Abstract
Glucocorticoids are used to treat respiratory dysfunction associated with premature birth but have been shown to cause neurodevelopmental deficits when used therapeutically. Recently, we established that acute glucocorticoid exposure at clinically relevant doses produces neural progenitor cell apoptosis in the external granule layer of the developing mouse cerebellum and permanent decreases in the number of cerebellar neurons. As the cerebellum naturally matures and neurogenesis is no longer needed, the external granule layer decreases proliferation and permanently disappears during the second week of life. At this same time, corticosterone (the endogenous rodent glucocorticoid) release increases and a glucocorticoid-metabolizing enzyme that protects the external granule layer against glucocorticoid receptor stimulation (11B-Hydroxysteroid-Dehydrogenase-Type 2; HSD2) naturally disappears. Here we show that HSD2 inhibition or raising corticosterone to adult physiological levels can both independently increase neural progenitor cell apoptosis in the neonatal mouse. Conversely, glucocorticoid receptor antagonism decreased natural physiological apoptosis in this same progenitor cell population suggesting endogenous glucocorticoid stimulation may regulate apoptosis in the external granule layer. We also found that glucocorticoids HSD2 can effectively metabolize generate less external granule layer apoptosis then glucocorticoids this enzyme is ineffective at breaking down. This finding may explain why glucocorticoids this enzyme can metabolize are clinically effective at treating respiratory dysfunction yet seem to produce no neurodevelopmental deficits. Finally, we demonstrate that both acute and chronic glucocorticoid exposure produces external granule layer apoptosis but without appropriate control groups this effect becomes masked. These results are discussed in terms of their implications for glucocortiocid therapy and neurodevelopment during the perinatal period.
Keywords: apoptosis, neural progenitor cell, cerebellum, glucocorticoid, dexamethasone, betamethasone, bronchopulmonary dysplasia, chronic lung disease, premature birth, neurodevelopment
Introduction
Recent clinical research has found that the postnatal use of glucocorticoid (GC) therapy can cause permanent neuromotor and cognitive deficits (Yeh et al., 2004) leading to the recommendation that this drug regimen not be used outside of placebo controlled clinical trials (Committee on Fetus and Newborn, 2002). Despite these concerns, GC therapy continues to be used throughout the world. For example, approximately 10% of prematurely born infants in North America and Europe still receive this treatment (Onland et al., 2009). Additionally, while prenatal GC therapy (which is given to approximately 7–10% of all pregnant women in North America and Europe; Matthews et al., 2004) has generally been considered safe, more recent clinical research has shown treatment regimens using multiple exposures produce decreased head circumference (a proxy measurement for brain size; Amaral et al., 2008), length, and weight in neonates at birth (Murphy et al., 2008). Unfortunately, despite the large percentage of humans exposed to this controversial therapy (Eventov-Friedman & Shinwell, 2008), surprisingly little is known about how GC exposure can iatrogenically produce the neurodevelopmental deficits seen clinically.
In previous research, we discovered that exposure to clinically relevant doses of dexamethasone (DEX) produced rapid neural progenitor cell (NPC) apoptosis (programmed cell death) in the external granule layer (EGL) of the immature mouse cerebellum (Noguchi et al., 2008). The window of vulnerability for this toxicity in the mouse corresponds to all time points during which perinatal GC therapy would occur in the human (Noguchi et al., 2008). One curious aspect of this toxicity is that it is selectively confined to the NPCs contained within the outer EGL, a transient proliferative region occupying the most superficial cerebellar layer. The EGL produces new neurons in its outermost layer that first congregate and mature in the inner EGL before migrating through the molecular and Purkinje cell layers to populate the internal granule layer as neurons (Figure 1A). The amount of EGL neurogenesis is quite extensive leading to the postnatal production of a homogenous population of internal granule layer cells so numerous they represent over half the neurons in the brain (Andersen et al., 1992, Harvey & Napper, 1988). During the second week of normal rodent life, the EGL is rapidly removed from the cerebellum as proliferation in the EGL decreases and neurogenesis ends.
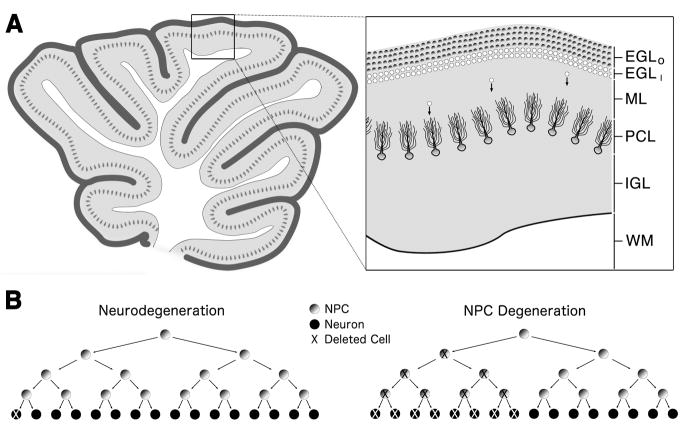
Figure 1. Cerebellar development and apoptotic cell death.
(A) The neonatal cerebellum is composed of white matter (WM) in its core surrounded by four cortical layers which include the internal granule layer (IGL) composed of granule cells, the Purkinje cell layer (PCL) which provides the sole output of the cerebellar cortex, the molecular layer (ML) which has few soma and mainly consists of synaptic connections between the Purkinje and granule cells, and the proliferative external granule layer (EGL). The EGL is further divided into the outer EGL (shaded circles; EGLO) composed solely of NPCs and the inner EGL (white circles; EGLI) composed of newly formed immature neurons that eventually migrate (cells with arrows) and populate the IGL. (B) The consequences of neural progenitor cell (NPC) apoptosis can be magnified due to the progenitor’s ability to exponentially expand. Whereas the apoptotic death of a neuron leads to a net loss of only one cell (left), the apoptotic death of a single NPC not only eliminates that cell but also deletes any NPCs or neurons that cell would have produced in the future (right).
During normal cerebellar development, the GC system is designed such that GC stimulation in the EGL is low while this proliferative region is undergoing massive amounts of neurogenesis and growth but high when this same region decreases proliferation and disappears from the cerebellum (Buresova, 1984, Dakine et al., 2000, Holmes et al., 2006, Pavlik & Robson et al., 1998). Based on the ability of GCs to produce progenitor cell apoptosis, changes in endogenous GC stimulation may affect EGL apoptosis thereby contributing to its natural disappearance. In addition, the increased cell death in an exponentially expanding population of NPCs may have a magnified effect by decreasing proliferation as a secondary effect. (Altman & Bayer, 1997, de la Rosa & de Pablo, 2000, Depaepe et al., 2005, Haydar et al., 1999, Kuida et al., 1996; Figure 1B). Based on this information, we examined how the GC system and its different regulating mechanisms might interact to effect NPC apoptosis in the developing cerebellum.
Materials and Methods
Animals and Drugs
Postnatal day (PND) 7 ICR mice (Harlan, IN, USA) were used unless otherwise indicated because previous research determined that this age was the middle of the window of vulnerability for this toxicity (Noguchi et al., 2008). Males and females were used, however, gender was not used as a factor during analysis since previous research found that it has no significant effect on this toxicity (Noguchi et al., 2008). Following final drug exposure for each drug regimen, all mouse pups were separated from the mother and maintained at 30° C until perfusion 6 hours later. All animal procedures used were in accordance with standards approved by the Washington University in Saint Louis Animal Studies Committee. DEX and betamethasone were administered using the preservative-free prodrug DEX sodium phosphate USP or betamethasone sodium phosphate USP respectively (Voigt Global Distribution LLC, Lawrence, KS, USA), the water soluble inorganic ester form of each drug typically administered clinically. DEX sodium phosphate and betamethasone sodium phosphate are expressed as molar equivalents to DEX and betamethasone respectively. Corticosterone, carbenoxolone, and mifepristone were obtained from Sigma-Aldrich (St. Louis, MO, USA). Doses of carbenoxolone and mifepristone were chosen based on previous research describing their ability to antagonize HSD2 (Heine & Rowitch, 2009) and the GC receptor (Mesripour et al., 2008) respectively. Corticosterone and mifepristone were dissolved in a sesame oil vehicle prior to injection whereas all other drugs were dissolved in sterile saline. The 100 mg/kg dose of mifepristone was administered in two 50 mg/kg doses. One 15 minutes prior to GC or carbenoxolone injection and the other 2 hours after. The 50 mg/kg mifepristone dose was administered as a single dose 15 minutes prior to GC exposure.
Activated caspase-3 immunohistochemistry
We previously established that activated caspase-3 immunohistochemistry is a sensitive marker for NPC apoptosis in the EGL by confirmation through the use of both electron microscopy (the gold standard for apoptotic detection) and the presence of pyknosis in plastic embedded semithin sections stained with methylene blue/Azure B (Noguchi et al., 2008). Animals in the current study were deeply anesthetized and transcardially perfused with 4% paraformaldehyde in 0.1 M Tris buffer six hours after last injection unless specified otherwise. Following postfixation, the cerebella were removed and sectioned at 75 um in the sagittal plane using a vibratome. Immunohistochemistry for activated caspase-3 was performed as described previously (Noguchi et al., 2008). Based on results from pilot experiments in ICR mice (data not shown), a 6 hr post-injection time interval (rather than 4 hr with C57BL/6 mice) after final drug exposure.
Semiquantitative evaluation of EGL degeneration
All scored slides were collected together and a rater, blind to treatment, randomly scored each slide based on the amount of degeneration in the EGL. A degeneration score for each animal was evaluated by examining the amount of cerebellar NPCs positive for activated caspase-3 from several different midsagittal levels of the outer EGL as described previously (Noguchi et al., 2008). The extent of damage was based on the following scale: 0 = no apoptotic profiles are seen in all EGL regions, 1 = apoptotic profiles are only seen in a minority of EGL regions, 2 = apoptotic profiles are seen in a majority of EGL regions, 3 = apoptotic profiles are seen in all regions of the EGL.
Unless otherwise indicated, statistical analysis was performed using a one-way ANOVA and Bonferroni posthoc analysis with treatment as a between groups factor. The four-parameter logistic equations were modeled using Prism software (Prism version 4.0a, Graphpad Software Inc., San Diego, CA, USA) based on degeneration scores and then used to produce dose response curves and 95% confidence intervals. Due to problems with solubility and the amount of vehicle that can be safely administered, the highest dose of corticosterone still failed to produce maximum degeneration scores comparable to DEX or betamethasone. As a result, the maximum (Top) values for the four-parameter logistic equations were all constrained to 2.595 (the highest Top value calculated for all GCs without constraint).
Results
GINA is mediated by the GC receptor
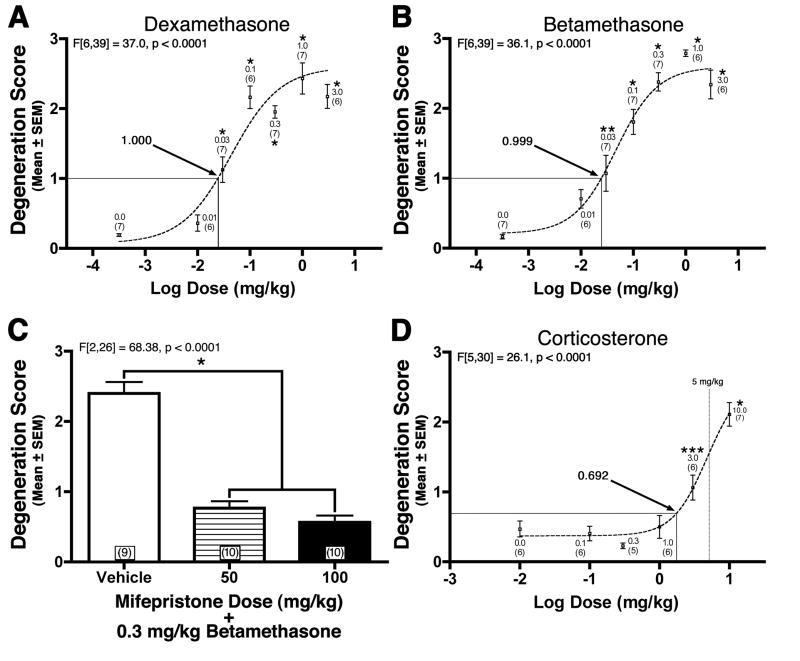
In previous research (Noguchi et al., 2008), we showed DEX can produce GC induced NPC apoptosis (GINA) in the inbred C57BL/6 neonatal mouse. The ICR strain is the most commonly used outbred mouse strain that offers several advantages in terms of docile disposition, reproductive characteristics, maternal care, and cost. In order to establish whether we can produce GINA in the ICR mouse strain, we exposed PND7 pups to the most common clinically used prenatal and postnatal GCs (i.e. betamethasone and DEX respectively) and semiquantitatively evaluated the amount of activated caspase-3 in the EGL. All degeneration scores were analyzed using a one-way ANOVA and Bonferroni post-hoc (verses vehicle control). Both GCs produced degeneration scores in a dose-dependent manner (p < 0.0001) with higher doses producing statistically significant increases in scores when compared to the control group (Figures 2A–B). To further clarify whether this toxicity is being mediated through the GC receptor, we treated animals with the GC receptor antagonist mifepristone (Jung-Testas & Baulieu, 1983) at a dose of 50 or 100 mg/kg prior to a 0.3 mg/kg betamethasone injection. Both doses of mifepristone significantly decreased degeneration scores in the EGL (Figure 2C; p < 0.0001) showing that GC receptor stimulation mediates GINA.
Figure 2. Glucocorticoid induced neural progenitor cell apoptosis (GINA) is mediated by the glucocorticoid receptor (A–C) and can be produced at physiologically relevant doses (D).
(A) Dexamethasone and (B) betamethasone produced a dose dependent increase in EGL degeneration scores following exposure. (C) The ability of the glucocorticoid receptor antagonist mifepristone (50 or 100 mg/kg) to block betamethasone induced degeneration indicates GINA is mediated through glucocorticoid receptor stimulation (D) In the neonate, corticosterone produces increased degeneration scores at levels below that seen physiologically in the normal adult (i.e. 5 mg/kg dose; indicated by vertical line). The numbers associated with each point represent the dose in mg/kg or, if in parenthesis, n per group. * p < 0.001, ** p < 0.01, and *** p < 0.05. Arrows indicate estimated degeneration scores produced by an equipotent dose (equivalent to 0.0245 mg/kg dexamethasone) of each glucocorticoid.
11 β-Hydroxysteroid Dehydrogenase Type 2 and corticosterone regulates GINA
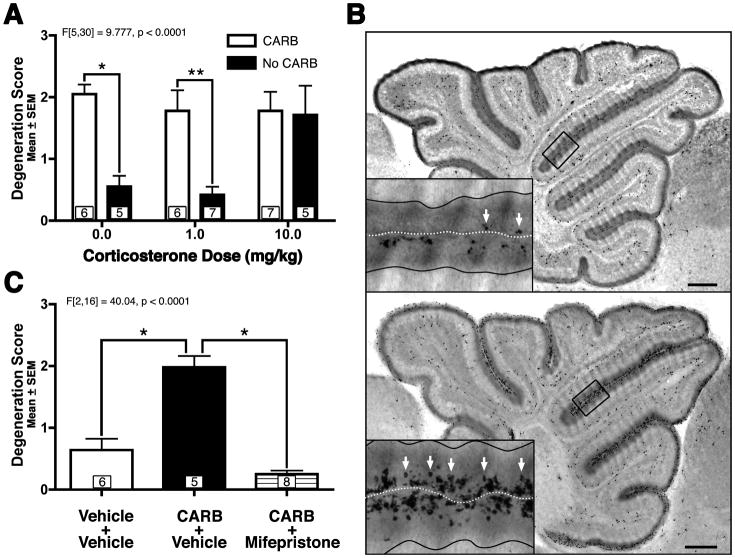
During cerebellar development, endogenous GC stimulation is highly regulated both temporally and regionally. The neonatal cerebellum contains the highest levels of GC receptors in the neonatal brain (Pavlik & Buresova, 1984) which are selectively located in the EGL (Noguchi et al., 2008). In addition, as the EGL is undergoing exponential expansion and massive proliferation, it is shielded from GC stimulation by dramatically lowered free GC levels (known as the stress hyporesponsive period; Dakine et al., 2000, Pavlik & Buresova, 1984, Schmidt et al., 2003) and protection from 11β-Hydroxysteroid Dehydrogenase Type 2 (HSD2), an enzyme that efficiently metabolizes endogenous GCs in local tissue and is almost exclusively isolated in the EGL at this age (Holmes et al., 2006, Robson et al., 1998). As the neonatal rodent ages and EGL proliferation decreases, endogenous GC levels surge to adult levels (Pavlik & Buresova, 1984) and HSD2 expression concomitantly decreases (Holmes et al., 2006, Robson et al., 1998) as the EGL disintegrates now that its job of producing new neurons is over. Interestingly, HSD2 has difficulty metabolizing the most common clinically used GCs (i.e. betamethasone and DEX) but can metabolize endogenous GCs such as hydrocortisone (cortisol) and corticosterone (the endogenous rodent GC similar to cortisol in the human). Therefore, we first tested whether a GC that the HSD2 enzyme can metabolize, is able to produce GINA by exposing PND7 (a period during which HSD2 expression is high) neonatal mouse pups to varying doses of corticosterone. Degeneration scores were analyzed using a one-way ANOVA and Bonferroni post-hoc (verses vehicle control). Corticosterone exposure produced significant increases in EGL degeneration scores at doses 3.0 mg/kg and above when compared to control animals (Figure 2D). Next, in order to test whether the HSD2 enzyme may protect the EGL against GINA, the HSD2 inhibitor carbenoxolone (100 mg/kg) or saline was administered 1 hour prior to a single corticosterone injection given at either 0.0, 1.0, or 10.0 mg/kg. (Figure 3A). It should be noted that carbenoxolone inhibits both HSD2 and 11B-Hydroxysteroid Dehydrogenase Type I, a related enzyme which is thought to regenerate GCs in intact cells. However, the latter enzyme is not expressed in the EGL (Heine & Rowitch, 2009) and, if it were to be inhibited by carbenoxolone, should reduce (not potentiate) GINA. A one-way ANOVA revealed a statistically significant difference (F[5,30] = 9.777, p < 0.0001) between groups which was followed by a Bonferroni post-hoc analysis comparing carbenoxolone treatment conditions for each corticosterone dose. When carbenoxolone was administered with the high dose of corticosterone, it could not further elevate the high degeneration scores suggesting a ceiling effect (p > 0.05). However, carbenoxolone pretreatment significantly elevated degeneration scores when administered with 1.0 mg/kg corticosterone injection (p < 0.01) suggesting inhibition of HSD2 might elevate corticosterone induced GINA. Surprisingly, carbenoxolone dramatically raised degeneration scores even when no corticosterone was administered at all (Figures 3A–B; p < 0.001). This toxicity was so profound that carbenoxolone alone produced degeneration scores comparable to carbenoxolone plus 10.0 mg/kg corticosterone.
Figure 3. 11β-Hydroxysteroid Dehydrogenase Type 2 (HSD2) inhibition alone produces GINA and is blocked by glucocorticoid receptor inhibition.
(A) The HSD2 inhibitor carbenoxolone (CARB; 100 mg/kg) dramatically potentiates EGL NPC apoptosis whether or not corticosterone is co-administered. (B) Photocomposite of activated caspase-3 immunoreactively reveals that, compared to saline (top), CARB treatment (bottom) dramatically increased the amount of activated caspase-3 positive NPCs (white arrows). Insets reflect magnified views of boxed regions with solid black lines defining the internal borders of each EGL and white dotted line defining the pial surface. Scale bars: 250 um. (C) The ability of the glucocorticoid receptor antagonist mifepristone to block CARB induced toxicity verifies that HSD2 mediates GINA. For all graphs the boxed numbers in each bar represents n per group. * p < 0.001, ** p < 0.01.
A variety of insults such as radiation (Altman & Bayer, 1997) or genotoxins (Noguchi et al., 2008) can additionally produce EGL NPC apoptosis. Therefore, we tested whether the apoptosis seen with carbenoxolone alone was due to endogenous GC stimulation by administering the GC receptor antagonist mifepristone (100 mg/kg) 15 minutes prior to carbenoxolone (100 mg/kg) or saline treatment. Mifepristone eliminated the elevated degeneration scores produced by carbenoxolone pretreatment (Figure 3C; p < 0.001). These results show that HSD2 is actually preventing the low amounts of endogenous GCs present during the stress hyporesponsive period from producing large amounts of GINA. They also suggest that endogenous GC levels may be responsible for some of the natural physiological apoptosis seen in the EGL as the HSD2 enzyme naturally disappears during development.
The endogenous GC corticosterone is less toxic at equipotent doses than DEX or betamethasone
While HSD2 potently protects against GINA produced from endogenous GCs, its limited ability to break down betamethasone and DEX, the most commonly used clinical GCs, suggests these two agents may be more toxic at equipotent doses than the endogenous hormone corticosterone. This hypothesis was tested in two ways. In the first analysis, all three GCs were converted to DEX equivalents based on their relative potency (Schimmer & Parker, 2006) and four-parameter logistic equations and 95% confidence intervals were calculated. A comparison (Table 1) revealed that the ED50 dose for corticosterone was over 30% higher than would be expected for both DEX (p < 0.01) and betamethasone (p < 0.0001). No significant differences in ED50 doses were seen between DEX and betamethasone.
Table 1.
Comparison of the dose of glucocorticoid needed to produce the ED50
| Drug | ED50 dose (mg/kg) | Standard deviation | Comparison verses dexamethasone (“Z”-score; p-value) | Comparison verses betamethasone (“Z”-score; p-value) |
|---|---|---|---|---|
| Dexamethasone | 0.044 | 0.068 | N/A | 0.33; p = 0.745 |
| Betamethasone | 0.047 | 0.038 | 0.33; p = 0.745 | N/A |
| Corticosterone | 0.067 | 0.014 | 2.65; p< 0.01 | 3.97; p< 0.0001 |
In addition to comparing ED50 values, a second analysis was performed examining the degeneration scores produced by these three GCs at equipotent doses. Based on the relative potencies of each GC, a dose was calculated which was equipotent to 0.025 mg/kg DEX (the dose at which DEX produces a degeneration score of 1.0). Degeneration scores and standard deviations were then calculated at these doses using the four-parameter logistic equations and 95% confidence intervals. A comparison (Table 2) revealed that equipotent dose of corticosterone produced degeneration scores more than 30% lower than DEX (p < 0.0001) and betamethasone (p < 0.0001). No differences in degeneration scores were seen between DEX and betamethasone.
Table 2.
Comparison of the amount of degeneration produced by equipotent doses of glucocorticoids
| Drug | Original dose | Potency relative to hydrocortisone | Dose normalized to dexamethasone equivalents | Associated degeneration score | Standard deviation | Comparison verses dexamethasone (“Z”-score; p-value) | Comparison verses betamethasone (“Z”-score; p-value) |
|---|---|---|---|---|---|---|---|
| Dexamethasone | 0.025 | 25.000 | 0.025 | 1.000 | 0.025 | N/A | 0.012; p = 0.991 |
| Betamethasone | 0.025 | 25.000 | 0.025 | 0.999 | 0.071 | 0.012; p = 0.991 | N/A |
| Corticosterone | 1.750 | 0.350 | 0.025 | 0.692 | 0.045 | 4.10; p < 0.0001 | 5.92; p < 0.0001 |
Chronic GC exposure produces GINA
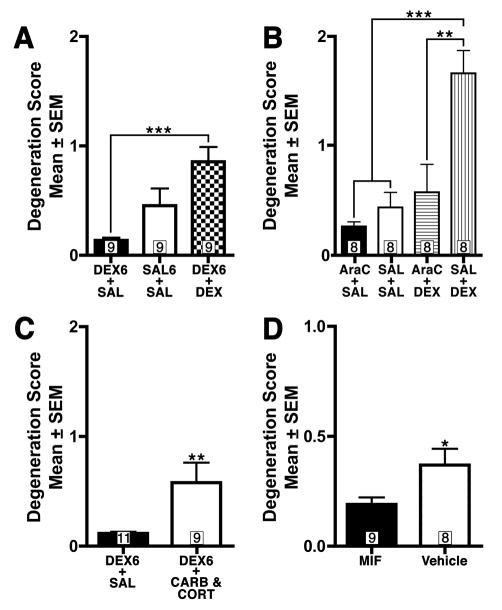
In a recent paper, Heine & Rowitch (2009) concluded that a single 0.1 mg/kg DEX injection on PND7 produces GINA but chronic administration (a single daily injection from PND0 to PND7) at this same dose produces no apoptosis as assessed through activated caspase-3 staining performed after the last injection. Unfortunately, we were concerned the control condition (a single daily saline injection from PND0 to PND7) used in this study for comparison was inadequate for the following reason. Since EGL NPCs are rapidly removed from the brain during apoptosis, they only remain activated caspase-3 positive for less than 12 hours after GC exposure (Noguchi et al., 2008). As a result, activated caspase-3 positivity after seven days of daily DEX exposure would not represent the cumulative number of NPCs undergoing apoptosis but would only capture dying cells produced by the last injection. This fact may be problematic since the first six days of DEX exposure may dramatically decrease NPC population size thereby biasing any results seen after the last injection. To address this issue, we replicated the chronic Heine & Rowitch (2009) dosing procedure by administering either 0.1 mg/kg DEX (DEX6-DEX group) or saline (SAL6-SAL group) daily from PND0 to PND7. Additionally, in order to control for all effects except those produced by the last DEX injection, an additional control group (DEX6-SAL group) was added which received 0.1 mg/kg DEX from PND0-PND6 but saline on PND7 (Table 3., top). After a one-way ANOVA revealed a statistically significant difference in degeneration scores between groups (F[2,24] = 8.838, p < 0.01) an all-pairwise Bonferroni post-hoc was performed. These results replicated the original Heine & Rowitch (2009) findings, showing a non-significant decrease in degeneration scores for the DEX6-DEX group when compared to SAL6-SAL. However, the DEX6-DEX group had a significant increase in GINA (p<0.001) compared to the DEX6-SAL group, indicating that chronic DEX exposure does indeed increase GINA (Figure 4A). This finding shows that both chronic and acute DEX exposure both produce GINA but without an appropriate control group the apoptosis from chronic exposure is masked.
Table 3.
Summary of Chronic Dosing Regimens
| Daily Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PND0 | PND1 | PND2 | PND3 | PND4 | PND5 | PND6 | PND7 | ||
| Groups | DEX6+SAL | D | D | D | D | D | D | D | S |
| SAL6+SAL | S | S | S | S | S | S | S | S | |
| DEX6+DEX | D | D | D | D | D | D | D | D | |
| AraC+SAL | AraC | AraC | AraC | S | |||||
| SAL+SAL | S | S | S | S | |||||
| AraC+DEX | AraC | AraC | AraC | D | |||||
| SAL+DEX | S | S | S | D | |||||
| DEX6+SAL | D | D | D | D | D | D | D | S | |
| DEX6+CARB&CORT | D | D | D | D | D | D | D | CARB+CORT | |
D = 0.1 mg/kg Dexamethasone Injection, S = Saline Injection, AraC = 25 mg/kg AraC Injection, CARB+CORT = 100 mg/kg Carbenoxolone Injection plus a 5 mg/kg Corticosterone Injection
Figure 4. Glucocorticoid stimulation produces GINA even after chronic exposure (A-C) and glucocorticoid receptor blockade decreases physiological NPC death (D).
(A) Chronic dexamethasone (DEX) exposure (0.1 mg/kg daily between PND0 through PND7) produces elevated degeneration scores when an appropriate control group is used. (B) Chronic exposure to the genotoxin AraC reduces GINA in a similar manner to chronic DEX. (C) The physiological changes which lead to heightened glucocorticoid stimulation (decreased HSD2 protection and increased corticosterone levels) as the neonate matures can be produced by carbenoxolone (CARB) and corticosterone (CORT) administration and lead to increased degeneration scores even after chronic DEX exposure. D) The 12-hour blockade of glucocorticoid stimulation by mifepristone (MIF) reduces physiological cell death in EGL NPCs. Numbers in boxes represent n per group. * p < 0.05, ** p < 0.01, *** p < 0.001.
Next we examined why chronic DEX pretreatment would mask the subsequent GINA seen on PND7. Heine & Rowitch (2009) proposed that chronic (as opposed to acute) exposure to GCs eliminated its ability to produce GINA leading to a treatment that can only effect EGL proliferation. Alternatively, we hypothesized that daily exposure to this drug is killing off NPCs in the EGL thereby dramatically decreasing the population that could be effected by GINA on PND7. If true, we would expect any chronic exposure to a drug that induces EGL apoptosis (regardless of whether or not it stimulates the GC receptor) would produce the same effect. In order to test whether this is true, we used AraC, a genotoxin with no affinity for the GC receptor that produces EGL NPC apoptosis through a different apoptotic intracellular signaling mechanism than GINA (Noguchi et al., 2008). Since chronic DEX exposure would start to produce GINA around PND4 (around when the window of vulnerability to GINA begins; Noguchi et al., 2008) we gave 25 mg/kg AraC daily from PND4-PND6 and then administered 0.1 mg/kg DEX (AraC-DEX group) or saline (AraC-SAL group) on PND7. For control groups we gave saline from PND4-PND6 and either gave 0.1 mg/kg DEX (SAL-DEX) or saline (SAL-SAL) on PND7 (Table 3., middle). After a one-way ANOVA revealed a statistically significant difference between groups (F[3,28] = 12.30, p < 0.0001) an all-pairwise Bonferroni post-hoc was performed. Our results revealed that chronic AraC, like chronic DEX, was also able to mask GINA (Figure 4B) showing this effect can be produced independent of GC stimulation.
During neonatal development, the EGL disappears and proliferation decreases around the same time the HSD2 enzyme rapidly disappears and endogenous levels of GCs dramatically increase to adult levels. It might be argued that these changes in the GC system during development represent a chronic, not acute, increase in GC stimulation. Based on this information, we tested whether two physiological events that occur during development, decreased HSD2 protection against endogenous GCs and the increase of endogenous GCs to adult levels, can still produce GINA after chronic exposure to GCs. We first administered 0.1 mg/kg DEX from PND0 to PND6 to all animals. On the following day (i.e. PND7), one group (DEX6-CORT+CARB group) received 5.0 mg/kg corticosterone (a dose which increases blood levels in the neonatal rodent pup to levels that are within the normal basal adult range; Gould et al., 1991) to mimic increased endogenous GC release and 100 mg/kg carbenoxolone to decrease HSD2 protection. A second control group (DEX6-SAL group) received the same chronic DEX exposure but only a vehicle injection on PND7 (Table 3., bottom). Despite this chronic DEX exposure, the DEX6-CORT+CARB group still exhibited increased degeneration scores (t[18] = 2.820, p < 0.01) showing that even with extreme chronic GC exposure the physiological changes which occur in the GC system during maturation can still produce GINA (Figure 4C).
Our findings suggest that endogenous GC stimulation may be sufficient to produce EGL NPC apoptosis in the neonate. As a result, the small amounts of EGL apoptosis we see in the untreated control animals may be partially the result of endogenous GCs producing physiological cell death. Previous research has established that physiological apoptosis in the EGL peaks on PND9 (Tanaka & Marunouchi, 1998). Based on this information, simply administering mifepristone (a GC receptor antagonist) on PND9 may further reduce endogenous physiological cell death. Since apoptotic NPCs remain activated caspase-3 positive for less than 12 hours (Noguchi et al., 2008), GC receptor antagonism should be present for at least this amount of time. Therefore, we exposed PND9 neonatal mouse pups to either mifepristone (50 mg/kg) or sesame oil vehicle every 200 minutes for three injections and perfused them 12 hours after the first injection. Since this treatment will provide at least 10 hours of GC antagonism and it takes more than 2 hours for activated caspase-3 to be detected, this drug regimen should eliminate much of the activated caspase-3 immunoreactivity due to endogenous GINA. Semiquantitative evaluation of EGL apoptosis revealed that mifepristone decreased degeneration scores (t[15] = 2.310, p < 0.05) indicating that endogenous GINA is responsible for a significant amount of physiological apoptosis in the EGL NPCs (Figure 4D).
Discussion
Clinical Perinatal Glucocorticoid Therapy
Clinically, DEX is the most commonly used postnatal GC and is often given chronically at doses that range between 0.25 to 1.0 mg/kg (Committee on Fetus & Newborn, 2002, Yeh et al., 2004). Our research found that this same drug increased GINA at doses 0.03 mg/kg and above in the mouse. Therefore, prematurely born infants are often exposed to DEX at doses that are a full order of magnitude greater then what we found to be toxic in the mouse. Since DEX is thought to be more potent in the human than the mouse (Keil et al., 1995, Pedraz et al., 1990), prematurely born infants exposed to GCs may also be experiencing GINA. These results may explain why correlational measurements following postnatal GC therapy revealed that the cerebellum is the most stunted brain region exhibiting a 20.6% smaller volume when compared to an untreated group (Parikh et al., 2007). While our findings may seem to produce a dilemma for the clinician, they also show that under normal conditions the HSD2 enzyme may provide a natural defense against GINA and suggests that the use of GCs HSD2 can efficiently break down may be preferred in the clinical setting. This finding is consistent with several clinical studies which have found that postnatal hydrocortisone therapy can be effective at reducing oxygen dependency yet produces none of the neurodevelopmental or structural deficits associated with DEX (Benders et al., 2009, Lodygensky et al., 2005, Peltoniemi et al., 2009, Rademaker et al., 2007, Rademaker & de Vries, 2009, Volpe et al., 2005, Watterberg et al., 2007). Interestingly, these findings have led some to speculate that HSD2 protection (particularly in the cerebellum) may be responsible for the lack of neurodevelopmental deficits and brain stunting seen with clinical hydrocortisone therapy (Benders et al., 2009, Rademaker & de Vries, 2009).
Our results are also the first to report that betamethasone, the most commonly used prenatal GC, can produce GINA in the mouse at doses that are 5.7 times lower than what is recommended for a 70 kg women (National Institutes of Health, 2000). If the same vulnerability exists in the developing human, there should also be significant concern that GINA is being produced during prenatal GC therapy. Despite our findings, there is a strong consensus that the benefits of acute prenatal betamethasone exposure dramatically outweigh the risks (Committee on Fetus and Newborn, 2002). Considering that postnatal GC therapy can produce permanent neurodevelopmental deficits why does acute prenatal therapy appear to be relatively safe? The answer may lie in the fact that GINA is producing apoptosis in a population of dynamically proliferating NPCs (rather than neurons) where early toxicity may be compensated for by ramping up subsequent neurogenesis by the remaining progenitors. Indeed, previous animal research has confirmed that the cerebellum can recover from the deleterious effects of early, but not late, exposure to GCs by increasing subsequent proliferation (Bohn & Lauder, 1980, Robson et al., 1998). As proliferation is ramped up, the intact developmental machinery will still faithfully incorporate any further granule cells produced thereby compensating for this early insult. Therefore, this research suggests GC therapy should be given early and briefly as possible to allow for recovery.
GC stimulation and cerebellar development
The spatial and temporal regulation of endogenous GC stimulation suggests GINA may play a critical role in the normal disappearance of the EGL. The neonatal cerebellum contains the highest density of GC receptors in the entire brain (Pavlik & Buresova, 1984) that are selectively located in the EGL (Noguchi et al., 2008). While the EGL undergoes rapid expansion and proliferation during early development this region is protected from GC stimulation by low levels of endogenous GCs and the selective expression of the GC metabolizing enzyme HSD2. As the animal matures, endogenous corticosterone suddenly increases to adult levels and HSD2 expression disappears as the EGL begins to disappear and proliferation wanes (Holmes et al., 2006, Pavlik & Buresova, 1984, Robson et al., 1998, Schmidt et al., 2002, Schmidt et al., 2003). One can mimic this natural increase in endogenous GC release by administering a 5.0 mg/kg corticosterone injection which increases blood levels in neonatal rodent pups that are within the normal basal adult range (Gould et al., 1991). Our corticosterone dose response curve showed that injections 3.0 mg/kg (40% lower than 5.0 mg/kg) and above produce increased degeneration scores (Figure 2D) suggesting that the natural increase in corticosterone levels seen during normal development may produce GINA. Additionally, we have also shown that simply inhibiting the HSD2 enzyme dramatically increases endogenous GINA to a much greater extent than a 5.0 mg/kg corticosterone injection. Therefore, the concomitant effect of eliminating HSD2 expression and increasing GC release conspires to increase GINA at the same time as the EGL disappears. Consistent with the idea, we also found that merely blocking GC receptors with mifepristone during the neonatal period reduces physiological EGL apoptosis.
Our findings are also bolstered by previous research from others suggesting GC stimulation and apoptosis play a critical role in the disappearance of the EGL. For instance, exogenous GC exposure leads to the premature disintegration of the EGL and decreases in cerebellar size (Benesova & Pavlik, 1985, Benesova & Pavlik, 1989, Bohn & Lauder, 1980, Ferguson & Holson, 1999) whereas decreasing endogenous GCs by adrenalectomy delays EGL disappearance until at least PND25 and increases cerebellar size despite producing smaller body size (Yehuda et al., 1989, Yehuda & Meyer, 1991). Therefore, it is clear that the correct amount of GC stimulation is needed for normal EGL disappearance. In other research, Kiuda et al (1996) produced transgenic mice in which NPC apoptosis is blocked but any direct effects endogenous GCs might have on EGL proliferation, migration, and/or differentiation were preserved. While the wild-type mice showed the normal disappearance of the EGL by PND16, transgenic mice displayed a robust mitotically active EGL with preserved migration of postmitotic neurons to an enlarged internal granule layer. Therefore apoptosis is critical for the normal elimination of the EGL despite any effect GC stimulation might have on migration, proliferation, and/or differentiation.
It is important to realize the effects of NPC apoptosis can have very different effects than neuronal apoptosis. In recent years, several important studies have been able to manipulate the amount of apoptosis occurring in NPCs during development. When NPC apoptosis was reduced, the offspring tended to die perinatally and displayed dramatically enlarged brain malformations that were not tumors (Kuida et al., 1996, Kuida et al., 1998). Alternatively, when NPC apoptosis was increased, the resulting brain showed dramatic decreases in brain size (Altman & Bayer, 1969, Depaepe, 2005). If the changes in apoptosis were isolated to a particular NPC pool (such as the EGL), only the brain regions receiving neurons from that pool were affected (Altman & Bayer, 1969, Depaepe, 2005). From this research the prevailing view is that premature NPC death deletes not only that cell but also the other NPCs and neurons that would have been produced by that NPC (Figure 1B). In this way, due to the exponential growth of this cell population during development (or lack thereof), the dysregulation NPC apoptosis can have a greatly magnified effect on proliferation and neurogenesis. Ultimately, the results of these studies have positioned the regulation of NPC apoptosis as a critical step in normal neurodevelopment and sparked numerous studies in this field (De la Rosa, 2000, De Zio et al., 2005, & Haydar et al., 1999). Our research suggests that endogenous GC stimulation may be a previously unknown endogenous signal regulating NPC apoptosis in the EGL. Such a discovery would be important since numerous signals have been found to effect neuronal apoptosis but relatively few are known to effect NPC apoptosis (De Zio et al., 2005). Based on this information, a similar effect of GINA on proliferation may explain the numerous studies showing that increased GC exposure inhibits cerebellar proliferation (Bohn & Lauder, 1980, Ferguson & Holson, 1999, Heine & Rowitch, 2009) but decreased GC exposure enhances proliferation (Yehuda, R., et al., 1989).
Chronic Verses Acute GC Stimulation
In a recent paper, Heine & Rowitch (2009) replicated our finding that acute DEX exposure produces GINA (Noguchi et al., 2008) but concluded that chronic exposure at the same dose only decreases EGL proliferation while producing no GINA. In light of this information, we replicated their chronic drug regimen but added an essential control group missing from the original design. The results proved that even chronic DEX exposure produces GINA but, without a proper control, this effect was masked due the transient nature of activated caspase-3 expression in apoptotic cells. Furthermore, we found that simply producing EGL apoptosis (in the absence of GC stimulation) similarly masks DEX induced GINA suggesting this is a general effect of chronic NPC loss rather than something distinctive about chronic GC stimulation. These results suggest that as chronic DEX is administered, it kills off the cells susceptible to GINA before on-going proliferation could replenish their numbers. By PND7, the loss of transient activated caspase-3 expression from previous injections and decreased NPC population would result in a misleading decrease in the number of activated caspase-3 positive cells per section resulting in artificially decreased measurements for apoptosis.
Conclusions
In previous research we showed that acute GC exposure in the neonatal rodent can produce rapid apoptosis in cerebellar NPCs and permanent decreases in the number of cerebellar granule cell neurons (Noguchi et al., 2008). Here we show that GINA is mediated through GC receptor stimulation and that several different types of GCs can produce this toxicity. It was also shown that GCs the HSD2 enzyme can efficiently break down are less toxic at equipotent doses in the normal mouse. Furthermore, we show that two mechanisms that increase GC stimulation as the EGL naturally disappears, HSD2 expression and corticosterone levels, critically regulate GINA expression and that endogenous GC stimulation is responsible for a significant amount of physiological apoptosis seen in the EGL. Taken as a whole, these results suggest the GC stimulation plays a role in cerebellar development by regulating the amount of NPC apoptosis in the EGL. Additionally, the neuroprotective effect HSD2 has against GINA indicates that drugs this enzyme can break down may be less toxic and the preferred choice in the perinatal clinical setting.
Acknowledgments
This study was supported by NIH grants MH083046, HD062171, ES012443, & HD055365
Abbreviations
- DEX
dexamethasone
- EGL
external granule layer
- GC
glucocorticoid
- GINA
glucocorticoid induced neural progenitor cell apoptosis
- HSD2
11β-Hydroxysteroid Dehydrogenase Type 2
- NPC
neural progenitor cell
- PND
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and functions. CRC Press; Boca Raton: 1997. [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosciences. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Andersen BB, et al. A quantitative study of the human cerebellum with unbiased stereological techniques. The Journal of comparative neurology. 1992;326:549–60. doi: 10.1002/cne.903260405. [DOI] [PubMed] [Google Scholar]
- Benders MJ, et al. Brain development of the preterm neonate after neonatal hydrocortisone treatment for chronic lung disease. Pediatric research. 2009;66:555–9. doi: 10.1203/PDR.0b013e3181b3aec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesova O, Pavlik A. Brain glucocorticoid receptors and their role in behavioural teratogenicity of synthetic glucocorticoids. Archives of toxicology. Supplement. = Archiv fur Toxikologie. 1985;8:73–6. doi: 10.1007/978-3-642-69928-3_7. [DOI] [PubMed] [Google Scholar]
- Benesova O, Pavlik A. Perinatal treatment with glucocorticoids and the risk of maldevelopment of the brain. Neuropharmacology. 1989;28:89–97. doi: 10.1016/0028-3908(89)90073-7. [DOI] [PubMed] [Google Scholar]
- Bohn MC, Lauder JM. Cerebellar granule cell genesis in the hydrocortisone-treated rats. Developmental neuroscience. 1980;3:81–9. doi: 10.1159/000112380. [DOI] [PubMed] [Google Scholar]
- Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330–8. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- Dakine N, et al. Effects of experimental hypothyroidism on the development of the hypothalamo-pituitary-adrenal axis in the rat. Life sciences. 2000;67:2827–44. doi: 10.1016/s0024-3205(00)00869-9. [DOI] [PubMed] [Google Scholar]
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends in neurosciences. 2000;23:454–8. doi: 10.1016/s0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- Depaepe V, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–50. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- De Zio D, Giunta L, Corvaro M, Ferraro E, Cecconi F. Expanding roles of programmed cell death in mammalian neurodevelopment. Semin Cell Dev Biol. 2005;16:281–94. doi: 10.1016/j.semcdb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Eventov-Friedman S, Shinwell ES. Current controversies in perinatal steroid therapy. Acta paediatrica. 2008;97:1492–501. doi: 10.1111/j.1651-2227.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Holson RR. Neonatal dexamethasone on day 7 causes mild hyperactivity and cerebellar stunting. Neurotoxicology and teratology. 1999;21:71–6. doi: 10.1016/s0892-0362(98)00029-4. [DOI] [PubMed] [Google Scholar]
- Gould E, et al. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. The Journal of comparative neurology. 1991;313:479–85. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Napper RM. Quantitative study of granule and Purkinje cells in the cerebellar cortex of the rat. The Journal of comparative neurology. 1988;274:151–7. doi: 10.1002/cne.902740202. [DOI] [PubMed] [Google Scholar]
- Haydar TF, et al. The role of cell death in regulating the size and shape of the mammalian forebrain. Cerebral cortex (New York, NY. 1999;9:621–6. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. The Journal of clinical investigation. 2009;119:267–77. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MC, et al. 11beta-Hydroxysteroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience. 2006;137:865–73. doi: 10.1016/j.neuroscience.2005.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Testas I, Baulieu EE. Inhibition of glucocorticosteroid action in cultured L-929 mouse fibroblasts by RU 486, a new anti-glucocorticosteroid of high affinity for the glucocorticosteroid receptor. Exp Cell Res. 1983;147:177–82. doi: 10.1016/0014-4827(83)90282-3. [DOI] [PubMed] [Google Scholar]
- Keil DE, et al. Differences in the effects of dexamethasone on macrophage nitrite production: dependence on exposure regimen (in vivo or in vitro) and activation stimuli. International journal of immunopharmacology. 1995;17:157–66. doi: 10.1016/0192-0561(95)00008-p. [DOI] [PubMed] [Google Scholar]
- Kuida K, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–72. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–37. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Lodygensky GA, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1–7. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- Matthews SG, et al. Fetal glucocorticoid exposure and hypothalamo-pituitary-adrenal (HPA) function after birth. Endocrine research. 2004;30:827–36. doi: 10.1081/erc-200044091. [DOI] [PubMed] [Google Scholar]
- Mesripour A, Hajhashemi V, Rabbani M. Metyrapone and mifepristone reverse recognition memory loss induced by spontaneous morphine withdrawal in mice. Basic Clin Pharmacol Toxicol. 2008;102:377–81. doi: 10.1111/j.1742-7843.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- Murphy KE, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372:2143–51. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Antenatal corticosteroids revisited: repeat courses. NIH consensus statement. 2000;17:1–18. [PubMed] [Google Scholar]
- Noguchi KK, et al. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell death and differentiation. 2008;15:1582–92. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onland W, et al. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics. 2009;123:367–77. doi: 10.1542/peds.2008-0016. [DOI] [PubMed] [Google Scholar]
- Parikh NA, Lasky RE, Kennedy KA, Moya FR, Hochhauser L, Romo S, Tyson JE. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119:265–72. doi: 10.1542/peds.2006-1354. [DOI] [PubMed] [Google Scholar]
- Pavlik A, Buresova M. The neonatal cerebellum: the highest level of glucocorticoid receptors in the brain. Brain research. 1984;314:13–20. doi: 10.1016/0165-3806(84)90171-8. [DOI] [PubMed] [Google Scholar]
- Pedraz JL, Lanao JM, Dominguez-Gil A. Interspecies pharmacokinetics of ketamine. In: Domino EF, editor. Status of Ketamine in Anesthesiology. Ann Arbor: NPP Books; 1990. pp. 285–295. [Google Scholar]
- Peltoniemi OM, et al. Trial of early neonatal hydrocortisone: two-year follow-up. Neonatology. 2009;95:240–7. doi: 10.1159/000164150. [DOI] [PubMed] [Google Scholar]
- Rademaker KJ, de Vries WB. Long-term effects of neonatal hydrocortisone treatment for chronic lung disease on the developing brain and heart. Seminars in fetal & neonatal medicine. 2009;14:171–7. doi: 10.1016/j.siny.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Rademaker KJ, et al. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. The Journal of pediatrics. 2007;150:351–7. doi: 10.1016/j.jpeds.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Robson AC, et al. 11 Beta-hydroxysteroid dehydrogenase type 2 in the postnatal and adult rat brain. Brain research. 1998;61:1–10. doi: 10.1016/s0169-328x(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Schimmer BP, Parker KL. Adrenocorticotropic Hormone; Adrenocortical Steroids And Their Synthetic Analogs; Inhibitors Of The Synthesis And Actions Of Adrenocortical Hormones. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11. Chapter 59. New York: The McGraw-Hill Companies, Inc; 2006. pp. 1587–1612. [Google Scholar]
- Schmidt MV, et al. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. International journal of developmental neuroscience. 2003;21:125–32. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, et al. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain research. 2002;139:39–49. doi: 10.1016/s0165-3806(02)00519-9. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Marunouchi T. Immunohistochemical analysis of developmental stage of external granular layer neurons which undergo apoptosis in postnatal rat cerebellum. Neurosci Lett. 1998;242:85–8. doi: 10.1016/s0304-3940(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–5. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. 2007;120:40–8. doi: 10.1542/peds.2006-3158. [DOI] [PubMed] [Google Scholar]
- Yeh TF, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. The New England journal of medicine. 2004;350:1304–13. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. Enhanced brain cell proliferation following early adrenalectomy in rats. Journal of neurochemistry. 1989;53:241–8. doi: 10.1111/j.1471-4159.1989.tb07320.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Meyer JS. Regional patterns of brain growth during the first three weeks following early adrenalectomy. Physiology & behavior. 1991;49:233–7. doi: 10.1016/0031-9384(91)90037-o. [DOI] [PubMed] [Google Scholar]