Abstract
Assisted reproduction technologies (ART) include in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), and are common treatments for infertility. Although generally successful, ART warrant further investigations due to emerging perinatal issues, especially low birth weight. Herein we extend our previous work demonstrating higher steroid clearance in murine ART placentas by examining steroid biosynthesis and the directional flow of steroids in the maternal-placental-fetal units. The activities of the major steroidogenic enzymes 3β-Hydroxysteroid Dehydrogenase (3β-HSD) and Cytochrome P450 17-αhydroxylase (CYP17) were assessed in maternal liver and ovaries and fetal livers as were levels of cholesterol, progesterone, estrone (E1), and estradiol (E2) in the maternal, placental and fetal units. No structural abnormalities were found in placentas from ART. Although ART increased 3β-HSD activity in maternal livers, there were no other changes in 3β-HSD- or CYP17-mediated steroidogenesis. Cholesterol levels were significantly lower in maternal livers of ICSI pregnancies and in placentas from both IVF and ICSI pregnancies but not altered in the fetal livers. Progesterone levels were higher in maternal and fetal livers in IVF and ICSI, respectively, but were significantly lowered in ICSI placentas, compared to normal fertilization. For estrogenic hormones, no differences in E1 or E2 levels were observed in maternal livers but ICSI significantly increased both E1 and E2 levels in placentas while both IVF and ICSI significantly lowered E1 but raised E2 levels in fetal livers. In summary, while steroid production was normal, steroid diffusion/flow from mother to fetus was altered in murine pregnancies conceived by ART. This appears to occur, at least in part; through placental mechanisms. Impaired cholesterol and steroid transfer may affect correct regulation of fetal growth and development.
Keywords: 3-beta hydroxysteroid dehydrogenase, cholesterol, Cytochrome P450 17, estrogen, placenta, progesterone
1.1 Introduction
The use of assisted reproduction technologies (ART) is increasing dramatically in the developed world [1]. While ART is considered a relatively safe and effective way to conceive, in vitro fertilization (IVF) with or without intra-cytoplasmic sperm injection (ICSI), confers a higher risk of adverse reproductive outcomes compared to couples who conceive naturally. These adverse outcomes include higher incidences of induced labor, cesarean section, premature birth, small-for-gestational age babies, pediatric cancer, imprinting disorders and congenital abnormalities [2-6]. In addition to neonatal outcomes; higher rates of placenta previa, placental abruption, premature rupture of the membranes, pre-eclampsia, unusual placental shape and umbilical cord insertion in humans and higher placental weights in mice are known to occur with ART [5, 7-10]. The etiology of such complications is unknown, but in addition to direct maternal causes, the placental and fetal origins of pregnancy outcomes should also be considered.
In human pregnancies, the major site of steroid production is the feto-placental unit, specifically the placenta, fetal adrenal and liver with the primary building-block (cholesterol) being the only major contribution from the maternal circulation. However, in mice pregnancies the maternal ovaries are the major site of sex steroid (estrone, estradiol, progesterone) production with a moderate contribution from placenta in the first half of gestation, and from the fetal tissues in the second half of gestation [11]. In both mice and humans, the production of these steroids from cholesterol is mediated upstream by the steroidogenic enzymes 3β hydroxysteroid dehydrogenase (3βHSD) and cytochrome P450 17α-hydroxylase (CYP17).
We have recently reported that functional changes in placental clearance of steroids occurred in mice when conception was achieved by ART [7] providing preliminary evidence that altered placental function may be responsible for some of the adverse reproductive outcomes reported for ART. Specifically we demonstrated bigger placental size in ART fetuses and dysregulation in steroid hormone metabolism and clearance across the placenta but no differences in maternal ovarian progesterone and estrogen levels in ART [7]. To expand our understanding of the mechanisms by which ART alters steroids, we wished to examine steroidogenesis and the directional flow of steroids in the maternal-placental-fetal units of normal and ART pregnancies. We generated a new cohort of ART and normal fertilization pregnancies, performed gross pathology and histological analyses on placental tissues, assessed activity of the steroidogenic enzymes 3βHSD and CYP17 in ovaries and maternal and fetal livers, and defined the levels of cholesterol, progesterone, estrone (E1) and 17β-estradiol (E2) in placentas and maternal and fetal livers. These tissues were chosen since they are the major site of production for estrone, estradiol, and progesterone during pregnancy as well as because of their major role in removing these steroids.
We demonstrated that IVF and ICSI do not alter placental structure nor do they alter ovarian or fetal liver steroid production by the enzymes 3βHSD and CYP17. However, we also showed that the net diffusion and/or transport of the steroids, progesterone, estrone and 17β estradiol, as well as their precursor cholesterol from the maternal circulation through the placenta to the fetus was altered in ART compared to normal pregnancies. This agrees with and strengthens our previous report showing higher steroid metabolism and clearance in the placenta [7] and further support our hypothesis that abnormal placental function may be responsible for adverse reproductive outcomes.
1.2 Materials and Methods
1.2.1. Reagents
Mineral oil was purchased from Squibb and Sons (Princeton, NJ); pregnant mares' serum gonadotrophin (eCG) and human chorionic gonadotrophin (hCG) were purchased from Calbiochem (Spring Valley, CA); assay kits for cholesterol were purchased from Cayman Chemical Company (Ann Arbor, MI); estrone (E1), estradiol (E2), and progesterone kits were purchased from ALPCO Diagnostics (Salem, NH); estradiol kits performed for CYP19 study were purchased from Calbiochem (Spring Valley, CA). All other chemicals were obtained from Sigma Chemical Co. (St Louis, MO) unless otherwise stated.
1.2.2 Animals
Mice of B6D2F1 (C57BL/6 × DBA/2) and CD-1 strains were obtained at 6 weeks of age from National Cancer Institute (Raleigh, NC) and Charles River Laboratories (Wilmington, MA), respectively. Mice B6D2F1 were used for mating and as sperm and oocytes donors for IVF and ICSI, and CD-1 mice were used as surrogate mothers and vasectomized males for embryo transfer. Mice were fed ad libitum with a standard diet and maintained in a temperature and light-controlled room (22°C, 14 h light/10 h dark), in accordance with the guidelines of the Laboratory Animal Services at the University of Hawaii and guidelines presented in National Research Council's (NCR) “Guide for Care and Use of Laboratory Animals” published by the Institute for Laboratory Animal Research (ILAR) of the National Academy of Science, Bethesda, MD, 1996. The protocol for animal handling and treatment procedures was reviewed and approved by the Animal Care and Use Committee at the University of Hawaii. In Vitro Fertilization (IVF), Intracytoplasmic Sperm Injection (ICSI), then subsequent embryo culture and transfer were performed as previously described [7].
1.2.3. Tissue Collection
Maternal livers and ovaries, placentas and fetal livers were collected after caesarian section performed at Day 18 of pregnancy, washed briefly in Dulbecco PBS (D-PBS), drained and placed singly into tubes. During collection all tissues were kept on ice for up to 30 min, and were subsequently frozen at −80 °C until use.
1.2.4. Tissue processing and histological examination
Maternal livers and ovaries, placentas and fetal livers were thawed, wet weight recorded, and were homogenized 1:4 in Tris-HCl buffer containing 5 mM MgCl2 and 2mM PMSF (pH 7.4). Homogenates were frozen at −80 °C until use. Before use, all tissue homogenates were normalized for protein concentration to 2.0 mg/mL using the Bicinchoninic acid method [12].
For structural analyses placentas (IVF n = 6, ICSI n = 4, normal reproduction n = 6, 2 per dam in each group) were embedded in OCT medium, re-frozen and cryotomed to 7 μm thickness. Masson's Trichrome Stain (Richard-Allan Scientific, Kalamazoo, MI) was used to visualize cellular structures, including: nuclei, cytoplasm, muscle fibers, and collagen. Slides were examined using bright field microscopy using an Olympus BX40 (Melville, NY) at 4X magnification. For analysis of the percentage area of the decidual, syncytial and labyrinth zones, the Image J program (http://rsb.info.nih.gov/ij/) was used on pictures taken at 4X magnification in the widest possible visual field. Pictures were then aligned and stitched together using Adobe Photoshop Professional CS4 (Adobe Systems Inc., San Jose, CA). For analysis, the area of each zone was identified in Image J and compared to area of the whole placenta. Percent areas were calculated from whole compound pictures and percentages presented are rounded to the nearest whole number.
1.2.5. Biochemical Assays for Steroid Enzymes
Assays for 3-β-hydroxysteroid dehydrogenase (3β-HSD) and cytochrome P450 17α-hydroxylase (CYP17) were performed using maternal livers (n = 3 each for IVF, ICSI and normal reproduction), ovaries (IVF n = 2, ICSI n = 4, normal reproduction n = 1), and fetal livers (IVF n = 35, ICSI n = 25, normal reproduction n = 26) using published protocols [13-15].
The assay for 3βHSD proceeded by addition of 10 μL of protein (0.05 mg /mL liver or 0.01 mg/mL ovaries), 79 μL of assay buffer (0.1M Tris-HCl buffer with 50 mM MgCl2, pH 7.4) and 1 μL of pregnenolone (500 μM stock) to a 1.7 mL microtube. An aliquot, 10 μL, of NAD+ (10 mM stock) was added to initiate the reaction, and tubes were covered and incubated in a 37 °C water bath for 10 min (maternal and fetal livers) or 5 min (ovaries). Reactions were terminated by plunging tubes into ice. Progesterone product in the supernatant of each reaction was quantified using a commercial ELISA as per manufacturer's instructions (Alpco, Salem NH). The optimal reaction conditions used here were determined by conducting protein (0.1 – 5 mg/mL), substrate concentration (5 nM – 5 mM) and incubation time (1 minute – 60 minutes) linearity studies. The protein, substrate and time concentrations used were within the linear rate of reaction, where less than 10% of substrate turnover occurred. The ELISA variance (coefficient of variation, CV) in our hands, using a positive control for 3β-HSD/progesterone, was 9.4%.
The published meta-dinitrobenzene assay for 17-ketosteroids [14] was adapted to specifically determine the conversion of 17α-hydroxypregnenolone to DHEA as an index of CYP17 activity. Briefly, in 1.7 mL microtubes the following were added: 10 μL of protein (0.05 mg/mL liver or 0.01 mg/mL ovary), 79 μL 0.1M Tris-HCl buffer containing 50 mM MgCl2, pH 7.4, 1μL of 17α-hydroxypregnenolone (50 mM stock) and 10 μL of NADPH (10 mM). Tubes were capped and incubated at 37 °C for 5 min or 2 min (livers and ovaries respectively) then reactions terminated by addition of 100 μL KOH (5 M). Immediately, 200 μL of 2% m-dinitrobenzene was added and mixed then solution transferred to an eppendorf tube and centrifuged for 2 min at 10, 000 RPM. Duplicate aliquots (100 μL) were transferred to a 96-well plate and optical density at λ = 520 nm determined in a Spectramax 340plus spectrometer (Molecular Devices, Sunnyvale, CA). The optimal reaction conditions used here were determined by conducting protein (0.1 – 5 mg/mL liver, 0.005 – 0.5 mg/mL ovary), substrate concentration (5 nM – 5 mM final concentration) and incubation time (30 seconds – 30 minutes) linearity studies. The protein, substrate and time concentrations used were within the linear rate of reaction, where less than 10% of substrate turnover occurred. Quantification of product from the supernatant of each reaction was performed with commercial DHEA ELISA as per manufacturer's instructions (Calbiotech, Spring Valley, CA). The limit of sensitivity for this assay was 1 μM and the variance of the ELISA in our hands was 6.1% (positive control, CV).
1.2.6. Quantification of Cholesterol and Steroid Hormone Levels
Cholesterol and three steroids (progesterone, estrone (E1) and estradiol (E2) were detected using commercial ELISAs. Hormone levels were determined in maternal livers (n = 3 each for IVF, ICSI and normal reproduction), placentas (IVF n = 30; ICSI n = 21 and normal reproduction n = 20) and fetal livers (IVF n = 35; ICSI n = 25 and normal reproduction n = 26). The inter-assay variance of the ELISAs, using positive control samples to calculate coefficient of variation (standard deviation/mean*100) was: cholesterol: 2.5%, progesterone: 7.6%, E1: 3.7% and E2: 0.8%.
A spiking and recovery experiment was performed for each ELISA to check for recovery rates and applicability between tissues. Pooled tissue lysates from mouse maternal ovary, placenta or fetal liver were generated, comprising of samples from 3, 14 and 14 individuals, respectively with mixed normal, IVF or ICSI pregnancies. Lysates were normalized to 1 mg/mL (ovary) or 2 mg/mL (placenta and fetal liver) then assessed in triplicate for native steroid concentrations. Additionally, each sample was spiked with pure steroid standard (manufacturer supplied) using 3 concentrations from the standard curve that fell in the linear portion of the curve. A further set of dilutions into assay buffer (manufacturer supplied) were also performed. Recovery was assessed by comparison to the standard curve.
1.2.7. Statistical Analyses
Statistical analyses were performed using Prism 5.0 with statistical significance set at α = 0.05. (GraphPad Prism, San Diego CA). Parametric statistics were performed since all data approximated Gaussian distributions (as assessed in Prism using the D'Agostino-Pearson test) and two-tailed student-t tests were used to assess differences between groups with an F-test to compare variances. All data of n = 4 or more are presented as means ± standard errors of the mean (SEM). For data sets containing n = 3, means ± standard deviations (SD) are presented. For discrete single tests, student's t-tests were performed.
Steroid levels were compared between different pregnancy types (normal reproduction, IVF, ICSI) for the same organ. We also compared levels of hormones between different organs from the same pregnancy (e.g. maternal liver, placenta, fetal liver). For these comparisons one-way ANOVA was performed to reveal the effects of ART (in the former set of tissues) or the flow of hormones (the latter tissue comparison). Bland-Altman tests for variance were carried out on raw means and, when it revealed that standard deviation increased as mean increased, the natural log of each data point was taken and one-way ANOVA of the natural logs performed with Dunnet's post-hoc multiple comparison tests [16]. The purpose of this log transformation was to de-correlate variances from the means so that data were normalized, thereby making means appropriate. This was necessary because basic statistics operate under the assumption that variance is random about the mean and additionally for ANOVA, that variances in groups should be independent and additive.
1.3. Results
1.3.1. Production of Fetuses after Mating, IVF and ICSI
Fetuses were obtained after normal reproduction, IVF and ICSI (Table 1). Three females were mated and all of them became pregnant, providing a total of 26 fetuses. When embryos produced by IVF and ICSI were transferred into the oviducts of pseudopregnant females, all females (3 per group) became pregnant, and 37 and 25 fetuses were obtained from IVF and ICSI groups, respectively. There were no significant differences in the number of fetuses, abortion sites or total embryos implanted between IVF and ICSI. However IVF and ICSI were associated with 8 and 11 abortion sites while only 1 abortion site was noted after caesarian section naturally mated females. There were no significant differences in the number of fetuses per dam between normal reproduction and ART pregnancies, nor between IVF and ICSI, hence the effects observed in this study cannot be attributed to simple overcrowding.
Table 1.
Summarized reproductive outcomes of Mating, IVF and ICSI.
| Dam ID | No of 2-cell embryos transferred |
No (%) of fetuses |
No (%) abortion sites |
No (%) total implants |
|
|---|---|---|---|---|---|
| M1 | n/a | 10 | 0 | 10 | |
| Mated | M2 | n/a | 8 | 0 | 8 |
| M3 | n/a | 8 | 1 | 9 | |
| V1 | 16 | 13 (81) | 1 (6) | 14 (88) | |
| IVF | V2 | 16 | 10 (63) | 6 (38) | 16 (100) |
| V3 | 16 | 14 (88) | 1 (6) | 15 (94) | |
| I1 | 16 | 8 (50) | 6 (38) | 14 (88) | |
| ICSI | I2 | 16 | 11 (69) | 4 (25) | 15 (94) |
| I3 | 16 | 6 (38) | 1 (6) | 7 (44) |
1.3.2. Fetal Liver and Maternal Ovary Weights
We have previously shown differences in placental size in ART compared to normal reproduction [7]. In this study, fetal livers weights in ICSI pregnancies were significantly higher than those from IVF and normal reproduction (P < 0.001, Figure 1A) but maternal ovary weights did not differ between mating types (Figure 1B). The ovary weight data represent measurements of ovaries collected for both the present and our previous study [7], which were performed exactly the same way. We have not previously presented ovarian weight data.
Figure 1. Organ weights and placental characteristics from ART dams and fetuses.
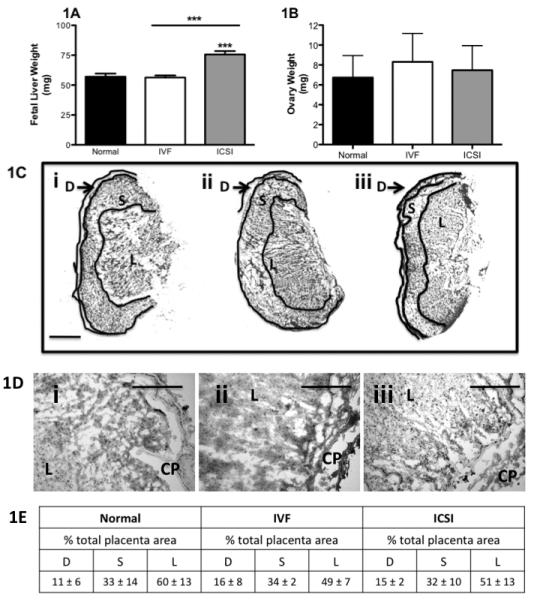
A) Fetal liver weights are higher in ART as assessed from n = 26, 37 and 26 weights respectively for normal, IVF and ICSI fetal livers. B) Ovarian weights do not differ between normal reproduction (n = 7 ovaries from four different mice), IVF (n = 8 ovaries from five different mice) and ICSI (n = 10 ovaries from six different mice). Bars are means ± SEM. *** P < 0.001 vs. normal except where indicated by a line, then significance is between indicated bars. C) Micrographs of placentas showing decidual (D), syncytial (S) and labyrinth (L) zones for Ci) normal, Cii) IVF and Ciii) ICSI placentas. Objective power was 4X and the scale bar is 1 mm. D) High magnification micrographs of the labyrinth zones from Di) normal, Dii) IVF and Diii) ICSI placentas. Pictures from 1D are representative of all slides, but are not taken directly from photomicrographs in 1C. Microscope objective power was 10X, and the scale bar is 100 μm. CP = chorionic plate, L = Labyrinth. 1E) Table demonstrating the percent area of the entire placenta, represented by each zone. Percentages are average ± SD, and rounded to the nearest whole number of n = 6, n = 4 and n = 6 placentas from each of 3, 2 and 3 separate dams for normal, IVF and ICSI pregnancies.
1.3.3. Placental Histopathology
Masson's Trichrome was used to visualize placental structures and to analyze fibrosis, changes in vascularity or other structural abnormalities that may influence fetal growth. Placentas from normal reproduction, IVF and ICSI showed no differences in structural features (Figure 1C). Vascularity, levels of fibrin, mesenchyme and other structural proteins were normal in all examined groups (Figure 1D). Additionally, there were no significant differences in the areas of the decidua, syncytium or labyrinth zones when each was calculated as a percentage of whole placenta area (Figure 1E).
1.3.4. Biochemical Assays for Steroidogenic Enzymes
ART increased 3β-HSD activities in maternal livers (IVF and ICSI, P < 0.05 vs. normal reproduction, Figure 2A) but not in maternal ovaries or fetal livers (Figures 2B and 2C). Additionally 3β-HSD activity was the highest in maternal ovaries (271 ± 1, 279 ± 16 and 265 ± 14 fmol/min/mg protein) and lower, but at similar levels in fetal livers (196 ± 45, 194 ± 16 and 191 ± 36 fmol/min/mg protein) and maternal livers (162 ± 3, 179 ± 7 and 215 ± 24 fmol/min/mg protein), for normal reproduction, IVF and ICSI, respectively (Figure 2A-C).
Figure 2. ART does not alter steroidogenesis in maternal or fetal tissues.
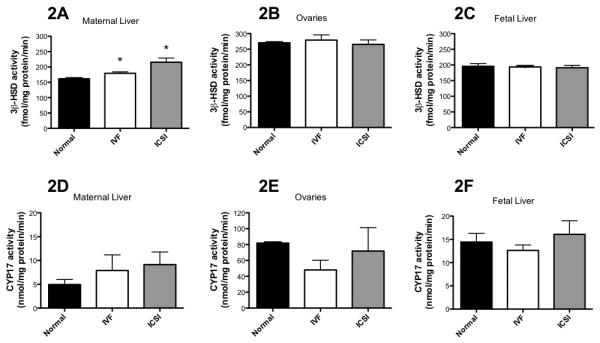
A-C) ART slightly raises 3β-HSD activities in maternal livers in ART, but has no effect in ovaries or fetal livers. D-F) ART does not affect CYP17 activity. * = P < 0.05 vs. normal reproduction. Bars are means ± SD for maternal livers (n = 3 each) and ovaries (n = 2, n = 2 and n = 4 for normal reproduction, IVF and ICSI respectively) and means ± SEM for fetal livers (n = 26, n = 35 and n = 25 for normal reproduction, IVF and ICSI respectively). Where there are less placentas and fetal liver tested for estrogenic hormones than those produced, this was due to sample processing and loss.
No significant differences in CYP17 activity were noted in ovaries and maternal and fetal livers in IVF and ICSI groups as compared to natural reproduction (Figure 2D-F). CYP17 activity was the highest in maternal ovaries (81.3 ± 0.02, 48.1 ± 17.2, 71.8 ± 41.8 nmol/min/mg protein), moderate in fetal livers (14.4 ± 9.3, 12.6 ± 6.8 and 16.1 ± 13.9 nmol/min/mg protein) and the lowest maternal livers (4.9 ± 1.5, 7.8 ± 5.7 and 9.1 ± 4.6 nmol/min/mg protein) in normal reproduction, IVF and ICSI, respectively (Figure 2D-F). Care is needed in the interpretation of maternal ovary data since we only report normal reproduction and IVF ovary data from n = 2 per group, although for ICSI n = 4.
1.3.5. ELISA Quantification of Steroid Hormone Levels
The commercial ELISAs were validated for use in mouse ovary, placenta and tissue lysates as described in the Materials and Methods Section. Results are presented in Table 2. Spikes of manufacturer-supplied controls into Buffer were at or around 100% recovery for all four hormones. For tissue lysates three hormones: DHEA, Progesterone and Estrone were quantifiable with recoveries within 75 – 125 % (inclusive of standard deviations) across all spiked concentrations for each tissue type tested. Since the manufacturer's positive controls are “accepted” when the derived concentration is ± 25 % of the theoretical concentration, these data indicate that for DHEA, Progesterone and Estrone these tissue lysates, at the concentrations used, are appropriate for quantification and comparison within this work and with other studies. For Estradiol, derived concentrations were routinely 200 % of theoretical concentrations in tissue lysates. However, the spiking experiments demonstrated that increasing concentrations of pure standard could still be differentiated across the linear range of the standard curve. Indeed, if results are divided by “two” for tissue lysates, results would be within acceptable ranges. This result suggests that tissue lysates, at the concentration used, contain a non-specific compound that increases background in the Estradiol ELISA, but does not interfere with quantification. Since quantification is to a standard curve in Assay Buffer, for true quantification, the manufacturer's standard curve for Estradiol should be performed in tissue lysates for absolute quantification. However, within this study, since the increase in Estradiol results was consistent (200% across all tissue types) and differences in concentration were still linear within the standard curve, the results derived can be compared across tissue types and concentrations as performed in this paper. For absolute quantification of estradiol or to compare levels to other studies and tissues, a scaling factor of 2 should be applied.
Table 2. Validation of commercial ELISAs for use in murine tissue lysates.
Spiking and recovery experiments using pure (manufacturer supplied) DHEA, progesterone, estradiol and estrone were performed across the linear portion of the standard curve. Results demonstrate that hormones can be quantified within and compared between the tissues in this study. Percentages and standard deviations are rounded to the nearest whole number.
| DHEA (ng/mL) |
PROGESTERONE (ng/mL) |
ESTRADIOL (pg/mL) |
ESTRONE (pg/mL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked Concentration | 1 | 5 | 15 | 2 | 10 | 30 | 80 | 150 | 320 | 80 | 400 | 1000 |
|
BUFFER Measured concentration |
0.80 | 7.2 | 21.6 | 2.2 | 11.7 | 35.0 | 80.5 | 150 | 299 | 74.0 | 345 | 898 |
| Recovery (%±SD) | 80 ±8 |
144 ±0.8 |
144 ±22 |
110 ±20 |
117 ±2 |
117 ±17 |
101 ±38 |
100 ±15 |
94 ±19 |
92 ±2 |
86 ±20 |
90 ±22 |
| Average (%± SD) | 122±10 | 115±13 | 98±24 | 89±15 | ||||||||
|
OVARY LYSATE Measured concentration |
1.4 | 6.6 | 19.7 | 2.0 | 11.9 | 26.7 | 143 | 334 | 512 | 75.6 | 526 | 1325 |
| Recovery ( %±SD) | 136 ±17 |
132 ±2 |
131 ±3 |
106 ±7 |
131 ±15 |
93 ±11 |
178 ±18 |
223 ±27 |
160 ±26 |
94 ±5 |
129 ±9 |
133 ±10 |
| Average Recovery (%±SD) | 133±7 | 102±25 | 210±25 | 119±8 | ||||||||
|
PLACENTAL LYSATE Measured concentration |
1.4 | 5.7 | 18.4 | 2.7 | 14.5 | 44.0 | 163 | 297 | 488 | 80.2 | 373 | 960 |
| Recovery (%±SD) | 140 ±18 |
114 ±8 |
122 ±16 |
137 ±30 |
145 ±24 |
137 ±20 |
205 ±37 |
198 ±21 |
153 ±38 |
100 ±24 |
93 ±3 |
96 ±8 |
| Average Recovery (%±SD) | 136±14 | 143± 19 | 185±32 | 96±11.8 | ||||||||
|
FETAL LIVER LYSATE Measured concentration |
0.86 | 6.7 | 21.0 | 1.5 | 9.8 | 34.5 | 196 | 265 | 758 | 74.0 | 344 | 898 |
| Recovery (%±SD) | 86 ±30 |
131 ±3 |
137 ±6 |
76 ±39 |
98 ±15 |
115 ±41 |
246 ±44 |
177 ±22 |
237 ±51 |
93 ±27 |
83 ±1 |
71 ±7 |
| Average (%± SD) | 118±13 | 96±35 | 220±39 | 82±12 | ||||||||
Cholesterol was significantly lower in maternal liver in the ICSI group (P < 0.05 vs. normal reproduction, Figure 3A). In the placenta, cholesterol was significantly lower in both IVF and ICSI pregnancies compared to normal reproduction (P < 0.01 both, Figure 3B). There were no differences in the fetal livers (Figure 3C). The comparison of cholesterol levels across examined tissues showed significant decline from maternal to placental to fetal compartments within each mating group (one-way ANOVA, P < 0.01)
Figure 3. ART alters cholesterol and progesterone levels in the murine maternal-placental-fetal unit.
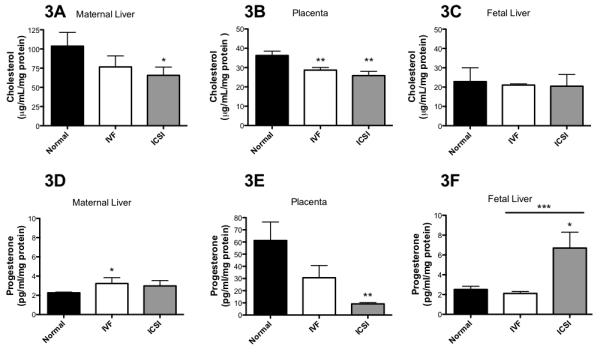
A-C) ART lowers cholesterol in the maternal livers and leads to its higher clearance in the placenta. D-F) ART affects progesterone levels in maternal and fetal livers and leads to its higher clearance across the placenta. * = P < 0.05, ** = P < 0.01, *** = P < 0.001 vs. normal reproduction except where indicated by a line, then significance is between indicated bars. Bars are means ± SD for maternal livers, n = 3 each. Bars are means ± SEM for placenta (n = 20, n = 30 and n = 21 for normal reproduction, IVF and ICSI, respectively) and fetal livers (n = 26, n = 35 and n = 25 for normal reproduction, IVF and ICSI, respectively). Where there are less placentas and fetal livers tested for estrogenic hormones than those produced, this was due to sample processing and loss.
ART increased progesterone levels in maternal livers (IVF P < 0.05 and ICSI P = 0.07 vs. normal reproduction, Figure 3D) and in fetal livers (ICSI P < 0.05 vs. normal reproduction, Figure 3F). However, progesterone levels were significantly lower in ICSI placentas (P < 0.01 vs. normal reproduction, Figure 3E) and approached significance for IVF placentas (P = 0.08). Interestingly, there was a highly significant difference in progesterone levels in fetal livers between IVF and ICSI groups (P < 0.001, Figure 3F).
No differences in estrone (E1) levels were observed in maternal livers (Figure 4A). ART significantly raised E1 in the placentas in ICSI group (P<0.001 vs. normal reproduction); ICSI also had higher placental E1 than IVF (P < 0.001, Figure 4B). Conversely, significantly lower E1 was observed in the ART fetal livers (IVF P < 0.01 and ICSI P < 0.001 vs. normal reproduction) with ICSI fetal livers containing lower E1 than IVF (P < 0.05, Figure 4C). In normal reproduction, maternal and fetal livers had higher levels of E1 than the placenta (P < 0.05). But this was not true for ART, where IVF showed equitable levels of E1 in the placenta and fetal liver (P = 0.11) while ICSI had higher levels of E1 in the placenta than fetal liver (P < 0.01).
Figure 4. ART alters estrogenic hormone levels in the murine maternal-placental-fetal unit.
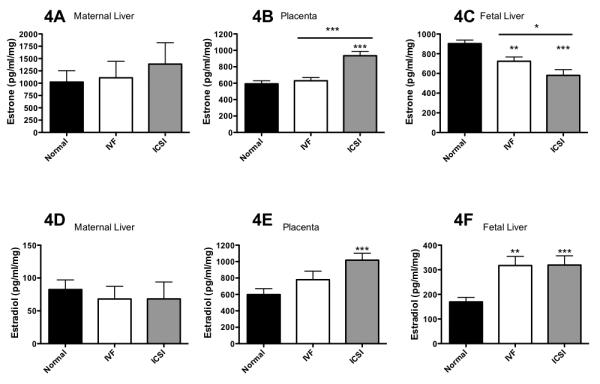
A-C) ART affects estrone levels in the placenta and fetal livers. D-F) ART raises estradiol in the placenta and fetal livers. * = P < 0.05, ** = P < 0.01, *** = P < 0.001 vs. normal reproduction except where indicated by a line, then significance is between indicated bars. Bars are means ± SD for maternal livers, n = 3 each. Bars are means ± SEM for placentas (n = 20, n = 29 and n = 21 normal reproduction, IVF and ICSI, respectively) and fetal livers (n = 26, n = 35 and n = 25 for normal reproduction, IVF and ICSI, respectively). Where there are less placentas and fetal liver tested for estrogenic hormones than those produced, this was due to sample processing and loss.
There were no differences in 17β-estradiol levels in maternal livers (E2, Figure 4D) from three types of pregnancy while in placentas, ICSI was associated with significantly higher levels of E2 (P < 0.001) compared to normal reproduction (Figure 4E). ART also significantly raised E2 in the fetal livers (IVF P < 0.01 and ICSI P < 0.001 vs. normal reproduction, Figure 4F). One-way analysis of variance for all mating types showed an increase in significance in order: normal reproduction (P = 0.861) < ICSI (P = 0.054) < ICSI (P = 0.0008) for estradiol transit from the maternal to placental to fetal unit.
1.4. Discussion
Despite playing a vital role in pregnancy and fetal development, placentas are seldom considered as a critical variable in obstetric and developmental research. Our previous data indicated that placental size and steroid clearance are altered by ART [7]. The aim of the current study was to determine if ART also caused changes in placental structure and/or steroidogenesis (3βHSD and CYP17) in the maternal, placental and fetal units that could be responsible for differences in steroid hormones. We also wished to assess net flow of hormones between the maternal, placental and fetal circulations.
Mice are a subject of ongoing debate regarding their suitability to model human diseases yet remain one of the most widely used laboratory models. In terms of placental research, mouse and human structures are very similar in function, although architecturally different. The murine placenta is hemotrichorial while the human placenta is hemomonochorial. In mice, the maternal decidua and spongiotrophoblast act as a pathway for nutrient delivery where maternal-fetal blood exchange occurs in the labyrinth, consisting of syncitiotrophoblasts, chorionic trophoblasts, stroma, and blood vessels [17]. In humans, this is similar, except that the chorionic villi are bathed directly in maternal blood, rather than the distinct pathways through the spongiotrophoblast observed in mice [17]. In spite of these differences placental function is similar since the organ is critical for the transport of substances (including steroids and other hormones, nutrients and essential minerals) to the fetus as well as removing wastes.
Here, we did not observe distinct differences in the architecture of maternal decidua, spongiotrophoblast or labyrinth zones, indicating that ART does not affect placental structural development which is in agreement with Delle Piane (2010) [18]. These previous authors studied morphology of placentas derived from normal mating and from IVF (although not ICSI) at the mid-gestational day 12.5. In contrast, here we report on the characteristics of normal, IVF and ICSI placentas at day 18 of gestation (term). Our data are similar to Delle Piane et. al. since we report that approximately 50 – 60% of the total placental area was occupied by the labyrinth zone although high magnification pictures of this zone seem to show larger fetal blood spaces than those reported by Delle Pianet et. al. [18]. This is not unexpected considering that our placentas were harvested at term, at the beginning of placental senescence. Despite this difference, our results were similar to the previous report since we saw no differences in the percentage of placental area that each zone occupies between normal, IVF or ICSI placentas and no marked structural or cellular differences. Although a delay in placental development for IVF was also reported previously (with no change in morphology), we cannot assess delays in placental development, since all placentas were harvested at term [18]. However, we did conclude that the mechanism for ART-induced changes in steroid dynamics in the murine placenta are not primarily structural.
Fetal liver size was higher in ICSI than IVF or normal reproduction fetuses. Since the liver usually grows at a proportional rate to the fetus, this would normally indicate greater fetal weights in ICSI. However, since in the ICSI group fetal livers also contained significantly higher progesterone and it is known that greater steroid levels can cause local hypertrophy, the use of fetal liver weight as an index for fetal weight is less certain. Maternal ovary weights did not differ between normal reproduction, IVF and ICSI pregnancies. Larger ovaries would contain more thecal and granulosa cells and therefore produce more steroid hormones. Since ovarian size did not increase in ART, this supports our findings that total steroid production by the mother is not increased. Essentially, neither steroidogenic enzymes are being up-regulated, nor are ovaries becoming larger and thereby producing more steroids (on a per milligram of protein basis).
In addition to placental structure, we determined the levels of steroid hormones in tissues from the maternal, placental and fetal compartments. Cholesterol is vital for steroid biosynthesis, being the first building-block in the steroid synthesis pathway, and the chemical from which all sex steroids are produced [19]. The mouse fetuses are dependent on transfer of maternal cholesterol throughout gestation for normal development, much more so than the human. A lack of maternal cholesterol transport, even at late gestational points can lead to fetal death [20-22]. Cholesterol in placentas and fetal tissues is mainly derived from production in the maternal liver with low levels of supplementary production by the fetus. It has also been demonstrated that cholesterol levels in the fetal compartment can be regulated through changes in placenta efflux in humans and rodents [23]. Here we report that cholesterol levels were significantly lower in ART placentas compared to placentas from normal reproduction, but there were no differences between ART and normal reproduction in respect to cholesterol levels in the fetal compartment. Since the concentrations of cholesterol in placentas and fetal livers were similar (but only about half that of the maternal liver) this may be explained in two ways: 1) less passage of cholesterol through the placenta due to its clearance and/or 2) equilibration of cholesterol concentrations where the placenta and fetal liver act as a combined compartment and equilibrate with the maternal system [7, 24]. Changes in cholesterol levels delivered to the fetus may be very important for body patterning and embryogenesis, as well as for the future development of pediatric cancers (many of which are believed to be of fetal origin) since cholesterol is intimately associated with the Wnt signaling pathway [19].
Furthermore, progesterone levels were significantly higher in placentas from normal reproduction than from IVF or ICSI, despite placenta not being a major site of production [11]. Coupled with no differences in progesterone concentrations in maternal or fetal livers, and very low levels of progesterone in both maternal and fetal systems (on a pg/mL/mg protein basis), we conclude that altered progesterone levels in ART placentas might have occurred through two mechanisms. First, it is possible that higher levels of metabolic clearance in the placenta accounted for low levels of progesterone on either side of that organ [7]. Second, a driving concentration gradient for progesterone into the fetal compartment might have occurred, where progesterone was rapidly used by the developing fetus, perpetuating the concentration gradient and further depleting placental stores. The latter is less likely since fetal liver progesterone levels were approximately equal to maternal levels for normal reproduction and IVF pregnancies, despite the fact that normal and IVF placentas had much higher progesterone levels than either the fetal or maternal circulations. Serious negative reproductive outcomes including placenta overgrowth and pre-term birth have been demonstrated when progesterone is over- or under-expressed [25-28]. Additionally, progesterone is important for inhibiting calcium-induced cell death in the fetal chorion and maternal decidua, and has also been implicated in suppressing fetal inflammatory response [27, 28]. Therefore, lower levels of progesterone may theoretically lead to inflammation in placental structures, which has been associated with pre-eclampsia, a disease that is also correlated with ART. In the present study we did not observe placental inflammation in tissue sections, perhaps due to low method sensitivity. Future studies in this area should test for inflammatory cytokines, which are more sensitive and accurate for studying inflammation. Finally, both human and animal research has shown that lowered progesterone levels and steroid metabolite inhibition significantly induce parturition and may cause the onset of premature labor [29, 30], and progesterone withdrawal can cause fetal growth restriction [31, 32]. Again, these are all primary concerns in the application and outcomes of ART.
After cholesterol and progesterone, estrogenic hormones are some of the most important factors in fetal development. Decreased levels of E1 hormones were detected in fetal livers in ART groups despite being high in placentas. Similarly, the absolute amounts of E2 in fetal livers were much lower than the levels quantified in placentas. Since placenta is not a site of steroid production, the foregoing arguments relating to clearance of steroids seems logical, while the concept of a driving gradient of steroid into the placenta that does not flow through to the fetus seems counter-intuitive. An alternative explanation would be that since E1 and E2 readily interconvert, ART tips this enzyme reaction towards more E1 production. This may be plausible since although E1 and E2 are found in the placenta in comparable concentrations for all conditions, the concentrations of E1 are much higher than E2 in fetal liver from normal pregnancies. However, in IVF and ICSI fetal livers, as levels of E1 decline significantly with ART, levels of E2 concurrently increase, although the mass balance of total estrogenic hormones is not completely preserved, indicating some loss of hormones presumably through clearance metabolism. One of the most important functions of E2 is its coordinated action with progesterone aiming to preparing the endometrium for implantation, and its overall role in the onset of labor during pregnancy. The long-term effects of ART are unknown, but the E2 dysregulation found in this study provides a plausible area of focus for studying development of ART offspring.
The observed differences in steroid hormone levels between compartments of the maternal-placental-fetal unit become even more interesting in light of our findings that steroidogenic enzymes do not generally differ. In fact the only significant difference was an increase in 3βHSD activities in maternal livers in ART groups. Since steroid production by the ovaries (measured herein by assessing enzyme activity and previously reported by us with levels of E1 and E2 in ovaries [7]) is so much higher than that of the maternal livers, the increases in 3βHSD activities observed for ART maternal livers are not likely to be significant systemically in terms of the steroid hormone levels in the maternal circulation. However, local dysregulation of liver steroid levels may affect hepatic cell function and signaling with down-stream effects on liver pathophysiology. The liver is a good proxy tissue for assessing levels of steroids in each compartment. The liver of both mice and humans receives the entire body's blood supply every 4-6 minutes. Since steroid hormones are highly fat soluble and diffuse across membranes very quickly, and since the liver is extremely vascular, we expect that steroid levels in the liver are representative of systemic blood levels. The caveat to this statement is that liver (and placenta) also metabolizes and removes steroids. Thus steroid levels in these organs are representative of systemic hormone levels, inclusive of clearance and elimination. The latter is an important consideration since we have previously identified differences in elimination of steroids in ART [7].
To summarize, in this study we present clear evidence of altered steroid hormone levels between the maternal, placental and fetal units in ART pregnancies, which are not caused by changes in steroidogenesis nor by pathological changes in placental structures. These results, coupled with our previously reported data showing higher placental clearance of steroid hormones in ART [7], indicate that increase in placental metabolism and clearance enzymes are critical to sex-steroid hormone dysregulation by ART. Our study extends the understanding of steroid dynamics in ART and presents a potential candidate mechanism: the pivotal role of the placental clearance of steroids. The cellular and molecular basis for this mechanism should be further elucidated to determine its importance for the developing fetus and to establish strategies to restore normal steroid balance.
Acknowledgement
We thank Dr. David Raunig, Pfizer Global Research and Development, Groton, CT, for useful conversations and assistance with statistical analyses in this paper.
Funding Acknowledgement:
This work was supported by NIH RR024206 (Project 4) to ACC and NIH RR024206 (Project 2) and NIH HD058059 to MAW.
References
- 1.De Geyter C, De Geyter M, Steimann S, Zhang H, Holzgreve W. Comparative birth weights of singletons born after assisted reproduction and natural conception in previously infertile women. Hum Reprod. 2006;21(3):705–712. doi: 10.1093/humrep/dei378. [DOI] [PubMed] [Google Scholar]
- 2.Sinnett D, Labuda D, Krajinovic M. Challenges identifying genetic determinants of pediatric cancers--the childhood leukemia experience. Fam Cancer. 2006;5(1):35–47. doi: 10.1007/s10689-005-2574-4. [DOI] [PubMed] [Google Scholar]
- 3.Allen VM, Wilson RD, Cheung A. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can. 2006;28(3):220–250. doi: 10.1016/S1701-2163(16)32112-0. [DOI] [PubMed] [Google Scholar]
- 4.Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110(4):885–891. doi: 10.1097/01.AOG.0000284627.38540.80. [DOI] [PubMed] [Google Scholar]
- 5.Kallen B, Finnstrom O, Nygren KG, Olausson PO. In vitro fertilization in Sweden: child morbidity including cancer risk. Fertil Steril. 2005;84(3):605–610. doi: 10.1016/j.fertnstert.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, Hankins GD, Eddleman K, Dolan S, Dugoff L, Craigo S, Timor IE, Carr SR, Wolfe HM, Bianchi DW, D'Alton ME. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106(5 Pt 1):1039–1045. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- 7.Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. Assisted reproduction technologies impair placental steroid metabolism. J Steroid Biochem Mol Biol. 2009;116(1-2):21–28. doi: 10.1016/j.jsbmb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavriil P, Jauniaux E, Leroy F. Pathologic examination of placentas from singleton and twin pregnancies obtained after in vitro fertilization and embryo transfer. Pediatr Pathol. 1993;13(4):453–462. doi: 10.3109/15513819309048235. [DOI] [PubMed] [Google Scholar]
- 9.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12(6):747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21(9):2353–2358. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Zimra M, Koler M, Melamed-Book N, Arensburg J, Payne AH, Orly J. Uterine and placental expression of steroidogenic genes during rodent pregnancy. Mol Cell Endocrinol. 2002;187(1-2):223–231. doi: 10.1016/s0303-7207(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 12.Juchau MR. Drug biotransformation in the placenta. Pharmacol Ther. 1980;8(3):501–524. doi: 10.1016/0163-7258(80)90074-1. [DOI] [PubMed] [Google Scholar]
- 13.Bauer HC, Bauer H. Micromethod for the determination of 3-beta-HSD activity in cultured cells. J Steroid Biochem. 1989;33(4A):643–646. doi: 10.1016/0022-4731(89)90054-x. [DOI] [PubMed] [Google Scholar]
- 14.Holtroff AF, Koch FC. The colorimetric estimation of 17-ketosteroids and their application to urine extracts. The Journal of Biological Chemistry. 1940;135(2):377–392. [Google Scholar]
- 15.Lephart ED, Simpson ER. Assay of aromatase activity. Methods Enzymol. 1991;206:477–483. doi: 10.1016/0076-6879(91)06116-k. [DOI] [PubMed] [Google Scholar]
- 16.Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; 1989. pp. 282–299. [Google Scholar]
- 17.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 18.Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, Maltepe E, Rinaudo PF. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. 2010 doi: 10.1093/humrep/deq165. P.,F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porterfield SP. Endocrine physiology. Mosby; St. Louis, Mo.: 1997. [Google Scholar]
- 20.Farese R, Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B genes results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proceedings of the National Academies of Sciences, USA. 1995;92:1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herz J, Farese RV. The LDL receptor gene family, apolipoprotein B and cholesterol in embryonic development. Journal of Nutrition. 1999;129:473S–475S. doi: 10.1093/jn/129.2.473S. [DOI] [PubMed] [Google Scholar]
- 22.Raabe M, Flynn LM, Zlot CH, Wong JS, Véniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice - reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proceedings of the National Academies of Sciences, USA. 1998;95:8686–8691. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woollett LA. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. American Journal of Clinical Nutrition. 2005;82:1155–1161. doi: 10.1093/ajcn/82.6.1155. [DOI] [PubMed] [Google Scholar]
- 24.Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, Mitchell MD, Keelan JA. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem Pharmacol. 2002;63(3):409–419. doi: 10.1016/s0006-2952(01)00890-5. [DOI] [PubMed] [Google Scholar]
- 25.Atallah AN, Guimaraes JA, Gebara M, Sustovich DR, Martinez TR, Camano L. Progesterone increases glomerular filtration rate, urinary kallikrein excretion and uric acid clearance in normal women. Braz J Med Biol Res. 1988;21(1):71–74. [PubMed] [Google Scholar]
- 26.le Roux PA, Tregoning SK, Zinn PM, van der Spuy ZM. Inhibition of progesterone secretion with trilostane for mid-trimester termination of pregnancy: randomized controlled trials. Hum Reprod. 2002;17(6):1483–1489. doi: 10.1093/humrep/17.6.1483. [DOI] [PubMed] [Google Scholar]
- 27.Murtha AP, Feng L, Yonish B, Leppert PC, Schomberg DW. Progesterone protects fetal chorion and maternal decidua cells from calcium-induced death. Am J Obstet Gynecol. 2007;196(3):257 e251–255. doi: 10.1016/j.ajog.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz N, Xue X, Elovitz MA, Dowling O, Metz CN. Progesterone suppresses the fetal inflammatory response ex vivo. Am J Obstet Gynecol. 2009;201(2):211 e211–219. doi: 10.1016/j.ajog.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Lopez Bernal A, Anderson AB, Turnbull AC. Cortisol:cortisone interconversion by human decidua in relation to parturition: effect of tissue manipulation on 11 beta-hydroxysteroid dehydrogenase activity. J Endocrinol. 1982;93(2):141–149. doi: 10.1677/joe.0.0930141. [DOI] [PubMed] [Google Scholar]
- 30.Anderson AB, Flint AP, Turnbull AC. Mechanism of action of glucocorticoids in induction of ovine parturition: effect on placental steroid metabolism. J Endocrinol. 1975;66(1):61–70. [PubMed] [Google Scholar]
- 31.Mucci LA, Lagiou P, Tamimi RM, Hsieh CC, Adami HO, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States) Cancer Causes Control. 2003;14(4):311–318. doi: 10.1023/a:1023966813330. [DOI] [PubMed] [Google Scholar]
- 32.Mark PJ, Smith JT, Waddell BJ. Placental and fetal growth retardation following partial progesterone withdrawal in rat pregnancy. Placenta. 2006;27(2-3):208–214. doi: 10.1016/j.placenta.2005.01.004. [DOI] [PubMed] [Google Scholar]


