Abstract
Organogenesis involves a series of dynamic morphogenesis and remodeling processes. Since feathers exhibit complex forms, we have been using the feather as a model to analyze how molecular pathways and cellular events are used. While several major molecular pathways have been studied, the roles of matrix degrading proteases and inhibitors in feather morphogenesis are unknown. Here we addressed this knowledge gap by studying the temporal and spatial expression of proteases and inhibitors in developing feathers using mammalian antibodies that cross react with chicken proteins. We also investigated the effect of protease inhibitors on feather development employing an in vitro feather bud culture system. The results show that antibodies specific for mammalian MMP2 and TIMP2 stained positive in both feather epithelium and mesenchyme. The staining co-localized in structures of E10 to E13 developing feathers. Interestingly, MMP2 and TIMP2 exhibited a complementary staining pattern in developing E15 and E20 feathers and in maturing feather filaments. Although they exhibited a slight delay in feather bud development, similar patterns of MMP2 and TIMP2 staining were observed in in vitro culture explants. The broad spectrum pharmacological inhibitors AG3340 and BB103 (MMP inhibitors) but not Aprotinin (a plasmin inhibitor) showed a reversible effect on epithelium invagination and feather bud elongation. TIMP2, a physiological inhibitor to MMPs, exhibited a similar effect. Markers of feather morphogenesis showed that MMP activity was required for both epithelium invagination and mesenchymal cell proliferation. Inhibition of MMP activity led to an overall delay in the expression of molecules that regulate either early feather bud growth and/or differentiation and thereby produced abnormal buds with incomplete follicle formation. This work demonstrates that MMPs and their inhibitors are not only important in injury repair, but also in development tissue remodeling as demonstrated here for the formation of feather follicles.
Keywords: skin appendage, feather morphogenesis, MMP, TIMP, plasmin, epithelium, mesenchyme
Introduction
Epithelial organ formation involves the interplay of epithelia and mesenchyme and usually involves a series of dynamic cellular process, including epithelia folding and tissue remodeling before it reaches the final stable form. To engineer stem cells to form epithelia organs we will need to fully understand the molecular pathways involved in these processes. Skin appendage development serves as an excellent model to study molecular mechanisms underlying this process. This experimental model enables us to explore how a flat layer of epidermis, interacting with the underlying dermis, is topologically transformed into complex structures, which protrude out of the skin surface (ie., hairs and feathers) or invaginate into dermis (i.e., mammary or sweat glands; Chuong, 1998; Wu et al., 2004). We have focused primarily on avian feather development as our experimental model to elucidate these mechanisms. Several key molecules controlling fundamental aspects of these processes have been identified. These include growth factors and their receptors, cell adhesion molecules and their ligands some of which are extracellular matrix (ECM) components, and signal transduction molecules and transcription factors (Widelitz et al., 2003). These components interact in a highly orchestrated fashion during the developmental process to form feathers. For instance, FGF, BMP, and beta-catenin signaling pathways are essential in the induction of feather buds. SHH controls the growth of feather buds. N-CAM, tenascin, integrin and fibronectin mediate dermal/mesenchymal condensation. Notch/delta signaling establishes the anterior-posterior axis during short feather bud formation while Wnt7a is involved in establishing the proximal-distal axis in long feather bud formation (Lin et al., 2006).
One molecular pathway that has not been studied involves the matrix degrading proteases and their inhibitors, which include the matrix metalloproteinases (MMPs) and TIMPs (tissue inhibitors of metalloproteinases), as well as members of the plasminogen activator family and plasmin system. Subject to regulation by growth factors and cytokines, these molecules have been shown not only to degrade ECM components during cell migration and tissue remodeling, but also to activate growth factors and cell membrane receptors during cell signaling (Parks and Robert, 1998; Stetler-Stevenson and Seo, 2005). MMPs are zinc-dependent endopeptidases composed of 25 known species, Twenty four of these species are found in mammals (Parks, et al., 2004). MMPs target a wide range of substrates including components of the ECM, cell surface receptors, latent forms of growth factors, and zymogens and therefore may mediate many biological activities. In the area of skin appendage morphogenesis, several species of MMPs are expressed during appendage development in human fetal skin (Karelina et al., 2000), and are secreted in vitro by cells derived from the dermal papilla and fibrous sheath of the human scalp (de Almeida et al., 2005). MMP2, also called gelatinase A, has been shown to promote the invasive behavior of cells during tissue remodeling, inflammation, development and cancer (Brooks et al., 1996). Together with MMP9 (gelatinase B) it can effectively degrade multiple species of matrix proteins including tenascin C (Jian et al., 2001; Siri et al., 1995; Cai et al., 2002), which is present in a distinct pattern at the leading edge of epithelial invagination. Beyond this, there is little known about the expression and function of these molecules in skin appendage morphogenesis.
There is a tremendous amount of cell migration and tissue remodeling during feather development that may depend on proteases and their inhibitors. There also are several adhesion molecules present during feather development, i.e., N-CAM, tenascin, integrin and fibronectin (Jiang and Chuong,1992) that may be substrates of proteases (Jovanova-Nesic and Shoenfeld, 2006; Hancox et al., 2009; Svineng, 2008; Malemud, 2006). Therefore, we hypothesized that the protease and protease inhibitor systems play an essential role in the formation of feather follicles. We addressed the hypothesis by studying the temporal and spatial expression of proteases and inhibitors in developing feather buds. We then investigated the effect of broad spectrum protease inhibitors on feather bud development employing an in vitro explant culture system. Additional studies of feather morphogenesis markers were carried out to reveal intervening steps that required protease activity during feather bud morphogenesis.
Materials and methods
Materials and Reagents
Fertilized eggs were purchased from SPAFAS, Preston, CT. Aprotinin was purchased from Sigma Co., and the broad spectrum MMP inhibitors AG3340 and BB3103 were gifts from British Biotechnology Company (Oxford, United Kingdom). Antibodies to MMP-2 (AB19167), TIMP-2 (AB2965), uPA, PAI-1, MMP-1, PCNA were purchased from Chemicon Co/Millipore (Billerica, MA).
Explant cultures
Chicken embryos were staged according to the method described by Hamburger and Hamilton (1951). Briefly, E8 dorsal skin between the lower neck to the tail region was dissected and placed on the membrane of tissue culture insert (0.4um pore size, Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) of 6-well tissue culture plate and supplemented with 2ml Dulbecco’s Modified Eagle’s Medium (DMEM) with 2 % fetal bovine serum (FBS). The explants were incubated in a humidified 5% CO2 incubator at 37°C for the indicated time period. The protease inhibitors were added in the culture medium at concentrations of 10, 25, 40, 100, and 400uM for MMP inhibitor AG3340 and BB3103, and at 10ug/ml and 20ug/ml for Aprotinin. Culture media were refreshed every other day for 7 days. At the end of 7days, skin samples were fixed for histology.
Western Blot
Skin explants or skin dissected from chicken embryos were rinsed with PBS three times before being homogenized in a buffer containing 50 mM sodium pyrophosphate, 50 mM NaCl, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, and 100μg/ml leupeptin (pH 7.4). Protein concentration was determined using the BioRad protein assay system (Bio-Rad, Richmond, CA). Equal amounts of proteins were treated with non-reducing sample buffer and loaded and separated by 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, proteins were transferred to nitrocellulose membranes using a transblotter (Bio-Rad, Richmond, CA). Nonspecific binding was blocked by using 5% fat-free milk powder and 0.1% Tween 20 in Tris-buffered saline. Membranes were incubated with primary antibodies (diluted 1:1000) for 2 hours in 0.2 M Tris-HCl (pH 7.5), 0.5 M NaCl buffer containing 5% fat-free milk powder and 0.1% Tween 20. Blots were washed and incubated for another hour with a goat anti-rabbit horseradish peroxidase–conjugated antibody (1:1000). Membrane blots were developed using a chemiluminescence detection kit (Lumi-Glo; Kirkegaard & Perry, Gaithersburg, MD).
Zymogram
Protein extracts were mixed with SDS-PAGE sample buffer (non-reducing) and subject to SDS-PAGE using 10 % polyacrylamide gel containing 0.1% (w/v) gelatin (Han et al., 2001). Electrophoresis was performed at 4°C for 16 hours. After electrophoresis, SDS in the slab gel was removed by incubating the gel with 2.5 % Triton X-100 prior to a 16 hour incubation in buffer containing 5 mM CaCl2, 150 mM Nacl, and 50 mM Tris, pH 7.5 to develop enzyme activity. The gelatinolytic activities were visualized by staining the gel with Coomassie Blue R-250.
Immunochemistry and in situ hybridization
Skin samples were dissected and fixed with 4% paraformaldehyde in PBS for 4 hours at 4°C followed by procedures described by Jiang and Chuong (1992) for immunohistochemistry. Paraffin sections were used for immunoalkaline phosphatase and peroxidase staining after primary antibody treatment with procedures also described by Jiang and Chuong (1992).
Non-radioactive in situ hybridization was performed according to procedures described by Chuong et al., (1996) with slight modifications. Briefly, skin samples were rinsed in RNAse-free PBS and fixed in 4% paraformaldehyde in PBS (pH 7) overnight at 4°C. The fixed tissues were dehydrated and then rehydrated through a series of methanol (25%, 50%, 75%, and 100%) in PBT (PBS containing 0.1% Tween-20) washes. The specimens were bleached in 6% hydrogen peroxide for one hour, treated with proteinase K (10ug/ml in PBS) for 20 min, re-fixed with 0.2% glutaraldehyde/4% paraformaldehyde, and rinsed with PBT. The tissues were then prehybridized in hybridization buffer (containing 50% formamide, 5x sodium citrate/sodium chloride buffer, 1% sodium dodecyl sulfate, 50ug/ml heparin, 50 ug/ml tRNA) at 70°C for one hour. After prehybridization, specimens were placed in new prehybridization buffer containing 1-3 ug/ml digoxigenin-labeled riboprobes and hybridized overnight at 70°C. Finally, specimens were incubated with alkaline phosphatase conjugated anti-digoxigenin Fab (Roche, Indianapolis, IN) overnight. Positive signals were detected by incubating the specimens with NBT/BCIP substrates (Promega, Madison WI).
Results
The in vitro explant culture of feather buds
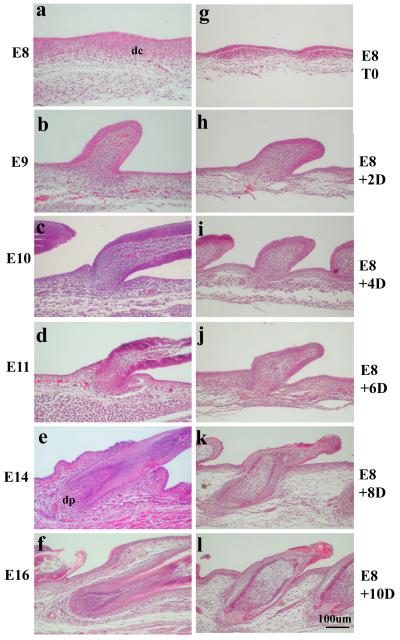
The major stages of feather development include: feather induction, mesenchymal condensation and epidermal placode formation, development of anterior-posterior asymmetry, proximal-distal elongation, follicle invagination, and branch formation (Lin et al., 2006). We have established an in vitro explant method to study feather bud development (Materials and Methods). A side-by-side comparison between in vivo and in vitro feather bud development is shown in Figure 1. In vivo, at E6.5, epidermal placodes form over areas of dermal condensations. Feather buds begin to develop along the dorsal midline and progress bilaterally. At E8, epidermal placode formation is still occurring laterally, developing approximately 5 rows from the midline (Fig.1a). The process is followed by the formation of a short feather bud (Fig. 1b, E9), which extends to form a long bud and establishes anterior-posterior asymmetry (Fig. 1c, E10). Subsequently, the long bud invaginates into the dermis (Fig. 1d, E11), wraps around the underlying dermal papilla (dp) (Fig. 1e, E14), and forms a primordial feather follicle (Fig. 1f, E16).
Fig.1.
A. Comparison of in vivo (a to f) and in vitro (g to l) feather follicle development (H & E staining). a) Epidermal placode is present over a dermal condensation (dc) at E8; b). Feather bud forms at E10; c-d). Feather bud elongates and invaginates into underlying dermis from E11 to E12; e) Feather follicle forms at E14 (dp: dermal papilla); f) A complete feather is developed at E16. Panels g to l: g). Fresh explant of E8 chicken skin (E8T0); h). 2-day in vitro culture (E8T2D); i). 4-day in vitro culture (E8T4D); j). 6-day in vitro culture (E8T6D), k). 8-day in vitro culture (E8T8D); l). 10-day in vitro culture (E8T10D). In vitro skin appears to develop slower, but feather follicles certainly form.
Overall, in vitro culture conditions are able to support the development of E8 feather buds to form primordial feather follicles in 10 days, the longest time observed. Nonetheless, there is a clear delay in the timing of each stage of development. Albeit shorter, the feather bud is formed in the 2-day explant culture (Fig. 1h, E8 + 2D). It proceeds to establish anterior-posterior asymmetry similar to that of in vivo E10 skin feather bud (Fig. 1c) after four days in culture (Fig. 1i, E8 + 4D). The invagination of feather buds and formation of primordial feather follicles occur after 6, 8, and 10 days, respectively after culture in vitro (Fig. 1j, E8 + 6D; Fig. 1k, E8 + 8D; Fig. 1l, E8 +10D).
MMP2 and TIMP2 are expressed in structures of developing feather follicles
Our effort to identify the MMP species present in tissues of developing feathers in vivo was challenged by the fact that the chicken homologs of MMPs/TIMPs were yet characterized. MMPs are encoded by 24 human and 23 mouse genes (Rivera et al. 2010). The sequences for chicken MMP2 and TIMP2 are available through PubMed. A nucleotide Blast revealed that chicken MMP2 and TIMP2 are both evolutionarily conserved with 95% and 90% homology with their mouse counterparts. Both mouse and chicken MMP2 have a molecular weight of 72kDa. The molecular weights of mouse and chicken TIMP2 are 22kDa and 21kDa, respectively. Nonetheless, antibodies to mouse MMP1, MMP2, MMP9, MT1-MMP, TIMP1, uPA, tPA, PAI-1 and TIMP2 were tested in developing chicken skin using immunohistochemistry (IHC). Among them only antibodies to MMP2 and TIMP2 gave clear positive staining and were subsequently used to study MMP2 and TIMP2 expression/staining in the developing feathers in a temporal and spatial fashion.
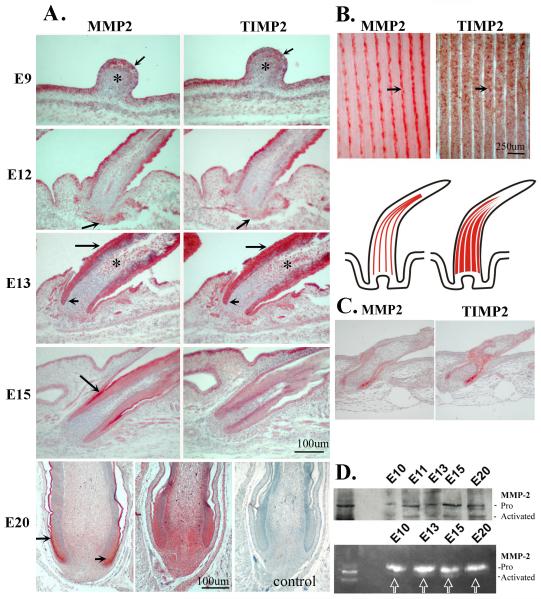
The results show that, MMP2 and TIMP2 staining were co-localized in the feather epithelium (arrow) and distal mesenchyme “*” of the short bud stage at E9 (Fig. 2A, E9). At E12, when feather buds started to elongate and invaginate downward into the dermis to form feather follicles, MMP2 and TIMP2 were co-localized in the epithelium/shaft as well as the mesenchymal region, with a greater intensity in the epithelium and beneath the leading edge of follicle epithelium (Fig. 2A, E12, arrow). At E13 when feather follicles have formed, MMP2 and TIMP2 were expressed strongly in the epithelial structures including the collar region of the feather follicle and feather sheath (Fig 2A, short and long arrows, respectively). Meanwhile, both MMP2 and TIMP2, albeit at low expression levels, were found in the pulp mesenchyme (Fig. 2A, E13, “*”). At E15, both MMP2 and TIMP2 showed diminished expression in most epithelial structures but MMP2 was present in the feather sheath (Fig. 2A, E15, MMP2, arrow) and TIMP2 was found in the skin epithelium (Fig. 2A, E15, TIMP2). At E20, MMP2 was expressed distinctly at the intermediate layer of the proximal follicle (Fig. 2A, E20, short arrow), the feather sheath (Fig. 2A, E20, MMP2, long arrow), and the distal feather sheath (not shown). In contrast, TIMP2 was expressed more diffusely in the dermal papilla and the pulp mesenchyme (Fig. 2A, E20, TIMP2). There is no staining in the negative control without the primary antibody (Fig. 2A, E20, right panel). Interestingly, MMP2 and TIMP2 formed a complementary pattern of expression in the mature feather with MMP2 present in marginal plates and TIMP2 in the barb ridges (Fig. 2B, MMP2 and TIMP2, arrows). The lower panel of Fig. 2B is a schematic representation of longitudinal feather follicles that express MMP2 and TIMP2 in marginal plates and in the barb ridges, respectively. Marginal plate cells later undergo apoptosis (Chang et al., 2004) while barb epithelia continue to grow into barb branches. The section of E8 skin +8 day in vitro culture showed a similar MMP2 and TIMP2 staining pattern in feather follicles although the follicles developed more slowly than in vivo (Fig. 2C).
Fig.2.
Temporal and spatial expression of MMP2 and TIMP2 proteins during feather development.
A. Chicken embryos at the indicated stages were sacrificed and processed for immunohistochemistry using antibodies against MMP2 (left panels) or TIMP2 (right panels). A negative control lacking the primary antibody is shown (E20, right panel).
B. Adult feather follicle filament. MMP2 is in the marginal plate whereas TIMP2 is in the barb plate epithelium. Lower panel is a schematic representation of MMP2 and TIMP2 expression in marginal plates and in the barb ridges of adult feather follicle.
C. MMP2 and TIMP2 staining of a section from E8 +8days in vitro cultured skin.
D. Western Blot and gelatin zymogram for MMP2 expression in chicken skin development. Note the presence of activated MMP2 in the various stages during follicle formation. Left lane, MMP2 from human fibroblasts.
Skin extracts from developing feathers (from E10 to E20) were also analyzed by Western Blot for MMP2 protein expression and by gelatin zymogram for MMP2 activity. An equal amount of proteins was loaded in each lane (Fig. 2D). The left lane of Fig. 2D, both upper and lower panels, are MMP-2 from control human skin fibroblasts. Both pro- and activated forms of MMP-2 are recognized by the MMP-2 specific antibody (AB19167, Chemicon Co/Millipore). The results show that, although the level varied, the Western blot detected both the pro- and activated forms of MMP-2 at all stages of skin extracts (Fig. 2D upper panel). Expression of the active form of MMP-2 is also shown by a zymogram (Fig. 2D, bottom panel “arrows”). The bands are clearly present at E13 and E15, but are present at much lower levels at E10 and E20. Gelatin zymography is a very sensitive assay to analyze the activity of MMP-2 and MMP-9 proteins (gelatinases). Besides detecting the active MMP, the activation of the latent MMPs (the pro-form of MMP-2 in our case, Fig. 2) during zymography was caused by SDS used during the procedure (Snoek-van Beurden and Von den Hoff, 2005).
MMP inhibitors block the formation of feather follicles and epithelia invagination
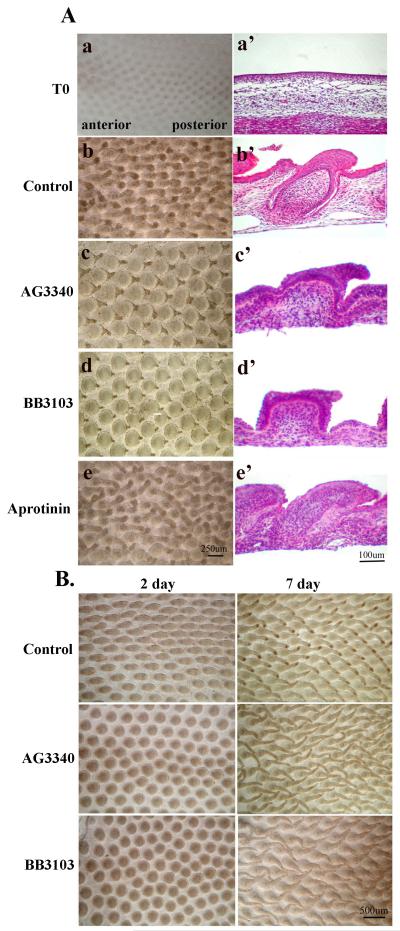
Broad spectrum MMP inhibitors and Aprotinin, an inhibitor to members of the plasminogen activator and plasmin systems, were used to investigate if proteases were involved in feather development. We cultured skin explants from E8 chick embryos to better control the dose of the inhibitors applied (Figure 3A). At the end of the 7th day, feather buds in the control group formed and were elongated oriented toward the posterior end of the skin (Fig. 3A.B). Longitudinal sections of the culture explants revealed that the epithelium flanking the feather buds had invaginated into the dermis and wrapped around the dermal structure to form the primary feather follicle (Fig. 3A.b’). In contrast, samples treated with pharmacological MMP inhibitors, AG3340 (10 uM) or BB3103 (100 uM), formed feather buds with enlarged and rounded bases and pointed tips (Fig. 3A.c, d). Longitudinal sections of these samples showed that in comparison to the control, the feather buds were enlarged at the base and were short. In addition, the epithelium failed to invaginate into the dermis (Fig. 3A.c’, d’). The development of feather buds was inhibited completely with 40 uM of AG 3340 and 400 uM of BB3103 (data not shown). Aprotinin-treated skin explants showed a similar morphology as the control group (Fig. 3A.e, e’). These results demonstrate that MMP activity, but not plasminogen/plasmin, might be essential in the formation, elongation, and invagination of feather buds.
Fig.3.
Effect of MMP inhibitors on feather explant cultures.
A. The broad spectrum MMP inhibitors AG3340 and BB3103 suppress feather development while Aprotinin does not. Explants of E8 chick skin were established in vitro and cultured for 7 days. Left panels: photographs of chick skin explant cultures. Right panels: corresponding vertical section of the explant culture (H&E staining). Top panels: Explant of E8 skin when culture explants were just established. The rest of the explants were cultured in 2% FBS/DMEM plus AG3340 (10uM), BB3103 (100uM), or Aprotinin (10ug/ml) for 7 days. Control: cultured in 2% FBS/DMEM for 7 days.
B. The effect of MMP inhibitors on feather development was reversible. Explants of E8 chick skin were established in vitro and cultured for 2 days in 2% FBS/DMEM plus AG3340 (10uM) or BB3103 (100uM). Cultures were then rinsed thoroughly (5 times) with 2% FBS/DMEM and subject to an additional 5-day culture in 2% FBS/DMEM. Control: cultured in 2% FBS/DMEM only. Left hand panels: images of explant cultures at the end of 2 days. Right hand panels: images of explant cultures at the end of 7 days.
To investigate if the effect of AG3340 or BB3103 on feather development was due to the toxicity of these inhibitors, feather skin explants were established as described previously and treated with the inhibitors, AG3340 (10 uM) or BB3103 (100 uM), for 2 days, washed, and then cultured for an additional 5 days in the absence of inhibitors. The results showed that a delay in feather bud development was evident for explants cultured in the presence of the inhibitors after 2 days (Fig. 3B, Control vs. AG3340 or BB3103, 2 Day). As the feather buds in the control group had elongated morphology, the inhibitor treated feather buds appeared round and flattened. After the removal of the inhibitors, however, these feather buds began to elongate with morphology similar to the control feather buds, more so in the AG3340-treated than in BB3103-treated groups (Fig. 3B, Control vs. AG3340 or BB3103, 7 Day). Hematoxylin and eosin (H&E) staining shows that AG3340 or BB3103 treated skins also have follicle formation just like control skin. (data not shown). Therefore, the inhibitory effect of AG3340 and BB3103 on feather bud development was not due to toxicity and was reversible.
The effect of TIMP2, a physiological inhibitor to several species of MMPs, was also tested to confirm the role of MMP in feather development. TIMP2 at the concentration of 20ug/ml was added to the E8 skin explants established as described above. The results in Figure 4 reveal that skin explants treated with TIMP2 (Fig. 4b) had a similar morphology as those treated with AG3340 or BB3103 (Fig. 3). A wholemount view shows that feathers in each treated group have an enlarged and rounded base. H&E staining of sections show that the feathers in TIMP2 treated skin also had no epithelial invagination or follicle formation (Fig. 4d), whereas follicles had already formed in control skin (Fig. 4c). Collectively, the results demonstrate that the activity of MMPs is essential in the early stages of feather development. Inhibition of MMP activity with either the pharmacological or physiological inhibitors blocks feather development.
Fig.4.

TIMP2 exhibits similar inhibitory effect as AG3340 or BB3103 on feather development. Upper panels: photographs of chicken skin explant culture. E8 chick skin explants were cultured for 7 days in 2% FBS/DMEM plus TIMP2 (20ug/ml). Control: cultured in 2% FBS/DMEM only (a-b). Low panels: corresponding vertical section of the explant culture (H&E staing)
MMP inhibitors reduce the proliferation of mesenchymal tissue
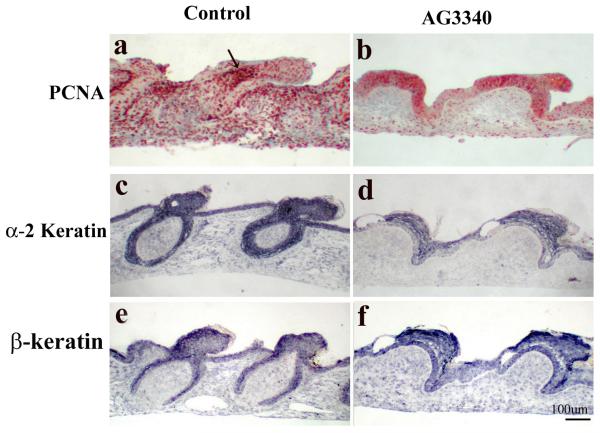
To identify the structural components that may require MMP activity during feather development, the expression level of proliferating cell nuclear antigen (PCNA) and markers of epithelial differentiation (α-2 keratin and feather specific β-keratin) were investigated. Explants of E8 dorsal skin were cultured with or without MMP inhibitors AG3340 or BB3103 for 7 days and processed for section and PCNA staining using immunohistochemistry or for α-2 keratin or β-keratin using in situ hybridization.
Results show that PCNA was present in both epithelium and mesenchyme of the developing feather bud, and the staining was exceptionally strong in the distal bud mesenchyme (Fig. 5a, arrow). With a delay in development and without epithelial invagination, the MMP inhibitor treated groups showed a strong PCNA expression in the epithelium but a weak staining in the mesenchyme (Fig. 5b). The expression of both α-2 keratinin and β-keratin, however, seemed to be comparable between the control and the inhibitor-treated groups (Fig. 5c-f). Similar results were obtained using BB3103 (data not shown).
Fig.5.
Characterization of proliferation and differentiation in MMP2-inhibited feather buds. Explants of E8 chick skin were established in vitro and cultured for 7 days in the presence AG3340 (10uM), or absence (Control) of the inhibitor. At the end of the 7 day culture, explants were processed for section and PCNA staining using IHC or for α-2 keratin or feather keratin using in situ hybridization. Note feather keratins are still expressed in epidermal, but cell proliferation in dermis is reduced in MMP2 inhibited feather buds.
MMP inhibition results in altered expression of molecular markers of feather bud differentiation
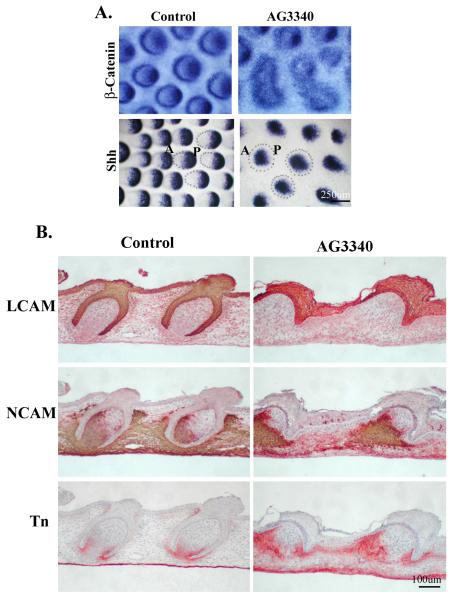
The effect of MMP inhibitors on the expression of molecules known to be involved in cell adhesion or signal transduction during feather development was also examined. Explants of E8 dorsal skin were cultured with or without the MMP inhibitors, AG3340 or BB3103, for 2 days for in situ hybridization or 7 days for section and immunohistochemistry. Samples were subsequently processed for β-catenin and SHH expression using whole mount in situ hybridization, and LCAM, NCAM, and tenascin protein expression using immunohistochemistry. The β-catenin signaling pathway plays an essential role in regulating epithelial morphogenesis (Widelitz et al., 2000). The result showed that β-catenin was localized at the posterior tip of the developing feather bud as well as at appearing as a circle at the peripheral border of control feather buds (Fig. 6A, Control, β-catenin). In contrast, MMP inhibitor treatment resulted in a diminished or diffused expression of β-catenin at the tip of the feather buds (Fig. 6A, AG3340, β-catenin). The expression was also diffuse at the peripheral feather bud border, and some of the feather buds seemed to fuse with their neighboring buds (Fig. 6A, AG3340, β-catenin). SHH is known to be involved in cell proliferation, cell size regulation, and condensation, and the polarization of mesenchymal tissue (Ting-Berreth et al., 1996). In the experiment, SHH showed an expected and well-defined distribution at the posterior-distal end of feather buds in control explants (Fig. 6A, Control, SHH). In contrast, MMP inhibitor treatment resulted in not only a less defined SHH expression in the feather bud, but also a reduction in the number of feather buds that had developed (Fig. 6A, AG3340, SHH). These molecular expression data suggest that feather development is delayed in the MMP inhibitor treated samples.
Fig.6.
Characterization of molecular expression in MMP2-inhibited feather buds.
A) Whole mount in situ hybridization for β-catenin or Shh in E8 explants of chick skin cultured 2 days in the presence or absence of AG3340 (10uM). The expression patterns of both molecules suggest that feather bud development is delayed.
B) Expression of adhesion molecules in E8 explants of chick skin cultured 7 days in the presence or absence of AG3340 (10uM). NCAM and Tn-C show expression patterns of delayed dermal papilla development. L-CAM shows a thickened epidermis.
LCAM and NCAM are cell-cell adhesion molecules with defined temporal and spatial patterns of expression during feather bud development. LCAM is expressed in the epithelium during feather follicle formation/epithelium invagination and NCAM is located in the distal bud epithelium plus mesenchyme surrounding the developing follicle (Jiang and Choung, 1992). In the current study, the control skin explants (E8 plus 7 days) exhibited the staining of LCAM at the epithelium, which had invaginated into the dermis to form a feather follicle, while NCAM was at the anterior mesenchyme surrounding and inside the feather follicle (Fig. 6B, Control, LCAM and NCAM). In the MMP inhibitor treated group, however, LCAM was present in the thickened epithelium, which failed to invaginate while NCAM was present only in the anterior mesenchyme beneath each feather; not in the interfollicular region (Fig. 6B, AG3340, LCAM and NCAM).
Tenascin C is an ECM molecule present in the anterior bud mesenchyme during early stages of feather bud development. As the feather bud elongates (long bud stage), its expression is localized to the mesenchyme beneath the angled junction of the anterior bud and inter-bud epithelium (Jiang and Chuong, 1992). This pattern was also seen in the control group of this study (Fig. 6B, Control, Tn). In the MMP inhibitor treated group, however, tenascin expression was present beneath the anterior and posterior region of bud epithelium, which was without invagination into the mesenchyme (Fig. 6B, AG3340, Tn). Its expression was more widely dispersed in the anterior mesenchyme than in controls. Expression was also seen in the inter-follicular mesenchyme. The effect of MMP inhibitor BB3103 on the expression of β-catenin, SHH, LCAM, NCAM, and tenascin was also tested and showed similar results (data not shown). Collectively, the results demonstrate that skin explants show similar patterns of markers of feather bud development as that seen in vivo and MMP inhibition leads to a delay and/or disruption of feather bud development identifiable by the altered pattern in the expression of these markers.
Discussion
The topological transformation from a feather bud or a hair peg into a feather or hair follicle is a profound process because the resultant follicle structure allows skin appendages to maintain progenitor cells in a specialized niche. This then ensures that growth occurs at the proximal end and also enables episodic regeneration (Wu et al., 2004). The chicken skin explant model has proven to be useful for analyzing cellular and molecular events during feather morphogenesis, i.e., from feather placode to short bud and then long bud formation (Lin et al., 2006). The transition from the short bud to the long bud stage requires the establishment of anterior-posterior asymmetry and epithelial invagination into the dermis at both the anterior and poster regions of the feather bud, which encases a portion of dermal mesenchyme and forms the feather follicle (Widelitz. et al. 2003). The difference in the speed of invagination between the anterior and posterior regions of the epithelium may contribute also to the establishment of anterior-posterior asymmetry of the feather bud. Although the driving force for epithelial invagination is still to be elucidated. The major stages of feather morphogenesis along with key cellular events and morphogenesis-related signaling molecules (e.g., Shh, Wnt, BMP) have been described (Ting-Berreth et al., 1996; Chang et al., 2004a; Jung et al., 1998; Noramly et al., 1999; Morgan et al., 1998; Noramly et al., 1998). They reveal the essential role of cell adhesion and interactions between cell and ECM in feather morphogenesis (Chuong and Edelman, 1985, Jiang and Chuong, 1992). Yet to be addressed, however, is the role of the ECM degrading proteases and their inhibitors, which modulate cell-cell and cell-ECM interactions.
In mammals, MMPs and TIMPs have been shown to be involved in hair development and regeneration. A large body of literature has documented the role of MMPs and TIMPs in wound healing and tumor invasion in mammals. Their function during embryonic tissue morphogenesis is less understood. Knockout studies in mice have not been very fruitful in this aspect probably due to redundancy in substrate specificity (Gill et al., 2010). During embryonic mouse development, MMP 2 and MMP9 are present in the lower part of the inner hair root sheath (Jarrousse et al., 2001). TIMP expression is primarily expressed in the hair follicle during mid-anagen (growth phase of the hair cycle), suggesting that MMPs may be more active during early anagen, catagen and telogen, and are suppressed during the active growing phase (mid-anagen) (Kawabe et al., 1991). In fact, MMP and TIMP may be a involved in hair follicle morphogenesis since MMP2 activity is associated with the disappearance of collagen VII during the invasion of epithelial cords of hair follicles and sweat glands in human skin (Karelina et al., 2000). A recent report showed that MMP9 is important in extracellular matrix remodeling and involved in the regulation of hair canal formation (Sharov et al 2011). The other MMP member that is involved in skin appendage morphogenesis is MMP-7 or matrilysin that is expressed in the basal layer of epidermis and hair placode keratinocytes during mouse hair follicle development (Botchkarev and Sharov 2004). Additionally, TIMP3 is expressed in hair structures during the anagen but not catagen phase of the hair cycle (Airola et al., 1998).
To carry out this study in the chicken skin, we have surveyed the expression of chicken MMPs and TIMPs using immunostaining. Many of the existing antibodies against mammalian MMPs do not cross reach with chicken. Among them, MMP2 and TIMP2 gave the most striking pattern. Since our goal is to study how the MMP pathway and its inhibitor are used in tissue remodeling, rather than characterizing chicken MMPs, we think MMP2 / TIMP2 can work as an antagonistic pair to demonstrate how the activity of this pathway is fine tuned and balanced to achieve the control of developmental tissue remodeling. Therefore we mapped the levels and distribution of MMP2 and TIMP2 protein in developing chicken skin using these available cross reacting antibodies. MMP2 and TIMP2 proteins are co-expressed in the posterior leading edge of the follicle epithelium at E12. The presence of MMP2 activity at the leading edge, ascertained by zymography, suggests that it might be involved in epithelial invagination. It is noteworthy, however, TIMP2, being a MMP2 inhibitor, co-localizes with MMP2 in the epithelial and mesenchymal structures of the developing feather from E10 to E13. It is unclear at this time what role TIMP2 is playing here. It has been shown to serve as an inhibitor or mediator of MMP activity in a concentration dependent fashion during wound healing in mouse skin (Madlener et al., 1998).
Using broad spectrum inhibitors to either MMPs or plasmin, we demonstrated that MMP activity, but not plasmin, was required for feather bud formation. Blocking MMP activity resulted in a disruption of feather morphogenesis, specifically the size of the feather buds, epithelial invagination and mesenchymal proliferation. Feather buds which are normally elongated and tapered become blunted/rounded at the tip. The phenotype is coupled with diminished mesenchymal proliferation and comparable keratin expression with control skin. The results indicate that MMPs are necessary for epithelial invagination and probably cell proliferation in the mesenchymal tissue in the early stages of feather development, but not epithelial differentiation. These data illustrate the importance of MMP in tissue remodeling. Thus, the MMP pathway is not only used in tissue remodeling after wounding, but is present and functional in physiological process of organogenesis during developmental tissue remodeling, in this case, feather morphogenesis.
β-catenin signaling might be involved in the altered feather morphogenesis due to MMP inhibition in the present study, because beta-catenin signaling was found to be upstream to MMP expression in both physiological and pathological tissue morphogenesis (Crawford et al., 1999; Brabletz et al., 1999; Garg et al., 2010; Sonderegger et al., 2010). Indeed, β-catenin was found to regulate the expression of MMP2 in T-lymphocytes (Wu et al., 2007). In chicken skin development, β-catenin expression is dynamic; initially enriched in the feather germ, then restricted to the posterior region (Widelitz et al., 2000). In the present study, β–catenin expression is altered in the feather buds of MMP inhibitor treated groups with delayed feather bud development.
In summary, through this study we can appreciate that during organogenesis there is large scale developmental tissue remodeling, which shapes the organ forms. MMP2 and TIMP2 are expressed at low levels in the early feather bud and are expressed at high levels at stages where feather follicles form. Upon completion of follicle formation, however, TIMP2 becomes more diffusely expressed through the follicle while MMP2 becomes localized at sites of ongoing tissue rearrangements, suggesting that the relative strength of MMP and TIMP can predict localized tissue remodeling activity during development (Schwertfeger et al., 2001). Furthermore, the controlled ECM degradation is likely to be a downstream event of such activities, exemplified in the study by the differential and restrictive presence of tenascin C. Using the unique model of feather morphogenesis, our future goals are to understand how topological tissue remodeling and organ morphogenesis are accomplished through the intricate up and down regulation of MMPs and TIMPs. We also anticipate this will strengthen our knowledge in managing the many processes involving protease pathways during tissue remodeling in repair and regeneration.
Acknowledgement
We thank the grant support of AR 42177, 47364 (to CMC) and 60306 (to CMC and TXJ) from NIAMS, and GM055081 (to TLT) from NIGMS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airola K, Ahonen M, Johansson N, Heikkila P, Kere J, Kahari VM, Saarialho-Kere UK. Human TIMP-3 is expressed during fetal development, hair growth cycle, and cancer progression. J Histochem Cytochem. 1998;46:437–447. doi: 10.1177/002215549804600403. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–26. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Cai M, Onoda K, Takao M, Kyoko IY, Shimpo H, Yoshida T, Yada I. Degradation of tenascin-C and activity of matrix metalloproteinase-2 are associated with tumor recurrence in early stage non-small cell lung cancer. Clin. Cancer Res. 2002;8:1152–1156. [PubMed] [Google Scholar]
- Chang CH, Jiang TX, Lin CM, Burrus LW, Chuong CM, Widelitz R. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech Dev. 2004a;121:157–171. doi: 10.1016/j.mod.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Yu M, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. Sculpting skin appendages out of epidermal layers via temporally and spatially regulated apoptotic events. J Invest Dermatol. 2004;122:1348–55. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Edelman GM. Expression of cell-adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. J Cell Biol. 1985;101:1009–1026. doi: 10.1083/jcb.101.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM. Feather Morphogenesis: A Model of the Formation of Epithelial Appendages. In: Chuong, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience; Austin: 1998. pp. 3–14. [Google Scholar]
- Chuong CM, Widelitz RW, Ting-Berreth SA, Jiang TX. Early events during the regeneration of skin appendages: Dependence of epithelial-mesenchymal interaction and order of molecular reappearance. J Invest Dermato. 1996;107:639–646. doi: 10.1111/1523-1747.ep12584254. [DOI] [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- de Almeida H, Jr, Zigrino P, Müller F, Krieg T, Korge B, Mauch C. Human scalp dermal papilla and fibrous sheath cells have a different expression profile of matrix metalloproteinases in vitro when compared to scalp dermal fibroblasts. Arch Dermatol Res. 2005;297:121–126. doi: 10.1007/s00403-005-0587-3. [DOI] [PubMed] [Google Scholar]
- Garg P, Sarma D, Jeppsson S, Patel NR, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res. 2010;70:792–801. doi: 10.1158/0008-5472.CAN-09-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SE, Kassim SY, Birkland TP, Parks WC. Mouse models of MMP and TIMP function. Methods Mol Biol. 2010;622:31–52. doi: 10.1007/978-1-60327-299-5_2. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morpho. 1951;88:49–92. [PubMed] [Google Scholar]
- Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS, Shaw JA, Walker RA, Pringle JH, Jones JL. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res. 2009;11:R24. doi: 10.1186/bcr2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrousse F, Boisnic S, Branchet MC, Beranger JY, Godeau G, Breton L, Bernard BA, Mahe YF. Identification of clustered cells in human hair follicle responsible for MMP-9 gelatinolytic activity: consequences for the regulation of hair growth. Int J Dermato. 2001;40:385–392. doi: 10.1046/j.1365-4362.2001.01239.x. [DOI] [PubMed] [Google Scholar]
- Jian B, Jones PL, Li Q, Mohler ER, 3rd, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-C in calcific aortic stenosis. Am J Pathol. 2001;159:321–327. doi: 10.1016/S0002-9440(10)61698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TX, Chuong CM. Mechanism of feather morphogenesis: I. Analyses with antibodies to Adhesion Molecules Tenascin, N-CAM and Integrin. Dev Biol. 1992;150:82–98. doi: 10.1016/0012-1606(92)90009-6. [DOI] [PubMed] [Google Scholar]
- Jovanova-Nesic K, Shoenfeld Y. MMP-2, VCAM-1 and NCAM-1 expression in the brain of rats with experimental autoimmune encephalomyelitis as a trigger mechanism for synaptic plasticity and pathology. J Neuroimmunol. 2006;181:112–121. doi: 10.1016/j.jneuroim.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Karelina TV, Bannikov GA, Eisen AZ. Basement membrane zone remodeling during appendageal development in human fetal skin. The absence of type VII collagen is associated with gelatinase-A (MMP2) activity. J Invest Dermatol. 2000;114:371–375. doi: 10.1046/j.1523-1747.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Kawabe TT, Rea TJ, Flenniken AM, Williams BR, Groppi VE, Buhl AE. Localization of TIMP in cycling mouse hair. Development. 1991;111:877–879. doi: 10.1242/dev.111.4.877. [DOI] [PubMed] [Google Scholar]
- Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Curr. Opin. Cell Biol. 2006;18:730–741. doi: 10.1016/j.ceb.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CM, Jiang TX, Baker RE, Maini PK, Widelitz RB, Chuong CM. Spots and stripes: pleomorphic patterning of stem cells via p-ERK-dependent cell chemotaxis shown by feather morphogenesis and mathematical simulation. Dev Biol. 2009;1334:369–382. doi: 10.1016/j.ydbio.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–210. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Orkin RW, Noramly S, Perez A. Stage-specific effects of sonic hedgehog expression in the epidermis. Dev Biol. 1998;201:1–12. doi: 10.1006/dbio.1998.8969. [DOI] [PubMed] [Google Scholar]
- Noramly S, Freeman A, Morgan BA. beta-catenin signaling can initiate feather bud development. Development. 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development. 1998;125:3775–3787. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- Parks WC, Robert PM. Matrix Metalloproteinases. Academic Press; New York: 1998. p. 362. [Google Scholar]
- Parks C, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci. 2010;17:15337–57. doi: 10.1523/JNEUROSCI.3467-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri A, Knauuper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem. 1995;270:8650–8654. doi: 10.1074/jbc.270.15.8650. [DOI] [PubMed] [Google Scholar]
- Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol Endocrinol. 2001;15:867–881. doi: 10.1210/mend.15.6.0663. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Schroeder M, Sharova TY, Mardaryev AN, Peters EM, Tobin DJ, Botchkarev VA. Matrix Metalloproteinase-9 Is Involved in the Regulation of Hair Canal Formation. J Invest Dermatol. 2011;131:257–260. doi: 10.1038/jid.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek-van Beurden PAM, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, Knöfler M. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151:211–20. doi: 10.1210/en.2009-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends. Mol Med. 2005;11:97–103. doi: 10.1016/j.molmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Svineng G, Ravuri C, Rikardsen O, Huseby NE, Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res. 2008;49:197–202. doi: 10.1080/03008200802143166. [DOI] [PubMed] [Google Scholar]
- Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev Dyn. 1996;207:157–170. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Yu M, Shen T, Shen JY, Wu P, Yu Z, Chuong CM. Molecular biology of feather morphogenesis: A testable model for evo-devo research. J Exp Zool B Mol Dev Evol. 2003;298:109–122. doi: 10.1002/jez.b.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Lu J, Chuong CM. beta-catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated beta-catenin. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong CM. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]