Abstract
Background
Self-care is believed to improve heart failure (HF) outcomes, but the mechanisms by which such improvement occurs remain unclear.
Methods
We completed a secondary analysis of cross-sectional data collected on adults with symptomatic HF to test our hypothesis that effective self-care is associated with less myocardial stress and systemic inflammation. Multivariate logistic regression modeling was used to determine if better HF self-care reduced the odds of having serum levels of NT proBNP and soluble TNFα receptor type 1 at or above the sample median. HF self-care was measured using the Self-Care of Heart Failure Index.
Results
The sample (n=168) was predominantly male (65.5%) and most (50.6%) had NYHA III HF (mean LVEF= 34.9%±14.0%); mean age was 58.8±11.5 years. Self-care management was an independent factor in the model (block χ2 =14.74, p=.005) after controlling for pertinent confounders (model χ2 =52.15, p<.001). Each one-point increase in self-care management score (range 15–100) was associated with a 12.7% reduction in the odds of having both biomarkers at or above the sample median (adjusted odds ratio =0.873, 95% CI=0.77–0.99, p=.03).
Conclusion
Better self-care management was associated with reduced odds of myocardial stress and systemic inflammation over and above pharmacologic therapy and other common confounding factors. Teaching HF patients early symptom recognition and self-care of symptoms may decrease myocardial stress and systemic inflammation.
Keywords: heart failure, self-care, biomarkers, adherence
Introduction
According to commonly-accepted pathogenic hypotheses, hormones1 and pro-inflammatory cytokines2 produced in response to heart failure (HF) contribute to progressive deterioration of cardiac performance. In support of these hypotheses, poor HF outcomes have been linked to elevated serum levels of biomarkers of myocardial stress and inflammation, including cardiac hormones3,4 and pro-inflammatory mediators.5,6 In addition to optimal medical management, engaging in effective self-care may be one way of reducing myocardial stress and limiting systemic inflammation in patients with HF.7
Self-care of HF involves behaviors that help prevent HF exacerbations such as following treatment advice (self-care maintenance).8 Importantly, self-care of HF also involves the decision-making process in which patients evaluate and address HF symptoms when they occur (self-care management).8 For HF clinicians, teaching and promoting effective self-care is a core component of disease management.9 Yet, there is limited evidence to support the assumption that effective HF self-care improves health outcomes.10 A highlight of the recent American Heart Association scientific statement on promoting HF self-care was the need to establish mechanisms by which self-care of HF may influence neurohormonal and inflammatory function.11 Although we have proposed hypothetical mechanisms by which self-care may influence both processes in the HF population,7 these mechanisms remain untested.
The purpose of this study was to describe the relationship between participant-reported HF self-care and serum biomarkers of myocardial stress and systemic inflammation. We hypothesized that patients who reported better HF self-care would be less likely to have elevated serum levels of amino terminal pro-B-type natriuretic peptide (NT-proBNP), an index of myocardial stress,12 and soluble tumor necrosis factor alpha receptor 1 (sTNFR1), an index of systemic inflammation and marker of HF pathogenesis.12 Using established prognostic cut-off values for NT-proBNP3 and sTNFR1,6 we tested the influence of HF self-care on the odds of having serum levels of NT-proBNP and sTNFR1 at or above the sample median.
Methods
We completed a secondary analysis of cross-sectional data collected at the time of enrollment in two parent studies13,14 conducted by a single group of HF investigators. Measures of HF self-care and biomarker data were available on 168 community-dwelling subjects, all of whom reported having HF symptoms within the past 3 months and did not have an implanted re-synchronization device. Subjects were recruited from academic medical centers in the East South Central region of the United States between 2004 and 2008. Data were collected by research staff members who were rigorously trained and retrained periodically by senior scientists and observed to meet the highest standards in every aspect of data collection. The parent studies were reviewed and approved by the institutional review boards at each participating center. Written informed consent was obtained from all study participants.
Measurement
Baseline sociodemographic characteristics were measured during enrollment. Age, gender, and ethnicity were collected using patient interview and medical record review. Height and weight, measured using a stadiometer and beam scale, were used to calculate body mass index (BMI). Comorbid conditions were assessed with the interview format of the Charlson Comorbidity Index.15 A list of 17 comorbid diseases was evaluated with the possible score ranging from 0 to 30; all participants had a score of at least 1 because all had HF. Clinical HF characteristics including duration of HF, etiology, and left ventricular ejection fraction (LVEF) were extracted from the medical record. Functional status was measured using clinician-rated New York Heart Association (NYHA) functional class obtained through chart review.
HF self-care was measured by participant-report using the Self-Care of Heart Failure Index (SCHFI) v.4.8 In most cases, SCHFI data were collected through interview; some participants opted to complete the SCHFI themselves. The SCHFI contains 15 items measured on a four-point Likert scale and captures self-care maintenance (adherence behaviors that maintain physiologic homeostasis and prevent HF symptoms and exacerbations), self-care management (symptom evaluation, symptom treatment, and treatment evaluation behaviors), and self-care confidence (perceived ability to engage in each phase of the self-care management process). HF patients were asked to think about the past 3 months when completing the SCHFI. Scores on each of these three SCHFI scales were standardized to range from 0–100, with higher scores indicating better self-care. Reliability (alpha) of the SCHFI v.4 maintenance, management, and confidence scores have been reported previously as 0.56, 0.70, and 0.82 respectively; construct validity of the SCHFI scales also has been supported by confirmatory factor analysis.8
Serum samples were collected during enrollment into the parent studies. Enzyme immunoassays kits were used to determine serum concentrations of NT-proBNP (ALPCO Diagnostics, Salem NH), and sTNFR1 (R &D Systems, Minneapolis MN). All samples were run in duplicate with an average inter-sample coefficient of variation (CV) < 2%; samples with a CV ≥ 10% were repeated. Serum levels of NT-proBNP were quantified in fmol/mL, and serum levels of sTNFR1 were quantified in pg/ml. We used NT-proBNP and sTNFR1, instead of B-type natriuretic peptide and tumor necrosis factor alpha, respectively, because of the previously established prognostic cutoff values of these biomarkers in prior research.3,6
Myocardial Stress and Systemic Inflammation
Our interest focused on the influence of HF self-care on the odds of having higher levels of both NT-proBNP and sTNFR1 for four reasons. First, using multiple markers of HF pathogenesis improves the prediction of poor cardiovascular outcomes.16 Second, there is significant intra-individual variability in serum levels of both NT-proBNP17 and sTNFR118 in persons with HF that interjects random error and can limit estimates of association,19 especially with cross-sectional data. Third, according to the most recent categorization of HF biomarkers, sTNFR1 is an index inflammation and HF pathogenesis,12 whereas NT-proBNP is considered an index of myocardial stress12 and should only be used as a confirmatory biomarker.20 Thus, fulfilling a fundamental requirement for effective multiple biomarker strategies21 and a recommendation for biomarker profiling in patients with HF,12 NT-proBNP and sTNFR1 represent distinct classes of biomarkers and reflect separate processes. Fourth and most importantly, we have hypothesized previously that HF self-care influences health outcomes through multiple cardioprotective means concurrently.7
Statistical Analysis
All analyses were performed using Stata/MP version 10.0 (StataCorp, College Station, TX). Statistical significance was predetermined at p < .05. Continuous normally distributed data are expressed as mean values (±SD). Non-normally distributed data (i.e. continuous biomarker data) are expressed as the median [25th, 75th percentile]. Multivariate logistic regression modeling was used to determine if better self-care reduced the odds of patients having levels of both NT pro-BNP and sTNFR1 above the sample median. For reference, we also present modeling of each biomarker separately. Median, as opposed to mean, values were used based on the work of Gardner et al.3 and Ueland et al.,6 and due to the skewed distribution of both biomarkers.
Rationale for Model Covariates
Patient characteristics of age, gender, BMI, and Charlson comorbidity category (low, medium, high), and HF characteristics of LVEF, NYHA functional class, and HF etiology were included in the first block of the model. Levels of NT-proBNP increase with age22 and are higher in women.22 On average, plasma levels of NT-proBNP are inversely proportional to values of BMI23 and vary considerably by comorbid conditions.24 Plasma levels of NT-proBNP are inversely proportional to values of LVEF25 and directly proportional to NYHA functional class.25 Levels of sTNFR1 vary significantly by HF etiology.26 We also controlled for commonly prescribed HF medications with neurohomonal blocking and/or anti-inflammatory effects (β-adrenergic blockers,27 angiotensin converting enzyme [ACE] inhibitors28 or angiotensin II receptor blockers [ARB]s,29 and cholesterol lowering agents.30
The Additive Influence of Heart Failure Self-Care
To determine the additive influence of measures of HF self-care in explaining the odds of having higher levels of both biomarkers, the three SCHFI scales (continuous data) were added to the second block of the model. Based on observed nonlinear bivariate relationships between self-care management and both biomarkers (data not shown), we also tested the quadratic effect of self-care management. Using the most common method of testing for a nonlinear relationship,31 we included both the SCHFI self-care management score and self-care management score-squared as independent variables in the second block of the model. The influence of each model block was assessed by evaluating the significance of the block and overall model χ2. Model fit was assessed using model χ2, Nagelkerke psedo-R2, and correct classification rates. The significance of individual characteristics was evaluated by calculating adjusted odds ratios (OR) with 95% confidence intervals (CI).
Results
The sample (n = 168) was predominantly male, Caucasian, and in middle adulthood (Table 1). Most (58.4%) had at least a moderate Charlson comorbidity score and HF of ischemic origin. Coefficient alpha for the SCHFI scales for patients included in this analysis were .68 (maintenance), .89 (management), and .86 (confidence). Average levels of self-care were generally low, with mean values of each SCHFI scale below the previously established cut-point of adequacy (70 out of 100 points).32 The median serum level of NT-proBNP was 582.8 fmol/mL and the median serum level of sTNFR1 was 1710.1 pg/mL.
Table 1. Sociodemographic and Clinical Characteristics of the Sample (N=168).
ACE=angiotensin converting enzyme, ARB=angiotensin II receptor blocker, NT-proBNP=amino terminal pro B-type natriuretic peptide, NYHA=New York Heart Association, SCHFI=Self-Care of Heart Failure Index, SD=standard deviation, sTNFR1=soluble tumor necrosis factor alpha receptor type 1.
| Variable | Mean ± SD, n (%), or Median [25th,75th percentile] |
|---|---|
| Patient Characteristics: | |
| Age (years) | 58.8 ± 11.4 |
| Female | 58 (34.5%) |
| Ethnicity: | |
| African America | 30 (17.9%) |
| Caucasian | 136 (81.0%) |
| Mixed | 2 (1.2%) |
| Education: | |
| < High school graduate | 35 (20.8%) |
| High school graduate | 50 (29.8%) |
| Some college | 42 (26.2%) |
| Associate degree or greater | 38 (22.6%) |
| Body Mass Index (kg/m2) | 32.8 ± 7.1 |
| Charlson Comorbidity Category: | |
| Score of 1 or 2 (low) | 70 (41.7%) |
| Score of 3 or 4 (medium) | 48 (28.6%) |
| Score of 5 or more (high) | 50 (29.8%) |
| Heart Failure Characteristics: | |
| Left Ventricular Ejection Fraction (%) | 34.8 ± 14.0 |
| NYHA Functional Class: | |
| Class I | 5 (3.0%) |
| Class II | 49 (29.2%) |
| Class III | 85 (50.6%) |
| Class IV | 29 (17.3%) |
| Heart Failure Etiology: | |
| Ischemic | 88 (52.4%) |
| Idiopathic | 32 (19.0%) |
| Hypertensive | 18 (10.7%) |
| Other (known) | 30 (17.9%) |
| Prescribed a β-blocker (%) | 148 (88.1%) |
| Prescribed an ACE or ARB (%) | 135 (80.4%) |
| Prescribed a cholesterol lowering agent (%) | 113 (67.3%) |
| Self-Care of Heart Failure: | |
| SCHFI Maintenance Score | 63.0 ± 16.2 |
| SCHFI Management Score | 61.1 ± 19.2 |
| SCHFI Confidence Score | 64.6 ± 15.0 |
| Biomarkers: | |
| NT-proBNP (fmol/mL) | 582.8 [431.7,845.6] |
| range | 59.9 to 3301.5 |
| sTNFR1 (pg/mL) | 1710.1 [1390.8,2234.5] |
| range | 795.3 to 9718.0 |
Full multivariate models are presented in Table 2. Only participant age and self-care management score consistently influenced the likelihood of having higher biomarker levels across the three models. That is, older age was associated with increased odds of having higher NT-proBNP, higher levels of sTNFR1, and higher levels of both NT-proBNP and sTNFR1. Pointedly, although not consistently significant, better self-care management was associated with lower odds of higher biomarker levels. Moreover, the relationship between self-care management and the odds higher biomarker levels was consistently nonlinear.
Table 2. Adjusted Odds (and 95% CI) of Having Heart Failure Biomarkers at or Above the Sample Median: Self-Care Management and Other Factors Retaining Individual Significance.
All results (displayed in adjusted odds ratios and 95% confidence intervals) are adjusted for age, gender, body mass index, left ventricular ejection fraction, New York Heart Association functional class, heart failure etiology, prescription of a β-adrenergic blocker, prescription of an angiotensin converting enzyme inhibitor or angiotensin receptor blocker, prescription of a cholesterol lowering agent, Charlson Comorbidity Index Category, and Self-Care of Heart Failure Index maintenance and confidence scale scores.
| NT-proBNP | sTNFαR1 | NT-proBNP & sTNFαR1 | |
|---|---|---|---|
| Age | 1.051 (1.009–1.095) | 1.077 (1.035–1.121) | 1.104 (1.050–1.159) |
| Body Mass Index | 0.922 (0.864–0.984) | 1.080 (1.016–1.149) | - |
| NYHA Class | - | 2.044 (1.113–3.754) | - |
| ACE or ARB | - | 0.218 (0.074–0.641) | - |
| Charlson Category | - | 1.754 (1.069–2.878) | 2.109 (1.163–3.824) |
| SC Management | 0.908 (0.803–1.032)† | 0.945 (0.836–1.067)† | 0.873 (0.771–0.988) |
| SC Management × SC Management * |
1.001 (1.0001–1.002) | 1.001 (1.000–1.002) | 1.001 (1.0003–1.002) |
| Model Fit: | |||
| χ2 | 33.06; p=.003 | 58.52; p<.001 | 52.15; p<.001 |
| Nagelkerke R2 | .299 | .404 | .410 |
| CCR | 68.5% | 76.0% | 86.9% |
Abbreviations ACE= angiotensin converting enzyme inhibitor, ARB= angiotensin receptor blocker, CCR = correct classification rate, CI=confidence interval, NT-proBNP=amino terminal pro B-type natriuretic peptide, NYHA= New York Heart Association, SC=self-care, sTNFR1=soluble tumor necrosis factor alpha receptor type 1.
quadratic function of self-care management score.
did not retain individual significance.
A minority of subjects (n=37, 22.0%) had serum levels of both NT-proBNP and sTNFR1 above the sample median. Patient characteristics (age, gender, BMI, and Charlson comorbidity category), and HF characteristics (LVEF, NYHA, etiology, and HF medications) added significantly to the model (block χ2 = 37.41, p < .001). In the second block, the three self-care scales and the quadratic effect of self-care management also added significance to the model (block χ2 = 14.74, p = .005); the full model was significant (χ2 = 52.15, p < .001; pseudo R2 = 41%; 86.9% of subjects correctly classified).
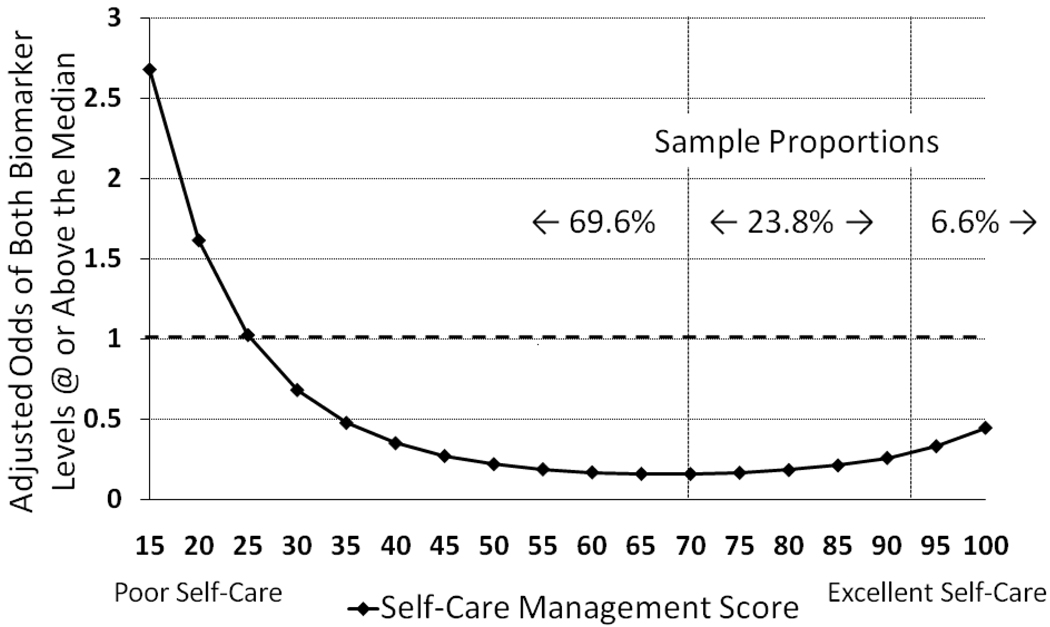
In the full multivariate model of both biomarkers, for every one-year difference in patient age there was a 10% change in adjusted odds of having higher levels of both biomarkers. The adjusted odds of having both biomarkers at or above the median were increased by 110% and 220% for medium and high comorbidity categories respectively. In addition, self-care management and the quadratic effect of self-care management were significant determinants of having levels of both biomarkers at or above the sample median. This finding indicated that the nonlinear relationship between self-care management and biomarker levels was also prominent in the model. Each one-point difference in self-care management score was associated with a 12.7% change in the odds of having both biomarkers at or above the sample median. However this relationship was nonlinear, meaning that there was reduction in the adjusted odds of having both biomarkers above the sample median for every one-point increase in self-care management score up to 70 (sample range from 15–100) (Figure 1). In contrast, and because of the nonlinear relationship, the adjusted odds of having both biomarkers above the sample median tended to be higher for every increase in self-care management score above 70. This effect was more prominent in the 6.6% of patients reporting the highest levels of self-care management.
Figure 1. The Influence of Self-Care Management on the Adjusted Odds of Having Levels of NT-proBNP and sTNFR1 Above the Sample Median.
The relationship between self-care management and levels of biomarkers of myocardial stress and systemic inflammation varied across levels of self-care management. Horizontal dashed line indicates neutral odds; for 69.6% of the sample, better self-care management was associated with lower odds of having levels of both biomarkers above the sample median. For the remaining 30.4% (those with self-care management scores of 70 or above), higher levels of self-care management were associated with increasing trend in the odds of having higher levels of both biomarkers. Results shown are adjusted for the influence of age (sample mean), gender (female), body mass index (sample mean), Charlson comorbidity category (low), left ventricular ejection fraction (sample mean), New York Heart Association functional class (I), non-ischemic etiology, and commonly prescribed heart failure medications.
Discussion
To the best of our knowledge, this is the first clinical investigation of the relationship between participant-reported self-care and serum biomarkers of myocardial stress and systemic inflammation in persons with HF. In support of our hypothesis, our most important finding was that in this sample of 168 patients with HF, better decision-making in response to HF symptoms (self-care management) was associated with reduced odds of having higher levels of NT-proBNP and sTNFR1. Interestingly and because of a nonlinear relationship, patients who reported the highest levels of self-care management had a trend toward increasing odds of having higher levels of both biomarkers.
The Influence of Heart Failure Self-Care Management
Our previous work has provided evidence that patients who reported better HF self-care management had a reduced risk of one-year mortality or hospitalization compared with those who practice poor self-care management.33 We have hypothesized previously that mechanisms through which effective HF self-care practices complement optimal medical management include neurohormonal and inflammatory homeostasis.7 In patients with HF, higher serum levels of NT-proBNP generally reflect greater synthesis and secretion of BNP due to myocardial distension and stress.34,35 Higher levels of sTNFR1 reflect elevated levels of active TNFα36 triggered by direct antigenic stimulation,37 endothelial disruption,38 and direct hemodynamic stress.39 Patients with HF may be able to manage their symptoms by making small adjustments to fluid or sodium intake or by taking low-dose diuretics7 and thereby limit the risk of reactive increases in neurohormonal activation that can occur with large changes in sodium intake40 or high-dose diuretics.41 Self-management of congestion may also limit the translocation of gut bacteria into the blood stream42 and help limit wall tension that impedes myocardial perfusion43 and causes hemodynamic stress, tissue ischemia, and inflammation in HF.
We also found that the relationship between self-care management and HF biomarkers was nonlinear. For the 70% of the sample with self-care management scores equal to or below 70, incremental improvements in self-care management were associated with significantly lower odds of having both biomarkers above the sample median holding other factors constant (Figure 1). For the remaining 30% of the sample, and particularly for the 7% of patients reporting very high levels of self-care management, incremental increases in self-care management were associated with an increase in the odds of having higher levels of both biomarkers. While the odds of having both biomarkers above the sample median for patients reporting the best self-care management did not approximate the high likelihood observed in patients who reported the poorest self-care management, this observation is quite intriguing.
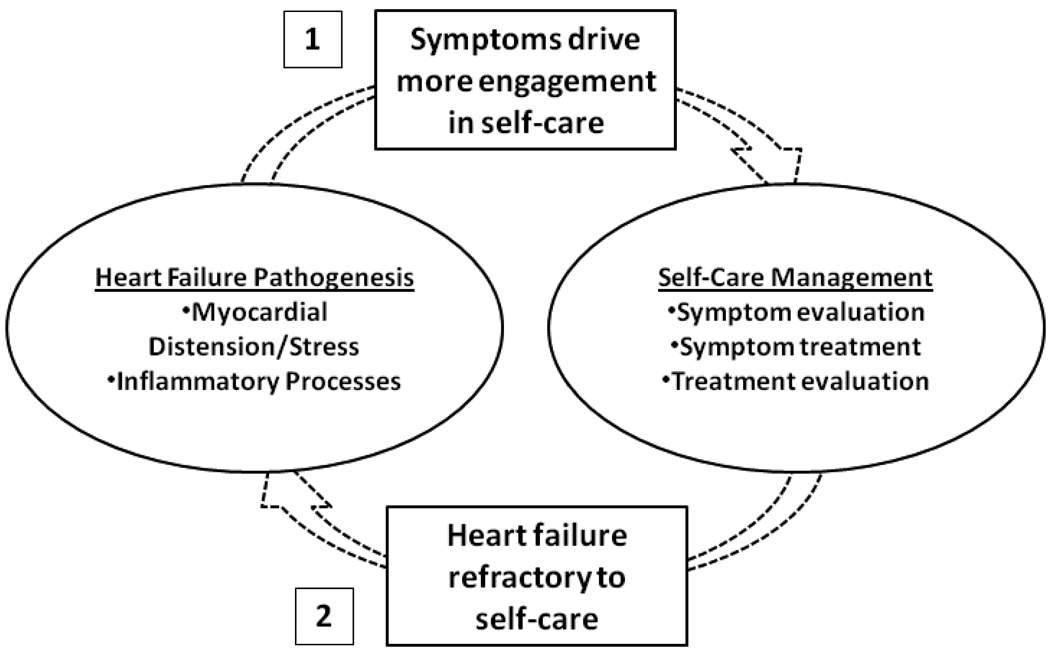
We postulate two main reasons why patients who reported the highest levels of self-care management were more likely to have higher biomarker levels. First, signs and symptoms of HF progression and/or cytokine-induced symptoms like malaise and general aching44,45 may drive patients to become more engaged in self-care (Figure 2). Lorig and Holman46 argue that by nature, self-care management is problem-based. That is, HF symptoms may indicate changes in underlying HF pathogenesis about which patients must make decisions and engage in problem-solving behavior. In previous research, HF patients who experienced more symptoms also were more engaged in HF self-care maintenance behaviors.47,48 In addition to driving better self-care maintenance, HF symptoms that are not addressed effectively may persist or become more burdensome and serve as a “wake up call” for patients to develop skill in detecting and managing HF symptoms earlier in their development or to try additional strategies to ameliorate symptoms.47
Figure 2. Two Potential Reasons Why Patients Who Reported the Best Self-Care Had Higher Levels of Biomarkers of Myocardial Stress and Systemic Inflammation.
1) Signs and symptoms of HF progression and/or cytokine-induced symptoms may serve as a “wakeup call” and drive patients to engage in better self-care management. 2) Heart failure pathogenesis may be refractory to even the most effective self-care practices (e.g. self-care is not enough), and the intervention of heart failure expert clinicians is what is needed to reduce myocardial stress and systemic inflammation.
Despite the appeal of this seemingly intuitive explanation, however, there are two empirical gaps that remain problematic. First, results from previous researchers suggest that HF symptoms are only loosely related to measures of pathogenesis. For example, Rector et al.49 observed that measures of HF pathogenesis, including LVEF, BNP, jugular venous distension, rales, peripheral edema, systolic blood pressure, creatinine, and hemoglobin, were not strongly related to HF symptoms. In addition, Shah et al.50 reported that changes in hemodynamic measures do not predict concomitant changes in underlying HF pathogenesis. These results demonstrate that there is considerable dissociation between indices of HF pathogenesis and HF symptoms. Others also have found no difference in self-care between patients who experience consistent or inconsistent symptoms over time.51 Thus, assumptions about the link between HF pathogenesis and HF symptoms, and the link between HF symptoms and improved self-care management are not well supported. As the measurement of self-care management in particular is relatively new, very little is known about the determinants of this aspect of HF self-care.
The second possible explanation of why patients who reported the highest levels of self-care management were more likely to have higher biomarker levels is that HF pathogenesis may become refractory even to the best self-care management. That is, perhaps HF has progressed beyond the effectiveness of even expert level decision-making, experience, and skill in evaluating and ameliorating HF symptoms. Instead, increased involvement of clinical experts and advanced monitoring and treatment therapies may be needed to decrease myocardial stress and systemic inflammation and minimize the risk of the associated clinical outcomes. Zambroski articulated the importance of promoting self-care of HF even in palliative care settings.52 But, the progressive nature of this multisystem disorder will at some point dominate over self-care in all persons with HF.
Morrow and Lemos53 argue that cardiovascular biomarkers must have clinical application, including risk stratification or monitoring of therapeutic response. In previous HF research, levels of NT-proBNP above the sample median have been associated with a greater risk of mortality,3,4,54 urgent ventricular assist device implantation, and urgent heart transplantation.3,4 Patients with higher levels of sTNFR1 also have an increased risk of mortality.5,6 Thus, patients with levels of NT-proBNP and sTNFR1 above the sample median have greater myocardial stress and systemic inflammation and are at-risk for poor HF outcomes. Importantly, having higher levels of both biomarkers also may indicate ineffectiveness of HF self-care. That is, HF progression may be refractory to self-care or these patients may not be engaged sufficiently in self-care management. Either way, patients with elevated levels of multiple HF biomarkers are a group primed for aggressive intervention and may require strategies beyond those focused on promoting self-care.
Other Factors and Heart Failure Biomarkers
Patient age was significant in our multi-marker model, confirming the results of previous research.22,55 Comorbidity category also was a determinant of having higher levels of both biomarkers confirming the results of others.24 Although confidence in self-care was helpful in explaining the nonlinear relationship between self-care management and health outcomes in our previous research,56 self-care confidence was not a factor in explaining the odds of having high levels of both biomarkers. Similarly, self-care maintenance, which most view as analogous to treatment adherence, was not a factor in our model. Therefore, we strongly recommend that the decision-making process of self-care management be measured and included as a determinant of HF biomarkers and health outcomes in future research, not just self-care maintenance or treatment adherence behaviors.
Strengths and Limitations
This was a secondary analysis of cross-sectional study data. Thus, it is not possible to make inferences about temporal aspects of the relationship between self-care management and circulating biomarkers.57 Although we controlled for pertinent confounders, other factors, such as the influence of the family or other caregivers, could influence the relationship between HF self-care and the biomarkers studied. In accordance with our objective, self-care was measured by participant-report. Although there are limitations to collecting data on self-care via participant report, the SCHFI is the only available instrument that measures HF self-care management.58 Follow-up studies in which self-care behaviors are measured objectively may either support or refute our results. The relatively young age and high BMI of the sample makes it more difficult to extend inferences from these results to all other HF patient populations. Lastly, as we studied the relationship between self-care and circulating biomarkers in self-identified African American and Caucasian persons with HF, our findings may not be supported in all cultural, ethnic, or racial groups. Future prospective and experimental studies are needed to gain more insight into the complex relationship between HF self-care and these biomarkers and to determine in whom HF self-care is most effective in reducing myocardial stress and systemic inflammation.
Conclusion
In summary, incremental increases in decision-making about HF symptoms and treatment were associated with lower odds of having higher levels of both NT-proBNP and sTNFR1, indicating less extensive myocardial stress and systemic inflammation. For patients reporting the best self-care management, however, there was a trend favoring greater odds of higher biomarker levels. This pattern may suggest that HF symptoms drive engagement in self-care management or that for some patients HF pathogenesis is refractory to even the most effective self-care practices. Patients with higher levels of multiple HF biomarkers need innovative intervention and concentrated future research.
What is New/ Summary and Implications
Patients with heart failure (HF) who reported better decision-making about HF symptoms and treatment were less likely to have levels of biomarkers of myocardial stress and systemic inflammation shown by others to be associated with associated greater risk of mortality, urgent ventricular assist device implantation, and urgent heart transplantation.
Effective HF self-care practices related to symptom recognition, treatment, and treatment evaluation, may complement optimal medical management in maintaining neurohormonal and inflammatory homeostasis.
Evaluating, teaching, and promoting effective self-care behaviors in persons with HF are evidence-based practices.
Self-care maintenance, which most view as analogous to treatment adherence, was not a factor in our model, and we strongly recommend that the decision-making process of self-care management be measured and included as a determinant of HF biomarkers and health outcomes in future research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher S. Lee, Oregon Health & Science University School of Nursing, Portland, OR.
Debra K. Moser, University of Kentucky College of Nursing, Lexington, KY.
Terry A. Lennie, University of Kentucky College of Nursing, Lexington, KY.
Nancy C. Tkacs, University of Pennsylvania School of Nursing, Philadelphia, PA.
Kenneth B. Margulies, University of Pennsylvania School of Medicine, Philadelphia, PA.
Barbara Riegel, University of Pennsylvania School of Nursing, Philadelphia, PA.
References
- 1.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992 Jul;20(1):248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 2.Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996 Sep;2(3):243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]
- 3.Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003 Oct;24(19):1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Rothenburger M, Wichter T, Schmid C, et al. Aminoterminal pro type B natriuretic peptide as a predictive and prognostic marker in patients with chronic heart failure. J Heart Lung Transplant. 2004 Oct;23(10):1189–1197. doi: 10.1016/j.healun.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000 Dec 19;102(25):3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 6.Ueland T, Kjekshus J, Froland SS, et al. Plasma levels of soluble tumor necrosis factor receptor type I during the acute phase following complicated myocardial infarction predicts survival in high-risk patients. J Am Coll Cardiol. 2005 Dec 6;46(11):2018–2021. doi: 10.1016/j.jacc.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Lee CS, Tkacs NC, Riegel B. The Influence of Heart Failure Self-care on Health Outcomes: Hypothetical Cardioprotective Mechanisms. J Cardiovasc Nurs. 2009 Mar 11;24(3) doi: 10.1097/JCN.0b013e31819b5419. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004 Aug;10(4):350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Executive summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):10–38. doi: 10.1016/j.cardfail.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. Mar;78(3):297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009 Sep 22;120(12):1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 12.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008 May 15;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 13.Heo S, Lennie TA, Moser DK, Riegel B, Chung M. Gender differences in and factors related to self-care behaviors: A cross-sectional, correlational study of patients with heart failure. International Journal of Nursing Studies. 2008;45(12):1807–1815. doi: 10.1016/j.ijnurstu.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser DK, Chung ML, Riegel B, Rayens MK, Lennie TA. Nonadherence is a mediator of the link between depressive symptoms, and rehospitalization or mortality in patients with heart failure. Circulation. 2006;144 suppl:II-518. [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008 May 15;358(20):2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 17.Bruins S, Fokkema MR, Romer JW, et al. High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem. 2004 Nov;50(11):2052–2058. doi: 10.1373/clinchem.2004.038752. [DOI] [PubMed] [Google Scholar]
- 18.Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J Am Coll Cardiol. 1999 Jun;33(7):1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Dietrich M, Norkus E, et al. Intraindividual variability of plasma antioxidants, markers of oxidative stress, C-reactive protein, cotinine, and other biomarkers. Epidemiology. 2006 Jul;17(4):404–412. doi: 10.1097/01.ede.0000220655.53323.e9. [DOI] [PubMed] [Google Scholar]
- 20.Apple FS, Wu AH, Jaffe AS, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine practice guidelines: Analytical issues for biomarkers of heart failure. Circulation. 2007 Jul 31;116(5):e95–e98. doi: 10.1161/CIRCULATIONAHA.107.185266. [DOI] [PubMed] [Google Scholar]
- 21.Allen LA, Michael Felker G. Multi-marker strategies in heart failure: clinical and statistical approaches. Heart Fail Rev. 2009 May 3; doi: 10.1007/s10741-009-9144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loke I, Squire IB, Davies JE, Ng LL. Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender and heart rate. Eur J Heart Fail. 2003 Oct;5(5):599–606. doi: 10.1016/s1388-9842(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 23.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005 Oct 4;112(14):2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 24.Cowie MR, Jourdain P, Maisel A, et al. Clinical applications of B-type natriuretic peptide (BNP) testing. Eur Heart J. 2003 Oct;24(19):1710–1718. doi: 10.1016/s0195-668x(03)00476-7. [DOI] [PubMed] [Google Scholar]
- 25.Tang WH, Girod JP, Lee MJ, et al. Plasma B-type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation. 2003 Dec 16;108(24):2964–2966. doi: 10.1161/01.CIR.0000106903.98196.B6. [DOI] [PubMed] [Google Scholar]
- 26.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001 Apr 24;103(16):2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 27.Gundogdu F, Bozkurt E, Kiziltunc A, et al. The effect of beta-blocker (carvedilol) therapy on N-terminal pro-brain natriuretic peptide levels and echocardiographic findings in patients with congestive heart failure. Echocardiography. 2007 Feb;24(2):113–117. doi: 10.1111/j.1540-8175.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 28.Gullestad L, Aukrust P, Ueland T, et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J Am Coll Cardiol. 1999 Dec;34(7):2061–2067. doi: 10.1016/s0735-1097(99)00495-7. [DOI] [PubMed] [Google Scholar]
- 29.Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004 Aug 31;110(9):1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 30.Sola S, Mir MQ, Rajagopalan S, Helmy T, Tandon N, Khan BV. Statin therapy is associated with improved cardiovascular outcomes and levels of inflammatory markers in patients with heart failure. J Card Fail. 2005 Oct;11(8):607–612. doi: 10.1016/j.cardfail.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Allison PD. Multiple Regression: A Primer. Thousand Oaks, CA: Pine Forge Press; 1999. [Google Scholar]
- 32.Riegel B, Dickson VV. A situation-specific theory of heart failure self-care. J Cardiovasc Nurs. 2008 May–Jun;23(3):190–196. doi: 10.1097/01.JCN.0000305091.35259.85. [DOI] [PubMed] [Google Scholar]
- 33.Lee CS, Moser DK, Lennie TA, Riegel B. Event-free survival in adults with heart failure who engage in self-care management. Heart & Lung. doi: 10.1016/j.hrtlng.2009.12.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goetze JP. Biochemistry of pro-B-type natriuretic peptide-derived peptides: the endocrine heart revisited. Clin Chem. 2004 Sep;50(9):1503–1510. doi: 10.1373/clinchem.2004.034272. [DOI] [PubMed] [Google Scholar]
- 35.Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr Rev. 2003 Jun;24(3):341–356. doi: 10.1210/er.2003-0006. [DOI] [PubMed] [Google Scholar]
- 36.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004 Apr;90(4):464–470. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005 Jun 6;95(11A):3C–8C. doi: 10.1016/j.amjcard.2005.03.006. discussion 38C–40C. [DOI] [PubMed] [Google Scholar]
- 38.Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann Med. 2005;37(2):74–85. doi: 10.1080/07853890510007232. [DOI] [PubMed] [Google Scholar]
- 39.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004 Jun 25;94(12):1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 40.Alvelos M, Ferreira A, Bettencourt P, et al. The effect of dietary sodium restriction on neurohumoral activity and renal dopaminergic response in patients with heart failure. Eur J Heart Fail. 2004 Aug;6(5):593–599. doi: 10.1016/j.ejheart.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 41.Patel J, Smith M, Heywood JT. Optimal use of diuretics in patients with heart failure. Curr Treat Options Cardiovasc Med. 2007 Aug;9(4):332–342. doi: 10.1007/s11936-007-0028-z. [DOI] [PubMed] [Google Scholar]
- 42.Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003 Oct;5(5):609–614. doi: 10.1016/s1388-9842(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 43.Katz AM. Physiology of the Heart. Forth ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 44.Kelley KW, Bluthe RM, Dantzer R, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003 Feb;17 Suppl 1:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 45.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007 Feb;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003 Aug;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 47.Rockwell JM, Riegel B. Predictors of self-care in persons with heart failure. Heart Lung. 2001 Jan–Feb;30(1):18–25. doi: 10.1067/mhl.2001.112503. [DOI] [PubMed] [Google Scholar]
- 48.Schnell-Hoehn KN, Naimark BJ, Tate RB. Determinants of self-care behaviors in community-dwelling patients with heart failure. J Cardiovasc Nurs. 2009 Jan–Feb;24(1):40–47. doi: 10.1097/01.JCN.0000317470.58048.7b. [DOI] [PubMed] [Google Scholar]
- 49.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients' perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006 Mar;12(2):87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Shah MR, Hasselblad V, Stinnett SS, et al. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. Eur J Heart Fail. 2002 Jun;4(3):297–304. doi: 10.1016/s1388-9842(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 51.Musil CM, Morris DL, Haug MR, Warner CB, Whelan AT. Recurrent symptoms: well-being and management. Soc Sci Med. 2001 Jun;52(11):1729–1740. doi: 10.1016/s0277-9536(00)00293-8. [DOI] [PubMed] [Google Scholar]
- 52.Zambroski C. Self-care at the end of life in patients with heart failure. J Cardiovasc Nurs. 2008 May–Jun;23(3):266–276. doi: 10.1097/01.JCN.0000317425.19930.c9. [DOI] [PubMed] [Google Scholar]
- 53.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007 Feb 27;115(8):949–952. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 54.Bettencourt P, Frioes F, Azevedo A, et al. Prognostic information provided by serial measurements of brain natriuretic peptide in heart failure. Int J Cardiol. 2004 Jan;93(1):45–48. doi: 10.1016/s0167-5273(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 55.Clerico A, Del Ry S, Maffei S, Prontera C, Emdin M, Giannessi D. The circulating levels of cardiac natriuretic hormones in healthy adults: effects of age and sex. Clin Chem Lab Med. 2002 Apr;40(4):371–377. doi: 10.1515/CCLM.2002.060. [DOI] [PubMed] [Google Scholar]
- 56.Lee CS, Suwanno J, Riegel B. The relationship between self-care and health status domains in Thai patients with heart failure. Eur J Cardiovasc Nurs. 2009 Oct;8(4):259–266. doi: 10.1016/j.ejcnurse.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Boston: Houghton Mifflin Company; 2002. [Google Scholar]
- 58.Cameron J, Worrall-Carter L, Driscoll A, Stewart S. Measuring self-care in chronic heart failure: a review of the psychometric properties of clinical instruments. J Cardiovasc Nurs. 2009 Nov–Dec;24(6):E10–E22. doi: 10.1097/JCN.0b013e3181b5660f. [DOI] [PubMed] [Google Scholar]