Abstract
Patients with follicular lymphoma (FL) typically experience an indolent course, however, the disease is rarely curable with conventional chemotherapy. Autologous hematopoietic cell transplantation HCT can extend progression-free survival (PFS) and overall survival (OS) but relapse is the primary cause of failure. Allogeneic HCT confers lower relapse rates due to a graft vs lymphoma effect. Reduced intensity conditioning (RIC) allows allogeneic HCT to be done with lower toxicity. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducted a prospective multicenter trial comparing these 2 strategies in FL patients with relapsed, chemotherapy sensitive disease. Patients were assigned a treatment arm based on the availability of an HLA-matched sibling donor (MSD). Those with a MSD underwent allogeneic HCT (n=8) with the FCR preparative regimen (fludarabine, cyclophosphamide(Cy), rituximab(RTX)) and received tacrolimus and methotrexate for graft vs host disease(GVHD) prophylaxis. Patients without a MSD (n=22) underwent mobilization with Cy, RTX, and filgrastim and received a conditioning regimen of either CBV (Cy, carmustine, VP16) or total body irradiation with Cy and VP16. Autologous HCT patients received 4 doses of weekly maintenance RTX (375 mg/m2) starting at day+42 post autoHCT. Sixteen patients were in complete remission (CR), 10 patients were in partial remission (PR), and 1 had stable disease after salvage therapy and prior to HCT. Median follow-up was 36 months (range, 1–51 months). OS was 73% vs 100% and PFS was 63% vs 86%, after autologous versus allogeneic HCT respectively. No patients had grade 2–4 acute GVHD; 2 patients developed extensive chronic GVHD. Three autologous recipients died from non-relapse causes. This trial closed early due to slow accrual. We show that the FCR regimen is well tolerated and that both allogeneic and autologous HCT result in promising 3-year OS and PFS in patients with relapsed FL.
INTRODUCTION
Follicular NHL (FL) is the second most common type of non-Hodgkin's lymphoma with an incidence of ~15,000 new cases/year in the U.S. When treatment is indicated, most patients achieve a remission with initial chemotherapy. However, a continuous pattern of relapse typically occurs resulting in progressively shorter remission durations with patients invariably succumbing to their disease1.
Three randomized trials demonstrated that early intensive therapy including autologous hematopoietic stem cell transplantation (HCT) in patients with newly diagnosed FL or patients in first remission yielded high response rates but did not confer an improved overall survival compared to conventional chemotherapy in part due to the higher incidence of myelodysplastic syndrome (MDS) in the transplanted patients2–4. For FL patients with relapsed disease, one randomized trial, known as the `CUP' trial, showed a survival advantage for patients who received high dose chemotherapy compared to conventional therapy at relapse5. However, relapse/progression after autologous HCT continues to be the leading cause of treatment failure6.
Allogeneic HCT after myeloablative conditioning regimens is sometimes offered to patients with recurrent FL with the goal of harnessing a graft-versus-lymphoma effect and to circumvent the tumor cell contamination associated with autologous hematopoietic stem cell harvests7–10. Retrospective data indicate a significantly lower risk of relapse compared to autologous HCT but this benefit is invariably offset by the treatment-related mortality associated with this approach8–9.
Allogeneic HCT with reduced intensity conditioning (RIC) regimens are increasingly used with the goal of reducing non relapse mortality while still taking advantage of graft-versus-lymphoma effects. Results appear promising with event free survivals ranging from 51% – 85% in studies with follow-up times ranging from two to 6 years11–16. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducted a prospective study comparing the efficacy of autologous HCT vs RIC allogeneic HCT for FL patients beyond first complete response or first partial response. Treatment allocation was by biological assignment. Patients with an available HLA-matched sibling were assigned to the allogeneic HCT arm while patients without an HLA-matched sibling received autologous HCT followed by rituximab maintenance therapy. Unfortunately, due to slow accrual, this trial closed prior to completing enrollment. We now report the outcomes of the 30 patients enrolled on this multicenter trial.
Methods
Patients
Patients up to 75 years of age with histologically confirmed grade I or II REAL classification17 follicular non-Hodgkin lymphoma were eligible for enrollment if they were in first relapse or beyond. All patients were required to have chemotherapy sensitive disease defined as: 1) less than 20% marrow involvement; and, 2) either all lymph nodes smaller than 3 cm in axial diameter or demonstrate more than a 75% reduction in total lymph node volume by bi-dimensional measurements with the most recent chemotherapy. Patients were ineligible if they had received a prior autologous or allogeneic HCT or more than 4 prior treatment regimens, excluding involved-field radiation or single agent monoclonal antibodies. Patients with evidence of transformation were excluded.
Other eligibility criteria included adequate organ function defined as a cardiac ejection fraction of 45% or greater; total bilirubin less than twice the upper limit of normal (ULN); aspartate (AST) and alanine (ALT) serum transaminases less than thrice ULN; a creatinine clearance more than 40mL/min; and diffusion capacity of carbon monoxide, forced expiratory volume in 1 minute, and forced vital capacity all more than 50% of normal after adjustment for hemoglobin. Patients had to be seronegative for human immunodeficiency virus (HIV) and could not be pregnant or breast-feeding. Patients with uncontrolled infections, defined as progressing on appropriate antimicrobial therapy, were ineligible. Donors of allogeneic cells were ineligible if they were seropositive for Hepatitis B, C, or HIV infections; were pregnant or breast-feeding; or were otherwise medically ineligible to receive filgrastim (Neupogen, AMGEN, Thousand Oaks, CA) and undergo leukapheresis of peripheral blood progenitor cells.
Study Design
This was a multi-center biological assignment study18 in which patients with an HLA-identical sibling received a RIC allogeneic HCT and all other patients received autologous HCT after high-dose therapy. HLA match was defined as a minimum of low resolution match at HLA-A and HLA-B and high-resolution match at HLA-DRB1. The protocol and informed consents were approved by the Protocol Review Committee and Data and Safety Monitoring Board of the National Heart Lung and Blood Institute and the Institutional Review Boards of all participating institutions. All patients provided informed consent in accordance with the Declaration of Helsinki. This study is registered at http://www.clinicaltrials.gov as NCT00096460.
Study Treatment
Cytoreduction/Mobilization
All patients, regardless of planned autologous or allogeneic HCT, received chemotherapy with rituximab (Genentech, San Francisco, USA) 375mg/m2 intravenously on day 1 and day 8 and cyclophosphamide 4,000 mg/m2 intravenously on day 2 within 6 weeks following enrollment. Patients assigned to the autologous HCT arm received filgrastim10 mcg/kg/day subcutaneously as a single daily or divided twice daily starting two days after cyclophosphamide and continuing until leukapheresis was completed. Collection of a minimum of 1.0 × 106 CD34+ cells/kg (goal: 2.0 × 106 CD34+ cells/kg) was required to proceed to high dose chemotherapy. Patients with a matched sibling donor received filgrastim 5 mcg/kg/day after cyclophosphamide until the absolute neutrophil count (ANC) was above 500/mm3 for 3 days.
Autologous HCT Conditioning
Patients received either a total body irradiation (TBI) based conditioning regimen or a chemotherapy only conditioning regimen. Centers declared the conditioning regimen at the time of study activation. Patients 60 years and older received only a chemotherapy based regimen.
Conditioning was either with: 1) BCNU 15mg/kg (not to exceed 550mg/m2) on day −6, VP-16 60mg/kg on day −4, and cyclophosphamide 100mg/kg on day −2 [n = 6]; or 2) fractionated TBI 1200 cGy on day −8, −7, −6, and −5, VP-16 60mg/kg on day −4, and cyclophosphamide 100mg/kg on day −2 [n = 14]. Patients then received autologous peripheral blood progenitor cell infusion on day 0 and then filgrastim 5 mcg/kg/day starting on day +5 until the ANC was above 500/mm3 for 3 days. Patients received rituximab maintenance therapy (dose 375 mg/m2/day intravenously) weekly for 4 doses starting between day +42 and day +75 following autologous HCT provided they had adequate renal and liver function tests; were fully recovered from mucositis; and did not have active cytomegalovirus (CMV) or fungal infections.
Allogeneic HCT Conditioning
Patients received conditioning with both fludarabine 30mg/m2/day intravenously and cyclophosphamide 750mg/m2/day intravenously on day −6, −5, and −4, and rituximab 375mg/m2/day intravenously on days −13, −6, +1, and +8. Peripheral blood progenitor cells were infused on day 0.
All sibling donors received filgrastim 16mcg/kg/day subcutaneously for 5 doses. Leukapheresis commenced on day −1 of the recipient's conditioning regimen and continued until at least 2 × 106 CD34+ cells/kg were collected, or a maximum of three aphereses. If fewer than 1 × 106 CD34+ cells/kg were collected after the third leukapheresis, the patient was managed at the discretion of the treating physician. Although this did not occur, these patients were to be removed from the trial therapy and followed for relapse, progression, and survival.
Graft –versus – Host Disease (GVHD) Prophylaxis after Allogeneic HCT
Tacrolimus 0.9 mg/kg/day orally, based on actual body weight, was initiated on day −2 with adjustment to maintain a serum trough level of 5 – 15 ng/mL and methotrexate 5mg/m2/day intravenously on day +1, +3, and +6 were administered for GVHD prophylaxis. A taper of tacrolimus was initiated on day +90 and completed by day +180 unless grade 3 or 4 acute GVHD developed.
Supportive Care
All patients received antimicrobial prophylaxis and blood product support in concordance with the BMT CTN Manual of Procedures19. Use of hematopoietic growth factors following HCT, supplemental intravenous immune globulin, and immunizations were administered per institutional guidelines.
Follow-up and Disease Response
Disease response was assessed based on the Cheson criteria20. Prior to initiation of cytoreduction/mobilization chemotherapy, all patients had disease staging with CT scans and a bone marrow biopsy. These tests were repeated within two weeks prior to initiation of conditioning. Patients receiving an autologous HCT were restaged prior to the initiation of rituximab maintenance. Thereafter, all patients, regardless of donor source were restaged at 12 weeks, 6 months, 1 year, 2 years, and 3 years following HCT.
Additional assessments for toxicity and GVHD were performed per protocol requirements. Specifically, toxicity was assessed at 4 weeks following the rituximab administered during the cytoreduction/mobilization phase of the study, as well as at 4, 8, and 12 weeks following HCT. Additional toxicity assessments occurred at 6 months following HCT and every 6 months thereafter until 3 years after HCT. All toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v321. GVHD was assessed in patients receiving allogeneic HCT weekly from day 0 through 14 weeks post-HCT and then at 6 months, 1 year, 2 years, and 3 years following transplantation. Acute GVHD and chronic GVHD were scored using consensus criteria22–23.
Statistical Analysis
The primary objective of this trial was to compare progression-free survival (PFS) at 3 years between patients receiving high-dose therapy with autologous HCT and those with an HLA-identical sibling receiving a RIC allogeneic HCT. It was estimated that 80 patients would be accrued to the allogeneic HCT arm and between 250 and 400 patients accrued to the autologous HCT arm in the same time period, based on the likelihood of having a suitable sibling donor. This study was terminated early due to slow accrual and the analysis is limited to descriptive outcomes of the two arms without comparisons or multivariate analyses. The cumulative incidence function with competing risks analysis was used to estimate relapse and transplant related mortality (TRM)24. TRM is defined as death without evidence of disease relapse or progression. Estimates of overall survival (OS) and progression-free survival (PFS) used the Kaplan-Meier method25.
Results
PATIENT CHARACTERISTICS
A total of 30 patients were enrolled on this trial between August 2004 and February 2006. Of these, 8 (27%) patients had an HLA-identical sibling and were assigned to the allogeneic HCT arm and 22 to the autologous HCT arm. Three patients (10%) did not receive transplant: 2 patients (1 autologous, 1 allogeneic) withdrew consent prior to the start of any therapy; 1 patient on the autologous arm failed to collect the minimum 1 × 106 CD34+ cells/kg in three leukapheresis procedures. Pertinent patient characteristics are shown in Table 1.
Table 1.
Characteristics of patients enrolled on the BMT CTN 0202 clinical trial by treatment arm
| Autologous (n = 22) | Allogeneic (n = 8) | |
|---|---|---|
| Age, years; median (range) | 50 (36 – 66) | 48 (40 – 64) |
|
| ||
| Gender, male; n (%) | 10 (45) | 5 (63) |
|
| ||
| KPS; n (%) | ||
| 100% | 8 (36) | 1 (13) |
| 90% | 12 (55) | 5 (63) |
| ≤ 80% | 2 (9) | 2 (25) |
|
| ||
| Disease status at enrollment; n (%) | ||
| Relapse | 2 (9) | 0 |
| First | 1 (5) | 0 |
| Second | 1 (5) | 0 |
| Third | ||
| Partial Remission | 8 (36) | 3 (38) |
| Second | 3 (14) | 1 (13) |
| Third | ||
| Complete Remission | 6 (27) | 3 (38) |
| Second | 1 (5) | 1 (13) |
| Third | ||
|
| ||
| Number of prior therapies | 2 (1 – 3) | 2 (1–3) |
|
| ||
| Time from diagnosis to transplant, months; median (range) | 39.8 (9.8 – 110.5) | 28.9 (16.2 – 64.7) |
|
| ||
| Follow-up from transplant, months; median (range) | 36.1 (1.0 – 50.8) | 36.2 (33.2 – 48.8) |
ENGRAFTMENT AND CHIMERISM
Data on CD34+ cell dose infused and time to neutrophil engraftment was available in 19 patients receiving autologous HCT and all patients receiving allogeneic HCT. The median cell doses infused were 2.9 × 106 CD34 cells/kg and 6.8 × 106 CD34+ cells/kg for autologous and allogeneic transplant recipients, respectively. Neutrophil engraftment occurred at a median of 11 days (range, 9 – 14 days) after autologous HCT and at a median of 10 days (range, 5 – 13 days) for patients receiving allogeneic HCT.
Patients in the allogeneic HCT arm had chimerism assessments at day 28, day 56, day 84, 6 months, and 1 year post-transplant. At 28 days post transplant, a median of 88% (66 – 100) of cells were donor-derived; this increased to 95% (68 – 100) by day 84 and remained greater than 95% through the 1 year time-point. There were no engraftment failures.
RESPONSE TO TRANSPLANT
Disease status was collected at the time of enrollment (Table 1) as well as immediately prior to the transplantation. Among the 20 patients who received autologous HCT, 12 (60%) were in complete remission (CR), 7 (35%) had a partial remission (PR), and 1 (5%) patient had stable disease at the time of transplantation. At the time of allogeneic HCT, four patients were in CR (57%) and three patients were in PR (43%). Disease responses following transplantation were assessed at three time points during the first year. Disease response to therapy was not evaluable in 2 patients receiving autologous HCT due to death prior to day 28 post-transplant. Two patients in the autologous HCT arm—1 in CR and 1 in PR at transplant—had progression of disease as the best response to therapy. Following autologous HCT for either a PR or stable disease (n = 8), seven (88%) patients achieved a CR. Among the 3 patients receiving allogeneic HCT in PR, all obtained a CR.
TOXICITIES
Fifteen autologous HCT recipients (75%) and 4 allogeneic HCT recipients (57%) had grade 3 – 5 toxicities reported in the first year post-transplant. Toxicity events (Table 2) included elevations of liver enzymes and bilirubin, vascular leak, hemorrhage, pulmonary symptoms including pneumonitis, mucositis, and delayed neutropenia. Following autologous HCT, there was 1 case of fatal pneumonitis that occurred prior to day 28. The estimated cumulative incidences of transplant related mortality (TRM) following autologous HCT were 15% (95% CI: 0 – 31) at 1 year and 21.8% (95% CI: 0 – 35) at 3 years. There were no deaths following allogeneic HCT.
Table 2.
Summary of grade 3/4 toxicities reported during the first year following autologous and allogeneic transplant.
| Time-Period | Days 0 – 28 | Days 29 – 56 | Days 57 – 84 | Day 85 – 6 months | 6 months – 1 year | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Transplant Type | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo | Auto | Allo |
| Event | ||||||||||
| Increase ALT | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Increase AP | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Increase bilirubin | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Dyspnea | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 |
| Hypoxia | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Hemorrhage | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular Leak | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypotension | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Mucositis | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 1 | 0 | 4 | 0 | 2 | 0 | 1 | 0 | ||
ALT = alanine aminotransferase, AP = alkaline phosphatase Neutropenia was not considered an event until after day 28.
GVHD
Acute GVHD was assessed weekly after allogeneic HCT. None of the 7 patients receiving allogeneic HCT developed grade 2 – 4 acute GVHD. Three patients were diagnosed with grade 1 acute GVHD. Chronic GVHD occurred in 4 patients at a median of 244 days (161 – 368) post-HCT. The maximum severity of chronic GVHD was mild in two patients and moderate in 2 patients.
RELAPSE
The cumulative incidences of relapse at 3 years were similar in the autologous and allogeneic HCT arms [autologous 15.4% (95% CI (0 – 26.5); allogeneic: 14.3% (95% CI: 0 – 28)]. However, relapses after autologous HCT occurred earlier after transplantation with all relapses observed in the first two years. The only patient who relapsed following allogeneic HCT did not relapse until nearly 3 years after transplantation.
SURVIVAL
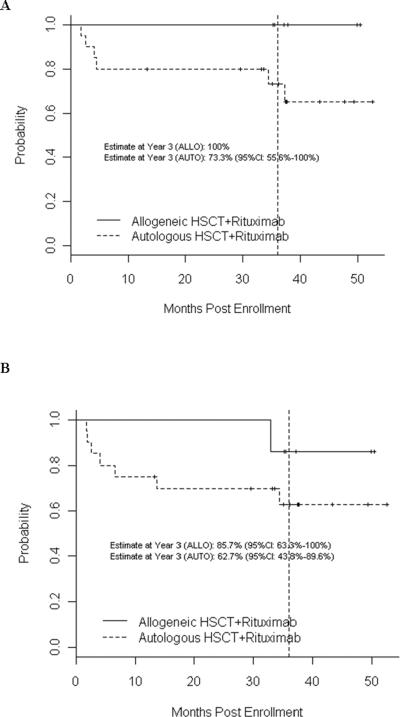
Median follow-up was 36 months after HCT (range, 1 – 51 months). The three-year probability of PFS following allogeneic HCT was 85.7% (95% confidence interval (CI): 63.3 – 100%); three-year PFS following autologous HCT was 62.7% (95% CI: 43.8 – 89.6) (Figure 1). Three-year probabilities of OS were 100% after allogeneic HCT and 73.3% (95% CI: 55.6 – 100) after autologous HCT. See Figure 1.
Figure 1.
Overall (A) and Progression-Free (B) survival following allogeneic and autologous HCT for follicular lymphoma
DISCUSSION
We report the outcomes of 30 patients with chemo-sensitive, relapsed FL who underwent either high-dose therapy with autologous HCT or RIC allogeneic HCT with assignment to treatment arm based on the availability of an HLA-matched sibling donor. This represents the first and only trial to date that attempted to prospectively compare the efficacy of autologous HCT vs RIC allogeneic HCT for patients with relapsed FL. Unfortunately, this trial closed prematurely due to slow patient accrual. Analysis of the reasons for poor accrual indicated lack of equipoise for the two treatments being tested. There was also a general concern about the potential for treatment-related morbidity and mortality following allogeneic HCT in this older patient population. Consequently, some clinicians favored use of allogeneic HCT for patients they perceived at very high risk of recurrence and autologous HCT for others, making them reluctant to enroll patients in a trial that could assign to either therapy.
With approximately 3 years of median follow-up, one patient in the allogeneic HCT arm vs 3 patients in the autologous HCT arm have relapsed. This observation adds to a growing literature supporting the existence of a robust graft vs lymphoma effect against FL. Additionally, there were no cases of TRM in the allogeneic HCT arm and no observed grade 2–4 acute GVHD. Three patients in the autologous HCT arm died from TRM, 1 from pneumonitis and 2 patients from infection. All allogeneic HCT recipients remain alive at a median of 36 months (range, 33 – 49) post-HCT. However, definitive conclusions comparing the efficacy of one treatment arm over the other cannot be drawn due to the limited sample size.
There are many treatment options available to patients with FL but allogeneic HCT remains the only known cure. Two large retrospective registry analyses demonstrated lower relapse rates among FL patients after allogeneic versus autologous HCT but prohibitive TRM after myeloablative allogeneic HCT has impeded long term survival8. Another separate registry analysis from the CIBMTR compared the outcome of FL patients after HCT using a myeloablative regimen versus an RIC regimen11. Interestingly, there were no significant differences in OS, PFS or TRM between the two types of conditioning regimens but there was a significantly increased risk of disease progression in the RIC patients. Compromised performance status and chemo-resistant disease adversely affected survival and increased the risk of TRM. A report from the Princess Margaret Hospital of 37 FL patients who received allogeneic HCT with a myeloablative regimen reported 5 year TRM of only 15% with OS and PFS of 79% and 76%, respectively10. This is an exceptionally low TRM considering that an ablative regimen was employed and could be explained in part by the fact that all patients were chemo-sensitive and the median age was only 45 years old.
RIC allogeneic regimens are increasingly offered to FL patients with the goal of utilizing the graft vs lymphoma effect while ameliorating the prohibitive TRM associated with myeloablative allogeneic HCT. Four prospective studies have reported favorable and encouraging results with some studies also including patients who had failed a prior autologous HCT12–15. All four studies incorporated fludarabine-based preparative regimens. The most favorable data so far originated from the M.D. Anderson Cancer Center in which 47 patients with relapsed FL with chemo-sensitive disease received the FCR conditioning regimen as utilized in our study (fludarabine, cyclophosphamide, rituximab)14. With a median follow up of 60 months, the OS and EFS were 85% and 83%, respectively. The TRM was 15% with infection being the leading cause of death. Only 2 patients relapsed and no patients died of progressive disease. In a recently published report, the GEL-TAMO group detailed the outcomes of 37 patients with FL who received allogeneic HCT after fludarabine and melphalan as the conditioning regimen13. Remission status at the time of HCT significantly impacted both OS and TRM. The 4 yr OS for patients in CR, PR and progressive disease was 71%, 48% and 29% with an TRM of 26%, 33% and 71%, respectively. The relapse incidence was only 8% for all patients. Several retrospective studies of RIC allogeneic HCT for FL patients have reported PFS ranging from 38% to 77% with follow-up intervals of ~1–3 years16, 26–29. TRM, however, has been considerably higher than in the above mentioned prospective studies. Such disparities can be explained in part by patient selection as some studies included a higher proportion of refractory patients while other studies allowed only chemo-sensitive patients.
The 3 yr OS and PFS rates after autologous HCT in this study were 73% and 63%, respectively, which compares favorably to other published autologous reports with relapsed FL patients. The use of rituximab represents a distinctive feature of our trial. We incorporated rituximab during mobilization as in vivo purging of the graft and as maintenance therapy after autologous HCT as a means of eradicating minimal residual disease30–31. Three patients have relapsed to date with all 3 relapses occurring within 2 years of autologous HCT.
Previous reports have consistently shown improved DFS with autologous HCT compared to conventional salvage therapy but only one study so far has shown a benefit in OS5. The `CUP' trial from Europe was the first randomized trial that prospectively addressed the role of autologous HCT vs conventional salvage chemotherapy. One hundred and forty patients with relapsed, chemo-sensitive follicular NHL were randomized to chemotherapy alone, autologous HCT with a purged graft or autologous HCT with an unpurged graft. The OS at 4 years for the 3 groups were 46%, 71% and 77%, respectively. There was a significant reduction in hazard rates for both PFS and OS when comparing the chemotherapy patients and the combined groups of autologous HCT. There were too few patients in the 2 HCT arms to assess the effect of ex vivo purging. In contrast to our study, the patients in the CUP trial were rituximab-naïve and did not receive rituximab during the peri-transplant period as this trial was initiated prior to routine rituximab use. In summary, our results show promising outcomes with both autologous HCT and RIC allogeneic HCT for FL patients with relapsed disease. Unfortunately, this trial suffered early closure due to slow accrual as did the `CUP' trial, the only other published randomized trial for relapsed FL patients. The numerous other choices of non-transplant therapies for patients with advanced FL most likely explain the poor accrual. Based on our results, we cannot definitively conclude which arm is more efficacious but the low relapse rate and TRM seen in the allogeneic HCT arm and previously published RIC allogeneic studies lends compelling support to further study of this modality. The BMT CTN therefore, has embarked on a phase II multicenter trial for relapsed FL patients with the goal to validate the highly promising results using the FCR regimen in the allogeneic HCT setting. This ongoing trial (BMT CTN #0701, Clinicaltrials.gov NCT00912223) is being conducted with the upfront participation of three National Cancer Institute cancer cooperative groups (Cancer and Leukemia Group B, Southwest Oncology Group and Eastern Cooperative Oncology Group).
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute and the National Cancer Institute (NIH grant U01-8L069294).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Blood and Marrow Transplant Clinical Trials Network
REFERENCES
- 1.Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin's lymphomas. N Engl J Med. 1984;311:1471–1475. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- 2.Deconinck E, Foussard C, Milpied N, et al. High-dose therapy followed by autologous purged stem-cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by GOELAMS. Blood. 2005;105:3817–3823. doi: 10.1182/blood-2004-10-3920. [DOI] [PubMed] [Google Scholar]
- 3.Lenz G, Dreyling M, Schiegnitz E, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:2667–2674. doi: 10.1182/blood-2004-03-0982. [DOI] [PubMed] [Google Scholar]
- 4.Sebban C, Mounier N, Brousse N, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA) Blood. 2006;108:2540–2544. doi: 10.1182/blood-2006-03-013193. [DOI] [PubMed] [Google Scholar]
- 5.Schouten HC, Qian W, Kvaloy S, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21:3918–3927. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Montoto S, Canals C, Rohatiner AZ, et al. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukemia. 2007;21:2324–2331. doi: 10.1038/sj.leu.2404850. [DOI] [PubMed] [Google Scholar]
- 7.Hosing C, Saliba RM, McLaughlin P, et al. Long-term results favor allogeneic over autologous hematopoietic stem cell transplantation in patients with refractory or recurrent indolent non-Hodgkin's lymphoma. Ann Oncol. 2003;14:737–744. doi: 10.1093/annonc/mdg200. [DOI] [PubMed] [Google Scholar]
- 8.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 9.van Besien K, Loberiza FR, Jr., Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 10.Kuruvilla J, Pond G, Tsang R, Gupta V, Lipton JH, Messner HA. Favorable overall survival with fully myeloablative allogeneic stem cell transplantation for follicular lymphoma. Biol Blood Marrow Transplant. 2008;14:775–782. doi: 10.1016/j.bbmt.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–45. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shea TC, Johnston J, Walsh W, et al. Reduced intensity allogeneic transplantation provides high disease-free and overall survival in patients with advanced idolent NHL and CLL: CALGB 109901. Blood. 2007;110:150a. doi: 10.1016/j.bbmt.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinana JL, Martino R, Gayoso J, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris E, Thomson K, Craddock C, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104:3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 16.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 17.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 18.Logan B, Leifer E, Bredeson C, et al. Use of biological assignment in hematopoietic stem cell transplantation clinical trials. Clin Trials. 2008;5:607–616. doi: 10.1177/1740774508098326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blood and Marrow Transplantation Clinical Trials Network Manual of Procedures. 2010 https://webemmescom/study/bmt2/MOPhtml.
- 20.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez J, Caballero MD, Gutierrez A, et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience. Ann Oncol. 2003;14:1768–1775. doi: 10.1093/annonc/mdg459. [DOI] [PubMed] [Google Scholar]
- 22.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1994;1995;15:825–828. [PubMed] [Google Scholar]
- 24.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 26.Vigouroux S, Michallet M, Porcher R, et al. Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC) Haematologica. 2007;92:627–634. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 27.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 28.Kusumi E, Kami M, Kanda Y, et al. Reduced-intensity hematopoietic stem-cell transplantation for malignant lymphoma: a retrospective survey of 112 adult patients in Japan. Bone Marrow Transplant. 2005;36:205–213. doi: 10.1038/sj.bmt.1705027. [DOI] [PubMed] [Google Scholar]
- 29.Tomblyn M, Brunstein C, Burns LJ, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–545. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magni M, Di Nicola M, Devizzi L, et al. Successful in vivo purging of CD34-containing peripheral blood harvests in mantle cell and indolent lymphoma: evidence for a role of both chemotherapy and rituximab infusion. Blood. 2000;96:864–869. [PubMed] [Google Scholar]
- 31.Ladetto M, Zallio F, Vallet S, et al. Concurrent administration of high-dose chemotherapy and rituximab is a feasible and effective chemo/immunotherapy for patients with high-risk non-Hodgkin's lymphoma. Leukemia. 2001;15:1941–1949. doi: 10.1038/sj.leu.2402302. [DOI] [PubMed] [Google Scholar]