Abstract
Glycosylation plays an important role in several types of recognition processes associated with fertilisation and development, allergies, pathological events and cell death. Whereas the amino acid sequence of a protein is fixed by the DNA, the glycosylation abilities depend on enzymes and substrates currently present in the cell.
During the last decades our knowledge on glycosylation – the structure of glycans as well as the corresponding biochemical pathways including the responsible enzymes - especially on glycans of mammalian origin increased enormously. The glycosylation capabilities of other species were under investigation only if their glycans were for any reason connected to human life (e.g. some recognition processes of pathogens or allergy on food or plant glycans) or if they were potent candidates for cell culture systems for the expression of therapeutic agents (some insect, yeast and plant cells). However, in the meantime there is an increasing interest also in invertebrate glycosylation.
Snails in particular show a broad spectrum of glycosylation abilities within their N-glycosylation pattern. In one case this has been shown to be involved in an intermediate host – parasite recognition process. For other snail species, it was found that they share many structural elements of N-glycans with mammals, plants, insects or nematodes. Sometimes several of these elements are present within one single structure.
Here we present an overview of the current knowledge of N-glycosylation of snails, the glycan structures and the corresponding enzymes involved in the biosynthetic glycosylation pathway.
Keywords: gastropod, snail, N-glycan, glycosyltransferase
INTRODUCTION
The structure of protein-linked glycans determines recognition events, decides about binding or not and influences the speed or quality of biological processes. Differences within the oligosaccharide structures appear not only between different species but also between individuals of the same species. Furthermore the current glycosylation pattern highly depends on the developmental and physiological status of the cell.
So, in medicine, the microheterogeneity of glycans has to be kept in mind in transfusion and transplantation events, especially, of course, in xenotransplantation. Since changes in the glycosylation pattern can be observed in the course of several diseases, this can be used for diagnoses or as control of therapy success. Different glycosylation features also play an important role in allergic processes against plant extracts, pollen or some food [1-3].
A number of pharmaceutically relevant products are already produced in cell-culture. Whereas the amino acid sequence of a protein is fixed by the cloned DNA, the posttranslational modifications depend on the current presence of enzymes and substrates in the expression cell-line. Amongst all possible modifications, the glycosylation pattern of the recombinant glycoprotein has to be analysed especially carefully. Glycosylation contributes not only to physical properties, such as conformational stability, protease resistance, charge or hydrophilicity, but glycans may also function as recognition determinants in host-pathogen relationships, protein targeting and cell-cell interactions [4,5]. Incorrect glycosylation may result, in the worst case, in a complete loss of activity or in the formation of allergenic structures [1,6]. Economic reasons force industry more and more to establish easier and cheaper cell culture systems for the production of recombinant glycoproteins for therapeutic purposes, but nevertheless special care has to be taken that the glycan moieties of the produced proteins are highly similar to those of the natural counterparts in order to keep their function. At the moment many of the pharmaceutically relevant glycoproteins, where a correct glycan structure is essential for their function, are produced in mammalian cell cultures because of their similarity to the human system; however, these are expensive and sensitive systems with some risks of virus transfer and so non-mammalian sources are under closer investigation. Therefore the investigation of glycosylation capacities combined with the analysis of substrate specificity and the order of involved enzymes is a growing field in this area.
Bacteria do not N-glycosylate in the same way as eukaryotes and yeasts often produce very large oligomannosidic glycans but lack the ability to synthesise complex ones. The glycosylation potential of insect cells is, due to their advantage in terms of costs as well as of biosafety, of pharmaceutical interest. Baculovirus expression systems as well as non-lytic systems are already used for the expression of recombinant proteins with small complex glycans, but they seem to lack the ability to produce negatively charged complex N-glycans in an appreciable amount, if at all [7,8]. Currently the engineering of transgenic insect cell lines is under investigation in order to express mammalian glycosyltransferases and enzymes synthesising the appropriate activated sugar molecules but up to now these systems are not in use for large scale production [9,10]. Furthermore other non-mammalian features, such as α1,3-fucosylation of the inner GlcNAc-residue of N-glycans, may occur on recombinant glycoproteins derived from insect cell culture. This structural feature is also typical for plants and has been shown to be highly immunogenic [1,11]. For this reason also plant cells have to be modified before they can be used as production system for pharmaceutical relevant glycoproteins. Another deficit of plant glycans, the production of too small antennae lacking terminal GlcNAc-residues, seems to be solved by the co-expression of a human N-acetylglucosaminyltransferase III which prevents the degradation of the antennae [12].
So, up to now, no optimal system has been found which allows the production of recombinant glycoproteins including all types of N-glycosylation in large quantities and constantly high quality. This is the reason why other non-mammalian sources are under investigation for their glycosylation abilities. The current knowledge on gastropod N-glycosylation is described here.
BIOSYNTHESIS OF N-GLYCANS
N-Glycosylation starts in the Endoplasmatic Reticulum by formation of a lipid linked precursor, containing two GlcNAc residues, nine mannoses and three glucoses, which is transferred en bloc onto the growing polypeptide chain. The processing of the N-glycans from Glc3Man9GlcNAc2 to complex structures is based on a strict order of the action of glycosidases and glycosyltransferases [13]. The individual glycosylation pattern produced by a cell is based on an interplay between substrates and enzymes, as well as their current availability and location within this cell. These factors are responsible for the creation of an individual glycosylation pattern in each specific cell, yielding species-, organ- and tissue-specific glycans.
While the first steps of the biosynthetic pathway of N-glycans seem to be similar in nearly all kinds of organisms, only very small modifications have been found so far there, the final modification steps are highly heterogeneous and lead to the wide range of various glycan structures.
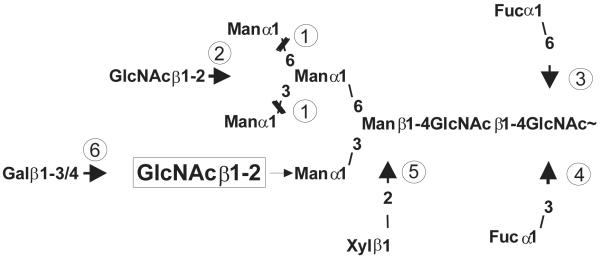
The key enzyme for the formation of complex glycans is the GlcNAc-transferase I, which forms the GlcNAcMan5GlcNAc2-structure [14]. After previous action of GlcNAc-transferase I, the glycan is a suitable substrate for a number of further modifying enzymes such as core α1,6-fucosyltransferase, core α1,3-fucosyltransferase, β1,2-xylosyltransferase, β1,2-GlcNAc-transferase II and β1,3 – or β1,4- galactosyltransferase which build up larger and more complex glycan structures (Fig 1). Most of the known modifying glycosyltransferases depend on the previous action of GlcNAc-transferase I, only few exceptions have been found up to now [15]. In some tissues the glycans are also further modified by additional non-sugar structural features, such as methylation, sulphation, phosphorylation or others to create even more variations.
Fig. 1.
Possible modifications of a glycan after the action of GlcNAc-transferase I. (1) Mannosidase II; (2) GlcNAc-transferase II; (3) Core α1,6-fucosyltransferase; (4) Core α1,3-fucosyltransferase; (5) β1,2-xylosyltransferase; (6) β1,3- or β1,4-galactosyltranferase.
ANALYSIS OF THE N-GLYCOSYLATION POTENTIAL
The glycosylation pathway of a specific cell or organism is usually elucidated on one hand by the analysis of the glycan structures and on the other hand by the determination of the enzymes which are responsible for the formation of these glycans.
First the glycan structures are released from the protein-backbone by specific glycopeptidases followed by labelling with a fluorescent dye. Routine analysis is carried out by two-dimensional HPLC-technique (separation by size and hydrophobicity) and mass spectrometry with or without fragmentation. Sometimes also digestion with specific exoglycosidases is used to get further information on type and linkage of terminal sugar residues [16,17]. However, even though this approach sounds quite simple, the strategy and the purification protocols have to be optimised for each kind of tissue separately, due to the highly complex sample matrices. With permanently evolving methods a continuous increase in detection sensitivity can be ensured [18].
Mammalian enzymes involved in the biosynthesis of glycans show a relatively high genetic similarity throughout different species and therefore it is often possible to identify further enzymes by homology search. Even though invertebrates and plants contain several enzymes with quite similar substrate specificity, the genetic homology of these enzymes with the mammalian ones is usually not high enough for determination by homology search.
N-GLYCANS OF MOLLUSCS
Hemocyanin, the oxygen-carrier of arthropods and molluscs, was the first target of investigation in the elucidation of the gastropod proteome. There, the occurrence of N-glycans in molluscs was detected [19,20] and soon a novel modification of glycans was identified: the methylation of hexoses (mannoses and galactoses) [21]. This kind of modification does not occur in mammals. Up to now it has been found in the kingdom of animals only in nematodes (2-O-methylated terminal fucose as well as 4-O-methylated galactose) [22] and in molluscs (see later in detail). All other studies on gastropods at that time were dealing with the analysis of the biochemical parameters of exoglycosidases or the binding specificities of lectins derived from snails.
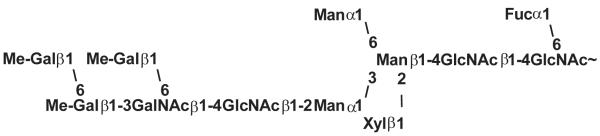
Since the nineteen-eighties the increase of advanced technical opportunities facilitated a more accurate and precise investigation of glycans. With this impact the detailed analysis of gastropod structures started again with the hemocyanin of some selected snails. It was shown that Helix pomatia hemocyanin includes complex structures containing a common core with a fucose α1,6-linked to the reducing GlcNAc and a xylose β1,2-linked to the β-mannose residue. One or both α-mannose residues may be substituted by GalNAcβ1,4GlcNAcβ1,2-elements which contain two to four β1,3- or β1,6-linked galactoses with or without 3- or 4-O-methylgroups (Fig 2) [12,24]. A recent study shows that especially the xylose and terminal 3-O-methylated galactoses seem to be responsible for the cross-reactivity of Helix pomatia glycoproteins [25].
Fig. 2.
The most complex N-glycan-chain of Helix pomatia [24].
Lymnaea stagnalis hemocyanin contains low and high molecular mass biantennary oligosaccharides. They lack the α1,6-linked fucose at the inner GlcNAc-residue but some antennae are terminated by an α1,2-linked fucose. The basic element of the antennae is a Galβ1,3GalNAcβ1,4GlcNAc unit [26,27]. The glycans of the Unio elongatulus gp273, which is the ligand for sperm-egg interaction in this mollusc bivalve, are of the oligomannosidic type (Glc1Man9GlcNAc2 and Man9GlcNAc2) [28].
Megathura crenulata (keyhole limpet) hemocyanin glycans carry a novel type of modification with galactose directly linked in β1,6-linkage to the α1,3- or the α1,6-linked mannose residues or even the β-linked mannose of the N-glycan core [29]. Some of the glycans are decorated at the α1,3-antenna of the trimannosyl core with the Fucα1,3GalNAcβ1,4[Fucα1,3]GlcNAc motif, which also occurs in schistosomal glycoconjugates mediating cross reactivities between these two organisms [30]. For the first time an elongation by up to two galactose residues of the fucose α1,6-linked to the inner core N-acetylglucosamine was found [31]. The two N-glycans of the functional unit RvH1-a of Rapana venosa hemocyanin are biantennary non-fucosylated oligosaccharides with terminal 3-O-methylated β1,3-linked galactose residues. One of them carries a sulphate group on the α1,6-linked core mannose and a 3-O-methylated GlcNAc residue linked β1,2 to the β-mannose of the core [32]. Furthermore, an unusually branched fucose residue substituted by a hexosamine and a hexouronic acid has been found here [33]. In Rapana thomasiana a similar fucose residue is elongated by 3-O-methylgalactose and N-acetylgalactosamine [34].
In Biomphalaria glabrata a core structure terminated by two 3-O-methylated mannose residues linked to the major soluble protein of the organic shell matrix was initially identified [35].
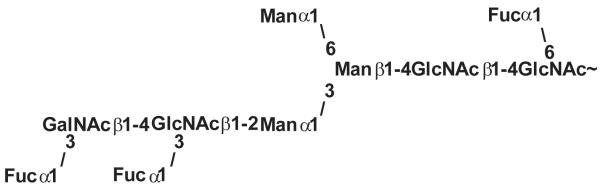
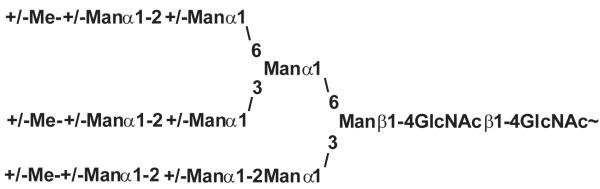
Later, several carbohydrate determinants such as terminal Fucα1,3GalNAc units or β1,2-linked xylose which are common to this intermediate host snail, as well as to the corresponding parasite, Schistosoma mansoni, were found (Fig. 3) [36,37]. In the course of our own previous projects we analysed the N-glycosylation patterns of whole tissue extracts derived from Arion lusitanicus, Limax maximus, Cepaea hortensis, Planorbarius corneus, Arianta arbustorum and Achatina fulica. There we found glycans with all structural features mentioned above and some more. The snails contain a broad spectrum of elements which are already known from different organisms [38-40]. Besides the common high mannosidic structures (Man3GlcNAc2 to Man9GlcNAc2) the same structures with up to three additional methyl groups occur (Fig 4). Xylosylation at the β-mannose and core fucosylation in α1,6-linkage are frequent modifications, but also core α1,3 fucosylation, terminal α1,3 fucosylation and terminal α1,2 fucosylation were found. The larger, complex glycans are terminated by galactose residues with or without a methyl group. A study on Achatina fulica eggs and young snails of different ages (up to 120 days) showed that at these young ages structures with several terminal GlcNAc-residues are dominant [41]. These changes of the N-glycan pattern during development may be due to different expression levels of various glycosyltransferases and glycosidases.
Fig. 3.
One example of a N-glycan of Biomphalaria glabrata hemolymph [36].
Fig. 4.
High mannosidic N-glycans with three to nine mannose residues and up to three methyl groups [39,40].
SNAIL ENZYMES INVOLVED IN GLYCOSYLATION
The information given on enzymes which are involved in glycan biosynthesis is restricted in most cases to enzyme specificity in vitro and some biochemical parameters. Lymnaea stagnalis has been shown to express the key enzyme for the formation of complex N-glycans, GlcNAc-transferase I, the prerequisite for the action of further modifying glycosyltransferases [42]. This water snail also expresses GlcNAc-transferase II and xylosyltransferase [42], a β1,4-GalNAc-transferase, which shows high homology to mammalian β1,4-galactosyltransferase [43], a β1,3-galactosyltransferase and an α1,2-fucosyltransferase, both located in the connective tissue [44,45]. Another β1,3-galactosyltransferase purified from the albumen gland is not involved in N-glycan biosynthesis but in the formation of Galβ1,3Galβ1,4Glc units. This enzyme may be essential for the production of galactogen, the main polysaccharide of Lymnaea stagnalis [46].
Hybridization experiments using the cDNA of bovine β1,4-galactosyltransferase as probe, revealed a β1,4-GlcNAc-transferase which requires a different nucleotide sugar but is similar to the mammalian galactosyltransferase in acceptor specificity and definitely not involved in the biosynthesis of the chitobiose core of N-glycans [47,48]. This enzyme was the first glycosyltransferase described where an exon duplication seems to have taken place during evolution (exons 6, 7 and 8 show extremely high sequence similarity) [49].
Furthermore an α1,3-fucosyltransferase catalysing the transfer of fucose from GDP-fucose to a Galβ1,4GlcNAc acceptor forming the Lewisx -unit has been found in the connective tissue of Lymnea stagnalis [45] and an α1,3-fucosyltransferase catalysing the transfer of fucose from GDP-fucose to the asparagine-linked GlcNAc has been found in the albumin and prostate glands of the same snail [50]. However, neither Lewisx-containing structures nor core α1,3-fucosylated structures have been detected in this snail so far. Another enzyme of which the in vivo products are still unknown, is the β1,4-glucosyltranferase present in the prostate gland of Lymnaea stagnalis. It catalyses the transfer of glucose from UDP-glucose to terminal GlcNAc-residues which are linked β1,6 to a galactose or a GalNAc residue in O-glycans, or, in N-glycans to a terminal GlcNAc which is β1,2-linked to mannose [51]. In Helix pomatia an α1,2-L-galactosyltransferase which seems to be involved in the elongation of the storage polysaccharides of the snail was found [52]. Besides its galactose transferring ability, this galactosyltransferase catalyses the transfer of a fucose from GDP-fucose into α1,2-linkage to a Galβ1,3Gal-O-Me substrate in vitro as well; nothing is known about this ability in vivo. Other galactosyltransferases with β1,6-specificity building linear chains of D-galactoses or creating branching points in galactans were identified in Biomphalaria glabrata, Helix pomatia and Arianta arbustorum [53,54].
Besides the glycosyltransferases some glycosidases (glucosidase I and II, mannosidase I and II) are involved in the biosynthetic pathway of N-glycans too. So far none of these specific exoglycosidases have been determined. Those glycosidases which have been found in snails seem to be more likely part of the degradation system for galactans, mannans and cellulose [55,56]. However, these enzymes may be of economical interest and some are checked for a use in food industry [57]. From Pomacaea canaliculata three exoglycosidases have been characterised. The α-mannosidase has a broad specificity cleaving α1,2-, α1,3- and α1,6-mannosidic linkages [58]. The fucosidase and the xylosidase are able to release the corresponding sugar residues from plant-derived oligosaccharides, α1,3-linked fucose and β1,2-linked xylose respectively, but have not been tested with other glycans [59]. For some exoglycosidases also genetic information is available. An endo-β-mannosidase from Haliotis discus hannai has been isolated and cloned and two cellulases from Pomacaea canaliculata were investigated in detail for their genomic organization and their expression in snails of different ages [60,61].
The genetic information on snails in general is marginal. There are some data mainly on enzymes involved in the oxidative phosphorylation process which are known to be extremely conserved. So far there are no data on glycosyltransferases. Because of its medical relevance as an intermediate host a genome initiative on Biomphalaria glabrata is ongoing which supplies at least some genetic information on this snail (http://biology.unm.edu/biomphalaria-genome/).
FUNCTION
Due to their diversity, glycans provide the most perfect instruments for each single cell to assign its communication and other recognition processes. These processes are well investigated in mammals and somehow in plants but until now not in gastropods. However, there is no doubt that glycans are involved in these processes in snails too. For gastropods only medical relevant recognition events are under investigation. They can be divided into two groups: one, where the interaction of the intermediate host gastropod with a parasite is analysed. It has been known for about twenty years that snails and parasites share some epitopes which cause cross-reactivity in vitro and antibody production in vivo [62]. This reaction can be used on one hand for the diagnosis and on the other hand for the design of drugs against the parasites [63-68].
And the second one, where gastropod glycoproteins are studied for their use in cancer therapy. Some gastropod lectins bind specifically to certain types of cancer cells allowing diagnoses and prognoses [69]. Also natural or modified gastropod glycoproteins are utilised against cancer cells by stimulating the human immune response [70-72].
CONCLUSION
The analysis of gastropod glycosylation is an emerging field in glycobiology, but we are still at the very beginning. A number of structure analyses have already been performed and several completely new glycan modifications have been detected. However, only few enzymes which are involved in the glycosylation process have been isolated and characterised so far and none have been cloned. Lacking these genetic data, not much can be said about the evolution of the enzymes involved in glycosylation. From the specificity data of the enzymes and the structural analysis of the glycans no distinctive differences between land and water snails or snails with and without shell can be made out yet.
Currently the main driving force for this field of research is the interest in new model organisms with a broad glycosylation spectrum to understand the biosynthesis of all different kinds of glycans. This new information could than be applied to medicine for the establishment of new diagnosis and therapy tools as a consequential reward.
ACKNOWLEDGEMENTS
Our work was funded by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung projects P13928-BIO and P20393-B11.
ABBREVIATIONS
- Fuc
fucose
- Gal
galactose
- GalNAc
N-Acetylgalactosamine
- Glc
glucose
- GlcNAc
N-Acetylglucosamine
- Man
mannose
- Xyl
xylose
REFERENCES
- [1].Tretter V, Altmann F, Kubelka V, März L, Becker WM. Fucose α1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int. Arch. Allergy Immunol. 1993;102:259–266. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- [2].Wilson IBH, Harthill JE, Mullin NP, Ashford DA, Altmann F. Core α1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology. 1998;8:651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]
- [3].Altmann F. The role of protein glycosylation in allergy. Int. Arch. Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- [4].Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu. Rev. Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- [5].Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu. Rev. Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- [6].Aeed PA, Elhammer ÅP. Glycosylation of recombinant prorenin in insect cells: the insect cell line Sf9 does not express the mannose 6-phosphate recognition signal. Biochemistry. 1994;33:8793–8797. doi: 10.1021/bi00195a022. [DOI] [PubMed] [Google Scholar]
- [7].Altmann F, Staudacher E, Wilson IBH, März L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- [8].Choi O, Tomiya N, Kim JH, Slavicek JM, Betenbaugh MJ, Lee YC. N-glycan structures of human transferrin produced by Lymantria dispar (gypsy moth) cells using the LdMNPV expression system. Glycobiology. 2003;13:539–548. doi: 10.1093/glycob/cwg071. [DOI] [PubMed] [Google Scholar]
- [9].Hollister J, Conradt H, Jarvis DL. Evidence for a sialic acid salvaging pathway in lepidopteran insect cells. Glycobiology. 2003;13:487–495. doi: 10.1093/glycob/cwg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aumiller JJ, Hollister J, Jarvis DL. A transgenic insect cell line engineered to produce CMP-sialic acid and sialylated glycoproteins. Glycobiology. 2003;13:497–507. doi: 10.1093/glycob/cwg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Van Ree R, Cabanes-Machetau M, Akkerdaas J, Milazzo JP, Loutelier-Bourhis C, Rayon C, Villalba M, Koppelman S, Aalberse R, Rodriguez R, Faye L, Lerouge P. J. Biol. Chem. 2000;275:11451–11458. doi: 10.1074/jbc.275.15.11451. [DOI] [PubMed] [Google Scholar]
- [12].Rouwendal GJA, Wuhrer M, Florack DEA, Koeleman CAM, Deeelder AM, Bakker H, Stoopen GM, Van Die I, Helsper JPFG, Hokke CH, Bosch D. Efficient introduction of a bisecting GlcNAc residue in tobacco N-glycans by expression of the gene encoding human N-acetylglucosaminyltransferase III. Glycobiology. 2007;17:334–344. doi: 10.1093/glycob/cwl078. [DOI] [PubMed] [Google Scholar]
- [13].Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- [14].Longmore GD, Schachter H. Product-identification and substrate-specificity studies of the GDP-L-fucose:2-acetamido-2-deoxy-β-D-glucoside (FUC goes to Asn-linked GlcNAc) 6-α-L-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydr Res. 1982;100:365–392. doi: 10.1016/s0008-6215(00)81049-6. [DOI] [PubMed] [Google Scholar]
- [15].Paschinger K, Staudacher E, Stemmer U, Fabini G, Wilson IB. Fucosyltransferase substrate specificity and the order of fucosylation in invertebrates. Glycobiology. 2005;15:463–474. doi: 10.1093/glycob/cwi028. [DOI] [PubMed] [Google Scholar]
- [16].Geyer H, Geyer R. Strategies for analysis of glycoprotein glycosylation. Biochim Biophys Acta. 2006;1764:1853–1869. doi: 10.1016/j.bbapap.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [17].Domann PJ, Pardos-Pardos AC, Fernandes DL, Spencer DI, Radcliffe CM, Royle L, Dwek RA, Rudd PM. Separation-based glycoprofiling approaches using fluorescent labels. Proteomics. 2007;7(Suppl 1):70–76. doi: 10.1002/pmic.200700640. [DOI] [PubMed] [Google Scholar]
- [18].North SJ, Hitchen PG, Haslam SM, Dell A. Mass spectrometry in the analysis of N-linked and O-linked glycans. Curr. Opin. Struct. Biol. 2009;19:1–9. doi: 10.1016/j.sbi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dijk J, Brouwer M, Coert A, Gruber M. Structure and function of hemocyanins. VII. The smallest subunit of alpha- and beta-hemocyanin of Helix pomatia: size, composition, N- and C-terminal amino acids. Biochim Biophys Acta. 1970;221:467–479. doi: 10.1016/0005-2795(70)90217-5. [DOI] [PubMed] [Google Scholar]
- [20].Waxman L. The structure of arthropod and mollusc hemocyanins. J. Biol. Chem. 1975;250:3796–3806. [PubMed] [Google Scholar]
- [21].Hall RL, Wood EJ, Kamerling JP, Gerwig GJ, Vliegenthart JFG. 3-O-Methyl sugars as constituents of glycoproteins. Biochem. J. 1977;165:173–176. doi: 10.1042/bj1650173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khoo KH, Maizels RM, Page AP, Taylor GW, Rendell NB, Dell A. Characterization of nematode glycoproteins: the major O-glycans of Toxocara excretory-secretory antigens are O-methylated trisaccharides. Glycobiology. 1991;1:163–171. doi: 10.1093/glycob/1.2.163. [DOI] [PubMed] [Google Scholar]
- [23].van Kuik JA, van Halbeek H, Kamerling JP, Vliegenthart JFG. Primary structure of the low molecular weight-carbohydrate chains of Helix pomatia α-hemocyanin. Xylose as a constituent of N-linked oligosaccharides in an animal glycoprotein. J. Biol. Chem. 1985;260:13984–13988. [PubMed] [Google Scholar]
- [24].Lommerse JPM, Thomas-Oates JE, Gielens C, Préaux G, Kamerling JP, Vliegenthart JFG. Primary structure of 21 novel monoantennary and diantennary N-linked carbohydrate chains from αD-hemocyanina of Helix pomatia. Eur. J. Biochem. 1997;249:195–222. doi: 10.1111/j.1432-1033.1997.00195.x. [DOI] [PubMed] [Google Scholar]
- [25].Siddiqui NI, Yigzaw Y, Préaux G, Gielens C. Involvement of glycans in the immunological cross-reaction between α-macroglobulin and hemocyanin of the gastropod Helix pomatia. Biochimie. 2009;91:508–516. doi: 10.1016/j.biochi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- [26].Van Kuik JA, Sijbesma RP, Kamerling JP, Vliegenthart JFG, Wood EJ. Primary structure of a low-molecular-mass N-linked oligosaccharide from hemocyanin of Lymnaea stagnalis. 3-O-methyl-D-mannose as a constituent of the xylose-containing core structure in an animal glycoprotein. Eur. J. Biochem. 1986;160:621–625. doi: 10.1111/j.1432-1033.1986.tb10083.x. [DOI] [PubMed] [Google Scholar]
- [27].Van Kuik JA, Sijbesma RP, Kamerling JP, Vliegenthart JFG, Wood EJ. Primary structure determination of seven novel N-linked carbohydrate chains derived from hemocyanin of Lymnaea stagnalis. 3-O-methyl-D-galactose and N-acetyl-D-galactosamine as constituents of xylose-containing N-linked oligosaccharides in an animal glycoprotein. Eur. J. Biochem. 1987;169:399–411. doi: 10.1111/j.1432-1033.1987.tb13626.x. [DOI] [PubMed] [Google Scholar]
- [28].Di Patrizi L, Capone A, Focarelli R, Rosati F, Gallego RG, Gerwig GJ, Vliegenthart JFG. Structural characterization of the N-glycans gp273, the ligand for sperm-egg interaction in the mollusc bivalve of the Unio elongatulus. Glycoconjugate J. 2001;18:511–518. doi: 10.1023/a:1019617728660. [DOI] [PubMed] [Google Scholar]
- [29].Kurokawa T, Wuhrer M, Lochnit G, Geyer H, Markl J, Geyer R. Hemocyanin from the keyhole limpet Megathura crenulata (KLH) carries a novel type of N-glycans with Gal(β1-6)Man-motifs. Eur. J. Biochem. 2002;269:5459–5473. doi: 10.1046/j.1432-1033.2002.03244.x. [DOI] [PubMed] [Google Scholar]
- [30].Geyer H, Wuhrer M, Resemann A, Geyer R. Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J. Biol. Chem. 2005;280:40731–40748. doi: 10.1074/jbc.M505985200. [DOI] [PubMed] [Google Scholar]
- [31].Wuhrer M, Robijn MLM, Koeleman CAM, Balog CIA, Geyer R, Deelder AM, Hokke CH. A novel Gal(β1,4)Gal(β1,4)Fuc(α1,6)-core modification attached to the proximal N-acetylglucosamine of keyhole limpet haemocyanin (KLH) N-glycans. Biochem. J. 2004;378:625–632. doi: 10.1042/BJ20031380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dolashka-Angelova P, Beck A, Dolashiki A, Beltramini M, Stevanovic S, Salvato B, Voelter W. Characterization of the carbohydrate moieties of the functional unit RvH1-a of Rapana venosa haemocyanin using HPLC/electrospray ionization MS and glycosidase digestion. Biochem. J. 2003;374:185–192. doi: 10.1042/BJ20030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sandra K, Dolashka-Angelova P, Devereese B, Van Beeumen J. New insights in Rapana venosa hemocyanin N-glycosylation resulting from on-line mass spectrometric analyses. Glycobiology. 2007;17:141–156. doi: 10.1093/glycob/cwl063. [DOI] [PubMed] [Google Scholar]
- [34].Gielens C, Idakieva K, van den Bergh V, Siddiqui NI, Parvanova K, Compernolle F. Mass spectral evidence for N-glycans with branching on fucose in a molluscan hemocyanin. Biochem. Biophys. Res. Commun. 2005;331:562–570. doi: 10.1016/j.bbrc.2005.03.217. [DOI] [PubMed] [Google Scholar]
- [35].Marxen JC, Nimtz M, Becker W, Mann K. The major soluble 19.6 kDa protein of the organic shell matrix of the freshwater snail Biomphalaria glabrata is an N-glycosylated dermatopontin. Biochim. Biophys. Acta. 2003;1650:92–98. doi: 10.1016/s1570-9639(03)00203-6. [DOI] [PubMed] [Google Scholar]
- [36].Lehr T, Geyer H, Maass K, Doenhoff MJ, Geyer R. Structural characterization of N-glycans from teh freshwater snail biomphalaria glabrata cross-reacting with Schistosoma mansoni glycoconjugates. Glycobiology. 2007;17:82–103. doi: 10.1093/glycob/cwl048. [DOI] [PubMed] [Google Scholar]
- [37].Lehr T, Beuerlein K, Doenhoff MJ, Grevelding CG, Geyer R. Localization of carbohydrate determinants common to Biomphalaria glabrata as well as to sporocysts and miracidia of Schistosoma mansoni. Parasitology. 2008;135:931–942. doi: 10.1017/S0031182008004514. [DOI] [PubMed] [Google Scholar]
- [38].Bürgmayr S, Grabher-Meier H, Staudacher E. Sialic acids in gastropods. FEBS Lett. 2001;508:95–98. doi: 10.1016/s0014-5793(01)03024-1. [DOI] [PubMed] [Google Scholar]
- [39].Gutternigg M, Ahrer K, Grabher-Meier H, Bürgmayr S, Staudacher E. Neutral N-glycans of the gastropod Arion lusitanicus. Eur. J. Biochem. 2004;271:1348–1356. doi: 10.1111/j.1432-1033.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- [40].Gutternigg M, Bürgmayr S, Pöltl G, Rudolf J, Staudacher E. Neutral N-glycan patterns of the gastropods Limax maximus, Cepaea hortensis, Planorbarius corneus, Arianta arbustorum and Achatina fulica. Glycoconjugate J. 2007;24:475–489. doi: 10.1007/s10719-007-9040-5. [DOI] [PubMed] [Google Scholar]
- [41].Park Y, Zhang Z, Laremore TN, Li B, Sim J-S, Im A, Ahn MY, Kim YS, Linhardt RJ. Variation of acharan sulfat ad monosaccharide composition and analysis of neutral N-glycans in African giant snail (Achatina fulica) Glycoconjugate J. 2008;25:863–877. doi: 10.1007/s10719-008-9149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mulder H, Dideberg F, Schachter H, Spronk BA, De Jong-Brink M, Kamerling JP, Vliegenthart JFG. In the biosynthesis of N-glycans in connective tissue of the snail Lymnaea stagnalis of incorporation GlcNAc by β2GlcNAc-transferase I is an essential prerequisite for the action of βGlcNAc-transferase II and β2Xyl-transferase. Eur. J. Biochem. 1995;232:272–283. doi: 10.1111/j.1432-1033.1995.tb20809.x. [DOI] [PubMed] [Google Scholar]
- [43].Mulder H, Spronk BA, Schachter H, Neeleman AP, Van den Eijneden DH, De Jong-Brink M, Kamerling JP, Vliegenthart JFG. Identification of a novel UDP-GalNAc:GlcNAcβ-R β1-4 N-acetylgalactosaminyltransferase from the albumen gland and connective tissue of the snail Lymnaea stagnalis. Eur. J. Biochem. 1995;227:175–185. doi: 10.1111/j.1432-1033.1995.tb20374.x. [DOI] [PubMed] [Google Scholar]
- [44].Mulder H, Schachter H, De Jong-Brink M, Van der Ven JGM, Kamerling JP, Vliegenthart JFG. Identification of a novel UDP-Gal:GalNAc β1-4GlcNAc-R β1-3-galactosyltransferase in the connective tissue of the snail Lymnaea stagnalis. Eur. J. Biochem. 1991;201:459–465. doi: 10.1111/j.1432-1033.1991.tb16303.x. [DOI] [PubMed] [Google Scholar]
- [45].Mulder H, Schachter H, Thomas JR, Halkes KM, Kamerling JP, Vliegenthart JFG. Identification of a GDP-Fuc:Gal β1-3GalNAc-R (Fuc to Gal) α1-2fucosyltransferase and a GDP-Fuc:Gal β1-4GlcNAc (Fuc to GlcNAc) α1-3 fucosyltransferase in connective tissue of the snail Lymnaea stagnalis. Glycoconjugate J. 1996;13:107–113. doi: 10.1007/BF01049686. [DOI] [PubMed] [Google Scholar]
- [46].Joziasse DH, Damen HCM, de Jong-Brink M, Edzes HT, Van den Eijnden DH. Identification of a UDP-Gal:β-galactoside β1,3-galactosyltransferase in the albumen gland of the snail Lymnaea stagnalis. FEBS Lett. 1987;221:139–144. doi: 10.1016/0014-5793(87)80368-x. [DOI] [PubMed] [Google Scholar]
- [47].Bakker H, Agterberg M, Van Tetering A, Koeleman CAM, Van den Eijnden DH, Van Die I. A Lymnaea stagnalis gene, with sequence similarity to that of mammalian β1-4-galactosyltransferases, encodes a novel UDP-GlcNAc:GlcNAc βR β1-4-N-acetylglucosaminyltransferase. J. Biol. Chem. 1994;269:30326–30333. [PubMed] [Google Scholar]
- [48].Bakker H, Schoenmakers PS, Koeleman CAM, Joziasse DH, Van Die I, Van den Eijnden DH. The substrate specificity of the snail Lymnea stagnalis UDP-GlcNAc:GlcNAcβ-R β4-N-acetylglucosaminyltransferase reveals a novel variant pathway of complex-type oligosaccharide synthesis. Glycobiology. 1997;7:539–548. doi: 10.1093/glycob/7.4.539. [DOI] [PubMed] [Google Scholar]
- [49].Bakker H, Van Tetering A, Agterberg M, Smith AB, Van den Eijnden DH, Van Die I. Deletion of two exons from the Lymneaea stagnalis β1,4-N-acetylglucosaminyltranferase gene elevates the kinetic efficiency of the encoded enzyme for both UDP-sugar donor and acceptor substrates. J. Biol. Chem. 1997;272:18580–18585. doi: 10.1074/jbc.272.30.18580. [DOI] [PubMed] [Google Scholar]
- [50].Van Tetering A, Schiphorst WECM, Van den Eijnden DH, Van Die I. Characterization of a core α1-3-fucosyltransferase from the snail Lymnaea stagnalis that is involved in the synthesis of complex-type N-glycans. FEBS Lett. 1999;461:311–314. doi: 10.1016/s0014-5793(99)01489-1. [DOI] [PubMed] [Google Scholar]
- [51].Van Die I, Cummings RD, Van Tetering A, Hokke CH, Koeleman CAM, Van den Eijnden DH. Identification of a novel UDP-Glc:GlcNAcβ1,4-glucosyltranferase in Lymnaea stagnalis that may be involved in the synthesis of complex-type oligosaccharide chains. Glycobiology. 2000;10:263–271. doi: 10.1093/glycob/10.3.263. [DOI] [PubMed] [Google Scholar]
- [52].Lüttge H, Heidelberg T, Stangier K, Thiem J, Bretting H. The specificity of an α(1-2)-L-galactosyltransferase from albumen glands of the snail Helix pomatia. Carbohydr. Res. 1997;297:281–288. doi: 10.1016/s0008-6215(96)00277-7. [DOI] [PubMed] [Google Scholar]
- [53].Stangier K, Lüttge H, Thiem JE, Bretting H. Biosynthesis of the storage polysaccharide from the snail Biomphalaria glabrata, identification and specificity of a branching β1,6-galactosyltransferase. J. Comp. Physiol. B. 1995;165:278–290. doi: 10.1007/BF00367311. [DOI] [PubMed] [Google Scholar]
- [54].Bretting H, Messer M, Bornaghi L, Kroger L, Mitschnick P, Thiem J. Galactan biosynthesis in snails: a comparative study of β1,6-galactosyltransferases from Helix pomatia and Biomphalaria glabrata. J. Comp. Physiol. 2000;170:601–613. doi: 10.1007/s003600000141. [DOI] [PubMed] [Google Scholar]
- [55].Bretting H, Jacobs G, Müller S, Breitfeld O. The galactan-degrading enzymes in the snail Biomphalaria glabrata. Comp. Biochem. Physiol. B. 1991;99:629–636. doi: 10.1016/0305-0491(91)90346-f. [DOI] [PubMed] [Google Scholar]
- [56].Yamaura I, Tatsumoto T. Purification and some properties of endo1,4-β-D-mannanase from a mud snail, Pomacea insularius (de Ordigny) Biosci. Biotech. Biochem. 1993;57:1316–1319. doi: 10.1271/bbb.57.1316. [DOI] [PubMed] [Google Scholar]
- [57].Tramice A, Andreotti G, Trincone A. Direct enzymatic glucosylation of naringin in grapefruit juice by α-D-glucosidase from the marine mollusc Aplysia fasciata. Biotechnol. J. 2008;3:545–554. doi: 10.1002/biot.200700161. [DOI] [PubMed] [Google Scholar]
- [58].Hirata K, Aso Y, Ishiguro M. Properties of α-mannosidase partially purified from the apple snail Pomacea canaliculata. Biosci. Biotechnol. Biochem. 1998;62:2242–2245. doi: 10.1271/bbb.62.2242. [DOI] [PubMed] [Google Scholar]
- [59].Hirata K, Nakahara Y, Kimura Y, Funatsu G. Purification and some properties of a β-xylosidase and an α-fucosidase from apple snails (Pomacea canaliculata) Biosci. Biotechnol. Biochem. 1996;60:249–254. doi: 10.1271/bbb.60.249. [DOI] [PubMed] [Google Scholar]
- [60].Ootsuka S, Saga N, Suzuki K, Inoue A, Ojima T. Isolation and cloning of an endo-β1,4-mannosidase from Pacific abalone Haliotis discus hannai. J. Biotechnol. 2006;125:269–280. doi: 10.1016/j.jbiotec.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [61].Imjongjirak C, Amparyup P, Sittipraneed S. Cloning, genomic organization and expression of two glycosyl hydrolase family 10 (GHF10) genes from golden apple snail (Pomacea canaliculata) DNA Seq. 2008;19:224–236. doi: 10.1080/10425170701517911. [DOI] [PubMed] [Google Scholar]
- [62].Dissous C, Grzych JM, Capron A. Schistosoma mansoni share a protective oligosaccharide epitope with freshwater and marine snails. Nature. 1986;323:443–445. doi: 10.1038/323443a0. [DOI] [PubMed] [Google Scholar]
- [63].Mansour MM, Ali PO, Farid Z, Simpson AJ, Woody JW. Serological differentiation of acute and chronic schistosomasis mansoni by antibody responses to keyhole limpet hemocyanin. Am. J. Trop. Med. Hyg. 1989;4:338–344. [PubMed] [Google Scholar]
- [64].Alves-Brito CF, Simpson AJ, Bahia-Oliveira LM, Rabello AL, Rocha RS, Lambertucci JR, Gazzinelli G, Katz N, Correa-Oliveira R. Analysis of anti-keyhole limpet haemocyanin antibody in Brazilians support its use for the diagnosis of acute schistosomiasis mansoni. Trans. R. Soc. Trop. Med. Hyg. 1992;86:53–56. doi: 10.1016/0035-9203(92)90439-j. [DOI] [PubMed] [Google Scholar]
- [65].Xue CG, Taylor MG, Bicklie QD, Savioli L, Renganathan EA. Diagnosis of Schistosoma haematobium infection: evaluation of ELISA using keyhole limpet haemocyanin or soluble egg antigen in comparison with detection of eggs or haematuria. Trans. R. Soc. Trop. Med. Hyg. 1993;87:654–658. doi: 10.1016/0035-9203(93)90275-u. [DOI] [PubMed] [Google Scholar]
- [66].Li Y, Rabello AL, Simpson AJ, Katz N. The serological differentiation of acute and chronic Schistosoma japonicum infection by ELISA using keyhole limpet haemocyanin as antigen. Trans. R. Soc. Trop. Med. Hyg. 1994;88:249–251. doi: 10.1016/0035-9203(94)90319-0. [DOI] [PubMed] [Google Scholar]
- [67].Verweij JJ, Polderman AM, Visser LG, Deelder AM. Measurement of antibody response to keyhole limpet haemocyanin was not adequate for early diagnosis of schistosomiasis in a group of Dutch visitors to Mali. Trans. R. Soc. Trop. Med. Hyg. 1995;89:48–50. doi: 10.1016/0035-9203(95)90654-1. [DOI] [PubMed] [Google Scholar]
- [68].El-Ansary A, Al-Daihan S. Important aspects of Biomphalaria snail-schistosome interactions as targets for antischistosome drug. Med. Sci. Monit. 2006;12:RA282–292. [PubMed] [Google Scholar]
- [69].Fenion S, Ellis IO, Bell J, Todd JH, Elston CW, Blamey RW. Helix pomatia an Ulex europeus lectin binding in human breast carcinoma. J. Pathol. 1987;152:169–176. doi: 10.1002/path.1711520305. [DOI] [PubMed] [Google Scholar]
- [70].Linn J, Black P, Derksen K, Rubben H, Thuroff JW. Keyhole limpet haemocanain in experimental bladder cancer: literature review and own results. Eur. Urol. 2000;37(Suppl3):34–40. doi: 10.1159/000052390. [DOI] [PubMed] [Google Scholar]
- [71].McFadden DW, Riggs DR, Jackson BJ, Vona-Davis L. Keyhole limpet hemocyanin, a novel immune stimulant with promising anticancer activity in Barrett’s esophageal adenocarcinoma. Am. J. Surg. 2003;186:552–555. doi: 10.1016/j.amjsurg.2003.08.002. [DOI] [PubMed] [Google Scholar]
- [72].Krug LM, Ragupathi G, Hood C, Kris MG, Miller VA, Allen JR, Keding SJ, Danishefsky SJ, Gomez J, Tyson L, Pizzo B, Baez V, Livingston PO. Vaccination of patients with small-lung cancer with synthetic fucosyl GM-I conjugated to keyhole limpet hemocyanin. Clin. Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]