Abstract
Maternal exposure to genital and reproductive infections has been associated with schizophrenia in previous studies. Impairments in several neuropsychological functions, including verbal memory, working memory, executive function, and fine-motor coordination occur prominently in patients with schizophrenia. The etiologies of these deficits, however, remain largely unknown. We aimed to assess whether prospectively documented maternal exposure to genital/reproductive infections was related to these neuropsychological deficits in offspring with schizophrenia and other schizophrenia spectrum disorders. The cases were derived from a population-based birth cohort; all cohort members belonged to a prepaid health plan. Cases were assessed for verbal memory, working memory, executive function, and fine-motor coordination. Compared to unexposed cases, patients exposed to maternal genital/reproductive infection performed more poorly on verbal memory, fine-motor coordination, and working memory. Stratification by race revealed associations between maternal G/R infection and verbal memory and fine-motor coordination for case offspring of African-American mothers, but not for case offspring of White mothers. Significant infection-by-race interactions were also observed. Although independent replications are warranted, maternal G/R infections were associated with verbal memory and motor function deficits in African-American patients with schizophrenia.
Keywords: infection, cognition, epidemiology, risk factors, environment, exposure, neurodevelopment
1. Introduction
Maternal exposure to genital/reproductive (G/R) infection is a known risk factor for congenital central nervous system anomalies (Kropp et al., 2006; Remington et al., 2006; Engman et al., 2008), and has been associated with schizophrenia in previous studies. In a follow-up of the Child Health and Development Study (CHDS) birth cohort, periconceptional exposure to maternal G/R infections was related to a significantly increased risk of schizophrenia and other schizophrenia spectrum disorders (SSD) among offspring (Babulas et al., 2006). Among G/R infections, herpes simplex virus type 2 (HSV-2), which is shed at genital sites, is one of the most common sexually transmitted diseases.
Previous studies have examined the relationship between maternal exposure to HSV-2 and risk of psychosis and schizophrenia in adult offspring. In the first two, based on the National Collaborative Perinatal Project (NCPP) birth cohort, elevated maternal IgG antibody levels to HSV-2, and seropositivity to this infection were related to a significantly increased risk of psychosis in adult offspring (Buka et al., 2001; Buka et al., 2008). In a third study, from the CHDS cohort, however, no relationships were observed between HSV-2 IgG antibody levels or seropositivity to this virus and risk of SSD (Brown et al., 2006). In particular, the NCPP birth cohorts in the latter study from this sample included a greater number of African-Americans than the CHDS cohort, and African-Americans have a considerably higher prevalence of HSV-2 infection, which in that study was over four times higher than in Caucasians (Buka et al., 2008). Other explanations for the differences in findings between the two birth cohorts are discussed in detail in Brown and Derkits (2010).
Perinatal exposure to HSV-2 is associated with adverse neuropsychiatric outcomes during childhood, including mental retardation, impaired attention, communication and language deficits, and gross and fine motor disability (Kropp et al., 2006; Engman et al., 2008). Other maternal G/R infections, including syphilis (Ingall, 2001) have also been associated with impaired neurocognitive outcomes among offspring. Impairment in neuropsychological functions is one of the hallmark features of schizophrenia and plays a considerable role in social and occupational impairment in this disorder (Goldberg et al., 2003; Kraus and Keefe, 2007). Hence, G/R infections during pregnancy may play a role not only in risk of schizophrenia but also in neurocognitive deficits in this disorder. Hence, in the present study, we investigated the relationship between maternal seropositivity to HSV-2 and other maternal G/R infections and neurocognitive outcomes in offspring with SSD from a population-based birth cohort. The sample comprised schizophrenia cases from the Developmental Insult and Brain Anomaly in Schizophrenia (DIBS) study, who were administered a comprehensive neuropsychological test battery. All cases were clinically stable adult schizophrenia outpatients who had been followed up for this disorder in an earlier study in a large birth cohort (Susser et al., 2000). Subjects who participated in the DIBS study represented a subsample of subjects who were followed up in the earlier study. The study featured prospectively collected data on GR infection that was acquired from testing of maternal sera for HSV-2 antibody and maternal obstetric records by physicians.
Based upon previous studies of neurocognition and neuromotor impairment in schizophrenia and in follow-up studies of maternal G/R infections, our evaluation focused on the relationship between this exposure and performance in 4 domains of functioning: verbal memory, working memory, executive function, and fine-motor coordination. Given the small number of controls with serologic data, the analysis was restricted to the SSD cases.
2. Methods
The methods of the DIBS study have been extensively described in a previous publication (Brown et al., 2009), which includes a flow diagram of the recruitment and selection of subjects for the DIBS study. Hence, these methods will be only summarized here.
Subjects were derived from a follow-up study of schizophrenia among offspring of mothers who were enrolled in the Child Health and Development Study (CHDS), a large birth cohort, from 1959–1966 (van de Berg, 1984). Nearly every pregnant woman received obstetric care from the Kaiser Permanente Medical Care Plan (KPMCP) in Alameda County, California. There were 12,094 live births in 1981, the beginning of case ascertainment from the KPMCP databases. Maternal sera were collected during pregnancy from virtually all mothers who participated in the CHDS.
2.1. Ascertainment and diagnosis
These methods are elaborated in detail in previous publications (Susser et al., 2000; Brown et al., 2004), and are summarized here. The main outcome was schizophrenia and other schizophrenia spectrum disorders (SSD), which was defined as: schizophrenia, schizoaffective disorder, delusional disorder, psychotic disorder not otherwise specified, and schizotypal personality disorder, in accord with previous studies (Kendler et al., 1995). Case ascertainment was based on computerized record linkage between the CHDS and KPMCP identifiers from inpatient and outpatient databases. Patients with ICD-9 diagnoses of 295–299 and/or patients treated with antipsychotics were administered the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994) by clinicians with a minimum of a master’s degree in a mental health field trained to reliability. Psychiatric diagnoses were made by DSM-IV criteria following consensus of three experienced research psychiatrists. Diagnostic chart reviews were conducted for potential cases who were not interviewed. These procedures resulted in 71 total SSD cases. There were 38 cases with schizophrenia, 15 with schizoaffective disorder, and 11 with other schizophrenia spectrum disorders. Therefore, the vast majority of cases had either schizophrenia or schizoaffective disorder.
2.2. Assessment of exposure to maternal genital/reproductive infections
We utilized two previously established sources of data on maternal G/R infection in the CHDS cohort. The first was based on seropositivity to IgG antibody for HSV-2. As in our previous study in this cohort (Brown et al., 2006), HSV-2 was quantified using the HerpesSelect ELISA assay (Focus Technologies, Cypress, CA). The second was systematically generated from CHDS records from physician diagnoses made during obstetric and medical visits to KPMCP, which provided treatment to all gravidas in the CHDS. All maternal G/R infections present in the database, and documented after 6 months prior to conception throughout pregnancy were included; these consisted of endometritis, cervicitis, pelvic inflammatory disease, vaginitis, syphilis, condylomata, “venereal disease,” and gonorrhea (Babulas et al., 2006). We included G/R infections occurring at any time during pregnancy, rather than only during the gestational period associated with schizophrenia in our previous study (Babulas et al., 2006) since we aimed to examine whether G/R infection both during and outside of that gestational period would be associated with impaired neurocognitive function. Subjects were considered to have been exposed to maternal G/R infection based on seropositivity to HSV-2 or diagnosis with any of the documented G/R infections. The rationale for combining these two definitions of exposure was threefold: first, HSV-2 is nearly always a G/R infection; second, all of these infections are localized to the maternal reproductive organs, with proximity to the fetal compartment, suggesting that they operate by common mechanisms to alter fetal brain development; third, combining these categories of infection improved statistical power. The prevalence of HSV-2 seropositivity was 25% and of database derived maternal G/R infection was 13% in the cases of the present study, excluding cases classified with both infections (12%) (see sec. 2.3 for description of cases).
2.3. Ascertainment of the DIBS study sample
The DIBS study is a nested case-control study based on the larger schizophrenia follow-up of the CHDS cohort described above. All subjects who met eligibility criteria were targeted for neuropsychological assessments. Subjects with major medical illnesses including neurologic disorders were excluded; see (Brown et al., 2009) for more details. Hospitalized cases or those deemed to be too severely psychotic for neurocognitive testing were also excluded. Selection of subjects was not determined by maternal antibody titers or maternal medical conditions.
Subjects were located and recruited using updated information from the CHDS birth cohort and KPMCP records. The flow of recruitment is described in detail in a flow chart from a previous publication from the DIBS (Brown et al., 2009). In summary, of the 71 cases, 3 were deceased, 15 were ineligible (lived out of the area, psychosis too severe to allow for testing, excluded medical conditions), 8 could not be contacted, and 19 refused participation. Hence, there were 26 cases of SSD. Complete data on neuropsychological performance and maternal G/R infection status were available on 25 SSD cases (13 with schizophrenia, 6 with schizoaffective disorder and 6 with other schizophrenia spectrum disorders). The cases in the DIBS sample were representative of the PDS sample with regard to diagnostic status, maternal age, maternal race, maternal education, parity, offspring sex, diagnostic distribution of schizophrenia spectrum disorders, and prevalence of infection (Table 1).
TABLE 1.
Characteristics of Case Subjects from the Prenatal Determinants of Schizophrenia (PDS) Study with Schizophrenia or Other Schizophrenia Spectrum Disorders who were Included and not Included in the Present Study
| Characteristic | Case Subjects in Present Study (N=25 1) | PDS Case Subjects not in Present Study (N=46 2) | P value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Maternal age3,4 | 0.61 | ||||
|
| |||||
| <=29 years | 14 | 56 | 28 | 62.2 | |
| >29 years | 11 | 44 | 17 | 37.8 | |
|
| |||||
| Maternal race4 | 0.47 | ||||
|
| |||||
| White | 14 | 56.0 | 20 | 44.4 | |
| African-American | 9 | 36.0 | 23 | 51.1 | |
| Other | 2 | 8.0 | 2 | 4.4 | |
|
| |||||
| Maternal education5 | 0.66 | ||||
|
| |||||
| <= High school graduate or trade school | 16 | 69.6 | 25 | 64.1 | |
| College graduate or some college | 7 | 30.4 | 14 | 35.9 | |
|
| |||||
| Maternal parity | 0.99 | ||||
|
| |||||
| 0 or 1 prior live births | 12 | 48.0 | 22 | 47.8 | |
| 2 or more prior live births | 13 | 52.0 | 24 | 52.2 | |
|
| |||||
| Infant Sex | 0.81 | ||||
|
| |||||
| Male | 17 | 68.0 | 30 | 65.2 | |
| Female | 8 | 32.0 | 16 | 34.8 | |
|
| |||||
| Diagnostic Breakdown | 0.32 | ||||
|
| |||||
| Schizophrenia | 13 | 52.0 | 30 | 65.2 | |
| Schizoaffective Disorder | 6 | 24.0 | 11 | 23.9 | |
| Other Schizophrenia Spectrum Disorder | 6 | 24.0 | 5 | 10.9 | |
|
| |||||
| Prevalence of G/R Infection6 | 11 | 44.0 | 12 | 31.6 | 0.32 |
26 PDS case subjects participated in DIBS, of which 25 case subjects had maternal G/R infection status
45 PDS case subjects who did not participate in DIBS and one DIBS case subject without maternal G/R infection status
Median maternal age was 29 years
Missing values: N=1 PDS case subject not in present study
Missing values: N=2 case subjects in present study, N=7 PDS case subjects not in present study
8 PDS case subjects not in present study without maternal G/R infection status
All subjects provided written informed consent. The study protocol was approved by the Institutional Review Boards of the New York State Psychiatric Institute, the Kaiser Foundation Research Institute, the University of California, San Francisco, and the San Francisco Department of Veterans Affairs Medical Center.
2.4. Neuropsychological assessments
Tests were administered by psychology doctoral students (minimum of master’s level), trained by WSK and JP. In order to assess whether the findings were due to diminished global intelligence, full scale IQ was assessed by a WAIS-III short form. Based on the literature, our primary focus was on four neuropsychological domains: Verbal memory (California Verbal Learning Test); Working memory (Wechsler Adult Intelligence Scale [WAIS-III] Digit Span, Letter-Number Sequencing Test, Auditory N-back (2-back, 0-back) Tests); Executive function (Wisconsin Card Sorting Test [WCST], Trail Making Test Parts A and B [Trails A, Trails B]); Verbal Fluency Test [letter & category fluency], Ruff Figural Fluency Test [figural fluency]), and Fine-motor coordination (Grooved Pegboard Test, dominant hand). Processing speed was assessed with the Symbol Search and Digit Symbol tests. These tests have been described in detail elsewhere (Lezak et al., 2004).
2.5. Analytic method
We compared performance in each neuropsychological domain between SSD case offspring of mothers who were exposed and unexposed to maternal G/R infection. Unequal variances were found for certain response variables in the comparisons of exposed and unexposed cases. Consequently, analyses were performed with generalized linear models (GLM) (McCullagh and Nedler, 1989). GLM is a flexible parametric class of models suitable for small datasets in which the conditional distribution of the response given the predictors is selected from an exponential family (e.g., Gaussian, Poisson, binomial, gamma) and can capitalize on specific structure in the response variables of the study. The variance structure is determined by the particular GLM, with only the Gaussian model having constant variance. The canonical link function was used throughout. Binomial regression was utilized for the WCST, WAIS-III Digit Span Test, and Letter-Number Sequencing Test, with the number of test items determining the number of trials in the binomial model. For the Grooved Pegboard Test, the response is an event time, and a gamma regression model was used. For the Verbal Fluency Test and Ruff Figural Fluency Test, the outcome is number of correct responses, which has no predetermined upper-bound, and a Poisson regression model was applied. Finally, for the CVLT, Trails B Test, Auditory N-back Test, WAIS IQ tests, Symbol Search Test, and Digit Symbol Test, a Gaussian model was used.
To mitigate the potential for type I error due to multiple comparisons, composite scores consisting of sums of z-scores over tests in a given domain were examined using the Gaussian model (fine-motor coordination involved only one test). Statistical significance was initially assessed using P<0.05; all tests were two-tailed. In addition, Bonferroni correction was applied; given 4 composite neuropsychological outcome measures, the corrected P was <0.0125. Given the small sample size, and the reduction of statistical power through adjustment for covariates, confounding and effect modification were initially evaluated by stratification of the exposure-outcome relationships on covariates (see Section 3.3); interaction terms were then added to the regression models based on visual inspection of stratified tables (see supplementary tables 1 and 2).
3. Results
3.1. Demographic comparisons between exposed and unexposed cases
Comparing the 11 cases exposed to maternal G/R infection to the 14 unexposed cases, there was no statistically significant association between exposure to G/R infection and maternal race (P=0.19). There was, however, a greater prevalence of maternal G/R exposure among offspring of African-Americans than Whites (Table 2). Given this result, and the previous literature indicating a higher prevalence of G/R infection in African-Americans, the lack of statistical significance may have been due to inadequate power given the small sample sizes. Hence, we investigated potential confounding and effect modification by maternal race (sec. 3.3). Maternal age and the proportions of exposed cases in each category of maternal education, parental socioeconomic status, child education, infant sex, antipsychotic medication use, and duration and severity of illness did not differ from one another. None of the cases had a maternal history of schizophrenia by the time of birth.
TABLE 2.
Characteristics of Case Subjects with Schizophrenia or Other Schizophrenia Spectrum Disorder who were Exposed and Unexposed to Maternal Genital/Reproductive Infection
| Characteristic | Exposed Case Subjects (n=11) | Unexposed Case Subjects (n=14) | Proportion Exposed Within Each Category (%) | P-value |
|---|---|---|---|---|
| Maternal age (years) | ||||
| Mean (SD) | 28.7(7.31) | 28.6(5.96) | - | 0.97 |
|
| ||||
| Maternal race | 0.19 | |||
| White | 4 | 10 | 29% | |
| African-American | 6 | 3 | 67% | |
| Other | 1 | 1 | 50% | |
|
| ||||
| Maternal education1 | 1.00 | |||
| ≤High school grad/trade school | 6 | 10 | 38% | |
| Some college/college grad/RN | 3 | 4 | 43% | |
|
| ||||
| Paternal occupation 2 | 0.47 | |||
| Non-Manual | 4 | 5 | 44% | |
| Manual | 3 | 8 | 27% | |
| Unemployed | 1 | 0 | 100% | |
|
| ||||
| Child Education (years) | ||||
| Mean (SD) | 13.4 (1.46) | 12.5 (1.62) | - | 0.18 |
|
| ||||
| Infant Sex | 1.00 | |||
| Male | 7 | 10 | 41% | |
| Female | 4 | 4 | 50% | |
|
| ||||
| Antipsychotic Medication3 | 1.00 | |||
| Presence | 6 | 8 | 43% | |
| Absence | 5 | 6 | 45% | |
|
| ||||
| BPRS score4 | ||||
| Mean (SD) | 42.1 (14.39) | 42.5 (13.10) | - | 0.94 |
|
| ||||
| Duration of illness (years) | ||||
| 14.3 (5.82) | 15.2 (4.74) | - | 0.66 | |
Missing values: N=2 exposed case subjects
Missing values: N=3 exposed case subjects, N=1 unexposed case subject
Antipsychotic medications: Clozaril, Trilafon, Zyprexa, Seroquel, Risperdal, Prolixin, Stelazine, Haldol, Thorazine, Navane
Brief Psychiatric Rating Scale, Missing value: N=1 for exposed case subject
3.2. Comparisons of neuropsychological functions between cases exposed and unexposed to maternal G/R infection during pregnancy
Table 3 depicts neuropsychological performance of SSD cases by maternal G/R exposure status. The results of the analyses by neuropsychological domain are summarized below.
TABLE 3.
Exposure to Maternal Genital/Reproductive Infection and Neuropsychological Performance in Case Subjects with Schizophrenia or Other Schizophrenia Spectrum Disorder
| Domain/Measure | Exposed Case Subjects (N=11) | Unexposed Case Subjects (N=14) | P-value from GLM | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
|
Verbal Memory
| |||||
| Composite score | −3.18 | 2.74 | 0.07 | 2.26 | 0.004 |
|
| |||||
| CVLT | |||||
| CVLT trial 5 z-score1 | −1.91 | 1.32 | −0.68 | 1.12 | 0.019 |
| CVLT total learning slope z-score1 | −1.27 | 1.46 | 0.75 | 1.33 | 0.001 |
|
| |||||
|
Working Memory
| |||||
| Composite score | −0.98 | 2.42 | 0.94 | 1.78 | 0.051 |
|
| |||||
| WAIS Digit Span | |||||
| Forward last correct2 | 5.00 | 1.73 | 5.07 | 1.14 | 0.897 |
| Backward last correct | 2.73 | 0.90 | 3.71 | 1.44 | 0.065 |
| Letter-Number Sequencing | |||||
| Letter-Number Sequencing scaled score | 7.09 | 3.18 | 8.21 | 2.78 | 0.207 |
| Auditory N-back | |||||
| Zero-back d-prime2 | 0.89 | 0.20 | 0.94 | 0.05 | 0.342 |
| Two-back d-prime | 0.51 | 0.34 | 0.69 | 0.21 | 0.164 |
|
| |||||
|
Executive Function
| |||||
| Composite score | 0.04 | 4.34 | −0.31 | 5.16 | 0.874 |
|
| |||||
| Wisconsin Card Sorting Test (WCST) | |||||
| Total errors | 14.91 | 10.59 | 15.43 | 13.05 | 0.689 |
| Perseverative errors | 4.91 | 6.44 | 6.36 | 7.42 | 0.084 |
| Non-perseverative errors | 10.00 | 6.99 | 9.07 | 7.22 | 0.342 |
| Trail Making Test | |||||
| Trails B time (sec.) | 92.56 | 37.16 | 112.69 | 60.25 | 0.304 |
| Verbal Fluency Test | |||||
| Letter fluency (total correct) | 12.18 | 3.95 | 12.50 | 3.61 | 0.822 |
| Category fluency (total correct) | 17.70 | 5.64 | 18.29 | 5.21 | 0.739 |
| Ruff figural fluency test | |||||
| Ruff figural fluency (total correct) | 56.73 | 26.60 | 65.86 | 23.05 | 0.004 |
|
| |||||
|
Fine Motor Coordination
| |||||
| Grooved pegboard | |||||
| Grooved pegboard dominant hand time (sec.) | 116.55 | 62.21 | 85.07 | 19.04 | 0.022 |
These are z-scored variables from the CVLT manual that are based on the CVLT normative sample.
Test scores were not part of working memory composite score
3.2.1. Verbal memory
Maternal G/R exposure was associated with significantly diminished performance on the verbal memory composite score (P=0.007), assessed by the CVLT trial 5 z-score and the total learning slope z-score (based on 5 trials), both of which were statistically significant. The association was statistically significant following Bonferroni correction.
3.2.2. Working memory
There was a strong trend for an association between maternal G/R exposure and the composite score for working memory (P=0.051). Among individual tests within this domain, a statistical trend was observed for poorer performance of exposed cases on the Digit Span Test, backward condition, but not the Digit Span, forward condition.
3.2.3. Executive functioning
Maternal G/R exposure was not associated with the composite score for executive functioning (P=0.87). The only test that demonstrated statistical significance within this domain was figural fluency, with exposed cases performing significantly more poorly than unexposed cases (P=0.004).
3.2.4. Fine-motor coordination
Maternal G/R exposure was associated with significantly poorer performance on the Grooved Pegboard Test (dominant hand) (P =0.022). This figure was slightly above the statistical significance threshold of P<0.0125 following Bonferroni correction.
3.2.5. Processing speed
No differences were observed between the groups on either of the two processing speed measures [Symbol Search: mean (SD)=8.1 (2.9) in exposed cases, 9.6 (3.1) in unexposed cases, P=0.23; Digit Symbol: mean (SD)=6.7 in exposed cases, 7.2 (2.7) in unexposed cases, P=0.67].
3.3. Confounding and effect modification by race
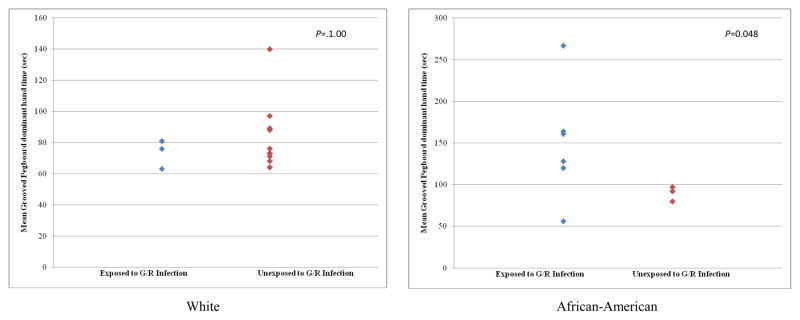
Stratification of the maternal G/R results by maternal race (White, African-American) revealed few significant or trend-level differences in neuropsychological outcomes between maternal G/R exposed and unexposed groups in the case offspring of White mothers, but group differences were observed in the case offspring of African-American mothers. G/R exposed case offspring of African-American mothers performed significantly more poorly than unexposed cases on verbal memory (composite P=0.009). This finding remained statistically significant following Bonferroni correction. There was a trend for poorer performance on fine-motor coordination (Grooved Pegboard, dominant, P=0.081). Further inspection of the distributions of the fine motor findings revealed that time to completion of the task was greater than 100 seconds in 5 of 6 G/R exposed case offspring of African-American mothers but in none of three unexposed cases (Fisher P=0.047) (Figure 1); there were no differences between the exposed and unexposed for offspring of White mothers. No differences were observed between exposed and unexposed case offspring of African-American or White mothers with regard to the composite measures of working memory or executive function performance (see supplementary tables 1 and 2), although whites unexposed to G/R infection committed more errors on the Wisconsin Card Sorting Test than exposed whites.
Figure 1. Grooved Pegboard Dominant Hand Time (sec.) in Case Subjects with Schizophrenia or other Schizophrenia Spectrum Disorders Exposed and Unexposed to Genital/Reproductive Infection1.
1Post Hoc analysis using 100 seconds as cutoff point, Fisher exact test
These findings suggest that maternal race acted as a potential effect modifier, rather than as a confounder. If confounding was present, the differences observed in the overall sample for verbal memory and fine-motor coordination are expected to have been eliminated following stratification by race. Hence, we analyzed whether there was statistical evidence of effect modification by race on the observed associations. Statistically significant interactions between race and G/R exposure on both verbal memory (P=0.047) and fine-motor coordination (P=0.042) were observed. Our results for the interaction between race and infection were not adversely affected by a potential lack of power to detect a main effect for race, as noted above (see sec. 3.1). The reason is that power is not simply a function of sample size, but also of effect size (as measured by the magnitude of the relevant regression parameter). In particular, if the true value of the interaction parameter is large, then, given a sufficiently large sample, there will be enough power to detect the interaction, but a much larger sample could be needed to detect a main effect if the corresponding regression parameter is small. These findings provide further evidence that the associations between maternal G/R infection and neuropsychological outcomes were present for case offspring of African-American mothers, but not for case offspring of White mothers.
3.4. Restriction to cases of schizophrenia and schizoaffective disorder
Similar results were obtained when the sample was restricted to a more narrow definition of case subjects (diagnosed with schizophrenia or schizoaffective disorder). Maternal G/R infection was significantly associated with diminished performance on the verbal memory standard composite score [mean (SD)= −4.0(2.2) in exposed cases and −0.5(2.3) in unexposed cases, P=0.003] and impaired performance on the Grooved Pegboard Test, dominant hand time [mean (SD) in exposed cases=126.4 (64.9) and 85.9 (21.4) in unexposed cases, P=0.018]. There was numerically poorer performance on working memory based on the composite score in G/R infection-exposed cases, compared to unexposed cases [mean (SD) in exposed cases=−0.95 (2.5) and 0.91 (1.9) in unexposed cases, P=0.14]. There was no difference in executive function composite scores between exposed and unexposed cases (P=0.7). There were too few subjects in this subsample to examine confounding and effect modification by race.
3.5. Separate analyses of maternal HSV-2 seropositivity and database derived maternal G/R infection
In order to examine the independent associations between maternal HSV-2 seropositivity and database derived maternal G/R infections, we excluded cases exposed to both categories of G/R infection; consequently, statistical power was reduced. Nonetheless, exposure to HSV2 (p=0.09) and to database derived G/R infection (p=0.054) were both associated with poorer verbal memory (albeit at trend levels), consistent with the finding which combined these two exposure variables. HSV2 (p=0.001), but not database derived GR infection (p=0.35), was associated with poorer performance on the grooved pegboard test, which was also observed with the combined G/R variable, as noted above (sec. 3.2). HSV2 exposure was significantly related to poorer working memory (p=0.02), a finding which was observed at a trend level (p=0.051) with the combined exposure variable.
4. Discussion
Maternal G/R infection was associated with impairments in verbal memory and fine-motor coordination in adults with schizophrenia. These effects appear to have been driven by the results in the African-American subsample of cases.
Verbal learning and memory, and working memory, are among the most robustly abnormal cognitive functions in schizophrenia, and are present in the earliest phases of the illness (Heinrichs and Zakzanis, 1998; Keefe et al., 2006; Lencz et al., 2006; Mesholam-Gately et al., 2009). Abnormalities have been observed in frontotemporal cortical networks during verbal working memory, word encoding, and recognition (Weiss and Heckers, 2001; Ragland et al., 2004). Impaired verbal memory is associated with poor community functioning and poor response to psychosocial treatment (Green et al., 2000; Evans et al., 2004).
Neuromotor dysfunction has been long associated with schizophrenia (Whitty et al., 2009). Neurological soft signs, impairment on tests of fine-motor coordination and other neuromotor functions, and abnormal involuntary movements have been demonstrated in antipsychotic-naïve patients, and neuromotor abnormalities have been observed in children destined to develop schizophrenia as early as 2 years of life (Walker et al., 1994; Isohanni et al., 2004). Given that G/R infection was not associated with processing speed in the present study, which has a strong psychomotor component, the findings of the present study suggest that the neuromotor abnormality was relatively specific to fine-motor coordination.
It is worth considering the reasons for the ethnic differences observed. Possible candidate risk factors include socioeconomic status, educational differences, and early life experiences. We demonstrated, however, that there were no relationships between maternal G/R infection and parental socioeconomic status, or maternal or child education. Although early life experiences were not evaluated in this cohort, this represents an important question to be addressed in future research.
These findings are consistent with previous epidemiologic studies indicating associations between maternal HSV-2 antibody and schizophrenia and other psychotic disorders in offspring (Buka et al., 2001; Buka et al., 2008). Since we did not observe an association between maternal HSV2 and schizophrenia in the birth cohort of the present study (Brown et al., 2006), and the definition of database derived maternal G/R infection included the entire pregnancy, rather than only the periconceptional risk period that was related to schizophrenia in our previous work (Babulas et al., 2006), these findings are consistent with a potential modifying role of maternal G/R infection on neuropsychological functioning, specifically verbal memory, in schizophrenia. Although one would expect that environmental risk factors that are related to risk of schizophrenia should also be related to neurocognitive and other core phenotypes of the disorder, it is plausible, in our view, that certain factors may be associated with these phenotypes, but not risk of disorder. For example, previous work has demonstrated that exposure to HSV1 as measured in adult patients with schizophrenia is related to impaired verbal memory (Dickerson et al., 2003) in schizophrenia but does not appear to be associated with risk of this disorder (Leweke et al., 2004). Taken more broadly, it is conceivable that different phenotypes of the syndrome of schizophrenia (e.g. positive symptoms, negative symptoms, neurocognitive dysfunction) may have at least some risk factors that differ from one another.
Maternal G/R infections represent a plausible risk factor for neurocognitive disruptions in schizophrenia. These microbes inhabit reproductive organs which are proximal to the developing fetus and placenta, including the endometrium, cervix, and vagina. Hence, they are capable of direct fetal transmission in the uterus, via ascension from the vagina or cervix, or during delivery; the last of these routes of transmission occurs during delivery for HSV-2 (Arvin et al., 2006). The incidence of cervical shedding of HSV2 in asymptomatic pregnant women is as high as 7.4%. CNS manifestations of neonatal HSV infection involve gross brain pathology, including cortical hemorrhagic necrosis. Chlamydia trachomatis is the most common bacterial cause of sexually transmitted infections in the USA, with prevalence as high as 12%, and is also transmitted to offspring during delivery (Darville, 2006). Gonorrhea, which is less common, is thought to be transmitted by ascending infection in instances of congenital infection (Embree, 2006) To date, no follow-up studies have been published on childhood or long-term neuropsychological or behavioral sequelae of these maternal infections, although studies of adults infected with HSV1 have revealed associations with neuropsychological impairment, particularly verbal memory deficits, which were shown in our study. Moreover, microbial invasion of the amniotic cavity can result from genital reproductive infections (Park et al., 2009), and chorioamnionitis has been associated with fetal inflammation and white matter damage, which has been related to impaired neurocognitive and neuromotor outcomes (Dammann et al., 2002).
Previous studies of healthy subjects administered the California Verbal Learning Test have localized novel and familiar word processing to activation of left medial temporal lobe regions, right hippocampus, and right frontal lobe (Johnson et al., 2001). We therefore speculate that maternal G/R infections may alter the developmental trajectory of these brain regions, giving rise to the observed deficits in verbal memory. The observed fine-motor coordination findings following maternal G/R infection may be related to disrupted activation in the precental/postcentral gyri, striatum, and cerebellum, which have been correlated with executed sequential hand movements (Lacourse et al., 2005).
In the only previous study to examine the relationship between in utero infection and neuropsychological function in schizophrenia, we observed associations between prenatal serologically documented exposure to influenza and toxoplasma infection and two executive function measures in schizophrenia, the Wisconsin Card Sorting Test and the Trails B Test (Brown et al., 2009). That study focused on measures of executive function, which was not related to maternal G/R infection, and did not include verbal memory or neuromotor functioning. In future work, we shall seek to assess whether other infections, including influenza and toxoplasma, also give rise to the abnormalities observed in the present study.
This work has the potential to shed light on the role of prenatal infections and other environmental factors in these abnormalities. Moreover, these findings suggest that straightforward and relatively inexpensive approaches, many of which exist presently, to prevent and treat maternal G/R infections prior to and during pregnancy may result in a reduction in core neurocognitive impairments in schizophrenia.
4.1. Limitations
We note several limitations. First, the sample size was small. Consequently, a larger sample size would have yielded greater power to reveal a potential association between maternal G/R infection and neuropsychological outcomes in offspring of White mothers, as well as other neurocognitive functions in the overall sample. However, it is worth noting in this regard that race has been strongly and independently related to genital/reproductive infections, including HSV-2, in numerous studies (Fleming et al., 1997). Second, because the maternal G/R infections identified from the database of the present study were heterogeneous and the numbers of exposed cases within each category of G/R infection were small, we were not able to identify which of these infections were associated with neuropsychological dysfunction. Third, the potential for type I error due to multiple comparisons needs to be considered. In this regard, we wish to emphasize that we aimed to test for a priori relationships between maternal G/R infections and a relatively small number of domains of neuropsychological functioning, and to further diminish type I error, we utilized composite scores for each domain. Moreover, the significant association between maternal G/R infection and verbal memory persisted after Bonferroni correction for multiple comparisons, the most conservative such method. Fourth, it is possible that residual confounding may have played a role in the observed findings. Although the present study did not identify relationships between maternal G/R infection and several potential confounders, it is possible that the impaired neuropsychological performance reflected inherited traits or lifestyle factors that co-segregate with G/R infection in the mother, or risk factors during childhood including infections. In addition, the possibility of selection bias should be considered. However, we found no differences with regard to several potential confounders, including maternal age, race, education, parity, infant sex, breakdown of schizophrenia spectrum diagnoses, and prevalence of infection, between the cases in the DIBS and those in the PDS sample but not the DIBS (see Table 1). Hence, there is no clear evidence for selection bias. Fifth, due to the lack of an adequate number of control subjects with serologically documented infection (N=8), we were not able to examine whether the observed associations were specific to schizophrenia. Nonetheless, our findings are consistent with a potential modifying role of maternal G/R infection on neuropsychological deficits in schizophrenia, regardless of whether or not the association is found in controls. Examining relationships between these infections and the same neurocognitive functions in control samples in future research would clearly contribute further valuable information, including evidence consistent with increased sensitivity of neuropsychological functions to these infections.
In summary, we have demonstrated that maternal G/R infection during pregnancy is associated with disruptions in verbal memory and fine-motor coordination in offspring with schizophrenia. The effect was modified by race, with relationships present in African-Americans but not in Whites. Strengths of the study include a well-characterized, representative birth cohort, prospective documentation of infection identified based on direct biomarkers from maternal sera and physician diagnoses, and follow-up in adulthood with a comprehensive neuropsychological test battery. Given the small sample size and the novelty of the finding, it will be essential to attempt to independently replicate this finding in larger samples.
Supplementary Material
Acknowledgments
The authors wish to thank Barbara Cohn, Catherine Schaefer, Christine Holland, Nicole Stephenson, P. Nina Banerjee, and Patric Prado for their contributions to this work.
This manuscript was supported by the following grants: National Institutes of Mental Health (NIMH) 1R01MH-60249 (A.S.B), NIMH 1K02-MH65422 (A.S.B.), a National Institutes of Mental Health (NIMH) Independent Investigator Award (A.S.B.), National Institute on Child Health and Development (NICHD) N01-HD-1-3334 (B.A. Cohn), and National Institute of Child Health and Development (NICHD) NO1-HD-6-3258 (B.A. Cohn), National Institute on Aging (NIA) 1R01 AG18386 (W.S.K.), 1R01 AG22381 (W.S.K.), and 1R01 AG22982 (W.S.K.), and the San Francisco Department of Veterans Affairs Medical Center (S.V.).
Role of the Sponsor: NIMH, NICHD, NIA, and the San Francisco Department of Veterans Affairs Medical Center had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript.
Footnotes
This study was presented in part at the Annual Meeting of the American College of Neuropsychopharmacology, December 8, 2009, Hollywood, Florida
Disclosures: Dr. Brown reports no competing interests
Dr. Vinogradov reports no competing interests
Dr. Kremen reports no competing interests
Dr. Poole reports no competing interests
Ms. Bao reports no competing interests
Mr. Kern reports no competing interests
Dr. McKeague reports no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvin AM, Whitley RJ, Gutierrez KM. Herpes Simplex Virus Infections. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious diseases of the fetus and newborn infant. 6. Elsevier Saunders; Philadelphia, PA: 2006. pp. 845–866. [Google Scholar]
- Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. American Journal of Psychiatry. 2006;163:927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of General Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. American Journal of Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Jr, Shen L, Susser ES. No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? American Journal of Psychiatry. 2006;163:2178–2180. doi: 10.1176/ajp.2006.163.12.2178. [DOI] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. American Journal of Psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Cannon TD, Torrey EF, Yolken RH. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biological Psychiatry. 2008;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Archives of General Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Dammann O, Kuban KCK, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- Darville T. Chlamydia infections. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious diseases of the fetus and newborn infant. 6. Elsevier Saunders; Philadelphia, PA: 2006. pp. 385–392. [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Archives of General Psychiatry. 2003;60:466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Embree JE. Gonococcal infections. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious diseases of the fetus and newborn infant. 6. Elsevier Saunders; Philadelphia, PA: 2006. pp. 393–402. [Google Scholar]
- Engman ML, Adolfsson I, Lewensohn-Fuchs I, Forsgren M, Mosskin M, Malm G. Neuropsychologic outcomes in children with neonatal herpes encephalitis. Pediatric Neurology. 2008;38:398–405. doi: 10.1016/j.pediatrneurol.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Evans JD, Bond GR, Meyer PS, Kim HW, Lysaker PH, Gibson PJ, Tunis S. Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophrenia Research. 2004;70:331–342. doi: 10.1016/j.schres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, St Louis ME. Herpes simplex virus type 2 in the United States, 1976 to 1994. New England Journal of Medicine. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, David A, Gold JM. Neurocognitive deficits in schizophrenia. In: Hirsch SR, Weingerger DR, editors. Schizophrenia. 2. Blackwell; Malden: 2003. pp. 168–186. [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Ingall D, Sanchez PJ. Syphillis. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. 5. WB Saunders; Philadelphia, PA: 2001. pp. 643–681. [Google Scholar]
- Isohanni M, Isohanni I, Koponen H, Koskinen J, Laine P, Lauronen E, Miettunen J, Maki P, Riala K, Rasanen S, Saari K, Tienari P, Veijola J, Murray G. Developmental precursors of psychosis. Current Psychiatry Reports. 2004;6:168–175. doi: 10.1007/s11920-004-0061-5. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Saykin AJ, Flashman LA, McAllister TW, Sparling MB. Brain activation on fMRI and verbal memory ability: functional neuroanatomic correlates of CVLT performance. Journal of the International Neuropsychological Society. 2001;7:55–62. doi: 10.1017/s135561770171106x. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophrenia Research. 2006;88:26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Walsh D. Evaluating the spectrum concept of schizophrenia in the Roscommon Family Study. American Journal of Psychiatry. 1995;152:749–754. doi: 10.1176/ajp.152.5.749. [DOI] [PubMed] [Google Scholar]
- Kraus MS, Keefe RS. Cognition as an outcome measure in schizophrenia. British Journal of Psychiatry. 2007;50:s46–51. doi: 10.1192/bjp.191.50.s46. [DOI] [PubMed] [Google Scholar]
- Kropp RY, Wong T, Cormier L, Ringrose A, Burton S, Embree JE, Steben M. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006;117:1955–1962. doi: 10.1542/peds.2005-1778. [DOI] [PubMed] [Google Scholar]
- Lacourse MG, Orr EL, Cramer SC, Cohen MJ. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage. 2005;27:505–519. doi: 10.1016/j.neuroimage.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biological Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Gerth C, Koethe D, Klosterkötter J, Ruslanova I, Krivogorsky B, Torrey EF, Yolken R. Antibodies to infectious agents in individuals with recent onset schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4. Oxford University Press; New York, NY: 2004. [Google Scholar]
- McCullagh P, Nedler JA. Generalized Linear Models. 2. Chapman Hall; London: 1989. [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Park CW, Moon KC, Park JS, Jun JK, Yoon BH. The frequency and clinical significance of intra-uterine infection and inflammation in patients with placenta previa and preterm labor and intact membranes. Placenta. 2009;30:613–618. doi: 10.1016/j.placenta.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. American Journal of Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington JS, Klein JO, Wilson CB, Baker CJ. Infectious diseases of the fetus and newborn infant. 6. Elsevier Saunders; Philadelphia, PA: 2006. [Google Scholar]
- Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophrenia Bulletin. 2000;26:257–273. doi: 10.1093/oxfordjournals.schbul.a033451. [DOI] [PubMed] [Google Scholar]
- van de Berg BJ. Handbook. Praeger; New York: 1984. The California Child Health and Developmental Studies. [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophrenia Bulletin. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scandinavian Journal of Psychology. 2001;42:239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophrenia Bulletin. 2009;35:415–424. doi: 10.1093/schbul/sbn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.