Abstract
The Functional Disability Inventory (FDI) is a well-established and commonly used measure of physical functioning and disability in youth with chronic pain. Further validation of the measure has been called for, in particular, examination of the clinical utility and factor structure of the measure. To address this need, we utilized a large multicenter dataset of pediatric patients with chronic pain who had completed the FDI and other measures assessing pain and emotional functioning. Clinical reference points to allow for interpretation of raw scores were developed to enhance clinical utility of the measure and exploratory factor analysis was performed to examine its factor structure. Participants included 1300 youth ages 8 to 18 years (M=14.2 years; 76% female) with chronic pain. Examination of the distribution of FDI scores and validation with measures of depressive symptoms and pain intensity yielded three distinct categories of disability: No/Minimal Disability, Moderate Disability and Severe Disability. Factor analysis of FDI scores revealed a two-factor solution representing vigorous Physical Activities and non-physically strenuous Daily Activities. The three-level classification system and factor structure were further explored via comparison across the four most commonly encountered pain conditions in clinical settings (head, back, abdominal and widespread pain). Our findings provide important new information regarding the clinical utility and validity of the FDI. This will greatly enhance the interpretability of scores for research and clinical use in a wide range of pediatric pain conditions. In particular these findings will facilitate use of the FDI as an outcome measure in future clinical trials.
Assessment of pain-related disability is an essential component in the evaluation and treatment of pediatric patients with chronic pain. The Functional Disability Inventory (FDI) [40] is one of the most widely used measures of functional impairment among children and adolescents with chronic pain, and its initial psychometric properties have been well established [3,26]. The FDI is a brief 15-item instrument originally developed to assess disability in children and adolescents with chronic abdominal pain [41,3]. Subsequently it has been used with a variety of pediatric chronic pain conditions such as fibromyalgia [20], headaches [24,22,26], back pain [28], and other non-disease specific musculoskeletal pain syndromes [23,12,11]. In a recently published consensus statement on measures recommended for use in clinical trials in pediatric chronic pain, the FDI was recommended for assessment of physical functioning outcomes [29].
The FDI is already a primary outcome measure in many clinical trials of cognitive-behavioral treatments for pediatric chronic pain [33,8,19]. Furthermore, it has been found to be an efficient and user-friendly tool for routinely tracking patient outcomes throughout the course of treatment and has been successfully integrated into busy outpatient clinic settings [27]. Despite its widespread use, there has been limited consideration of the measure’s clinical utility. For example, there are no established criteria for interpretation of FDI scores which presents a challenge for research and clinical use, both when comparing scores across individuals or within an individual patient before and after treatment [32,30]. In addition, there is limited information about construct validity of the FDI, and the underlying factor structure of the FDI has not been formally examined. Therefore, it is not clear whether the FDI measures the single domain of physical functioning or multiple underlying factors, which poses a limitation for accurate interpretation of treatment outcomes. Large datasets representing youth with diverse pain conditions provide an opportunity to develop a clinically-relevant classification system and examine the factor structure of the FDI.
The primary objective of this collaborative project was to use combined databases from four large pediatric pain treatment centers, comprising over 1000 patients to: 1) develop a classification system that is clinically useful in describing the severity of functional disability in children and adolescents with chronic pain, and 2) examine the factor structure of the FDI to determine whether it measures a uniform construct of physical impairment or multiple latent factors. Our secondary objective was to explore differences in functional disability levels and subscale/factor scores in four subgroups of patients representing the most common pain conditions in pediatric pain clinics (widespread pain, head pain, abdominal pain and back pain) to explore whether a single set of normative ranges could be applied across pain conditions. The FDI was initially developed and validated in a sample of children and adolescents with recurrent abdominal pain, and it is not clear how it performs in other pediatric pain populations, despite widespread use with diverse chronic pain conditions. We did not have specific a priori hypotheses regarding utility of the FDI across pain conditions.
Methods
Participants
Participants were 1300 children and adolescents presenting to four large multidisciplinary pediatric pain treatment centers between January 2000 and February 2010. Inclusion criteria were: 1) primary presenting complaint of chronic or recurrent pain (persistent pain ≥ 3 months), 2) 8-18 years of age, and 3) patient and parent ability to read and comprehend questionnaires in English. Patients were excluded if they had significant developmental delays or impairments that affected their ability to respond to self-report measures. Patients were typically referred from a variety of medical subspecialties (e.g., orthopedics, rheumatology, neurology, gastroenterology) and primary care pediatricians, usually after other treatment attempts failed to substantially reduce symptoms or pain was more severe than expected given the underlying medical condition. The data included from all study sites were collected as part of site-specific Institutional Review Board (IRB) approved protocols. Further, IRB permission was obtained for combining the de-identified databases from all four contributing sites (Children’s Hospital Boston, Children’s Hospital of Philadelphia, Cincinnati Children’s Hospital, and Oregon Health & Science University) for the purposes of this study.
Measures
Demographic Information
Parents completed a demographic information form including the child or adolescents’ age, sex, race and ethnicity, as well as information regarding the onset, duration, and frequency of pain.
Functional Disability Inventory (FDI)
The FDI [40] is a 15-item self-report inventory assessing difficulty with the performance of daily activities in home, school, recreational, and social domains such as “doing chores at home,” “being at school all day,” or “walking the length of a football field.” Participants are asked to rate how much difficulty they had completing various tasks, “in the past few days…” Items are rated on a 5-point Likert scale, ranging from 0 to 4 (“No Trouble” to “Impossible”) and summed to create a total score (range 0-60) with higher scores indicating greater pain-related disability. The FDI was initially created for use in children and adolescents 8-17 years old with recurrent abdominal pain [40] and has subsequently been used with a wide range of pediatric pain conditions in children and adolescents 8-18 years of age [33,41,17,20,8,24,22,23,28,12,26,11]. The FDI has been reported to have high internal consistency, moderate to high test-retest reliability, moderate cross-informant (parent-child) reliability, and good predictive validity [40,3].
Pain Intensity
Children and adolescents provided pain intensity ratings using one of two validated self-report pediatric pain measures. Three study sites utilized Visual Analog Scales and one site used the Faces Pain Scale – Revised.
Visual Analog Scale (VAS)
Visual analog scales have been well-researched and validated for children 8 years of age and older [35,29,39]. Separate 10-cm VAS lines, anchored with the terms “no pain” and “worst imaginable pain” at the end points were used to assess highest, lowest, and average pain intensity on a 0-10 scale (two of three study sites obtained ratings on current pain intensity as well).
Faces Pain Rating Scale-Revised (FPS-R)
The Faces Pain Scale – Revised (FPS-R; [14]) is the revised version of the scale originally developed by Bieri and colleagues [1] to measure pain intensity in children ages 4 to 16 years. The revised scale allows for scoring along the widely accepted 0-10 scale. Children choose one of six gender-neutral line drawings of faces to represent their level of pain or discomfort. The FPS-R is a well-established measure that has demonstrated good psychometric properties in multiple studies [2,39,4,6].
Children’s Depression Inventory (CDI)
The CDI is comprised of 27 items assessing self-reported symptoms of depression in children and adolescents 7-17 years of age [21]. The CDI is well-validated [34] and is frequently used to measure depressive symptoms in youth with chronic pain [17,20,5]. Although a few patients were older than the normative age limit of 17, the CDI is clinically used in 18-year-olds to maintain consistency of measures in pediatric pain clinics and has also been used in 18-year-old adolescents in prior pediatric pain research [17,16,18]. CDI raw scores and corresponding norm-based T-scores were calculated.
Procedures
A working group of pediatric psychologists was established representing four large pediatric pain treatment centers that utilized similar assessment tools in their clinical and research protocols. The working group engaged in multiple electronic communications and face-to-face meetings at professional conferences to achieve consensus on: 1) classification of pain locations and pain diagnoses, 2) measures to be included in the combined database, 3) data cleaning and transmission of de-identified data, and 4) the data analysis plan.
At each study site, the FDI was administered to children as part of an initial clinical or research study evaluation, prior to their receiving treatment. When data were obtained as part of a clinical protocol (Boston, Cincinnati and Philadelphia sites), completion of a battery of questionnaires was a standard part of the multidisciplinary pain assessment. At one site (Oregon) measures were administered as part of research protocols examining psychosocial factors and chronic pain in children and adolescents.
Statistical Analysis Plan
All data entry and data analyses were conducted using PASW 17.0 software.
Missing data
Patients who did not complete the FDI (n=5) were excluded from analyses. For the remaining patients, missing data on individual FDI items in the entire database were found to be less than .005% of items and therefore no adjustments for missing data were made.
Descriptive statistics
Descriptive statistics on patient demographics (age, race and sex), pain characteristics (duration, intensity, frequency, and location), functional disability and depressive symptoms were computed.
Classification of levels of disability
Scores corresponding to quartile cut-offs based upon the distribution of FDI scores on the entire sample were determined. This allowed an initial classification into 4 levels of disability (No/Minimal, Mild, Moderate and Severe) based upon total scores on the FDI. This strategy has been previously used to develop clinical reference points for levels of pain-related disability in a pediatric headache population on a different measure; the PedMIDAS scale [13]. To test the validity of the classification system, and assess whether using quartile reference points was clinically meaningful, one-way ANOVAs and post-hoc tests were conducted to examine whether there were significant differences in pain intensity and depressive symptoms across the four levels of disability. The goal was to refine the classification such that with each increasing level of disability the related constructs of pain intensity (an indicator of symptom severity) and depressive symptoms (an indicator of emotional distress) would also show a statistically significant increase, and thereby represent distinct FDI reference points that also were clinically meaningful.
Factor Analysis of the FDI
The FDI was designed as a single 15-item scale to broadly assess difficulties in daily functioning due to pain. Exploratory Factor Analysis (EFA) was conducted to examine the underlying factor structure of the FDI. Prior to the EFA, preliminary analyses were run to ensure that all assumptions (adequate sample size and correlation of items) and factorability criteria (inter-item correlation, anti-image correlation, KMO measure of sampling adequacy, and Bartlett’s test of sphericity) were met. The 15 FDI items were entered into a principal axis factor analysis with an oblique rotation with an eigenvalue set to one. An oblique rotation is most appropriate for circumstances in which it is highly likely that underlying factors are correlated with one another. FDI items were retained within a factor if they had a primary factor loading of ≥ .40 and a secondary factor loading of ≤ .30. Those items that did not meet the above criteria, that is, items that did not clearly load on one factor were determined to be complex items. Sequential EFA were run by removing the complex items one at a time (starting with the weakest item) to test if the EFA model could be improved.
Cronbach’s alpha was calculated for the factors identified in the final model to determine the internal consistency of the items within each factor. Validity of the factor structure was tested by computing correlations between each of the factor scores with pain and depressive symptoms.
Clinical Utility of FDI disability levels
For the four largest subgroups of pain conditions (widespread pain, head pain, abdominal pain, and back pain) that together represented 66.4% of the sample, we computed the proportion of individuals within each level of disability. Finally, the four subgroups were compared on their FDI factor scores using one-way ANOVAs.
Results
Sample characteristics
The majority of the participants were female (75.7%) and Caucasian (86.5%) (Table 1). Minority representation was 8.9% African-American, 2.3% Hispanic, .9% Asian, .2% Native American and 1.2% biracial. Mean age of the sample was 14.2 years (SD = 2.4). Most of the sample (77.1%) fell in the adolescent age range (13-18 years). Average pain duration was 118 weeks (SD = 135) or over 2 years, and mean rating on average pain intensity was 5.5 (SD = 2.3). Most children and adolescents (89%) reported pain frequency as daily or 4-5 days of pain per week. The most frequently reported primary complaints were widespread pain, head pain, abdominal pain, and back pain (see Table 2 for pain characteristics). The mean total score on the FDI for the entire sample was 21.1 (SD = 11.4), with adolescents (13-18 years) scoring significantly higher than children (8-12 years) (M = 21.73 versus 19.13; p< .001). There were no significant gender differences (females M = 20.96 and males M = 21.71; p = .31), or differences based upon race (minorities M = 20.16 and non-minorities = 21.30; p = .22). The average CDI raw score for the sample was 11.6 (SD = 7.72) corresponding to a T-score range of 50-53 (depending on age and gender norms) which is within the normative range for children and adolescents [21]. Of the entire sample, 204 youth (15.69%) fell above the normative cut-off, i.e., in the at-risk or clinical range for depressive symptoms (CDI raw score = 17; T-score range of 57-62 based on age/sex; [21]).
Table 1.
Demographic characteristics
| Mean | SD | |
|---|---|---|
| Age (8-18 years) | 14.22 | 2.42 |
|
|
||
| N | % | |
|
|
||
| Sex | ||
| Male | 316 | 24.3 |
| Female | 983 | 75.7 |
| Race | ||
| White | 1114 | 86.5 |
| African-American | 115 | 8.9 |
| Hispanic | 29 | 2.3 |
| Asian | 11 | .9 |
| Native American | 3 | .2 |
| More than one race | 16 | 1.2 |
Table 2.
Descriptive data on pain duration, intensity, frequency, and location
| Mean | SD | N † | |
|---|---|---|---|
| Duration of Pain (in weeks) | 118.5 | 134.8 | 1094 |
| Pain Intensity | |||
| Current Pain Rating | 5.0 | 3.0 | 745 |
| Highest Pain Rating | 8.3 | 2.0 | 1112 |
| Lowest Pain Rating | 3.1 | 2.4 | 1114 |
| Average Pain Rating | 5.5 | 2.3 | 1009 |
| N | % | ||
|---|---|---|---|
| Pain Location | |||
| Widespread* | 362 | 27.8 | |
| Head | 199 | 15.3 | |
| Abdominal/pelvic/flank | 162 | 12.5 | |
| Back | 140 | 10.8 | |
| Joint | 136 | 10.5 | |
| Leg | 85 | 6.5 | |
| Foot/ankle | 83 | 6.4 | |
| Arm | 29 | 2.2 | |
| Sickle Cell | 25 | 1.9 | |
| Chest | 23 | 1.8 | |
| Neck/Shoulder | 21 | 1.6 | |
| Hand/Wrist | 14 | 1.1 | |
| Hip | 12 | 0.9 | |
| Face | 9 | 0.7 | |
Sample size varies because of differences between sites with regard to specific pain information obtained.
Widespread pain was defined as pain in at least three separate body areas that were bilateral and included both the upper and lower half of the body.
Cut-offs for levels of disability
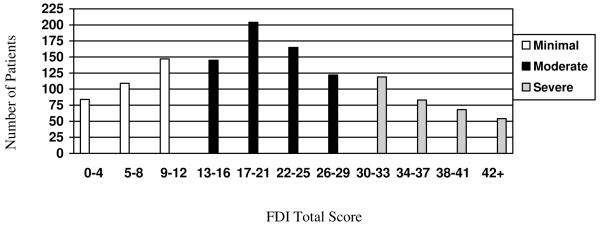
The four levels of disability based upon quartile reference points corresponded to the following total FDI scores: No/Minimal (0-12), Mild (13-20), Moderate (21-29) and Severe (≥30). To test the validity of these reference points and further refine the classification, we conducted one-way ANOVAs which showed that increasing levels of disability were significantly associated with increasing levels of depressive symptoms and pain intensity based upon the omnibus ANOVA (F = 34.32, p<.000; F = 60.25, p < .000). However, further post-hoc testing using Tukey’s and Scheffé comparisons showed that on both depressive symptoms and pain, the Mild and Moderate categories represented a homogenous category and could not be significantly distinguished from one another. Therefore, these groups were combined to arrive at the final 3-level classification system of No/Minimal Disability (0-12), Moderate Disability (13-29) and Severe Disability (≥30). Mean scores on the FDI, CDI and pain intensity ratings for each disability level are presented in Table 3. The distribution of FDI scores by level of disability are presented in Figure 2.
Table 3.
Average functional disability, pain intensity and depressive symptom scores for No/Minimal, Moderate, and Severe Disability categories
| I No/Minimal (FDI = 0-12) |
II Moderate (FDI = 13-29) |
III Severe (FDI ≥ 30) |
p | |
|---|---|---|---|---|
| Functional Disability (0-60) | 7.18 (3.63) |
20.81 (4.71) |
36.39 (5.14) |
.000 * |
| Average Pain Intensity (0-10) | 4.16 (2.68) |
5.56 (1.95) |
6.70 (1.90) |
.000 * |
| CDI Raw Score (0-54) | 8.20 (6.27) |
11.51 (7.28) |
14.67 (8.44) |
.000 * |
Omnibus ANOVA shows significant differences at the <.001 level. Post-hoc tests (Tukey and Sheffé comparisons) show that each disability level is significantly different from the others on functional disability, pain and depressive symptoms (ps < .001).
Figure 2.
Distribution of FDI scores within No/Minimal, Moderate, and Severe Disability categories
Factor Analysis
The EFA yielded a 15-item solution representing a 2-factor structure (see Table 4 for factor loadings). The two factors were determined to represent impairment in activities requiring physical exertion (such as - walking up stairs, walking the length of a football field, doing activities in gym class/playing sports, etc., termed Factor I: Physical Activities, 8 items) and non-physically strenuous daily activities (i.e., doing homework, watching television, eating regular meals etc., termed Factor II: Daily Activities, 7 items). Factor I explained 42.5% of the variance, and the addition of Factor II explained a combined 54.0% of the variance. There were two complex items (Item 4 – doing chores, and Item 15 – getting to sleep) that loaded on both factors but examination of the factor loadings after sequentially removing the complex items did not strengthen the model (combined R2= 53.9%). Based upon these results, it was decided to leave the two items in the 2-factor EFA model, allowing them to remain within the factor on which they loaded most highly.
Table 4.
Mean score on each FDI item and factor loadings based on Exploratory Factor Analysis
| FDI Item # | Mean | (SD) | Factor 1 (E = 6.38) |
Factor 2 (E = 1.79) |
|---|---|---|---|---|
| 1. Walking to the bathroom | .64 | (1.02) | .672 | .000 |
| 2. Walking up stairs | 1.38 | (1.21) | .864 | −.144 |
| 3. Doing something with a friend (For example, playing a game) |
1.35 | (1.16) | .473 | .298 |
| 4. Doing chores at home * | 1.33 | (1.18) | .540 | .316 |
| 5. Eating regular meals | .74 | (1.06) | −.031 | .447 |
| 6. Being up all day without a nap or rest | 1.45 | (1.33) | .086 | .462 |
| 7. Riding the school bus or traveling in the car |
1.03 | (1.11) | .291 | .414 |
| 8. Being at school all day | 1.96 | (1.27) | .278 | .559 |
| 9. Doing the activities in gym class (or playing sports) |
2.52 | (1.30) | .673 | .105 |
| 10. Reading or doing homework | .99 | (1.09) | −.117 | .744 |
| 11. Watching TV | .45 | (.75) | −.054 | .609 |
| 12. Walking the length of a football field | 1.59 | (1.36) | .874 | −.060 |
| 13. Running the length of a football field | 2.54 | (1.39) | .850 | −.086 |
| 14. Going shopping | 1.44 | (1.16) | .616 | .270 |
| 15. Getting to sleep at night and staying asleep * |
1.89 | (1.31) | .227 | .371 |
Complex Item
Items in bold represent those that clearly loaded on each Factor
Internal consistency reliability of items for each of the factors was high for Factor I (Cronbach α = .91) and adequate for Factor II (Cronbach α = .77). The mean score on Factor I was 12.62 (SD = 7.62, range = 0-32) and on Factor II was 8.48 (SD = 5.17, range = 0-26), of a possible range scores of 0-32 on Factor I and 0-28 on Factor II. The two factors were significantly correlated with one another (Pearson r = .58. p < .001). Correlations between average pain intensity and each of the two factors were significant (r = .36 with Factor I and r = .38 with Factor II; p values < .001). Correlations between depressive symptoms and each factor score also were significant (r = .23 with Factor I and r = .39 with Factor II; p values < .001).
Clinical utility of the classification system and factor scores
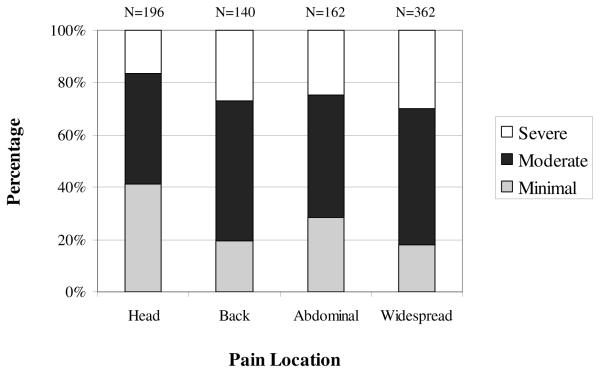
For the four pain subgroups (widespread pain, abdominal pain, back pain and head pain), the percentage of individuals in each disability level was calculated; Figure 1 displays the proportion of individuals with No/Minimal, Moderate and Severe Disability in each subgroup. The four most common pain complaints were then compared on total FDI scores and scores on the 2 factors, Factor I (Physical Activities) and Factor II (Daily Activities). Results showed that individuals with head pain scored significantly lower than the 3 other groups (back pain, abdominal pain and widespread pain) on total FDI score and on Factor I (see Table 5). There was no significant difference among the groups on Factor II. As shown in Table 6, with regard to average pain intensity ratings and depressive symptoms, results of the omnibus ANOVAs revealed no significant differences among the subgroups on pain intensity (F = .89, p = .45), but significant differences emerged in depressive symptoms (F = 2.92, p < .05). Specifically, patients with widespread pain had significantly higher levels of depressive symptoms than patients with head pain (p < .05).
Figure 1.
Percentage of patients in each disability category by pain location
Table 5.
Comparison of four common pain conditions on FDI total score and factor scores
| Pain Location | n | Total Score* Mean (SD) |
I: Physical Activities* Mean (SD) |
II: Daily Activities Mean (SD) |
|---|---|---|---|---|
| Head Pain | 199 | 17.37 * (11.15) |
8.52 * (6.80) |
8.77 (5.43) |
| Back Pain | 140 | 22.40 (10.41) |
13.68 (6.99) |
9.09 (4.52) |
| Abdominal Pain | 162 | 21.57 (11.76) |
12.13 (7.27) |
9.47 (5.59) |
| Widespread Pain | 362 | 23.27 (10.77) |
13.66 (7.09) |
9.61 (4.75) |
Omnibus ANOVAs show significant differences between groups on FDI total score and Factor I (p <.001) with those in the Head Pain category scoring significantly lower than all other groups (p<.001)
Table 6.
Pain intensity and depressive symptoms in four most common pain conditions
| Head | Back | Abdomen | Widespread | |
|---|---|---|---|---|
| VAS (0-10) | 5.88 (2.20) |
5.54 (2.14) |
5.56 (2.20) |
5.52 (2.08) |
| CDI (0-54) | 10.56* (8.28) |
11.97 (8.60) |
11.86 (8.03) |
13.01* (7.43) |
Omnibus ANOVAs showed a significant difference between groups on CDI total score (p < .05), with the widespread pain group showing a significantly higher level of depressive symptoms than the head pain group(p < .05).
Discussion
The FDI is a widely used measure in pediatric pain research, and the PedIMMPACT guidelines [29] recommend the FDI for assessment of physical functioning in clinical trials. However, the lack of empirically referenced interpretation of FDI scores currently limits its clinical utility and restricts its research applications. One prior study [15] described a 5- level classification system incorporating the FDI as part of a global pain severity assessment using a community-based sample. However, this classification system was not sufficiently sensitive for clinical use in a pediatric pain patient population, because the vast majority of pain patients fell in the top two levels of severity [9].
The primary contribution of the current large-scale collaborative multi-site study of functional disability assessment is to provide clinical reference points for direct interpretation of FDI scores for the pediatric chronic pain population. The large sample size using combined databases from four pediatric pain centers across the country increases the likelihood that these reference points are robust and generalizable across a wide variety of pediatric pain conditions seen in clinical settings. Scores on the FDI in this sample are consistent with numerous other studies using the measure with specialized outpatient pain clinic samples [40,17,16,23,10,12,11,42]. The empirically derived classification system describes three clinically distinct categories of patients with no/minimal disability, moderate disability and severe disability. Children who score in the moderately disabled category (FDI = 13-29) represent the most typical patients presenting to specialty pediatric pain clinics. Those who score in the no/minimal disability range (FDI ≤ 12) tend to be patients who are able to function quite well despite pain and tend not to exhibit elevated levels of depressive symptoms (clinically described as the “good copers”), in contrast to those who are severely disabled (FDI ≥ 30) and express high levels of pain and depressive symptoms. This evidence-based classification system will be useful for researchers and clinicians for uniform interpretation of FDI scores in a variety of pediatric pain conditions (beyond recurrent/functional abdominal pain, for which the measure was developed). Reference points also can be useful in clinical settings for developing concrete treatment goals for disability reduction in collaboration with patients and their parents, and also as important indicators of treatment efficacy in clinical trials.
Results of the factor analysis suggest that the FDI provides a good indication of physical functioning in pediatric chronic pain patients with the items on the Physical Activities domain forming a highly internally consistent factor and accounting for a large portion of the variance of the total score. Other items assessing non-strenuous Daily Activities (e.g. eating, watching television) formed a secondary factor which contributed less to the overall variance of the measure and had lower but still acceptable internal consistency. The two factors were significantly correlated with one another and each factor correlated significantly with measures of pain and depressive symptoms. Given that the FDI was initially designed as a broad measure of daily functioning, it is not surprising that the measure tapped into more than one underlying factor. In each of the pain diagnostic subgroups, total FDI scores and factor scores generally followed the same pattern (with the exception of the head pain group who had lower total scores on Physical Activity impairment than the other three groups but similar impairment in Daily Activities). Results suggest that the FDI appears to perform quite well as a unitary measure of functioning, leading this group to recommend an emphasis on total FDI scores in both clinical and research contexts. The findings reveal that two distinct factors may exist within the measure representing Physical Activity and less strenuous Daily Activities. However, further investigation is necessary to confirm the reliability of this factor structure.
It is useful to note that other measures assessing physical functioning in children with chronic pain do exist but to date, none of these alternative measures have empirically-derived classification systems for describing the extent of pain-related disability. Relevant measures include the Child Activity Limitations Interview self-report questionnaire version (CALI-21; [32]), the Bath Adolescent Pain Questionnaire (BAPQ; [7]), and the Pediatric Quality of Life Inventory (Peds-QL; [38,37,36]). Each of these measures has subscales that specifically assess physical functioning and/or pain related disability, which overlap considerably with the content and format of the FDI. In an exploratory factor analysis of the CALI-21 (the measure most comparable to the FDI in its brevity, primary focus on functional disability, and specificity to the pediatric chronic pain population), Palermo et al [31] also found two factors that represented Active and Routine domains and that differentiated between children with and without comorbid disease. Different patterns were found for child and parent report on the CALI-21 suggesting that future work with the FDI to explore factor structure in the parent report version may be an important next step.
Based on the wide variety of pain conditions in our sample, the FDI appears to be suitable for assessing pain-related disability across pediatric pain conditions, with initial evidence indicating that the proposed classification levels can be applied to a broad spectrum of pain conditions. Further support for the clinical utility of the measure was obtained by comparing the FDI scores of subgroups of the four most common pain conditions and correlating those scores with measures of pain intensity and depressive symptoms. In general the head pain group had the lowest levels of physical disability and those with widespread pain had the highest disability. Previous studies show similar patterns of differences between pain subgroups [17], [31], suggesting that the FDI may have utility in comparing physical functioning across pain conditions.
The results of the current study provide further support for the FDI and enhance the interpretability of the scores in clinical trials and clinical care of pediatric pain patients. The clinical reference points provide a foundation for future research to classify levels of disability and how they might be associated with different coping styles and response to treatment. It also may be possible to tailor treatment recommendations or develop clinical pathways based upon initial assessment of levels of pain-related disability.
Several limitations to the study exist. Although the multisite collaboration was a strength of the study, analyses were limited to measures that were collected uniformly across sites. Sites differed in the types and formats of information they collected from patients on other measures of interest. For example, school absences are an important indicator of functioning in youth with chronic pain [25] and would have served as a useful indicator of FDI validity, but approaches to measuring school attendance were so varied across sites that this information could not be included. More uniform guidelines for measuring important functional indictors such as school attendance are necessary for future clinical trials. The availability of indicators such as school and social functioning might also have enabled us to better distinguish between the two FDI factor scores; with the available data no differential associations emerged, providing insufficient support for the validity of the factor structure.
It is important to note that this study was based on clinical samples of youth seeking services for chronic pain problems and the findings may not generalize to community samples. Community-based studies may provide better sensitivity of the FDI at lower disability levels. For example, in one study of over 500 schoolchildren [15] which described a 5-level pain severity classification based upon pain intensity and FDI scores, the vast majority (about 95%) of school-aged children fell at the no/minimal disability level (FDI < 10) based upon the classification described in our current study. However, clinically small but statistically significant differences in FDI scores emerged between those reporting low pain intensity and those reporting high pain intensity. Future studies might extend FDI norms to community samples and address the possibility that some non-referred youth may experience pain and functional disability but fail to seek treatment due to other factors, for example lower levels of parental anxiety or concern or barriers to accessing treatment.
This study examined only the self-report version of the FDI; future studies will need to validate these levels of disability in the parent-report version of the measure. An additional direction for future research is to further validate these clinical reference points for self-perceived disability against objective assessment of physical functioning and against physician ratings of the extent of patients’ functional disability. Validating the FDI and its reference points against more objective indicators of disability would strengthen the support for the FDI in particular and for self-perceived disability more broadly. Given that negative response sets (including helplessness, low self-efficacy) may play a role in any self-report measure, it is important to establish correlations between the FDI self-report and other approaches to functional disability assessment.
In conclusion, this large multi-center collaborative study developed a three-level FDI classification system (No/Minimal Disability, Moderate Disability, Severe Disability) that provides distinct, clinically significant reference points for describing the extent of functional disability among pediatric patients with chronic pain. This classification system allows for uniform interpretation of FDI scores for both clinical care and clinical trials across diverse chronic pain complaints.
Acknowledgments
Funding and Acknowledgments
This project was partially supported by NIH K24 Midcareer Awards in Patient-Oriented Research (#AR056687 and #HD060068 to S. Kashikar-Zuck and T. Palermo, respectively). We would like to thank Drs. Kenneth Goldschneider, Charles Berde, John B. Rose, David D. Sherry and the faculty and staff of the Cincinnati Children’s Pain Management Clinic, Children’s Hospital Boston Pain Treatment Service, Children’s Hospital of Philadelphia Pain Programs and Pediatric Pain Management Center at Oregon Health and Science University for their assistance with patient referrals and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no financial or other relationship that might lead to a conflict of interest.
References
- [1].Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990;41(2):139–150. doi: 10.1016/0304-3959(90)90018-9. [DOI] [PubMed] [Google Scholar]
- [2].Chambers CT, Hardial J, Craig KD, Court C, Montgomery C. Faces scales for the measurement of postoperative pain intensity in children following minor surgery. Clin J Pain. 2005;21(3):277–285. doi: 10.1097/00002508-200505000-00011. [DOI] [PubMed] [Google Scholar]
- [3].Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121(1-2):77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Connelly M, Neville K. Comparative Prospective Evaluation of the Responsiveness of Single-Item Pediatric Pain-Intensity Self-Report Scales and Their Uniqueness From Negative Affect in a Hospital Setting. J Pain. 2010 doi: 10.1016/j.jpain.2010.04.011. [DOI] [PubMed] [Google Scholar]
- [5].Conte P, Walco G, Kimura Y. Temperament and stress response in children with juvenile primary fibromyalgia syndrome. Arthritis and Rheumatism. 2003;48(10):2923–2930. doi: 10.1002/art.11244. [DOI] [PubMed] [Google Scholar]
- [6].de Tovar C, von Baeyer CL, Wood C, Alibeu JP, Houfani M, Arvieux C. Postoperative self-report of pain in children: interscale agreement, response to analgesic, and preference for a faces scale and a visual analogue scale. Pain Res Manag. 2010;15(3):163–168. doi: 10.1155/2010/475907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eccleston C, Jordan A, McCracken LM, Sleed M, Connell H, Clinch J. The Bath Adolescent Pain Questionnaire (BAPQ): development and preliminary psychometric evaluation of an instrument to assess the impact of chronic pain on adolescents. Pain. 2005;118(1-2):263–270. doi: 10.1016/j.pain.2005.08.025. [DOI] [PubMed] [Google Scholar]
- [8].Eccleston C, Malleson PN, Clinch J, Connell H, Sourbut C. Chronic pain in adolescents: evaluation of a programme of interdisciplinary cognitive behaviour therapy. Archives of Disease in Childhood. 2003;88(10):881–885. doi: 10.1136/adc.88.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Flowers SR, Lynch-Jordan A, Desai A, Goldschneider KR, Ting TV, Szabova A, Kashikar-Zuck S. Utility of a global pain severity index in a pediatric chronic pain population; Presented at the International Association for the Study of Pain, 13th World Congress on Pain; Monteal, Quebec, Canada. August 2010. [Google Scholar]
- [10].Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: Patterns and predictors across different domains of functioning. Pain. 2007;131(1-2):132–141. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- [11].Guite JW, Logan DE, McCue R, Sherry DD, Rose JB. Parental beliefs and worries regarding adolescent chronic pain. Clin J Pain. 2009;25(3):223–232. doi: 10.1097/AJP.0b013e31818a7467. [DOI] [PubMed] [Google Scholar]
- [12].Guite JW, Logan DE, Sherry DD, Rose JB. Adolescent self-perception: associations with chronic musculoskeletal pain and functional disability. Journal of Pain. 2007;8(5):379–386. doi: 10.1016/j.jpain.2006.10.006. [DOI] [PubMed] [Google Scholar]
- [13].Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57(11):2034–2039. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- [14].Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- [15].Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9(3):226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- [16].Kashikar-Zuck S, Allen R, Noll R, Graham T, Ho I, Swain N, Crain B, Mullen S. Anxiety and depressive symptoms in adolescents with juvenile fibromyalgia and their mothers. The Journal of Pain. 2005;6(3 Suppl):31. [Google Scholar]
- [17].Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clinical Journal of Pain. 2001;17(4):341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- [18].Kashikar-Zuck S, Lynch AM, Slater S, Graham TB, Swain NF, Noll RB. Family factors, emotional functioning, and functional impairment in juvenile fibromyalgia syndrome. Arthritis and Rheumatism. 2008;59(10):1392–1398. doi: 10.1002/art.24099. [DOI] [PubMed] [Google Scholar]
- [19].Kashikar-Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. Journal of Rheumatology. 2005;32(8):1594–1602. [PubMed] [Google Scholar]
- [20].Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping and functional disability in juvenile primary fibromyalgia syndrome. Journal of Pain. 2002;3(5):412–419. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- [21].Kovacs M. Children’s Depression Inventory. 1992 Available from Multi-Health systems, Inc., 908 Niagara Falls Blvd., North Tonawanda, N.Y. 14120-2060. [Google Scholar]
- [22].Lewandowski AS, Palermo TM, Peterson CC. Age-dependent relationships among pain, depressive symptoms, and functional disability in youth with recurrent headaches. Headache. 2006;46(4):656–662. doi: 10.1111/j.1526-4610.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- [23].Logan DE, Guite JW, Sherry DD, Rose JB. Adolescent-parent relationships in the context of adolescent chronic pain conditions. Clin J Pain. 2006;22(6):576–583. doi: 10.1097/01.ajp.0000210900.83096.ca. [DOI] [PubMed] [Google Scholar]
- [24].Logan DE, Scharff L. Relationships between family and parent characteristics and functional abilities in children with recurrent pain syndromes: An investigation of mederating effects on the pathway from pain to disability. Journal of Pediatric Psychology. 2005;30(8):698–707. doi: 10.1093/jpepsy/jsj060. [DOI] [PubMed] [Google Scholar]
- [25].Logan DE, Simons LE, Stein MJ, Chastain L. School impairment in adolescents with chronic pain. J Pain. 2008;9(5):407–416. doi: 10.1016/j.jpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- [26].Long AC, Palermo TM, Manees AM. Brief report: using actigraphy to compare physical activity levels in adolescents with chronic pain and healthy adolescents. J Pediatr Psychol. 2008;33(6):660–665. doi: 10.1093/jpepsy/jsm136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lynch-Jordan AM, Kashikar-Zuck S, Crosby LE, Lopez WL, Smolyansky BH, Parkins IS, Luzader CP, Hartman A, Guilfoyle SM, Powers SW. Applying quality improvement methods to implement a measurement system for chronic pain-related disability. J Pediatr Psychol. 2010;35(1):32–41. doi: 10.1093/jpepsy/jsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Psychosocial risks for disability in children with chronic back pain. Journal of Pain. 2006;7(4):244–251. doi: 10.1016/j.jpain.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [29].McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9(9):771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [30].Palermo TM. Assessment of chronic pain in children: current status and emerging topics. Pain Res Manag. 2009;14(1):21–26. doi: 10.1155/2009/236426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Palermo TM, Lewandowski AS, Long AC, Burant CJ. Validation of a self-report questionnaire version of the Child Activity Limitations Interview (CALI): The CALI-21. Pain. 2008;139(3):644–652. doi: 10.1016/j.pain.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Palermo TM, Long AC, Lewandowski AS, Drotar D, Quittner AL, Walker LS. Evidence-based assessment of health-related quality of life and functional impairment in pediatric psychology. J Pediatr Psychol. 2008;33(9):983–996. doi: 10.1093/jpepsy/jsn038. discussion 997-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reid GJ, Lang BA, McGrath PJ. Primary juvenile fibromyalgia: psychological adjustment, family functioning, coping, and functional disability. Arthritis and Rheumatism. 1997;40(4):752–760. doi: 10.1002/art.1780400423. [DOI] [PubMed] [Google Scholar]
- [34].Sitarenios G, Kovacs M. In: Use of the Children’s Depression Inventory. Maruish ME, editor. Book Title|, Vol. Volume|. City|: Publisher|, Year|. p.^pp. Pages|. [Google Scholar]
- [35].Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125(1-2):143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [36].Varni JW, Burwinkle TM, Limbers CA, Szer IS. The PedsQL as a patient-reported outcome in children and adolescents with fibromyalgia: an analysis of OMERACT domains. Health Qual Life Outcomes. 2007;5:9. doi: 10.1186/1477-7525-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Varni JW, Burwinkle TM, Szer IS. The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: reliability and validity. J Rheumatol. 2004;31(12):2494–2500. [PubMed] [Google Scholar]
- [38].Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- [39].von Baeyer CL. Children’s self-report of pain intensity: what we know, where we are headed. Pain Res Manag. 2009;14(1):39–45. doi: 10.1155/2009/259759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of Pediatric Psychology. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- [41].Walker LS, Heflinger CA. In: Quality of life predictors of outcome in pediatric abdominal pain patients: findings at initial assessment and 5-year follow-up. Drotar D, editor. Book Title|, Vol. Volume|. City|: Publisher|, Year|. p.^pp. Pages|. [Google Scholar]
- [42].Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain--a randomized controlled trial. Pain. 2009;141(3):248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]