Abstract
Glutamate release is a root cause of acute and delayed neuronal damage in response to hypoxic/ischemic insults. Nevertheless, therapeutics that target the postsynaptic compartment have been disappointing clinically. Here we explored whether presynaptic silencing (muting) of glutamatergic terminals is sufficient to reduce excitotoxic damage resulting from hypoxia and oxygen/glucose deprivation. Our evidence suggests that strong depolarization, previously shown to mute glutamate synapses, protects neurons by a presynaptic mechanism that is sensitive to inhibition of the proteasome. Postsynaptic Ca2+ rises in response to glutamate application and toxicity in response to exogenous glutamate treatment were unaffected by depolarization preconditioning. These features strongly suggest that reduced glutamate release explains preconditioning protection. We addressed whether hypoxic depolarization itself induces presynaptic silencing, thereby participating in the damage threshold for hypoxic insult. Indeed, we found that the hypoxic insult increased the percentage of mute glutamate synapses in a proteasome-dependent manner. Furthermore, proteasome inhibition exacerbated neuronal loss to mild hypoxia and prevented hypoxia-induced muting. In total our results suggest that presynaptic silencing is an endogenous neuroprotective mechanism that could be exploited to reduce damage from insults involving excess synaptic glutamate release.
Keywords: synaptic plasticity, glutamate, hippocampus, ischemia, homeostasis, excitotoxicity
Introduction
For decades we have known that glutamate excitotoxicity (Olney et al., 1971) results in damage to neurons following a wide variety of insults including hypoxia, ischemia, seizures (Gidday, 2006; Goldberg and Choi, 1993; Rothman, 1984), and neuropsychiatric disorders (Olney, 2003; Zorumski and Olney, 1993). Therapeutic approaches have centered on blocking glutamate receptors and other downstream, postsynaptic targets involved in the excitotoxic cascade. Unfortunately, thesex approaches have proven disappointing in human studies because of poor efficacy or unacceptable side effects (Gidday, 2006; Moskowitz et al., 2010). Thus fresh approaches and new basic insights are needed. One alternative might be to study and exploit endogenous homeostatic synaptic mechanisms as strategies. By augmenting endogenous pathways of adaptive synaptic plasticity, such strategies might circumvent some of the problems that have arisen with previous interventions.
We have been studying a form of persistent presynaptic plasticity involving depression of glutamate vesicle availability in hippocampal neurons. This depression is characterized by presynaptic silencing, or muting, that outlives the inducing stimulus. Muting is induced at glutamate, but not γ-aminobutyric acid (GABA), synapses by strong depolarization (Moulder et al., 2004). It reverses over several hours (Moulder et al., 2008) and is characterized by involvement of inhibitory G-protein signaling (Crawford et al., 2011) and the ubiquitin/proteasome system (UPS)(Jiang et al., 2010).
Two issues relevant to excitotoxic pathophysiology emerge from our previous observations. First, because the source of glutamate during excitotoxic insults may not be purely synaptic (Rossi et al., 2000), it is unclear whether presynaptic interventions, such as induction of presynaptic muting, are effective neuroprotectants. Second, because presynaptic silencing has been induced only under controlled experimental conditions, it is unclear whether depolarizing insults such as hypoxia can induce muting rapidly and strongly enough to provide endogenous neuroprotection. If so, muting may normally help set the threshold for damage during insults. Support for these two ideas would help establish the plausibility of augmentation of presynaptic muting as a neuroprotective intervention and would offer new basic insights into the roles of synaptic plasticity in nervous system function and dysfunction.
Our results demonstrate that presynaptic silencing induced by strong depolarization protects neurons in in vitro hypoxia and oxygen-glucose deprivation (OGD) models, consistent with the idea that synaptic glutamate is important for the damage. Although depolarizing preconditioning paradigms have been shown to be neuroprotective in other models through postsynaptic mechanisms (Grabb and Choi, 1999; Grabb et al., 2002; Meller et al., 2008), we show that presynaptic mechanisms, most likely involving presynaptic muting, are most important in our paradigm. Further, our results suggest that a hypoxic insult induces muting to limit damage. Proteasome inhibition, among other likely effects, prevents hypoxia-induced silencing and exacerbates damage through a presynaptic mechanism, suggesting that muting helps set the threshold for hypoxic damage. These results yield insights and suggest new approaches that might be exploited for benefit in disorders involving dysfunction of glutamate synapses.
Materials and Methods
Cell culture
Hippocampal cultures were prepared as described previously (Mennerick et al., 1995). In brief, dissected postnatal (postnatal days 0–3) male and female rat hippocampi were incubated with papain, mechanically dissociated, and plated at 650 cells/mm2 on collagen substrate. Plating media consisted of Eagle's medium (Invitrogen) supplemented with heat-inactivated horse serum (5%), fetal bovine serum (5%), 17 mM glucose, 400 μM glutamine, 50 U/ml penicillin, and 50 g/ml streptomycin. Cultures were maintained at 37°C in a humidified incubator with 5% CO2/95% air. Cytosine arabinoside at 6.7 μM was added at 3–4 d after plating to inhibit cell division. At 1 d a media exchange was performed with Neurobasal medium (Invitrogen) plus B27 supplement. Cells were used for experiments at 13-15 days in vitro. Neurons exhibited increased sensitivity to all forms of glutamate toxicity with maturation.
Preconditioning
Fresh filtered Neurobasal (Invitrogen) medium without L-glutamine was used as a base for preconditioning media and media exchanges. The original conditioned media was removed and replaced with the preconditioning media. The cultures were returned to their original media just before hypoxic exposure. For depolarizing preconditioning, the preconditioning media contained Neurobasal plus 30 mM KCl. Control preconditioning media included 30 mM NaCl in place of KCl as an equimolar non-depolarizing control. In our typical protocol, this resulted in control and experimental preconditioning solutions that were ~60 mosmol hyperosmotic. To ensure that hypertonicity did not interact with the effects of depolarization, we performed a subset of preconditioning experiments in media that were isotonic (142 mM total monovalent cation concentration, KCl substitution for NaCl, n=5 experiments). These experiments produced similar preconditioning protection as our standard protocol (27.7 ± 9.66 % cell survival with hypoxia, 62.8 ± 3.58 % survival with hypoxia plus KCl preconditioning, compare with Fig. 1). We did not routinely perform experiments in the isotonic media because of the high expense of custom media preparation. Unless otherwise noted, all control and depolarizing preconditioning solutions also included 0.5 μM 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxyline (NBQX), and 25μM D-2-Amino-5-phosphonovalerate (D-APV) to prevent induction of glutamate-receptor dependent forms of preconditioning protection or plasticity during preconditioning. In some experiments we omitted extracellular Ca2+ (0.1 mM EGTA added to chelate residual Ca2+) or included Carbobenzoxy-L-leucyl-L-leucyl-L-leucinal (MG-132; Sigma, 3 μM) in preconditioning solutions. Unless otherwise noted, the media was exchanged immediately before hypoxic exposure to remove glutamate receptor antagonists and the preconditioning stimulus.
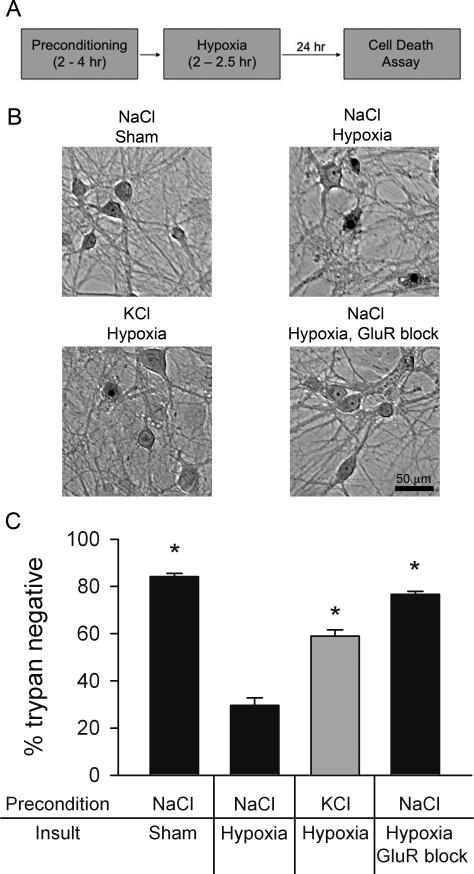
Figure 1.
Depolarization preconditioning protects against hypoxia. A. Schematic of preconditioning/hypoxic exposure paradigm. B. Photomicrographs from a single experiment demonstrating depolarization protection. Upper left: brightfield image from a normoxic control stained with trypan blue 24 hr post-preconditioning with 4 hr of 30 mM NaCl (control preconditioning) and 2.5 hr sham hypoxia. Upper right: A field from a dish preconditioned with 30 mM NaCl and subjected to 2.5 hr hypoxia. Trypan blue positive pyknotic nuclei are apparent. Lower left: a field from a dish preconditioned with 30 mM KCl for 4 hr and then subjected to 2.5 hr hypoxia. Lower right: a protection control in which 1 μM NBQX and 100 μM D-APV, ionotropic glutamate receptor (GluR) antagonists, were included in the hypoxia media. C. Summary of experiments like that depicted in panel B, showing protection afforded by KCl depolarization preconditioning. Cell survival is expressed as the percentage of trypan blue negative cells averaged over ten fields evaluated with a 20x objective (n=7 independent experiments). Asterisks designate p < 0.05 compared with NaCl hypoxia (unpaired, two-tailed t-tests with Bonferroni correction for multiple comparisons). Gray bar emphasizes the major hypothesized result of KCl preconditioning protection.
Hypoxia Exposure
A commercially available chamber (Billups-Rothenberg Company) was used for hypoxia induction and maintenance. Cells were incubated in a humidified environment saturated with 95% nitrogen and 5% CO2 at 37° C for the specified amount of time (2-2.5 hr depending on the experiment). The gas exchange was performed according to the specifications of the chamber manufacturer (flow of 20 L per minute for four minutes to achieve 100% gas exchange). After the insult, we removed cells from the chamber and incubated them under standard conditions until the cell death assay (24 hr). When used during the insult to demonstrate neuroprotection, the glutamate receptor antagonists were 1 μM NBQX and 100 μM D-APV, but antagonists were not routinely present during hypoxia or OGD insults. For OGD experiments the method of oxygen deprivation was the same, and just prior to oxygen deprivation culture media was switched to Neurobasal without L-glutamine and without glucose. Control cultures received the same media with glucose (25 mM). Procedures for sham insults indicated in figures included all media changes and incubation times relevant to the insult conditions. For experiments in which exogenous glutamate exposure was used in place of hypoxia, media was exchanged with fresh Neurobasal without L-glutamine and with the indicated concentration of glutamate. The cultures were then returned to their original media for 24 hr until the cell death assay was performed.
Cell Death Assay
To assess cell death, trypan blue dye (Sigma) was used. 24 hr after the insult, culture media was removed and replaced with 1 mL of 0.4% trypan blue dissolved in phosphate buffered saline. Cells were incubated in dye at 37° C for 5 min and washed with phosphate buffered saline. Cultures were then fixed with 4% paraformaldehyde and 0.2% glutaraldehyde at room temperature. Cells were visualized with a 20x objective using both phase-contrast and brightfield microscopy to confirm healthy neuronal profiles (phase-contrast) and verify trypan blue uptake (brightfield). In one experiment the designation of healthy neurons was verified 24 hr after hypoxia treatment by calcein AM (acetoxymethyl) uptake (2 μM calcein-AM incubation for 30 min). Cells deemed healthy by morphology under phase-contrast optics were always calcein positive (n = 5 fields, 20 - 40 cells per field). The total numbers of dead and intact neurons were counted and expressed as a percentage of trypan blue positive dead cells to total cells. The average of ten microscope fields for each condition was treated as a single data point for purposes of statistics.
Calcium indicators and imaging
Fluo4-AM (high affinity indicator; Invitrogen) and Fluo3-FF-AM (low affinity indicator; Teflabs) fluorescent calcium indicators were used. We incubated neurons in preconditioning solutions as noted above. After the 4 hr preconditioning period cells were incubated for 30 min. at 37° C in normal media containing 2 μM of the AM indicator. 5-7 fields per condition were imaged. Fluorescence images were obtained with a CoolSnap ES2 camera (Photometrics) every 600 ms, using a fluorescein filter set and metal halide light source. We selected 3 cells randomly from each field from a phase contrast image, without reference to fluorescence images, for a total of 15-21 cells per condition as indicated. Regions of interest were analyzed in the soma cytoplasm adjacent to the nucleus. Background fluorescence was subtracted from each image using a cell-free region of the field. Image acquisition and analysis were performed using Metamorph (MDS).
FM1-43fx labeling, immunochemistry, and imaging
Details have been previously published (Crawford et al., 2011). Briefly, cells were removed from preconditioning (Fig. 2) or the hypoxia chamber (Fig. 7), and after a saline wash (~30 s) FM1-43fx (10 μM; Invitrogen) was loaded into active presynaptic terminals during a brief (2 min) 45 mM KCl depolarization. vGluT-1 primary antibody (1:2000) was applied after aldehyde fixation, permeabilization, and block. Alexa Fluor 647 goat anti-guinea pig secondary antibody (Invitrogen) was used at 1:500. Fluorescence images were acquired using a Nikon C1 confocal microscope and analyzed using Metamorph software. Parameters for image acquisition and analysis were constant for each independent experiment.
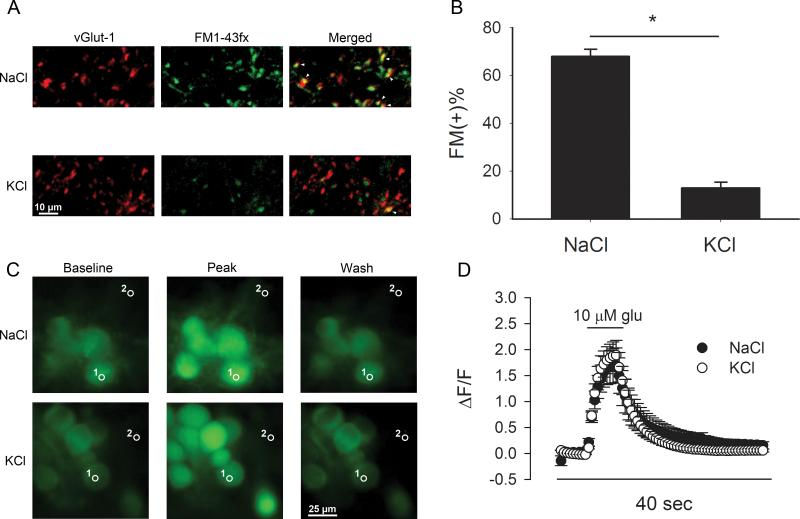
Figure 2.
Preconditioning induces presynaptic muting but no detectable postsynaptic changes. A. Images of vGluT-1 positive puncta from representative fields from cultures preconditioned by 4 hr KCl (30 mM) depolarization or by 30 mM NaCl control preconditioning. Middle panels show uptake of FM1-43fx during brief depolarization in the same field. The merged images reveal more inactive (FM1-43fx negative) vGluT-1 positive puncta after depolarizing preconditioning. Green = FM1-43fx. Red = vGluT-1. Red puncta with no green overlap are mute synapses, while yellow indicates overlap and active synapses. White arrowheads indicate examples of co-labeled, active glutamatergic synapses. B. The percentage of FM1-43fx positive (FM (+)) terminals is summarized from 5 experiments like that depicted in A. C. Representative fluorescence images from somatic Ca2+ signals measured in control (n = 18 cells in 6 fields) and depolarization-conditioned (n = 15 cells in 5 fields) cells. Cells were loaded with a 30 min bath application of 2 μM Fluo3- FF AM. Representative regions of interest (labeled with “1”) show typical locations of measurement in the soma, chosen based on a phase contrast image (not shown). Dendrites and axons are below the plane of focus. Images of baseline (prior to glutamate perfusion), peak fluorescence (5 s following 10 μM glutamate onset), and return to baseline (30 s wash) fluorescence are shown. Region 2 indicates a region devoid of cells and typical of regions from which background fluorescence was measured. D. Summary of glutamate-evoked somatic intracellular Ca2+ signals measured over the entire experiment (67 images over 40 seconds) with control-conditioned and depolarization-conditioned cells overlaid.
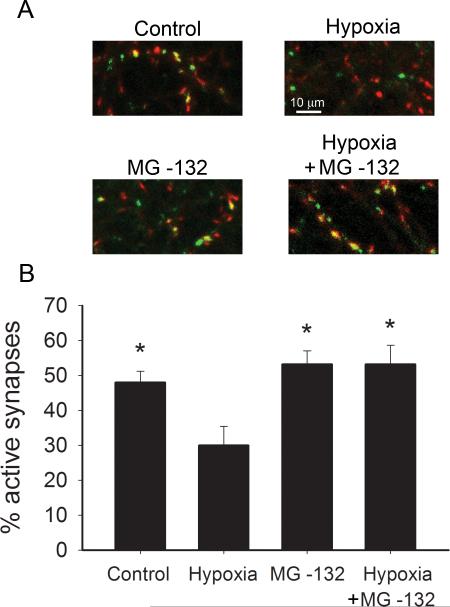
Figure 7.
Hypoxia induces proteasome-dependent presynaptic silencing. A. FM1-43fx/vGluT-1 correspondence assay. Green = FM1-43fx. Red = vGluT-1. Red puncta with no green overlap are mute synapses, while yellow indicates overlap and active synapses. A 2 hr preconditioning period with or without MG-132 (3 μM) was followed by 2 hr hypoxic insult (or sham) with immediately subsequent FM1-43fx assay. B. Summary of experiments depicted in panel A showing the percentage of active synapses after hypoxic insult with and without MG-132 co-incubation (n=25 fields from 5 independent experiments). Asterisk denotes p < 0.05 compared with hypoxia alone (Bonferroni corrected t-tests). Comparisons of control versus MG-132, control versus hypoxia plus MG-132, and MG-132 versus hypoxia plus MG-132 showed no differences.
To identify active glutamatergic synapses, an observer naïve to the experimental conditions identified 10 vGluT-1-positive puncta as regions of interest from 5 fields for each experimental condition. Next, FM1-43fx images were thresholded, after which vGluT-1 regions were loaded into the FM1-43fx image. Active synapses were defined as puncta that reached or exceeded 10 thresholded pixels in the FM1-43fx image (Crawford et al., 2011).
Data analysis
Data analysis was performed in Excel (Microsoft) and Sigma Plot 10.0 (Systat Software). Student's unpaired t-test was used to test for significance. Where indicated, the Bonferroni correction for multiple comparisons was used. Except where otherwise indicated, values for n in the text and figure legends represent independent experiments on separate cultures.
Materials
All reagents were obtained from Sigma Chemical Company unless otherwise indicated. Culture media were from Invitrogen, guinea pig vGluT-1 antibody was from Chemicon.
Results
Hypoxic damage is attenuated by depolarization preconditioning
We explored the hypothesis that strong depolarization, a stimulus that induces presynaptic muting in hippocampal neurons, protects neurons from subsequent hypoxic damage. Unlike other forms of preconditioning, which exhibit a latent period during induction of hours or days (Gidday, 2006; Kitagawa et al., 1991), presynaptic muting is induced by strong depolarization, and recovers within a few hours of removal of the inducing stimulus (Moulder et al., 2004). Therefore, we designed our paradigm to capture the likely protective effect of presynaptic muting immediately after induction, without invoking previously described mechanisms of preconditioning protection with longer latencies. We incubated synaptically mature cultured hippocampal neurons (13-15 days in vitro) with 4 hr of high KCl (30 mM), a stimulus that silences 75-80% of glutamate presynaptic terminals, or 4 hr of NaCl (30 mM) as an osmotic, non-depolarizing control (Moulder et al., 2004). Both control preconditioning and depolarizing preconditioning were always performed in the presence of D-APV and NBQX to prevent glutamate receptor activation. This ensured that other forms of synaptic plasticity dependent on glutamate receptors were not activated by preconditioning; presynaptic silencing is not glutamate receptor-dependent (Crawford et al., 2011; Moulder et al., 2006; Moulder et al., 2004). After preconditioning, the neurons were then removed from the preconditioning stimulus, including receptor antagonists, by media exchange and immediately exposed to hypoxia for 2.5 hr. In addition to terminating the preconditioning stimulus, this media exchange also eliminated glutamate receptor antagonists and any contributions of substances secreted during preconditioning (see Methods). Thus, only persisting cellular changes contributed to neuroprotection during hypoxia. We used trypan blue staining, a well-validated probe of membrane integrity, 24 hr after the insult to assess cell death (Fig. 1A). With sham treatment, we found only mild attritional cell death (84 ± 1.3% healthy neurons; Fig. 1B, C), consistent with previous work (Shute et al., 2005). In cells rendered hypoxic after control (NaCl) preconditioning, a 2.5 hr hypoxic insult resulted in 30 ± 3.2% survival (Fig. 1B, C). By contrast, depolarizing preconditioning strongly and reliably protected neurons from hypoxia (59 ± 2.6% survival, Fig. 1B, C). Postsynaptic glutamate receptor blockade during the insult nearly fully protected neurons (77 ± 1.4% survival, Fig. 1B, C), as previously shown (Rothman, 1984), and verifying the pivotal role of glutamate release and glutamate receptor activation in hypoxic cell loss.
Depolarization preconditioing works through a presynaptic mechanism
These results suggest that a stimulus known to induce presynaptic silencing protects neurons from damage by endogenous glutamate. To verify presynaptic muting by the preconditioning paradigm, we performed analysis of FM1-43fx labeling of glutamatergic presynaptic terminals (defined by vGluT-1 immunoreactivity). As previously observed (Crawford et al., 2011; Jiang et al., 2010; Moulder et al., 2004), 4 hr depolarization preconditioning silenced a majority of glutamate terminals (Fig. 2A, B). Because the FM1-43fx loading protocol used 2 min of strong depolarization to induce vesicle cycling, loading should overcome changes in vesicle release probability that could occur, for instance, by decreases in Ca2+ influx during dye loading. This protocol has been shown to label the entire pool of vesicles capable of exo/endocytosis (Mozhayeva et al., 2002). Therefore, terminals appear functionally silenced in response to the preconditioning stimulus, a state that would be expected to effectively reduce glutamate release during major insults such as hypoxia. In this set of experiments we also noted a mild decrease in FM1-43fx uptake at remaining, active synapses (Fig. 2A), which could indicate a graded change in the number of releasable vesicles (Murthy et al., 2001).
We have previously shown that changes in FM1-43fx uptake quantitatively match depression of EPSCs, suggesting that postsynaptic changes do not contribute significantly to the effects of depolarization conditioning (Moulder et al., 2004). Furthermore, depolarization preconditioning does not induce detectable postsynaptic changes measured by mEPSC analysis, response to exogenous glutamate agonists, or AMPA receptor immunoreactivity (Moulder et al., 2006; Moulder et al., 2004). These past experiments do not exclude the possibility that depolarization conditioning alters postsynaptic Ca2+ influx or handling downstream of receptor activation. To test whether depolarization preconditioning influences the postsynaptic Ca2+ signals presumably important for hypoxic damage (Choi, 1985; Choi, 1987), we loaded cells with Fluo3-FF and then challenged depolarization-preconditioned cells and control cells with brief applications of 10 μM glutamate (Fig. 2C, D). Because cells were stimulated by exogenous glutamate applied to somatodendritic postsynaptic receptors, Ca2+ signals measured near the soma should reflect any changes in receptor-mediated Ca2+ influx or intracellular handling that might participate in neuroprotection. Preconditioned neurons did not differ in amplitude or half decay time (t1/2) of somatic Ca2+ signals evoked by a 5 s application of 10 μM glutamate, indicating that preconditioning did not affect Ca2+ handling or buffering in the postsynaptic compartment (peak ΔF/F = 1.8 ± 0.4, and t1/2 = 3.3 ± 0.6 s for NaCl control; peak ΔF/F = 2.0 ± 0.3, and t1/2 = 3.2 ± 0.3 s for KCl preconditioning, p > 0.05, n = 18 cells for control, n = 15 cells for KCl preconditioning; Fig. 2C, D). We used the low-affinity dye Fluo3-FF to prevent dye saturation, but to ensure that the indicator was not saturated by our glutamate challenge, we applied 100 μM glutamate to 15 additional cells and found that peak ΔF/F from these cells was 4.5 ± 0.6, much larger than the 2.0 ± 0.3 ΔF/F average peak 10 μM glutamate signal, confirming the lack of dye saturation.
It is possible that the low-affinity indicator may not reveal differences in the decay phase of glutamate-induced Ca2+ rises, where the Ca2+ concentration falls below detection limits of the dye. Therefore, we examined decay t1/2 values using the high-affinity indicator Fluo4-AM. As expected, the decays using the high-affinity dye were slower than those observed with the low-affinity dye, reflecting the sensitivity to low Ca2+ concentrations. However, there was still no difference between depolarization-preconditioned neurons and control-preconditioned neurons (t1/2 = 17.4 ± 3.2 s for control cells, t1/2 = 15.1 ± 2.2 s for depolarization preconditioned cells, p > 0.05, n = 18 and 21 cells per respective condition).
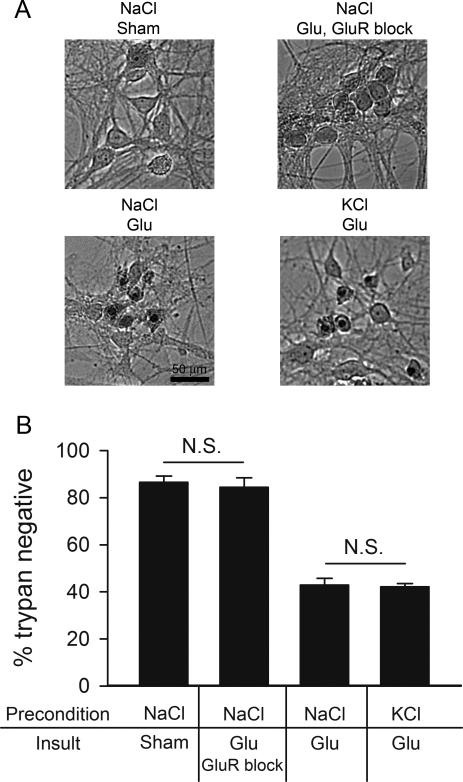
In a final test of whether preconditioning recruited postsynaptic adaptations, we evaluated the effect of depolarization preconditioning on toxicity induced by exogenous glutamate. This experiment exploited the pivotal role of glutamate receptor overstimulation in hypoxic cell death (Choi and Rothman, 1990). Direct overstimulation of postsynaptic receptors by-passes endogenous glutamate release contributing to hypoxic damage. Thus, if depolarization preconditioning acts through presynaptic mechanisms, it should be ineffective against exogenous glutamate toxicity. If, however, preconditioning works through postsynaptic mechanisms, it should retain effectiveness and protect against direct glutamate excitotoxicity. We found that depolarization preconditioning was not effective in protecting neurons against exogenously applied glutamate (Fig. 3). In control cultures preconditioned with 30 mM NaCl, application of 10 μM glutamate for 5 min killed over 60% of neurons, evaluated 24 hr post-insult (Fig. 3). This was indistinguishable from cell loss in cultures preconditioned with depolarizing KCl (Fig. 3). Glutamate-induced cell loss following control preconditioning was similar to or slightly milder than that achieved in our hypoxia model. This result strongly suggests that the neuroprotection from hypoxia by strong depolarization preconditioning is of presynaptic origin, consistent with a potential role for presynaptic muting.
Figure 3.
Exogenous glutamate toxicity is unaffected by depolarization preconditioning. A. Brightfield photomicrographs of trypan blue-stained fields from the indicated conditions in a single experiment. Exogenous glutamate (Glu; 10 μM for 5 min) replaced hypoxia in the preconditioning/insult paradigm. Upper left: control cells 24 hr post-preconditioning with 30 mM NaCl and sham glutamate exposure that included media exchange. Upper right: protection control cells preconditioned with 30 mM NaCl, subjected to 10 μM glutamate (5 min) with ionotropic GluR antagonists present (1μM NBQX + 100μM D-APV). Lower left: cells preconditioned with 30 mM NaCl, subjected to 10 μM glutamate for 5 min. Lower right: cells preconditioned with 30 mM KCl, then subjected to 5 min of 10 μM glutamate. B. Summary graph of the conditions shown in A (n=5). Glutamate significantly increased trypan blue staining compared with the NaCl sham condition in this dataset (p < 0.05, Bonferroni corrected t-test). However, depolarization preconditioning did not significantly alter the severity of cell loss. As with hypoxia, GluR block (1 μM NBQX, 100 μM D-APV) effectively protected neurons from glutamate-induced death, consistent with the pivotal role of excitotoxicity in both insults.
Preconditioning protection extends to oxygen/glucose deprivation
Figure 1 shows that depolarization preconditioning protects against a 2.5 hr hypoxic insult, which normally kills approximately 70% of neurons in our paradigm. Previous studies in cortical neurons have shown that deprivation of both oxygen and glucose may be a more severe insult than hypoxia alone (Goldberg and Choi, 1993). We wanted to test whether this potentially more severe insult is also attenuated by presynaptic muting induced by depolarization preconditioning. As in the hypoxia paradigm we exposed cells to 4 hr of high KCl (30 mM) or 4 hr of a non-depolarizing NaCl control. After preconditioning, the neurons were removed from the preconditioning stimulus and immediately subjected to OGD for 2.5 hr. The cells were then returned to their original media and were allowed to incubate under normal conditions for 24 hr, after which we performed trypan blue staining. As expected, OGD for 2.5 hr killed more cells than hypoxia alone (compare Fig. 4 to Fig. 1). Nevertheless, as with the milder insult, depolarization preconditioning significantly protected neurons from the more severe OGD insult (Fig. 4).
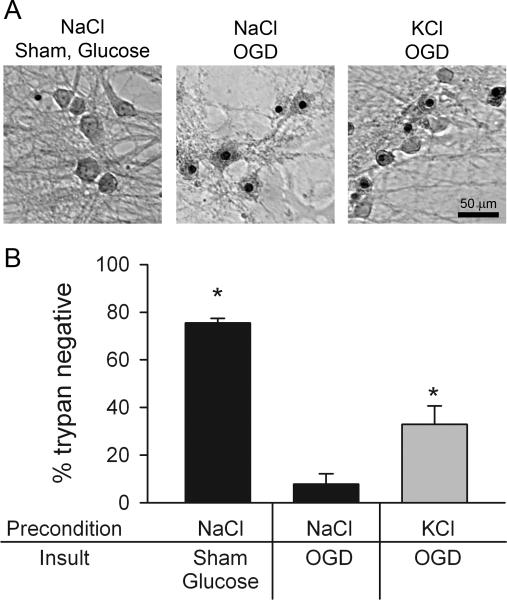
Figure 4.
Depolarization preconditioning protects against oxygen-glucose deprivation (OGD). A. Brightfield micrographs of trypan blue staining from each condition: (left to right) control dish 24 hr post-preconditioning with 30 mM NaCl and sham OGD; dish preconditioned with 30 mM NaCl, subjected to OGD for 2.5 hr; dish preconditioned with 30 mM KCl, then subjected to OGD for 2.5 hr. B. Summary graph showing protection afforded by KCl depolarization preconditioning (n=4). Asterisks denote p < 0.05 compared with the NaCl OGD condition (Bonferroni corrected t-tests). Gray bar emphasizes the major hypothesized result of KCl preconditioning protection.
Depolarization preconditioning protection does not involve GABAA receptor activation
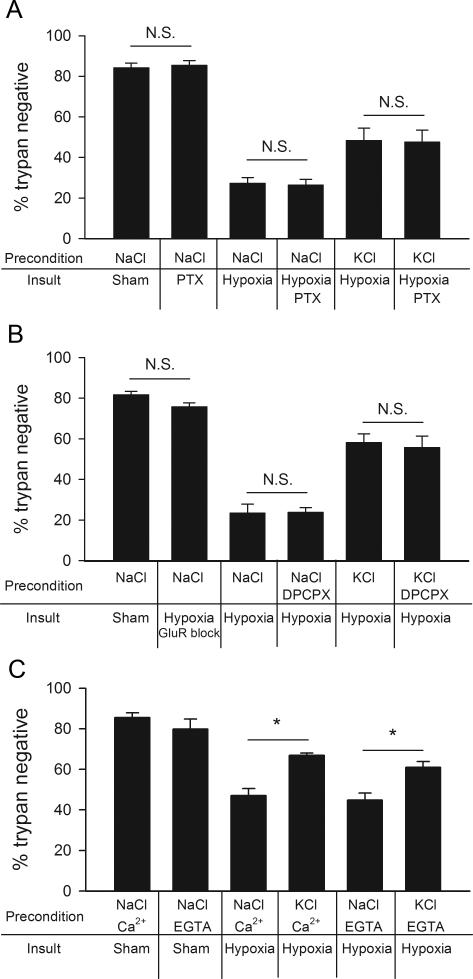
Previous work has suggested that a primary mechanism by which cortical neurons are desensitized to hypoxic damage after a depolarizing preconditioning stimulus is through enhancement of GABAA receptor activity during subsequent lethal ischemia (Grabb et al., 2002). On the other hand, some studies have suggested a more limited contribution of GABAA receptor activation in other forms of preconditioning (Lange-Asschenfeldt et al., 2005). Studies conducted in our lab have shown that muting is induced at glutamate synapses, but not GABA synapses, by strong depolarization (Moulder et al., 2004). Therefore, if we are correct that glutamate presynaptic muting is responsible for neuroprotection in our preconditioning paradigm, we expect no role for GABA in the protection. To test whether neuroprotection in our paradigm is dependent on GABAA receptor activity during hypoxia (Grabb et al., 2002), we preconditioned neurons, followed by blockade of GABAA activity during the insult. The non-competitive GABAA receptor antagonist picrotoxin (100 μM) was used to circumvent the possibility that competitive antagonists might be overwhelmed by endogenous GABA release. Blockade of GABAA receptors during hypoxia did not affect cell survival in our paradigm (Fig. 5A), and picrotoxin did not affect the protection afforded by KCl preconditioning (Fig. 5A). These results support the idea that reduction in presynaptic glutamate release, rather than altered GABA signaling, is key to the preconditioning protection from strong depolarization.
Figure 5.
Depolarization preconditioning protection from hypoxia does not depend upon GABAA receptor modulation, adenosine A1 receptor activation, or extracellular Ca2+ influx. A. The non-competitive GABAA antagonist picrotoxin (PTX ;100 μM), applied during hypoxia (2.5 hr), did not prevent depolarization protection. Summary of the indicated experimental conditions (n=6). “N.S.” indicates lack of significant difference in comparisons of the effect of PTX with the corresponding condition in the absence of PTX. B. The A1 receptor antagonists DPCPX (200 nM) did not block depolarization preconditioning protection. Summary of various 4 hr preconditioning conditions. Hypoxic insult was 2.5 hr (n=5). “N.S.” indicates a lack of difference for the indicated comparisons. C. Summary graph showing protection afforded by KCl depolarization preconditioning (n=5) independent of the addition of 1.8 mM extracellular Ca2+ to the Ca2+ free conditioning media. For all panels, neuronal survival was assessed 24 hr after insult with trypan blue exclusion. Asterisks denote p < 0.05 comparisons (Bonferroni corrected t-tests).
Depolarization preconditioning protection does not involve A1 receptors
A1 adenosine receptor activation may contribute to ischemic preconditioning tolerance (Nakamura et al., 2002; Perez-Pinzon et al., 1996) and selectively affect glutamate transmission in the hippocampus (Yoon and Rothman, 1991). Blockade of A1 adenosine receptors, however, does not abolish depolarization-induced presynaptic muting of glutamate terminals by high KCl exposure (Crawford et al., 2011). To distinguish depolarization-induced muting from adenosine-dependent forms of preconditioning, we co-administered the A1 adenosine receptor blocker 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX; 200 nM) during depolarization preconditioning. As before, the cells were subsequently challenged with 2.5 hr of hypoxia. The neuroprotective effects of elevated KCl were not altered by A1 receptor inhibition (Fig. 5B). This suggests that in our paradigm, unlike other forms of preconditioning (Nakamura et al., 2002; Perez-Pinzon et al., 1996), the protection from hypoxia by presynaptic silencing of glutamate release is unlikely to involve A1 adenosine receptor activation during preconditioning.
Depolarization preconditioning protection from hypoxia does not require Ca2+ influx
A unique aspect of the induction of presynaptic silencing by strong depolarization is that it is Ca2+-independent, unlike most other forms of synaptic plasticity (Crawford et al., 2011). Therefore, as an additional test of the involvement of presynaptic muting in preconditioning protection, we preconditioned cells with KCl in the absence of extracellular Ca2+ (plus 0.1 mM EGTA to chelate residual Ca2+; Fig. 5C). Removing Ca2+ did not affect the ability of depolarization preconditioning to protect from hypoxic excitotoxicity (compare Fig. 5C with Fig. 1C). These results demonstrate a signature feature of presynaptic muting and are additional support for the idea that muting plays a strong role in preconditioning protection. Furthermore, the finding excludes Ca2+-dependent secretion of neurotransmitters and neuromodulators during preconditioning in the protection.
Proteasome inhibition prevents depolarization preconditioning protection
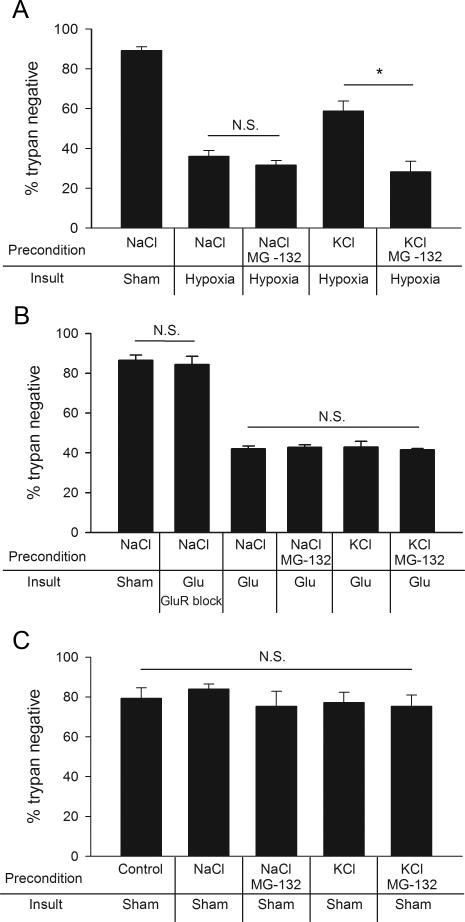
The UPS is involved in the regulation of synaptic function, including presynaptic development and function (Haas and Broadie, 2008; Hegde and DiAntonio, 2002; Willeumier et al., 2006). Recently a postsynaptic UPS-dependent mechanism induced by N-Methyl-D-aspartic acid (NMDA) receptor activation was implicated in rapid ischemic tolerance, a neuroprotective effect of subtoxic conditioning ischemia (Meller et al., 2008). Proteasome inhibition also prevents the induction of presynaptic silencing (Jiang et al., 2010). If presynaptic muting is involved in depolarization preconditioning-induced neuroprotection, proteasome inhibition should reverse the protection from depolarization preconditioning. To investigate the role of the UPS in preconditioning neuroprotection, we co-applied the proteasome inhibitor MG-132 (3 μM) during the preconditioning period of high KCl (30 mM) exposure. Protection from hypoxia after the high KCl preconditioning period was completely abolished by proteasome inhibition during preconditioning (Fig. 6A). Taken together with preceding evidence (Fig. 1-3) for a presynaptic locus of KCl neuroprotection, this result is consistent with the idea that UPS-dependent presynaptic silencing induced prior to the insult protects neurons from hypoxia. Because D-APV and NBQX were present in preconditioning solutions, it is unlikely that MG-132 acts through these postsynaptic receptor systems to reverse the presynaptic protection afforded by depolarization. Furthermore, when we by-passed presynaptic terminals and killed neurons by direct exogenous glutamate exposure, there was no difference in survival following control preconditioning, KCl preconditioning, MG-132 preconditioning, or a combination of KCl and MG-132 preconditioning (Fig. 6B). Although the effects on the UPS from hypoxia are likely complex and operate in both presynaptic and postsynaptic compartments, our results suggest that MG-132 reverses protection in our model by a presynaptic mechanism induced during preconditioning, upstream of postsynaptic receptor overstimulation. Control experiments also verified that neither KCl nor MG-132 affected cell survival in the absence of insult. A 4 hr application of 30 mM KCl or 3 μM MG-132 alone was not toxic to the neurons (Fig. 6C).
Figure 6.
Proteasome inhibition prevents depolarization preconditioning protection through a presynaptic mechanism. A. Summary of neuronal survival after several 4 hr preconditioning conditions and 2.5 hr hypoxic exposure (n=5). MG-132 (3 μM, co-applied for 4 hr with 30 mM KCl) prevented the protective effect of depolarization. Indicated comparisons represent the major hypothesized effects (asterisk denotes p < 0.05 with Bonferroni correction). Other comparisons that showed a significant difference from insult alone (NaCl hypoxia) were the NaCl sham control and the KCl hypoxia conditions. There was no difference between the NaCl hypoxia condition and NaCl hypoxia plus MG-132. B. Summary of cell survival after indicated preconditioning conditions followed by an insult of exogenously applied glutamate (Glu; 10 μM for 5 min). There was no effect of KCl or of MG-132 on cell survival using the glutamate insult (n=5), suggesting a presynaptic mechanism of depolarization preconditioning protection. No comparison of the NaCl/glutamate group with any of the other indicated groups yielded a significant difference. Glutamate treatment caused significant death relative to control (p < 0.05, Bonferroni corrected t-test for multiple comparisons). C. Control experiment testing direct effects of KCl and MG-132 on cell survival during various 4 hr preconditioning paradigms in the absence of hypoxia (n=5). No comparison with the control sham condition exhibited a significant difference (unpaired t-tests vs. control). For all experiments depicted in panels A, B, and C neuronal survival was evaluated with trypan blue 24 hr after the insult.
Hypoxia induces muting
The preceding results establish that, in principle, muting of presynaptic glutamate release induced prior to insult is capable of protecting neurons from subsequent hypoxic/OGD excitotoxicity, but do neurons employ muting of glutamate synapses during the insult itself? Depolarization of neurons is an important early consequence of hypoxic/ischemic insult and is reinforced by subsequent glutamate release and receptor activation (Moskowitz et al., 2010). If depolarization is rapid and strong enough to induce muting, presynaptic silencing may be an important endogenous regulator of damage threshold. To investigate this, we used FM1-43fx uptake to assess presynaptic function immediately after a 2 hr hypoxic event, without any preceding preconditioning. As shown in Figure 2, muted glutamate terminals immunolabel with an antibody against the vesicular glutamate transporter 1 (vGluT-1) but not with the activity-dependent FM dye (Moulder et al., 2004). Hypoxia exposure clearly increased the number of mute synapses (Fig. 7). The muting induced by hypoxia was completely prevented by the inclusion of MG-132 (3 μM) during the hypoxic challenge (Fig. 7B), suggesting that hypoxia and depolarization activate the same proteasome-dependent signaling cascade. As previously observed (Crawford et al., 2011; Jiang et al., 2010), MG-132 had no effect on the basal percentage of silent terminals (Fig. 7B). Further, we failed to find a significant effect of MG-132 on overall glutamate synapse density (97.8 ± 6.5 vGluT-1 positive synapses per field in control, 117.0 ± 17.2 puncta mm2 after MG-132 treatment, n = 5 experiments, p > 0.3). Finally, in two previously published papers by our group, we failed to detect any effect of MG-132 alone on excitatory postsynaptic currents (Crawford et al., 2011; Jiang et al., 2010). These results offer direct evidence that proteasome-dependent presynaptic silencing occurs during hypoxia and suggest that presynaptic muting is an endogenous cellular defense mechanism that may reduce damage even without prior preconditioning designed to invoke synaptic muting.
Proteasome inhibition exacerbates neuronal death during a hypoxic insult
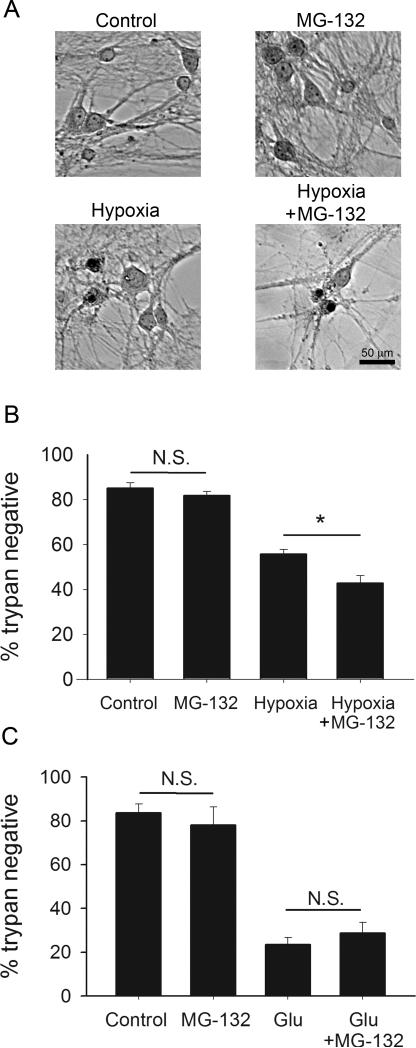
To test directly whether proteasome-dependent presynaptic muting observed in Figure 7 reduces the damage wrought by a moderate hypoxic insult, we tested the effect of proteasome inhibition on neuronal loss induced by hypoxia. We co-applied MG-132 during a 2 hr hypoxic event (Fig. 8A). We chose an insult that would provide approximately 50% neuronal death in order to ensure detection of either protection or exacerbation of cell death. MG-132 during hypoxia significantly increased neuronal death (Fig. 8B). Again, MG-132 did not affect neuronal death when applied to neurons for a total of 4 hr without hypoxia (Fig. 6C, 8B). Further, we found that MG-132 preincubation, followed by co-incubation with 10 μM glutamate to bypass presynaptic effects, did not alter direct, glutamate induced death (Fig. 8C). These results are consistent with a presynaptic action of MG-132. Taken together, the results of Figures 7 and 8 suggest that presynaptic silencing raises the threshold for neurotoxicity during a depolarizing insult.
Figure 8.
Proteasome inhibition during hypoxia exacerbates neuronal damage. A. Brightfield trypan blue-stained fields from a representative experiment. Neurons were incubated with or without MG-132 for 2 hr to allow drug penetration, then subsequently co-incubated with MG-132 for additional 2 hr with or without hypoxia. Culture media was then exchanged, and cells were incubated normally for 24 hr after which trypan blue staining was performed. Upper left: control with no MG-132 or hypoxia. Upper right: 4 hr total MG-132 exposure (no hypoxia). Lower left: 2 hr hypoxia alone. Lower right: 2 hr MG-132 followed by hypoxia/MG-132 co-incubation for 2 hr (4 hr total exposure to MG-132). B. Summary of A showing that MG-132 exacerbates hypoxia-induced death (n=7). Asterisk denotes p < 0.05 (Bonferroni corrected t-test). C. Summary of control for postsynaptic effects of MG-132 (n = 5 experiments). 10 μM glutamate (Glu) treatment was used as a surrogate for hypoxic insult. There was no significant exacerbation of glutamate-induced damage by MG-132. As with hypoxia experiments in B, cells were pre-incubated in MG-132 for 2 hr prior to insult.
Discussion
We have shown that a form of synaptic plasticity working through a presynaptic UPS-dependent mechanism significantly protects neurons from in vitro hypoxic/ischemic insults. Importantly, muting is endogenously invoked by hypoxia with no prior conditioning to limit the damage severity inflicted during a moderate insult. Protection is achieved by invoking an endogenous mechanism that mutes vesicular glutamate release at presynaptic terminals. Among a growing body of research into preconditioning and tolerance (Gidday, 2006; Sapolsky, 2001), presynaptic muting is a previously unknown UPS-dependent mechanism of tolerance. Because muting is part of a native cellular defense mechanism in response to stressful depolarization, it might be a good target for future therapeutics. Our insights into this UPS-dependent mechanism demonstrate presynaptic regulation of neuronal homeostasis and suggest new approaches that may benefit disorders involving glutamatergic synaptic dysfunction.
Presynaptic muting appears particularly well suited as an adaptation against strong insults like hypoxia/ischemia. Strong depolarization and associated sustained Ca2+ influx during these insults are likely to quickly overwhelm many presynaptic adaptive mechanisms, such as G-protein-mediated decreases in presynaptic Ca2+ influx (Brown and Sihra, 2008). Because muting effectively eliminates vesicle release competence to Ca2+ and other secretagogues (Moulder et al., 2006; Moulder et al., 2004), muting should be especially effective at disrupting the cycle of pathological depolarization during the insult. On the other hand, we acknowledge that strategies designed to disrupt glutamate signaling have to date proved disappointing in clinical settings (Gidday, 2006; Lipton, 2007; Moskowitz et al., 2010), and presynaptic muting may be ineffective during strong insults that recruit non-synaptic glutamate release (Rossi et al., 2000).
Proteasome inhibition exacerbated damage and also inhibited presynaptic silencing in response to hypoxic insult (Fig. 7 and Fig. 8). Proteasome activity is increased during strong depolarization, and proteasome inhibition also prevents presynaptic silencing during strong depolarization (Jiang et al., 2010). In the present study, proteasome inhibition prevented the neuronal protection from strong depolarization followed by hypoxia (Fig. 6A). Similar exacerbation of cell death was recently described in an ischemia model, where an NMDA receptor and UPS-dependent postsynaptic remodeling mechanism was implicated in a rapid ischemic tolerance (Meller et al., 2008). These previous results fit with known UPS roles in the postsynaptic compartment (Mabb and Ehlers, 2010; Tai and Schuman, 2008). On the other hand, the UPS is also important for aspects of presynaptic development and function (DiAntonio and Hicke, 2004; Jiang et al., 2010; Willeumier et al., 2006). Our observations fit more closely with this latter literature. Postsynaptic NMDA receptor activation is not required for our preconditioning protection, as D-APV was present in all preconditioning treatments in our experiments to block NMDA receptor-dependent forms of plasticity. Furthermore, a postsynaptic mechanism of protection is excluded by our experiments examining preconditioning effects on exogenous glutamate damage and postsynaptic Ca2+ handling in response to exogenously applied glutamate after preconditioning (Fig. 2C-E, Fig. 3, and Fig. 6B).
Our experiments were designed to isolate presynaptic contributions to self-defense mechanisms and therefore do not negate contributions of previously described postsynaptic mechanisms of adaptive neuroprotection under some conditions. These mechanisms may be most important in the most severe insults where non-synaptic glutamate release is recruited (Rossi et al., 2000). Further work is needed to elucidate the relative importance of presynaptic silencing and other adaptive, self protective mechanisms.
Although UPS-dependent neuroprotection in our model is mainly presynaptic and distinct from previously described UPS-dependent and NMDA-dependent forms of tolerance (Grabb and Choi, 1999), it is possible that complex changes in UPS function accompany insult. Although proteasome inhibition has been reported following brain ischemia and is associated with ATP depletion (Asai et al., 2002; Thompson et al., 2008), this does not exclude local persistence of or increases in UPS activity in certain tissue compartments, specific cell types, or specific subcellular compartments. We expect that UPS-dependent presynaptic muting will be particularly relevant to glutamate terminals in the penumbra of an insult where a certain degree of neuronal function is preserved.
Our results demonstrating a role of the UPS in presynaptic muting and cell survival (Fig. 7 and Fig. 8) also exclude a possible alternative explanation for the muting observed in response to hypoxia: cell death. Although cell loss was not evident for several hours after the hypoxic insult and there was little evidence of presynaptic damage at the time of synaptic imaging (Fig. 7), the presynaptic silencing observed might be a trivial result of dying neurons rather than a neuroprotective response of viable neurons. The effect of MG-132 clearly argues against this possibility. MG-132 application during hypoxia prevented muting (Fig. 7) but exacerbated neuronal damage (Fig. 8). This pattern of results excludes damage as a cause of muting and strongly suggests that presynaptic function is an important determinant of subsequent cell loss.
Additional study of the pathways responsible for presynaptic muting may guide strategies for therapeutic exploitation of these pathways. Upstream targets may include pertussis toxin-sensitive Gi/o-linked G-protein receptors (Crawford et al., 2011). Downstream effectors may include relevant targets of the UPS (Jiang et al., 2010). Depolarization-induced muting results in decreased levels of the vesicle priming proteins Rim1α and Munc13-1, and overexpression of Rim1α prevents presynaptic silencing (Jiang et al., 2010). However, the proteasome also potentially regulates a host of other synaptic proteins such as SNAP-25, syntaxin, and synaptophysin (Chin et al., 2002; Ma et al., 2005; Wheeler et al., 2002; Willeumier et al., 2006) among many other candidates, befitting the ubiquitous nature of the UPS system. Targeting the specific proteins responsible for muting may offer a viable, selective therapeutic strategy.
Conclusions
With these results we provide new insight into a homeostatic neuronal defense mechanism at glutamatergic presynaptic terminals. Pharmacological exploitation of a mechanism reducing presynaptic glutamate release could potentially treat excitotoxic damage resulting from stroke, neonatal hypoxia, epilepsy, and head trauma at the time of injury, where treatment options are quite limited and postsynaptic interventions have failed. Presynaptic muting appears to render presynaptic terminals incompetent to exocytose even when challenged with sustained Ca2+ rises. . The mechanism we report here does not require lengthy latent periods for its induction and is immediately available as a homeostatic mechanism. Additional study of presynaptic muting will increase our basic understanding of hippocampal synaptic function. Further study may also identify potential treatment targets related to muting that could benefit various neurological and psychiatric disorders involving glutamate dysfunction.
Acknowledgements
This work was supported by National Institutes of Health grants DA07261 (J.H.), NS066611 (D.C.C.), MH78823 (S.M.), and NS54174 (S.M). We thank Ann Benz and Amanda Taylor for assistance with cultures and members of the Zorumski and Mennerick labs for comments and discussion. We thank Kris Hyrc for the gift of Fluo3-FF aliquots.
Non-standard abbreviations
- GABA
γ-aminobutyric acid
- OGD
Oxygen-glucose deprivation
- NBQX
2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxyline
- D-APV
D-2-Amino-5-phosphonovalerate
- MG-132
Carbobenzoxy-L-leucyl-L-leucyl-L-leucinal
- AM
Acetoxymethyl
- vGluT-1
Vesicular glutamate transporter 1
- DPCPX
8-Cyclopentyl-1,3-dipropylxanthine
- UPS
Ubiquitin-proteasome system
- NMDA
N-Methyl-D-aspartic acid
- Glu
Glutamate
- GluR
Glutamate receptor
- t1/2
half-time
- F
Fluorescence
- ΔF
Change in fluorescence
- PTX
Picrotoxin
- N.S. (Not significant)
used in summary figure panels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asai A, et al. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2002;22:705–10. doi: 10.1097/00004647-200206000-00009. [DOI] [PubMed] [Google Scholar]
- Brown DA, Sihra TS. Presynaptic signaling by heterotrimeric G-proteins. Handb. Exp. Pharmacol. 2008;184:207–60. doi: 10.1007/978-3-540-74805-2_8. [DOI] [PubMed] [Google Scholar]
- Chin LS, et al. Staring, a novel E3 ubiquitin-protein ligase that targets syntaxin 1 for degradation. J. Biol. Chem. 2002;277:35071–9. doi: 10.1074/jbc.M203300200. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci. Lett. 1985;58:293–7. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987;7:369–79. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 1990;13:171–82. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Crawford DC, et al. Calcium-independent inhibitory G-protein signaling induces persistent presynaptic muting of hippocampal synapses. J. Neurosci. 2011;31:979–91. doi: 10.1523/JNEUROSCI.4960-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu. Rev. Neurosci. 2004;27:223–46. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006;7:437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993;13:3510–24. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J. Neurosci. 1999;19:1657–62. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabb MC, et al. Preconditioned resistance to oxygen-glucose deprivation-induced cortical neuronal death: alterations in vesicular GABA and glutamate release. Neuroscience. 2002;115:173–83. doi: 10.1016/s0306-4522(02)00370-6. [DOI] [PubMed] [Google Scholar]
- Haas KF, Broadie K. Roles of ubiquitination at the synapse. Biochim. Biophys. Acta. 2008;1779:495–506. doi: 10.1016/j.bbagrm.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN, DiAntonio A. Ubiquitin and the synapse. Nat. Rev. Neurosci. 2002;3:854–61. doi: 10.1038/nrn961. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. A role for the ubiquitin-proteasome system in activity-dependent presynaptic silencing. J. Neurosci. 2010;30:1798–809. doi: 10.1523/JNEUROSCI.4965-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, et al. ‘Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res. 1991;561:203–11. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, et al. Ischemic tolerance induction in organotypic hippocampal slices: role for the GABAA receptor? Neurosci. Lett. 2005;384:87–92. doi: 10.1016/j.neulet.2005.04.053. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat. Rev. Neurosci. 2007;8:803–8. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Ma Z, et al. Evidence that insulin secretion influences SNAP-25 through proteasomal activation. Biochem. Biophys. Res. Commun. 2005;329:1118–26. doi: 10.1016/j.bbrc.2005.02.086. [DOI] [PubMed] [Google Scholar]
- Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu. Rev. Cell Dev. Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, et al. Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J. Neurosci. 2008;28:50–9. doi: 10.1523/JNEUROSCI.3474-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, et al. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J. Neurophysiol. 1995;73:320–32. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, et al. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, et al. A specific role for Ca2+-dependent adenylyl cyclases in recovery from adaptive presynaptic silencing. J. Neurosci. 2008;28:5159–68. doi: 10.1523/JNEUROSCI.5317-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, et al. Physiological activity depresses synaptic function through an effect on vesicle priming. J. Neurosci. 2006;26:6618–26. doi: 10.1523/JNEUROSCI.5498-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, et al. Plastic elimination of functional glutamate release sites by depolarization. Neuron. 2004;42:423–35. doi: 10.1016/s0896-6273(04)00184-9. [DOI] [PubMed] [Google Scholar]
- Mozhayeva MG, et al. Development of vesicle pools during maturation of hippocampal synapses. J. Neurosci. 2002;22:654–65. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, et al. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–82. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nakamura M, et al. Rapid tolerance to focal cerebral ischemia in rats is attenuated by adenosine A1 receptor antagonist. J. Cereb. Blood Flow Metab. 2002;22:161–70. doi: 10.1097/00004647-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitotoxicity, apoptosis and neuropsychiatric disorders. Curr. Opin. Pharmacol. 2003;3:101–9. [PubMed] [Google Scholar]
- Olney JW, et al. Monosodium glutamate effects. Science. 1971;172:294. doi: 10.1126/science.172.3980.294. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, et al. Anoxic preconditioning in hippocampal slices: role of adenosine. Neuroscience. 1996;75:687–94. doi: 10.1016/0306-4522(96)00311-9. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, et al. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–21. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothman S. Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J. Neurosci. 1984;4:1884–91. doi: 10.1523/JNEUROSCI.04-07-01884.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Cellular defenses against excitotoxic insults. J. Neurochem. 2001;76:1601–11. doi: 10.1046/j.1471-4159.2001.00203.x. [DOI] [PubMed] [Google Scholar]
- Shute AA, et al. Astrocytes exert a pro-apoptotic effect on neurons in postnatal hippocampal cultures. Neuroscience. 2005;131:349–58. doi: 10.1016/j.neuroscience.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat. Rev. Neurosci. 2008;9:826–38. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- Thompson SJ, et al. Ubiquitin-proteasome system as a modulator of cell fate. Curr. Opin. Pharmacol. 2008;8:90–5. doi: 10.1016/j.coph.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler TC, et al. Regulation of synaptophysin degradation by mammalian homologues of seven in absentia. J. Biol. Chem. 2002;277:10273–82. doi: 10.1074/jbc.M107857200. [DOI] [PubMed] [Google Scholar]
- Willeumier K, et al. Proteasome inhibition triggers activity-dependent increase in the size of the recycling vesicle pool in cultured hippocampal neurons. J. Neurosci. 2006;26:11333–41. doi: 10.1523/JNEUROSCI.1684-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KW, Rothman SM. Adenosine inhibits excitatory but not inhibitory synaptic transmission in the hippocampus. J. Neurosci. 1991;11:1375–80. doi: 10.1523/JNEUROSCI.11-05-01375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Olney JW. Excitotoxic neuronal damage and neuropsychiatric disorders. Pharmacol. Ther. 1993;59:145–62. doi: 10.1016/0163-7258(93)90043-d. [DOI] [PubMed] [Google Scholar]