Abstract
Ghrelin, the natural ligand for the growth hormone (GH)-secretagogue receptor (GHS-R), is produced predominantly in the stomach. It is present in the circulation in two major forms, an acylated and an unacylated form, both of which have reported activities. Some of the best understood main effects of acylated ghrelin administration include anorexic effects, increased appetite and the stimulation of GH secretion. Ghrelin also seems to plays a role in glucose homeostasis, lipid metabolism and immune function. Based on its orexigenic and metabolic effects, ghrelin and ghrelin mimetics have potential benefit in antagonizing protein breakdown and weight loss in catabolic conditions such as cancer cachexia, renal, cardiac and pulmonary disease, and age-related frailty. Ghrelin also has potentially useful positive effects on cardiac function and gastric motility. Ghrelin antagonists may be of benefit to increase insulin sensitivity and potentiate weight loss. The following chapter presents some background on ghrelin and ghrelin assays and discusses some of the potential therapeutic approaches for the use of ghrelin, ghrelin mimetic compounds and ghrelin antagonists in clinical disease.
Keywords: ghrelin, growth hormone, ghrelin-mimetics, aging
Physiology
Ghrelin is a 28 amino acid peptide hormone secreted predominantly from the stomach that acts to regulate appetite and metabolism. The ghrelin peptide has an unusual post-translational modification; an 8 or 10 carbon fatty acid is ester linked to serine 3. This acylation is unstable, and the majority of ghrelin seen in the circulation is in an unacylated form (Kojima et al. 1999). Only acylated ghrelin has biological activity at the known ghrelin receptor (Smith 2005), though the des-acylated form is reported to have other activities (Delhanty et al. 2010). The main site of ghrelin synthesis is within the oxyntic glands in the fundus of the stomach, but ghrelin synthesis has also been documented in the small intestine, and to lesser extents in the pancreas, immune cells and other sites (Korbonits et al. 2004).
Experiments examining the structure-activity relationship of ghrelin (Guerlavais et al. 2003), showed that a bulky lipophilic residue at the Ser3 residue is necessary for its affinity towards its receptor. This receptor was originally named the growth-hormone-secretagogue receptor 1a (GHS-R), but after the discovery that its natural ligand was ghrelin, it is now often called the ghrelin receptor. The n-octanoyl group can be replaced by a decanoyl or palmitoyl group as well as a benzoyl or adamantyl group. The studies also showed that the ester function at Ser3 can be replaced by an amide function without loss in biological activity and that the four N-terminal residues of ghrelin are necessary for binding and activation of its receptor (Bednarek et al. 2000; Matsumoto et al. 2001).
Naturally occurring ghrelin is heterogeneous due to variation in an intron splice junction (desGln14), variation in proghrelin protease cleavage sites (desArg28), and variation in the nature of the acylation which can be either 8 carbon (C8, octanoyl) or 10 carbon (C10, decanoyl) and may contain a double bond (C8:0 or C8:1). In the stomach, these acylated ghrelin forms have been isolated and their molar ratios have been described (Hosada et al. 2000) as follows: ghrelin (C8:0) : ghrelin (1–27) (C8:0) : ghrelin (C10:0) : ghrelin (1–27)(C10:0) : ghrelin (C10:1) as 6:2:2:1:1.
Ghrelin has been shown to elicit multiple endocrine effects such as an increase in appetite, increase in food intake, and growth hormone (GH) release (Tschoep et al. 2000; van der Lely et al. 2004). Circulating ghrelin levels have also been shown to be associated with GH secretion during fed conditions, implying that circulating ghrelin levels modulate the pre-existing pulsatile GH pattern (Nass et al. 2008a), while GH seems to have no impact on circulating ghrelin levels (Nass et al. 2004). Besides its orexigenic and GH releasing effects, ghrelin has been shown to increase gastric motility (Levin et al. 2006), increase lean body mass as well as exert adipogenic effects and anti-inflammatory effects (Dixit et al. 2004; Kojima et al. 2001). Ghrelin is also thought to play a role in regulating glucose homeostasis possibly by modulating insulin secretion and insulin sensitivity, however to date it is unclear whether this is also the case under physiologic conditions (Tong et al. 2009; Tong et al. 2010). Recent animal studies also suggest that ghrelin might be necessary for triggering the GH response to prolonged nutritional deprivation that prevents hypoglycemia under conditions of long-term caloric restriction (Nass et al. 2010; Zhao et al. 2010). Des-acyl ghrelin is reported to itself have multiple functions that are independent of the known ghrelin receptor and sometimes appear to oppose the actions of acyl-ghrelin; these include enhancement of pancreatic β-cell function and survival (Granata et al. 2010), beneficial effects on cardiovascular function (van der Lely et al. 2004), and regulation of lipid and carbohydrate metabolism (Delhanty et al. 2010). In order to accurately assess the physiology of ghrelin and the regulation of the different forms of circulating ghrelin, the use of an assay which can distinguish between acylated and des-acylated forms of ghrelin is mandatory (Liu et al. 2008).
Ghrelin assays
Circulating ghrelin is heterogeneous (see above) and labile to degradation by both proteases and especially by esterases in blood (Hosada et al. 2000; Liu et al. 2008). This results in a mixture of ghrelin forms and degradation products. Assays for ghrelin must first start with a sample preparation method where further degradation has been stabilized, and then must characterize which forms of ghrelin or its fragments will be detected.
Sample preparation
Blood samples for ghrelin assay are usually collected in EDTA tubes. Chilling slows the rate of esterase activity. Esterase/protease inhibitors added directly to the collection tube further preserve ghrelin acylation. After prompt preparation of plasma, acidification with HCl can irreversibly denature endogenous esterase activity (Liu et al. 2008).
Assay Types
1. Single-site RIA or ELISA for “active” or “total” ghrelin with or without C18 extraction
These assays are dependent on the specificity of a single antiserum which may or may not require that the sample be extracted to remove interferences. This type of assay is conveniently used to measure “total ghrelin”, whether or not it is acylated, but is confounded by also detecting ghrelin fragments not detected by more specific assays (Prudom et al. 2010).
2. HPLC separation of ghrelin isoforms followed by single-site ELISA or RIA
This type of assay requires sample extraction and is tedious, but is able to separately quantitate acyl and des-acyl ghrelin without interference from fragments (Hosada et al. 2000).
3. Two site sandwich assays specific for acyl or des-acyl ghrelin
This type of assay uses the specificity of two separate antisera directed to the two ends of the ghrelin peptide in order to lower non-specific signals and select for full-length ghrelin. The values reported by this type of assay are generally lower than those seen in single site assays (Prudom et al. 2010).
4. Mass spectrometry
This method offers the ultimate specificity in that it can identify and distinguish all the ghrelin variants (Rauh et al. 2007), but is technically difficult, requires sample extraction, and detection sensitivity is very dependent on sample interferences. One assay protocol uses ghrelin immunoprecipitation to lessen sample interferences before mass spectrometry (Gutierrez et al. 2008).
Care must be taken in evaluating ghrelin assay results. For example, Groschl found a tenfold difference when the same set of samples were assayed for total ghrelin using kits from different manufacturers (Groschl et al. 2004) and Hotta et al. found that in a sample set from anorexia nervosa patients, acyl-ghrelin was either increased, decreased or unchanged relative to controls depending which of three assays was used (Hotta et al. 2004).
Potential use of ghrelin or ghrelin antagonists in disease
A discussion about modulating ghrelin receptor action must consider 2 different therapeutic approaches: a) blocking ghrelin receptor action and b) enhancing ghrelin receptor action. The following review will address some of the studies published.
A) Animal studies
a) Enhancing ghrelin receptor action
The orexigenic and GH releasing effects of ghrelin make it an excellent agent to be used in catabolic situations.
Cancer cachexia
In rodent models, ghrelin or ghrelin mimetic administration have been shown to improve weight gain and food intake, when compared to mice receiving placebo (DeBoer et al. 2007; Hanada et al. 2003; Wang et al. 2006). Garcia and colleagues could show that ghrelin administration resulted in the decrease in cisplatin-induced mechanical hyperalgesia, anorexia and cachexia in normal rats treated with cisplatin (Garcia et al. 2008).
End stage kidney disease (ESKD)
Administration of a ghrelin mimetic (DeBoer et al. 2008) in a surgical model of CKD resulted in improved food intake and gain in lean mass when compared to vehicle treated animals.
Cardiac cachexia
Data by Nagaya et al. (Nagaya et al. 2005) suggested a beneficial effect of chronic subcutaneous administration of ghrelin on LV dysfunction and LV remodeling and cardiac cachexia in rat model with chronic heart failure. Further studies are necessary to evaluate the cardiac effects of ghrelin or its mimetics.
Aging, sarcopenia
Administration of ghrelin over 2 weeks to senescent rats who underwent a surgical intervention prevented the 2–3% body weight loss, which was found in the control group. While the study did not find a difference in food intake, the results suggested a possible decrease in the proinflammatory cytokine response after surgery in the ghrelin treated animals (Nagaya et al. 2004). Increase in food intake and GH secretion in 27 months old rats was described by Toshinai et al. (Toshinai et al. 2007).
b) Blocking ghrelin action
Several clinical and animal studies suggest that ghrelin might play a role in the regulation of glucose homeostasis either through hepatic glucose production and or direct actions at the pancreas (Tong et al. 2009). In addition, GHS-R1a deficiency in ob/ob mice was shown to improve glucose tolerance and enhance insulin secretion (Sun et al. 2007). These observations lead to increased interest in developing GHS-R1a antagonists. Esler et al. (Esler et al. 2007) showed an improved glucose homeostasis in intraperitoneal glucose tolerance test in rats after a single dose of an oral GHS-R1a antagonist. The group could also show a reduction in food intake and increase in weight loss. Interestingly the antagonist had no effect on insulin secretion in the absence of ghrelin in an in vitro model experiment (Esler et al. 2007). Another approach to block ghrelin action was described by Helmling and colleagues (Helmling et al. 2004) who used a Spiegelmer, a stable RNA-based compound, which can specifically bind noctanoyl ghrelin. The compound was able to suppress ghrelin-induced GH release in rats by specifically binding acyl-ghrelin and interfering with its activation of the ghrelin receptor. So far no studies have been published showing long-term beneficial effects of blocking ghrelin action. The available data describing the effects of ghrelin receptor antagonists are currently too limited to allow conclusions to be drawn about their potential clinical uses.
B) Clinical studies
a) Enhancing ghrelin receptor action
1) Administration of ghrelin
Chronic heart failure (CHF)
Nagaya et al. (Nagaya et al. 2004) studied the effects of ghrelin on cardiac cachexia in patients with CHF. Daily administration of ghrelin (2ug/kg bid) for 3 weeks resulted in an increase in food intake and body weight. The study, albeit not placebo controlled, showed improvement in the exercise capacity and left ventricular function.
End stage kidney disease (ESKD)
Studies in malnourished dialysis patients suggest that short term ghrelin administration over 2 weeks can increase food intake (Ashby et al. 2009). Similar positive effects of ghrelin administration in peritoneal dialysis patients with mild to moderate malnutrition was described by Wynne et al. (Ashby et al. 2009). The authors found a doubling of the energy intake after a single subcutaneous injection of ghrelin (3.6 nmol/kgbw).
Cancer cachexia
Cancer anorexia/cachexia is characterized by a catabolic state, which includes weight loss, fat and muscle loss and hypermetabolism. Based on the preliminary data of ghrelin administration in cancer patients, Strasser et al. (Strasser et al. 2008) conducted a 2 weeks single-center, randomized, double-blind, placebo-controlled trial of IV ghrelin infusion in patients with advanced, incurable cancer and involuntary loss of weight and appetite. The administered infusion rate was 2 or 8 ug/kg bw for 60 minutes. While the infusion of ghrelin was safe and well tolerated, the study did not find an increase in nutritional intake or an increase in IGF-I levels in the treatment group. One of the possible explanations was thought to be the presence of ghrelin resistance. Other studies could show an orexigenic effect in cancer patients. Neary et al. (Neary et al. 2004) did find a 31% increase in energy intake after 2 ghrelin infusions, in a small number of mainly breast cancer patients. One of the major concerns is the possibility of either direct ghrelin mediated or indirect GH mediated stimulation of tumor growth in these patients. While some in vitro studies have suggested that ghrelin might enhance the proliferation of cancer cells (Duxbury et al. 2003; Yeh et al. 2005), others found an inhibition of proliferation and an increase in apoptosis (Cassoni et al. 2006) in lung cancer cells. To date there are no clinical studies suggesting an increase in tumor incidence with the administration of ghrelin, albeit the available studies are short term studies and include only a small number of patients.
Chronic obstructive pulmonary disease (COPD
Intravenous ghrelin treatment for 3 weeks in patients with COPD reduced the neutrophil count in sputum samples as well as the volume of sputum (Kodama et al. 2008). IV ghrelin administration over 3 weeks in a dose of 2 ug/kg b.i.d to seven cachectic patients with COPD resulted in a small but significant increase in body weight and an improvement in the 6 min walk (Nagaya et al. 2005). The authors also found a decrease in plasma norepinephrine levels.
Aging
Based on the expected demographic age shift of the world population, strategies to prevent and/or slow the development of frailty and the age-dependent muscle loss will be of increasing interest (Nass et al. 2009; Thorner 2009). Several studies have found low acyl-ghrelin levels in the elderly (Nass et al. 2008b; Rigamonti et al. 2002) supporting a role for ghrelin or ghrelin mimetics in this age group. In a small study in frail older women, a graded ghrelin infusion (2.5, 5, and 10 pmol/kg/min) over 180 minutes resulted in an increased food intake. Overall the women consumed 51% more calories after the ghrelin infusion when compared to placebo (Cappola 2009). Additional clinical studies in the aging field have been done with ghrelin mimetics which have the advantage of oral availability and are discussed below.
Gastric motility
The migrating motor complex is a pattern of electromechanical activity observed in the gastrointestinal tract. Tack et al. (Tack et al. 2006) showed in 9 healthy volunteers that administration of 40ug of ghrelin given IV, resulted in a gastric phase III migrating motor complex (MMC), which was independent of motilin. In accordance with the results of this study, Levin et al. (Levin et al. 2006) found an increase in the gastric emptying rate in healthy humans after a 180 minute IV infusion of ghrelin.
2) Administration of ghrelin mimetics
Age related muscle loss and frailty
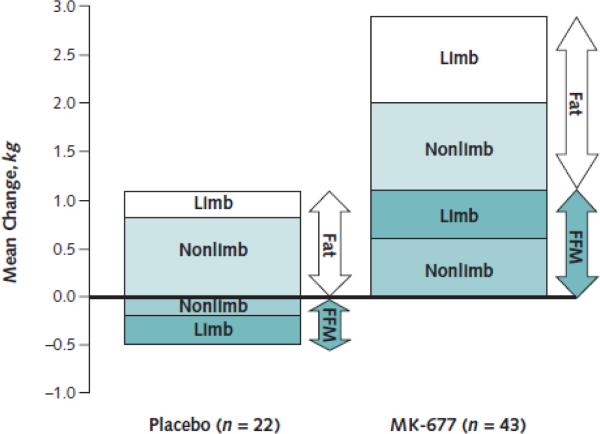
The first ghrelin mimetics were described in the 1970s by Bowers et al., (Bowers et al. 1977) about a quarter of a century before the ghrelin receptor (Howard et al. 1996) and ghrelin peptide (Kojima et al. 1999) were discovered. They were called GH releasing peptides (GHRP) and initial research focused on their GH releasing effects. In the 1990s the first orally available ghrelin mimetics were tested and were named GH secretagogues (Smith 2005). The number of clinical studies with ghrelin mimetics, some of them orally available, has been relatively small, when compared to the studies testing the ghrelin peptide itself. This is surprising as some of the ghrelin mimetics have the advantage that they are orally available and can be given once a day. One of the first oral ghrelin mimetics, which was tested for its effects under a catabolic situation, is the nonpeptide spiropiperidine MK-677 (Murphy et al. 1998). MK-677 is orally active and enhances the pre-existing pulsatile release of growth hormone (GH) (Chapman et al. 1997). The study showed that administration of MK-677 improves nitrogen balance within 2 weeks of caloric restriction. Short term studies with MK-677 showed its ability to enhance pulsatile GH release and IGF-I concentrations to levels seen in young adults (Chapman et al. 1997) and suggested that it could increase fat free mass in obese males (Svensson et al. 1998). In an 18 months study, in postmenopausal women, MK-677 had a positive effect on bone mineral density at the femoral neck when combined with alendronate vs alendronate alone; however this effect was not seen at the lumbar spine, total hip or total body (Murphy et al. 2001). Bach et al. (Bach et al. 2004) report the effects of MK-677 when given to hip fracture patients after hip surgery in a group of patients age 65 years and older. The study showed a greater improvement relative to placebo in 3 of four lower extremity functional performance measures. However the study did not find a statistically significant effect when measuring the ability to live independently or in the domain of the SIP-NH (Sickness Impact Profile for Nursing Homes) 6 weeks after the surgery. The longest study to date to assess the effects of MK-677 in healthy older adults was published in 2008 (Nass et al. 2008c). One year treatment with MK-677 resulted in an increase of fat free mass by 1.1 kg, while the placebo group showed a 0.5 kg muscle loss (see figure 1). This effect was sustained for a total of 2 years in the group which received MK-677. The increase in muscle mass did not result in a measurable change in muscle strength or function. This could be in part due to the fact that the study included mainly physically fit, active, healthy older adults.
Figure 1.
Mean changes in fat and fat-free mass (FFM) at 12 months. (From Nass et al, 2008c)
Limb = appendicular lean soft tissue and appendicular fat; nonlimb = total minus limb.
Treatment with the ghrelin mimetic capromorelin, a pyrazoline-piperidine, increased lean body mass by 1.4 kg vs 0.3 kg in the placebo group and resulted in an improvement in tandem walk and stair climb (White et al. 2009). The treatment group included older adults with mild functional limitation. However, as part of the analysis, pooling of the treatment group was required, because of different dosing groups.
Cancer cachexia, COPD and gastric paresis
Other ghrelin mimetics have been tested in patients with cancer cachexia (RC-1291) and in COPD patients with cachexia (Garcia et al. 2007; Gertner 2010; Nagaya et al. 2005; Nagaya et al. 2006) and have shown improvement in lean body mass. Administration of the ghrelin mimetic Ulimorelin (TZP-101) has shown positive effects on nauseas associated with gastric paresis in patients with diabetic gastric paresis (Lasseter et al. 2008). While these results are promising, most of these studies were not conducted long enough to provide information about the safety of the compounds used.
Diagnostic use in GH deficiency
Other ghrelin mimetic compounds are in the early stage of testing and have shown GH releasing effects in healthy adults, supporting a role for ghrelin mimetics also as a potential diagnostic tool to be used in the future in diagnosing GH deficiency (Zdravkovic et al. 2000).
Conclusion
Several conditions such as CHF, ESKD, COPD, cancer and the sarcopenia of aging are associated with significant weight loss and increased protein breakdown, which ultimately lead to increased morbidity and mortality. Based on its orexigenic effects, ghrelin and ghrelin mimetics have been shown to be of potential benefit in antagonizing protein breakdown and weight loss in catabolic conditions. Compared to ghrelin, ghrelin mimetics have the advantage that some can be given once daily and are orally active. Further research in this area is mandatory to assess the efficacy and safety of ghrelin mimetics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby DR, Ford HE, Wynne KJ, et al. Sustained appetite improvement in malnourished diaysis patients by daily ghrelin treatment. Kidney International. 2009;76:199–206. doi: 10.1038/ki.2009.114. [DOI] [PubMed] [Google Scholar]
- Bach MA, Rockwood K, Zetterberg C, et al. The effects of MK-0677, an oral growth hormone secretagogue, in patients with hip fracture. J Am Geriatr Soc. 2004;52(4):516–23. doi: 10.1111/j.1532-5415.2004.52156.x. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, Feighner SD, Pong SS, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. Journal of Medicinal Chemistry. 2000;43(23):4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- Bowers CY, Chang JK, Fong TTW. A synthetic pentapeptide which specifically releases GH, in vitro. Proc 59th Meeting of The Endocrine Society; Chicago. 1977. [Google Scholar]
- Cappola A. Effects of ghrelin administration in frail and healthy older women. P3-495, 91st Annual Meeting of the Endocrine Society; Washington, D.C.. 2009. [Google Scholar]
- Cassoni P, Allia E, Marrocco T, Ghe C, Ghigo E, Muccioli G, Papotti M. Ghrelin and cortistatin in lung cancer: expression of peptides and related receptors in human primary tumors and in vitro effect on the H345 small cell carcinoma cell line. J Endocrinol Invest. 2006;29:781–790. doi: 10.1007/BF03347371. [DOI] [PubMed] [Google Scholar]
- Chapman IM, Pescovitz OH, Murphy G, et al. Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adults. Journal of Clinical Endocrinology & Metabolism. 1997;82(10):3455–63. doi: 10.1210/jcem.82.10.4297. [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Zhu X, Levasseur PR, et al. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149:827–35. doi: 10.1210/en.2007-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer MD, Zhu XX, Levasseur P, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148:3004–12. doi: 10.1210/en.2007-0016. [DOI] [PubMed] [Google Scholar]
- Delhanty PJD, Sun Y, Visser JA, et al. Unacylated Ghrelin Rapidly Modulates Lipogenic and Insulin Signaling Pathway Gene Expression in Metabolically Active Tissues of GHSR Deleted Mice. PLoS ONE. 2010;5(7):e11749. doi: 10.1371/journal.pone.0011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr., Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114(1):57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury MS, Waseem T, Ito H, Robinson MK, Zinner MJ, Ashley SW, Whang EE. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem Biophys Res Commun. 2003;309:464–468. doi: 10.1016/j.bbrc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Esler WP, Rudolph J, Claus TH, et al. Small-Molecule Ghrelin Receptor Antagonist Improve Glucose Tolerance, Suppress Appetite, and Promote Weight Loss. Endocrinology. 2007;148:5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- Garcia JM, Cata JP, Dougherty PM, Smith RG. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149(2):455–60. doi: 10.1210/en.2007-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JM, Graham C, Kumor K, Polvino W. A phase II, randomized, placebo-controlled, double blind study of the efficacy and safety of RC-1291 for the treatment of cancer-cachexia. J Clin Oncol. 2007;S25 abstract. [Google Scholar]
- Gertner J. SUN 11031 (synthetic human Ghrelin) improves lean body mass and function in advanced COPD cachexia in a placebo controlled trial. 5th International Congress of the GRS and IGF-I Society; NYC. 2010. [Google Scholar]
- Granata R, Volante M, Settanni F, et al. Unacylated ghrelin and obestatin increase islet cell mass and prevent diabetes in streptozotocin-treated newborn rats. Journal of Molecular Endocrinology. 2010;45(1):9–17. doi: 10.1677/JME-09-0141. [DOI] [PubMed] [Google Scholar]
- Groschl M, Uhr M, Kraus T. Evaluation of the Comparability of Commercial Ghrelin Assays. Clin Chem. 2004;50(2):457–458. doi: 10.1373/clinchem.2003.025429. [DOI] [PubMed] [Google Scholar]
- Guerlavais V, Boeglin D, Mousseaux D, et al. New active series of growth hormone secretagogues. J Med Chem. 2003;46(7):1191–203. doi: 10.1021/jm020985q. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proceedings of the National Academy of Sciences. 2008;105(17):6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Toshinai K, Kajimura N, et al. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. 2003;7(301):275–9. doi: 10.1016/s0006-291x(02)03028-0. [DOI] [PubMed] [Google Scholar]
- Helmling S, Maasch C, Eulberg D, et al. Inhibition of ghrelin action in vitro and in vivo by an RNA-Spiegelmer. PNAS. 2004;101(36):13174–13179. doi: 10.1073/pnas.0404175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosada H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: Two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279(3):909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Hotta M, Ohwada R, Katakami H, Shibasaki T, Hizuka N, Takano K. Plasma Levels of Intact and Degraded Ghrelin and Their Responses to Glucose Infusion in Anorexia Nervosa. J Clin Endocrinol Metab. 2004;89(11):5707–5712. doi: 10.1210/jc.2004-0353. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato N. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther. 2008;21:774–779. doi: 10.1016/j.pupt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends in Endocrinology & Metabolism. 2001;12(3):118–22. doi: 10.1016/s1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin--a hormone with multiple functions. Frontiers in Neuroendocrinology. 2004;25(1):27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lasseter KC, Shaughnessy L, Cummings D, Pezzullo JC, Wargin W, Gagnon R, Oliva J, Kosutic G. Ghrelin agonist (TZP-101): safety, pharmacokinetics and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol. 2008;48(2):193–202. doi: 10.1177/0091270007310380. [DOI] [PubMed] [Google Scholar]
- Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. JCEM. 2006;91:3296–3302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- Liu J, Prudom CE, Nass R, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93(5):1980–7. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hosoda H, Kitajima Y, et al. Structure-activity relationship of ghrelin: pharmacological study of ghrelin peptides. Biochemical & Biophysical Research Communications. 2001;287(1):142–6. doi: 10.1006/bbrc.2001.5553. [DOI] [PubMed] [Google Scholar]
- Murphy MG, Plunkett LM, Gertz BJ, He W, Wittreich J, Polvino WM, Clemmons DR. MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol Metab. 1998;83(2):320–5. doi: 10.1210/jcem.83.2.4551. [DOI] [PubMed] [Google Scholar]
- Murphy MG, Weiss S, McClung M, Schnitzer T, Cerchio K, Connor J, Krupa D, Gertz BJ, MK-AS Group Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. Journal of Clinical Endocrinology & Metabolism. 2001;86(3):1116–25. doi: 10.1210/jcem.86.3.7294. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, Kangawa K. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128(3):1187–93. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Kojima M, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Intern Med. 2006;45(3):127–34. doi: 10.2169/internalmedicine.45.1402. [DOI] [PubMed] [Google Scholar]
- Nagaya N, Moriya J, Yasumura Y, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–9. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- Nass R, Farhy LS, Liu J, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008a;93(5):1988–94. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Johannsson G, Christiansen JS, Kopchick JJ, Thorner MO. The aging population--is there a role for endocrine interventions? Growth Horm IGF Res. 2009;19(2):89–100. doi: 10.1016/j.ghir.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Nass R, Liu J, Hellmann P, Coschigano KT, Gaylinn B, Berryman DE, Kopchick JJ, Thorner MO. Chronic changes in peripheral growth hormone levels do not affect ghrelin stomach mRNA expression and serum ghrelin levels in three transgenic mouse models. J Neuroendocrinol. 2004;16(8):669–75. doi: 10.1111/j.1365-2826.2004.01220.x. [DOI] [PubMed] [Google Scholar]
- Nass R, Liu J, Pezzoli S, Oliveri M, Gaylinn B, Thorner M. 24-h Mean acyl-ghrelin levels are decreased in older adults. 4th International Congress of the GRS and the IGF Society; Italy. 2008b. [Google Scholar]
- Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008c;149(9):601–11. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass RM, Gaylinn BD, Rogol AD, Thorner MO. Ghrelin and growth hormone: story in reverse. Proc Natl Acad Sci U S A. 2010;107(19):8501–2. doi: 10.1073/pnas.1002941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Wren AM, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(6):2832–6. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- Prudom C, Liu J, Patrie J, Gaylinn BD, Foster-Schubert KE, Cummings DE, Thorner MO, Geysen HM. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab. 2010;95(5):2351–8. doi: 10.1210/jc.2009-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh M, Groschl M, Rascher W. Simultaneous Quantification of Ghrelin and Desacyl-Ghrelin by Liquid Chromatography-Tandem Mass Spectrometry in Plasma, Serum, and Cell Supernatants. Clin Chem. 2007;53(5):902–910. doi: 10.1373/clinchem.2006.078956. [DOI] [PubMed] [Google Scholar]
- Rigamonti AE, Pincelli AI, Corra B, et al. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175(1):R1–5. doi: 10.1677/joe.0.175r001. [DOI] [PubMed] [Google Scholar]
- Smith RG. Development of growth hormone secretagogues. Endocr Rev. 2005;26(3):346–60. doi: 10.1210/er.2004-0019. [DOI] [PubMed] [Google Scholar]
- Strasser F, Lutz TA, Maeder MT, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98(2):300–8. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Garcia JM, Smith RG. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology. 2007;148(3):1323–9. doi: 10.1210/en.2006-0782. [DOI] [PubMed] [Google Scholar]
- Svensson J, Lonn L, Jansson JO, et al. Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab. 1998;83(2):362–9. doi: 10.1210/jcem.83.2.4539. [DOI] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64(10):1039–44. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]
- Tong J, Davis H, Nass R, D'Alessio D, Tschop MH. Effects of ghrelin administration in humans. Obesity and Metabolism. 2009;5(2):60–68. [Google Scholar]
- Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D'Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59(9):2145–51. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Shimbara T, Yamaguchi H, Date Y, Kangawa K, Nakazato M. Grelin stimulates growth hormone secretion and food intake in aged rats. Mech Ageing Dev. 2007;128:182–6. doi: 10.1016/j.mad.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tschoep M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Wang W, Andersson M, Iresjoe BM, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol. 2006;28:1393–400. [PubMed] [Google Scholar]
- White HK, Petrie CD, Landschulz W, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94(4):1198–206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- Yeh AH, Jeffery PL, Duncan RP, Herington AC, Chopin LK. Ghrelin and a novel preproghrelin isoform are highly expressed in prostate cancer and ghrelin activates mitogen-activated protein kinase in prostate cancer. Clin Cancer Res. 2005;11:8295–8303. doi: 10.1158/1078-0432.CCR-05-0443. [DOI] [PubMed] [Google Scholar]
- Zdravkovic M, Sogaard B, Ynddal L, Christiansen T, Agerso H, Thomsen MS, Falch JE, Ilondo MM. The pharmacokinetics, pharmacodynamcs, safety and tolerability of a single dose of NN073, a novel orally active growth hormone secretagogue in healthy male volunteers. Growth Horm IGF Res. 2000;10:193–8. doi: 10.1054/ghir.2000.0152. [DOI] [PubMed] [Google Scholar]
- Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107(16):7467–72. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]