys on MAD2 function, we used co-immunoprecipitation to assay the binding of MAD2 with CDC20 in MEFs expressing CHFR constructs. In agreement with previous data using siRNA reduction of CHFR [9], we observed a decrease in MAD2-CDC20 binding in CHFR-/- MEFs relative to wild type MEFs (Figure 4A). Notably, expression of CHFR in CHFR-/- MEFs increased the interaction between CDC20 and MAD2 (Figure 4B). Expression of both CHFRFHA and CHFRRing constructs were co-immunoprecipitated with MAD2 at levels similar to wild type, while CHFRCys was unable to increase the MAD2-CDC20 interaction (Figure 4B). This suggests that the cysteine-rich domain is necessary for CHFR to enhance MAD2-CDC20 binding, possibly through control of MAD2 localization during mitosis.
Discussion
The MAD2 spindle assembly checkpoint is the focus of a wide number of studies. While the complex mechanism by which MAD2 performs its function is not fully understood, the interaction between MAD2 and CDC20 has been established as a critical component of this pathway [7, 12]. Current models predict that MAD2 forms a diffusible complex with CDC20, BUBR1, and BUB3 at unattached kinetochores [7, 13, 14]. This binding impairs the CDC20-dependent activation of the anaphase-promoting complex and is abolished by an unknown mechanism when the spindle-assembly checkpoint is satisfied [7, 13, 14].
Our data support the hypothesis that CHFR may play a role in localization of MAD2 to one or more of its functional complexes, and loss of CHFR or the cysteine-rich domain of CHFR can disrupt the MAD2 spindle-assembly checkpoint pathway. In the absence of functional CHFR, MAD2 may not be transported to the kinetochores for activation (Figure 4C). This disruption would result in reduced inhibition of the anaphase-promoting complex, effectively repressing the spindle-assembly checkpoint and promoting anaphase progression. While CHFR has been defined as an E3 ubiquitin ligase [3], we could not see any measurable change in MAD2 or CDC20 protein levels in cells expressing CHFRRing, which is unable to ubiquitinate substrates (Figure 4B) [1]. However, our results suggest an important role for CHFR in MAD2 localization and activation.
The cysteine-rich domain appears to be a major protein-protein interaction domain for CHFR, as it has been identified as the domain required for interactions with Aurora A, Kif22, and HDAC1 in addition to MAD2 [4, 6, 15]. The cysteine-rich domain also contains the PAR (poly(ADP-ribose))-binding zinc finger (PBZ) motif, which binds to PAR-bound proteins [16]. Interestingly, PAR-proteins accumulate on the mitotic machinery, and PAR addition to proteins is critical for spindle function [16-18]. It is possible that the cysteine-rich domain deletion is unable to localize to PAR-enriched sites, which then results in reduced binding to MAD2. Alternatively, CHFR binding to MAD2 could precede localization to the mitotic machinery. Extensive future work will be required to decipher the exact mode of action of CHFR with MAD2.
The interaction between CHFR and MAD2 represents another layer of complexity in the mitotic checkpoint function of CHFR, and may have important implications in cancer treatment. Both MAD2 and CHFR have been implicated as tumor suppressors, and changes in expression of both proteins have been linked to tumorigenesis [4, 19]. Chfr expression is reduced by promoter methylation in a wide array of cancer types [2]. A small number of CHFR mutations have been identified in cancer cells [2, 20]. One polymorphism (V539M) located in the cysteine-rich domain was reported to be strongly associated with colorectal cancer risk [21]. Surprisingly, MAD2 overexpression was reported to lead to tumorigenesis in mice and was shown to correlate to shorter survival in lung and bone cancers, suggesting that MAD2 has multiple functions in mitosis, which are sensitive to changes in MAD2 dosage [22-24]. Clearly both MAD2 and CHFR represent interesting candidates for cancer biomarkers. Further understanding of the interaction between CHFR and MAD2 may lead to increased accuracy in cancer prognoses as well as more fine-tuned cancer treatments in the future.
Acknowledgements
The authors would like to thank Dr Xiaochun Yu for sharing reagents and equipment, and for critical reading of the manuscript. We also thank Dr Jiaxue Wu for technical guidance and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaturvedi P, Sudakin V, Bobiak ML, Fisher PW, Mattern MR, Jablonski SA, Hurle MR, Zhu Y, Yen TJ, Zhou BB. Chfr regulates a mitotic stress pathway through its RING-finger domain with ubiquitin ligase activity. Cancer Res. 2002;62:1797–801. [PubMed] [Google Scholar]
- 2.Privette LM, Petty EM. CHFR: A Novel Mitotic Checkpoint Protein and Regulator of Tumorigenesis. Transl Oncol. 2008;1:57–64. doi: 10.1593/tlo.08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scolnick DM, Halazonetis TD. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 2000;406:430–5. doi: 10.1038/35019108. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ, Ward IM, Saya H, Fang G, van Deursen J, Chen J. Chfr is required for tumor suppression and Aurora A regulation. Nat Genet. 2005;37:401–6. doi: 10.1038/ng1538. [DOI] [PubMed] [Google Scholar]
- 5.Privette LM, Gonzalez ME, Ding L, Kleer CG, Petty EM. Altered expression of the early mitotic checkpoint protein, CHFR, in breast cancers: implications for tumor suppression. Cancer Res. 2007;67:6064–74. doi: 10.1158/0008-5472.CAN-06-4109. [DOI] [PubMed] [Google Scholar]
- 6.Maddika S, Sy SM, Chen J. Functional interaction between Chfr and Kif22 controls genomic stability. J Biol Chem. 2009;284:12998–3003. doi: 10.1074/jbc.M900333200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H. Regulation of APC-Cdc20 by the spindle checkpoint. Curr Opin Cell Biol. 2002;14:706–14. doi: 10.1016/s0955-0674(02)00382-4. [DOI] [PubMed] [Google Scholar]
- 8.Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol Biol Cell. 2002;13:755–66. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Privette LM, Weier JF, Nguyen HN, Yu X, Petty EM. Loss of CHFR in human mammary epithelial cells causes genomic instability by disrupting the mitotic spindle assembly checkpoint. Neoplasia. 2008;10:643–52. doi: 10.1593/neo.08176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks L, 3rd, Heimsath EG, Jr., Loring GL, Brenner C. FHA-RING ubiquitin ligases in cell division cycle control. Cell Mol Life Sci. 2008;65:3458–66. doi: 10.1007/s00018-008-8220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang D, Chen J, Wong J, Fang G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J Cell Biol. 2002;156:249–59. doi: 10.1083/jcb.200108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang G, Yu H, Kirschner MW. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–83. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma HT, Poon RY. Orderly Inactivation of the Key Checkpoint Protein Mitotic Arrest Deficient 2 (MAD2) during Mitotic Progression. J Biol Chem. 2011;286:13052–9. doi: 10.1074/jbc.M110.201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suijkerbuijk SJ, Kops GJ. Preventing aneuploidy: the contribution of mitotic checkpoint proteins. Biochim Biophys Acta. 2008;1786:24–31. doi: 10.1016/j.bbcan.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Oh YM, Kwon YE, Kim JM, Bae SJ, Lee BK, Yoo SJ, Chung CH, Deshaies RJ, Seol JH. Chfr is linked to tumour metastasis through the downregulation of HDAC1. Nat Cell Biol. 2009;11:295–302. doi: 10.1038/ncb1837. [DOI] [PubMed] [Google Scholar]
- 16.Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, West SC. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451:81–5. doi: 10.1038/nature06420. [DOI] [PubMed] [Google Scholar]
- 17.Chang P, Jacobson MK, Mitchison TJ. Poly(ADP-ribose) is required for spindle assembly and structure. Nature. 2004;432:645–9. doi: 10.1038/nature03061. [DOI] [PubMed] [Google Scholar]
- 18.Oberoi J, Richards MW, Crumpler S, Brown N, Blagg J, Bayliss R. Structural basis of poly(ADP-ribose) recognition by the multizinc binding domain of checkpoint with forkhead-associated and RING Domains (CHFR). J Biol Chem. 2010;285:39348–58. doi: 10.1074/jbc.M110.159855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, Dobles M, Sorger PK, Murty VV, Benezra R. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–9. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 20.Mariatos G, Bothos J, Zacharatos P, Summers MK, Scolnick DM, Kittas C, Halazonetis TD, Gorgoulis VG. Inactivating mutations targeting the chfr mitotic checkpoint gene in human lung cancer. Cancer Res. 2003;63:7185–9. [PubMed] [Google Scholar]
- 21.Kang HC, Kim IJ, Jang SG, Hong SH, Hwang JA, Shin HR, Park JG. Coding region polymorphisms in the CHFR mitotic stress checkpoint gene are associated with colorectal cancer risk. Cancer Lett. 2008;260:170–9. doi: 10.1016/j.canlet.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato T, Daigo Y, Aragaki M, Ishikawa K, Sato M, Kondo S, Kaji M. Overexpression of MAD2 predicts clinical outcome in primary lung cancer patients. Lung Cancer. 2011 doi: 10.1016/j.lungcan.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Guo WC, Zhao SH, Tang J, Chen JL. Mitotic arrest defective protein 2 expression abnormality and its clinicopathologic significance in human osteosarcoma. APMIS. 2010;118:222–9. doi: 10.1111/j.1600-0463.2009.02583.x. [DOI] [PubMed] [Google Scholar]