Abstract
The neuropeptide bradykinin (BK) sensitizes nociceptor activation following its release in response to inflammatory injury. Thereafter, the bioactivity of bradykinin is controlled by the enzymatic activities of circulating peptidases. One such enzyme, the metalloendopeptidase EC3.4.24.15 (EP24.15), is co-expressed with bradykinin receptors in primary afferent neurons. In this study, utilizing approaches encompassing pharmacology, biochemistry, cell biology and behavioral animal models, we discover a crucial role for EP24.15 and the closely-related EP24.16 in modulating bradykinin-mediated hyperalgesia. Pharmacological analyses indicate that EP24.15 and EP24.16 inhibition significantly enhances bradykinin type-2 receptor activation by bradykinin in primary trigeminal ganglia cultures. In addition, bradykinin-induced sensitization of TRPV1 activation is increased in the presence of the EP24.15/16 inhibitor JA-2. Furthermore, behavioral analyses illustrate a significant dose-response relationship between JA-2 and bradykinin-mediated thermal hyperalgesia. These results indicate an important physiological role for the metallopeptidases EP24.15 and EP24.16 in regulating bradykinin-mediated sensitization of primary afferent nociceptors.
Keywords: EP24.15, EP24.16, bradykinin, trigeminal, pain, inflammation
Introduction
Bradykinin (BK) is a potent neuropeptide that stimulates numerous physiological processes through the activation of the constitutively-expressed bradykinin type-2 receptor (B2R) of the rhodopsin G-protein coupled receptor (GPCR) class [19]. The type-1 receptor subtype (B1R) is poorly expressed in normal (non-inflamed) tissue, and is activated by metabolic products of bradykinin, including des-Arg9-BK. However, B1R expression increases incrementally following inflammation [23]. Following exposure to full-length BK, B2R activation mediates important physiologic functions, including blood pressure regulation, smooth muscle contraction, and pain [19]. The predominance of these functions follow the activation of B2R-driven Gαq-signaling pathways, that increase the downstream activities of many molecules, including protein kinase C, phospholipase Cβ, ras, and MAPK [3]. B2R-mediated activation of PKC directs the sensitization and/or activation of various nociceptive receptor-channels, including TRPV1 and TRPA1 [1,10].
The neuropeptide BK is sensitive to extracellular degradation by both circulating and plasma membrane-bound peptidases. Although degradation produces bioactive agonists for B1R, it significantly reduces agonist availability for B2R activation. Peptidases capable of metabolizing BK include angiotensin converting enzyme I (ACE), prolyl endopeptidase, and metalloendopeptidases EP24.15 and EP24.16 [33]. Although the biological significance of ACE and its inhibitors have been explored, other peptidases capable of processing BK have received far less attention and are less understood. The metalloendopeptidases EC3.4.24.15 (also known as endopeptidase 24.15, or thimet oligopeptidase, referred to as EP24.15 here-on) and EC3.4.24.16 (also known as endopeptidase 24.16, or neurolysin, referred to as EP24.16 here-on) are two such enzymes capable of degrading BK [28]. EP24.15/16 cleave BK at the Phe5-Ser6 bond, yielding two inactive peptides: Arg1-Phe5 and Ser6-Arg9. The metabolic de-activation of BK regulates the bioactivity of this crucial neuropeptide.
Previous studies have characterized immunocytochemical co-localization of EP24.15/16 and B2R on primary afferent terminals [14], suggesting a potential physiologic role for EP24.15 in modulating BK activation and sensitization of nociceptors. Additional investigations concluded that EP24.15 significantly affects BK-signaling in multiple systems [25,30]. To investigate a potential relationship between EP24.15/16 and BK-mediated nociception, we utilized a combined strategy employing pharmacological, cellular and behavioral models to study the effects of EP24.15/16 inhibition on B2R activation and pharmacology. The results of these studies collectively identify EP24.15/16 as mediators of inflammatory pain.
Materials and Methods
Tissue culture
All procedures utilizing animals were approved by the Institutional Animal Care and Use Committee of UTHSCSA, and were conducted in accordance with policies for the ethical treatment of animals established by the National Institutes of Health. Trigeminal ganglia (TG) were dissected bilaterally from male Sprague-Dawley rats (200-250 g; Charles River Laboratories, Wilmington, MA) and disassociated by treatment with collagenase (Lot# S5K8219, Worthington, Lakewood, NJ) for 30 min, followed by treatment with trypsin (Sigma, St. Louis, MO) for 15 min. Cells were centrifuged, aspirated and re-suspended in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), 100 ng/ml nerve growth factor (Harlan Laboratories, Indianapolis, IN), 1% penicillin/streptomycin (Invitrogen) and 1% glutamine (Sigma), and then placed on poly-D-lysine coated plates. Cultures were maintained at 37°C and 5% CO2 and grown for 5-7 days.
Homogenization, fractionation, and Western blotting
Following Trypsin treatment (0.025%, Sigma), cultured neurons were homogenized by 20 strokes in a Potter-Elvehjem homogenizer in a hypotonic homogenization buffer (25 mM HEPES, 25 mM sucrose, 1.5 mM MgCl2, 50 mM NaCl, pH 7.2), the whole-cell homogenate was incubated on ice for 15 min and then centrifuged at 1000 g for 1 min at 4°C to remove nuclei and unlysed cells from the homogenate. The supernatant was isolated and centrifuged at 16 000 g for 30 min at 4°C, separating cytosolic proteins from cell membrane proteins. The pellet (crude plasma membrane fraction) was then re-suspended in 200 μL homogenization buffer containing 1% Triton. The protein contents of crude plasma membrane and cytosolic fractions were quantified using the Bradford assay [4] as recommended by the manufacturer (Sigma). Following protein quantification, crude plasma membrane and cytosolic fractions (20 μg) were resolved via 12.5% SDS-polyacrylamide and transferred to polyvinyl difluoride membrane (Millipore, Billerica, MA) for immunoblotting. Western blots were blocked in 5% non-fat milk in Tris-buffered saline/Tween 20 and incubated in anti-EP24.15 [14], anti-EP24.16 [14], or anti-caveolin-1 (clone N-20, Santa Cruz Biotechnology), followed by the appropriate horseradish peroxidase-conjugated secondary antisera (GE Healthcare, Piscataway, NJ). Enhanced chemiluminescence (GE Healthcare) was used following manufacturer’s instructions for visualization of antigen-antibody binding.
Calcium Imaging
To measure intracellular [Ca+2] levels, the dye Fura-2 AM (2 μM; Molecular Probes, Carlsbad, CA) was incubated with cells for 30 min at 37° C in the presence of 0.05% Pluronic (Calbiochem/EMD Biosciences, Gibbstown, NJ). Fluorescence was detected with a Leica DM IRB microscope fitted with a 20x/0.8 NA Fluor objective. Fluorescence images from 340 nm and 380 nm excitation wavelengths were collected and analyzed with MetaFluor Software (MetaMorph, Web Universal Imaging Corporation, Downingtown, PA). To assess for Ca+2 accumulation following TRPV1 activation, cells were pre-treated with standard extracellular solution (SES) buffer (140mM NaCl, 5mM KCl, 2mM CaCl2, 1mM MgCl2, 10mM D-(+)-glucose, 10mM HEPES, pH 7.4) with or without the EP24.15/16 inhibitor JA-2 (10 μM, generously provided by A.I. Smith, Monash University) for 3 min followed by a 30 sec vehicle or bradykinin (BK; 50 nM) application. Following pre-treatment, a 30 sec washout with SES was followed by a single application of capsaicin (CAP, 50 nM) for 30 sec. For analysis of TRPV1 desensitization, repeated CAP (50 nM) applications were interrupted by SES washout or JA-2 (10 μM) for 3 min followed by a 30 sec application of vehicle of BK (50nM). The net change in Ca+2 (ΔF340/380) was calculated by subtracting the basal from the peak F340/380 Ca+2 accumulations achieved.
Behavioral assay
Experiments were conducted on male Sprague Dawley rats (200-250g) from Charles River, Wilmington, MA. A 12-hour light to dark cycle was used with all testing occurring during the light phase. Animals were housed for one week before the experiment with food and water made available. Paw withdrawal latency to a thermal stimulus was measured by blinded observers on the Hargreaves’ apparatus [12], . Animals were allowed to acclimate to their environment for a period of 30 minutes. The rate of increase in temperature of the glass floor was adjusted so that the baseline paw withdrawal values were close to 10 seconds with a cutoff time of 20 seconds. Measurements were taken in triplicate with at least 30 seconds apart and the average was used for statistical analysis. After 30 minutes of baseline readings, the plantar surface of the animals’ hindpaw received a subcutaneous injection with either vehicle (50% DMSO/0.9% Saline) or JA-2 in vehicle (5μM) in a 25μl volume. After a 30-minute incubation period, paw withdrawal latencies were recorded. This was followed by an injection of BK (1μM or .1μM, 25μl volume) in the same paw, and after 5 minutes paw withdrawal latencies were recorded. Similarly, animals were also treated with Complete Freund’s Adjuvant (CFA, 25 μL of a 1:1 emulsion of oil:saline, approximately 0.5 mg/ml) for 24 hr, and paw withdrawal latencies were recorded 30 min following vehicle (50% DMSO/0.9% Saline) or JA-2 in vehicle (5μM) in a 25μl volume. n = 6-8 animals per genotype/treatment. All reagents utilized were obtained from Sigma unless otherwise indicated.
Inositol Phosphate (IP) Accumulation
IP accumulation was measured as described previously [14]. Trigeminal ganglia cells in normal medium were labeled with 1 μCi/ml [3H]-myo-inositol (25 Ci/mmol) for 24 h at 37°C. After the labeling period, cells were washed three times with Hanks Balanced Salt Solution (HBSS) containing calcium and magnesium, 20 mM HEPES, and 0.1% fatty acid-free bovine serum albumin (BSA). Between washes, the cells were incubated for 5 min in a 37°C water bath (15-min total wash and pre-incubation time). After the wash procedure, cells were incubated in 500 μl of experimental medium containing vehicle (H2O) or the indicated drug concentrations. Cells were pre-incubated with peptidase inhibitors for 5 min at 37°C, and then with bradykinin for 15 min at 37°C in the presence of LiCl (20mM). After incubation, the media samples were aspirated quickly and 1 ml of 10 mM formic acid (4°C) was added to extract the accumulated [3H]-IPs (IP1, IP2, and IP3, collectively referred to as IP).
Concentration-response data were fit to a logistic equation (Equation #1) using non-linear regression analysis to provide estimates of maximal response (Rmax), potency (EC50) and slope factor (n).
| Equation #1 |
where R is the measured response at a given agonist concentration (A), Ro is the response in the absence of agonist, Ri is the response after maximal inhibition by the agonist, EC50 = the concentration of agonist that produces half-maximal response, and n = slope index. Rmax (the maximal inhibition produced by the agonist) was calculated as Ro-Ri. Experiments were repeated at least 4 times, with total n corresponding to wells of cultured TG tested independently (approximately 18, 000 total neurons/well). Statistical differences in concentration-response curve parameters between groups were analyzed with Student’s paired t-test. p<0.05 was considered statistically significant.
Quenched Fluorescent Substrate (QFS) Assay
EP24.15/16 activity was measured as described previously [20]. Briefly, TG cultures were switched to serum-free DMEM media and treated for 1 min at 37°C with 0.025% Trypsin-EDTA (Gibco). Following treatment, cultures were rinsed once with ice-cold PBS, and homogenized in Homogenization Buffer (25mM HEPES, 25mM sucrose, 1.5mM MgCl2, 50 mM NaCl, pH to 7.2) by 20 passes through a Potter-Elvehjem homogenizer. Nuclei and non-lysed cells were pelleted following centrifugation at 1000g for 5 min, and crude plasma membrane fractions were isolated following centrifugation of the nuclear spin supernatant at 16,500g for 30 min. Plasma membrane fractions were quantified by Bradford Analysis [4], and 10 μg of protein sample were combined with the indicated drugs (as described in text) and QFS substrate with or without inhibitors, incubated at 37°C for 60 min, with the reaction stopped by the addition of sodium formate. Samples were analyzed in a 96-well plate by fluorescence spectroscopy on an Infinite M-200 microplate reader (Tecan US, Inc., Durham, NC) with an excitation wavelength of 314 nm and emission wavelength of 418 nm.
Results
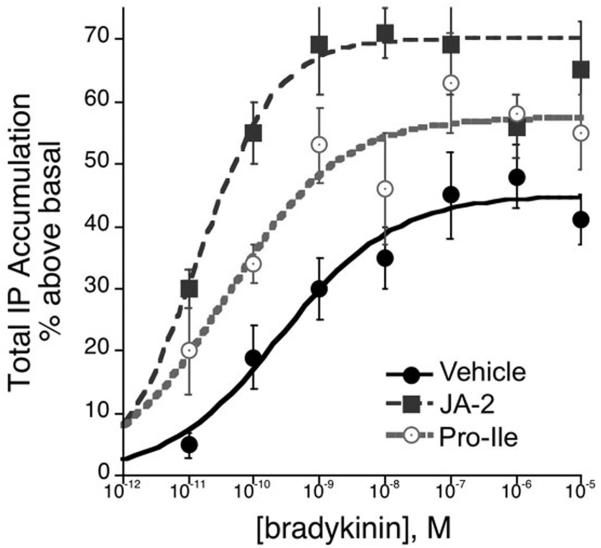
Metalloendopeptidase EP24.15 was originally characterized as one of several important enzymes that control peptide and hormonal bioactivities along the hypothalamo-pituitary-adrenal axis [13,28]. Previous work utilizing the EP24.15 inhibitor, cFP (N-[1(R,S)-carboxy-3-phenylpropyl]-Ala-AlalTyr-p-Aminobenzoate), demonstrated a significant regulatory role for EP24.15 in the activation of bradykinin type-2 receptors (B2Rs) by bradykinin (BK) [14]. However, cFP has a short half-life in vivo, prompting the design and synthesis of a similarly efficacious, non-peptidic inhibitor that provides greater stability in tissue and behavioral models [32]. This new inhibitor, JA-2, is 20-30 times more potent at inhibiting EP24.15 (Ki = 23 nM) than EP24.16, and retains its activity after prolonged incubation in various tissues [32]. Hence, we investigated the potential for this inhibitor to modulate B2R-stimulated inositol phosphate turnover by BK in primary cultures of rat trigeminal ganglia (TG). In Figure 1, pre-treatment of TG cultures with JA2 significantly increased the potency as well as the maximal response to BK for IP accumulation. The EC50 for BK shifted to the left more than 20-fold (veh + BK EC50 = 0.5 nM vs. JA-2 + BK EC50 0.013 nM; p< 0.05, n=6), and the Emax increased from 47 ± 3 % above basal (veh + BK) to 66 ± 3 % above basal (JA-2 + BK; p < 0.05, n=6). Similarly, specific inhibition of endogenously expressed EP24.16 with Pro-Ile increased the potency of BK 0.04 nM, p < 0.05, n=4) but did not significantly alter the maximal response to BK which was 58 ± 3% above basal in the presence of Pro-Ile. These results suggest an important role for EP24.15 and EP24.16 in B2R pharmacology.
Figure 1.
EP24.15/16 inhibition increases bradykinin efficacy in trigeminal neurons. Cultured TG neurons were treated in the presence of the EP24.16-specific inhibitor, Pro-Ile (200 μM, n=4), the EP24.15/16 inhibitor JA-2 (5 μM, n=6), or the vehicle (0.005% DMSO/H2O, n=10) prior to treatment with increasing concentrations of bradykinin. Total IP accumulation was measured and expressed as percent above basal IP accumulation in non-treated neurons.
Although many immunologically-based studies have demonstrated EP24.15 expression on the plasma membrane, there exists some ambiguity as to the inside-outside orientation of the peptidase from a functional standpoint [7,11,14,16,27,30,31]. To investigate this, we treated TG cultures with diluted trypsin, and measured EP24.15 and EP24.16 activity in cytoplasmic and plasma membrane fractions. Trypsin incubation reduced both EP24.15 and EP24.16 expression only in plasma membrane fractions without affecting cytoplasmic expression of the peptidases (Figure 2A). Furthermore, caveolin-1 expression in plasma membrane fractions was unaffected by trypsin incubation, indicating that proteins associated with the intracellular face of the plasma membrane were not affected by proteolytic degradation. The enzymatic activity of endogenous intrinsic EP24.15/16 activity was also measured from aliquots of the cytoplasmic and plasma membrane fractions (Figure 2B). Results indicate that trypsin treatment significantly reduced EP24.15/16 activity in plasma membrane fractions, but not in cytoplasmic fractions, while JA-2 inclusion in the enzyme assay effectively inhibited EP24.15. Taken together, these data identify EP24.15 on the extracellular face of the plasma membrane, where the peptidase would have the greatest opportunity to regulate the bioactivities of circulating neuropeptides that serve as substrates for the peptidase.
Figure 2.
Functional expression of EP24.15 on the extracellular surface of the plasma membrane in trigeminal neurons. Cultured TG neurons were treated with 0.025% Trypsin-EDTA in serum-free media conditions. Cultures were harvested, and fractionated to separate plasma membrane from cytoplasm. A. 35 μg samples were resolved by SDS-PAGE, and probed for EP24.15, EP24.16 and caveolin-1. Results are representative of three independent trials. B. 10 μg samples were combined with QFS reaction materials to measure EP24.15 activity. n=6, ** p<0.01, two-way ANOVA.
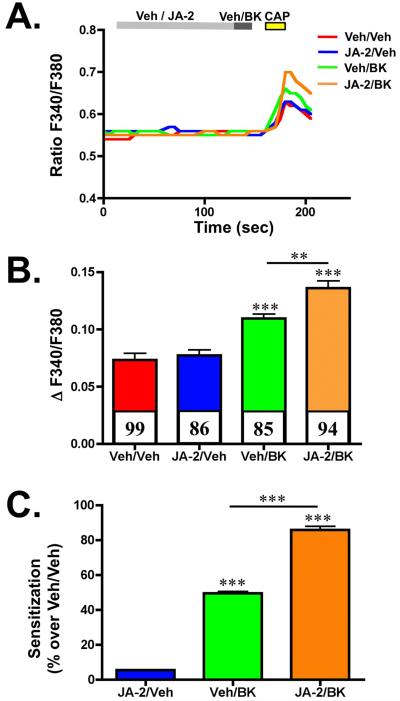
The TRPV1 receptor channel is expressed in a subset of primary afferent neurons that innervate the periphery and convey painful stimuli, including heat, protons, and capsaicin (CAP) [5,37]. The neuropeptide BK activates B2R that is co-expressed in TRPV1-positive neurons, sensitizing the channel to reduce activation thresholds, leading to increased sensitivity to painful stimuli [10]. We sought to investigate whether EP24.15 inhibition would affect BK-sensitization of TRPV1 to CAP. In Figure 3, TG cultures were pre-treated with vehicle or the JA-2 inhibitor for 3 min, then treated with BK for 30 sec, and then challenged with CAP to identify and measure TRPV1 activity in expressing neurons. Results from these studies indicate that EP24.15 inhibition following JA-2 pre-treatment significantly increases BK-mediated sensitization of TRPV1. Importantly, this effect must be mediated through the BK signaling pathway, since JA-2 alone had no effect on TRPV1 sensitivity to CAP application. Indeed, comparisons of sensitization between treatment paradigms indicate that JA-2 potentiates the sensitization of TRPV1 by BK almost two-fold (Figure 3C).
Figure 3.
EP24.15/16 inhibition potentiates bradykinin-sensitization of TRPV1 in trigeminal neurons. Cultured TG neurons were pre-treated with vehicle (0.005% DMSO/H2O) or JA-2 (5 μM, 3 min), then treated with vehicle (H2O) or BK (50 nM, 30 sec), and then stimulated with capsaicin (CAP, 50 nM, 30 sec). A. Representative traces of calcium accumulation following the illustrated experimental paradigm, treatment conditions indicated in legend. B. Quantified cumulative results of calcium accumulation, n for each treatment paradigm indicated along the X-axis. Colored bars correspond to colors in A. ** p<0.01, *** p < 0.005, as determined by one-way ANOVA, with Bonferroni correction ad-hoc. C. Quantified sensitization values for each treatment paradigm shown in A and B, normalized to veh/veh treatment, expressed as percentage. *** p < 0.005, as determined by one-way ANOVA, with Bonferroni correction ad-hoc.
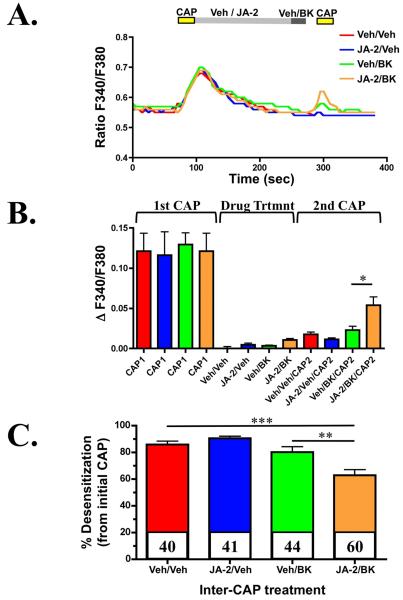
PKC activation reduces the pharmacological desensitization of TRPV1 in cellular models [21]. Given that B2R activation by BK stimulates PKC activation intracellularly [2], we next tested whether JA-2 co-treatment could potentiate the ability of BK to reduce pharmacological desensitization of TRPV1. TG neurons were cultured and treated multiple times with CAP, as shown in Figure 4, to induce a tachyphylactic response associated with pharmacological desensitization of the channel. In between CAP challenges, JA-2 and/or BK were administered, to test their effects on TRPV1 desensitization. The inhibition of EP24.15 by JA-2 significantly increased the effect of BK on the second CAP-administration. Additionally, when desensitized responses are normalized to their initial responses, the data indicates that JA-2 potentiates the reduction in TRPV1-desensitization following BK treatment. Taken together, these calcium-imaging studies support the hypothesis that EP24.15 inhibition significantly increases the effects of BK on TRPV1.
Figure 4.
EP24.15/16 inhibition potentiates bradykinin-mediated re-sensitization of desensitized TRPV1. Cultured TG neurons were pre-treated with vehicle (0.005% DMSO/H2O) or JA-2 (5 μM, 3 min), stimulated with CAP (50 nM, 30 sec), then treated with vehicle (H2O) or BK (50 nM, 30 sec), and again with CAP (50 nM, 30 sec). A. Representative traces of calcium accumulation following the illustrated experimental paradigm, treatment conditions indicated in legend. B. Quantified cumulative results of calcium accumulation, n=62-98 for each treatment paradigm indicated along the X-axis. Colored bars correspond to colors in A. * p<0.05, as determined by one-way ANOVA, with Bonferroni correction ad-hoc. C. Quantified desensitization percentages for each treatment paradigm shown in A and B, normalized to the initial CAP response. ** p<0.01, *** p < 0.005, as determined by one-way ANOVA, with Bonferroni correction ad-hoc.
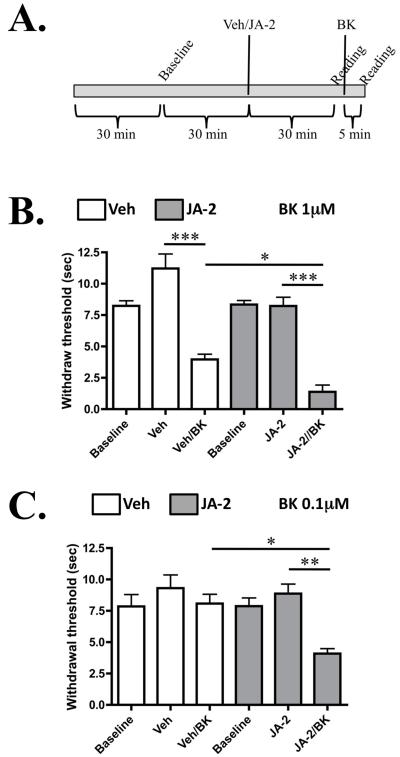
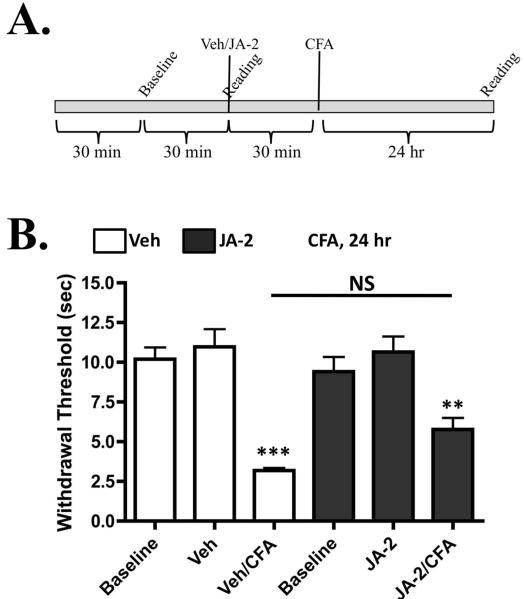
We next conducted studies on thermal nociception, since BK application is known to induce thermal hyperalgesia in multiple models [22,35]. As depicted in Figure 5A, animals were pre-treated with vehicle or JA-2, and then tested before and after BK administration, using the Hargreaves’ apparatus [12]. Results depicted in Figure 5B indicate that JA-2 significantly increases BK (1μM)-induced thermal hyperalgesia. Importantly, results in Figure 5C indicate that although the concentration of BK (0.1μM) was too low to induce hyperalgesia, pre-treatment with JA-2 resulted in a nociceptive response to the sub-threshold administration of BK. A dose-response relationship between JA-2 and BK is observed in this behavioral model, similar to the dose-response relationship seen in Figure 1. Complete Freund’s Adjuvant (CFA) was also utilized to determine whether JA-2 administration could inhibit induced thermal hyperalgesia that develops following the inflammatory insult that CFA induces (Figure 6, [40]). In contrast to the effects upon BK-mediated thermal hyperalgesia, JA-2 did not significantly reduce CFA-induced thermal hyperalgesia (Figure 5E).
Figure 5.
JA-2 pre-treatment increases bradykinin-induced thermal hyperalgesia. Male, adult rats were injected i.pl. with 25 μl of either vehicle (0.005% DMSO/0.9% Saline) or JA-2 (5 μM), and tested for paw withdrawal latency to a thermal stimulus following same-paw administration of BK (1μM or 0.1μM, 25μl). A. Experimental timeline that was followed for each animal injected with BK. B. Paw withdrawal latencies for animals treated with 1 μM BK, n = 6-8 per treatment group. C. Paw withdrawal latencies for animals treated with 0.1 μM BK, n = 6-10 per treatment group. * p < 0.05, ** p<0.01, *** p < 0.005 (as shown between indicated groups), as determined by one-way ANOVA, with Bonferroni correction ad-hoc.
Figure 6.
JA-2 pre-treatment does not significantly affect CFA-induced thermal hyperalgesia. A. Experimental timeline that was followed for each animal injected with complete Freund’s adjuvant (CFA). B. Male, adult rats were injected i.pl. with 25 μl of either vehicle (0.005% DMSO/0.9% Saline) or JA-2 (5 μM), and tested for paw withdrawal latency to a thermal stimulus 24 h after same-paw administration of CFA (0.5 mg/ml), n = 6 per treatment group. NS= no significance (as shown between indicated groups), ** p<0.01, *** p < 0.005 (compared to respective baseline readings), as determined by one-way ANOVA, with Bonferroni correction ad-hoc.
Discussion
The bradykinin (BK) neuropeptide is a significant component of the inflammatory reaction to tissue injury that results in sensitization and/or activation of peripheral nociceptors. BK is introduced to the affected area through either plasma extravasation, or release from adjacent cells. BK can then activate B2R expressed on primary afferent receptors, if it resists enzymatic degradation by extracellular peptidases. Two closely related peptidases, EP24.15 and EP24.16, negatively affect B2R activation by BK. Trypsin digestion of primary cultures of trigeminal ganglia support previous studies, demonstrating that EP24.15 expression on the plasma membrane is extracellular in nature. Additional investigations to assess the physiological importance of EP24.15 on TRP channel activation suggest that the peptidase can negatively regulate BK-sensitization of TRPV1 in trigeminal neurons. Additionally, BK-mediated thermal hyperalgesia was positively regulated in the presence of the EP24.15 inhibitor, JA-2. Taken together, these data indicate that EP24.15 and EP24.16 play a significant role in negatively modulating the activation of B2R by BK in peripheral nociceptors, thereby reducing inflammatory nociception.
Previous studies have investigated the potential for EP24.15 and EP24.16 to metabolize peptides such as BK but have focused solely on the tensive properties that would otherwise significantly alter physiologic systems. Smith et al. demonstrated that inhibitors of EP24.15 and EP24.16 potentiated hypotensive responses to BK in vivo [34]. Later studies confirmed that EP24.15 and EP24.16 are present in the cerebral vasculature and are capable of metabolizing BK in vivo [25,26]. Similarly, other peptidergic inhibitors of EP24.15 have demonstrated significant vasodilation effects when used in conjunction with BK, over using BK alone [29]. These findings are supportive of the work presented here, illustrating that EP24.15 and EP24.16 serve as important negative modulators of B2R activation by BK.
Plasma membrane-associated EP24.15 and EP24.16 are expressed on both the intracellular and extracellular faces of the plasma membrane. We sought to obtain functional results illustrated in Figure 2 that indicate that EP24.15 expression and activity associated with the plasma membrane in cultured trigeminal neurons is sensitive to trypsin treatment, indicating extracellular orientation. Multiple immunologically-based studies have demonstrated that EP24.15 is expressed on the extracellular face, citing immunofluorescence, and biotintylation as evidence [7,14,30]. However, additional studies suggest that EP24.16 expression on the extracellular surface of the plasma membrane in some cell lines is more abundant [11,38,39]. Taken together, these studies EP24.15 and EP24.16 are both expressed extracellularly, in an active, physiologically relevant conformation, and can metabolize substrates outside of the cell to reduce their bioavailability for receptor activation.
Angiotensin 1-converting enzyme (ACE) is related to EP24.15/16 in its affinity for metabolizing similar peptidergic substrates, and important regulation of BK signaling events. Until recently, it was believed that ACE inhibition, both in the plasma compartment and on the cell, led to increased B2R activation due to increased concentrations of BK available. While this paradigm is correct to a certain extent, new studies have discovered that ACE association with B2R causes ACE inhibitors to act as allosteric modulators of B2R activity, further affecting BK pharmacology [9]. Results presented in Figure 1 suggest that inhibition of EP24.15 and EP24.16 with JA-2 and Pro-Ile, respectively, also allosterically modulate B2R activation. Co-incubation with the inhibitors results in significant increases in Emax values for BK, suggesting intrinsic changes to B2R activation and/or internalization kinetics. Indeed, one study has characterized the strong association of EP24.15 and B2R in epithelial cells [31], similar to what has been shown for ACE and B2R [6]. Further analysis of this phenomenon could provide important avenues for further research on potential treatments for inflammatory hyperalgesia.
Inflammation that results from the administration of CFA induces hyperalgesia that can be quantified in a number of assays. In this study, we employed the Hargreaves’ apparatus [12] to determine thermal hyperalgesia in our rat model. Interestingly, CFA-induced thermal hyperalgesia was not as sensitive to JA-2 pre-treatment as BK-induced thermal hyperlagesia. It is possible that CFA-induced inflammation of the hindpaw did not result in a significant collection of peptidergic inflammatory mediators that EP24.15/16 can degrade, such as BK. Indeed, inflammatory insult can induce the release of numerous biologically active molecules capable of acting upon and sensitizing nociceptive afferent fibers, including nerve growth factor, histamine, serotonin, ATP, and BK (for review, see [8]). Of these, only BK serves as a substrate for EP24.15/16, thereby explaining the lack of a significant effect from JA-2 pre-treatment 24 hours-post CFA administration. However, it would be interesting to determine the potential role(s) of EP24.15/16 in mediating endogenous opioid synthesis in response to peripheral inflammatory insult.
There are other molecules that affect peripheral nociception, including opioid precursors, identified as substrates for EP24.15 and EP24.16. The affinity for EP24.15 and EP24.16 for opioid precursors including Dynorphin A1-8 indicates a potential role for the peptidases in controlling the release of endogenous opioid peptides such as enkephalins, endorphins, and dynorphins that act to provide antinociception during times of stress or pain. Indeed, intracerebroventricular administration of the EP24.15/16-specific inhibitor cFP (N-[1-(R,S)-carboxy-3-phenylpropyl]-Ala-Ala-Tyr-p-aminobenzoate), was reported to produce significant antinociception in time and dose-dependent mechanisms [18]. Additional studies demonstrated the ability of cFP to potentiate the antinociceptive effects of Met-enkephalin-Arg-Gly-Leu and dynorphin A1-8, when co-administered [17]. Furthermore, EP24.15 has also demonstrated enzymatic activity towards nociceptin/orphanin FQ agonists of the opioid receptor-like 1 orphan receptor (ORL1), such that the administration of an EP24.15 inhibitor helped to potentiate the reduction in motor activity resulting from nociceptin/orphanin FQ treatment [24]. Therefore, EP24.15 and EP24.16 are involved in multiple pathways that modulate the sensation of peripheral pain.
The increased in vivo stability of the peptidase inhibitor JA-2 proved significant in generating thermal hyperalgesia in animals that did not produce nociceptive responses to the low dose of BK administered in Figure 5. Importantly, it must be noted that JA-2 is capable of inhibiting not only plasma membrane-bound enzymes, but also secreted forms of the enzymes, known to be present in the periphery in circulating amounts [16]. Indeed, previous investigations that utilized first-generation inhibitors of EP24.15 and EP24.16, including cFP, have demonstrated in vivo activities that are the likely result of angiotensin converting enzyme (ACE) inhibition [36]. The 20-30 fold selectivity of JA-2 inhibiting EP24.15 preferentially over EP24.16 [32], coupled with Pro-Ile, a specific EP24.16 inhibitor, and its stability in vivo collectively indicate that this inhibitor could help to discern the distinct roles of EP24.15 and EP24.16 in regulating BK-mediated hyperalgesia in the periphery.
Acknowledgements
We wish to acknowledge Dr. A. Ian Smith for generously providing JA-2, and Dr. James Roberts for his critical reading of this manuscript. This work was supported by NIH grants NS039892 (MJG), NS55835 (WPC), DA26619 (KAB), and NS061884 (NAJ).
Abbreviations
- ACE
angiotensin converting enzyme I
- B1R
bradykinin type-1 receptor
- B2R
bradykinin type-2 receptor
- BK
Bradykinin
- BSA
bovine serum albumin
- CAP
capsaicin
- EP24.15
metalloendopeptidase EC3.4.24.15
- EP24.16
metalloendopeptidase EC3.4.24.16
- GPCRs
G-protein coupled receptors
- H2O
Water
- HBSS
Hanks Balanced Salt Solution
- IP
Inositol Phosphate
- QFS
Quenched Fluorescent Substrate
- SES
standard extracellular solution
- TG
Trigeminal ganglia
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- [2].Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther. 2007;321:839–847. doi: 10.1124/jpet.106.116681. [DOI] [PubMed] [Google Scholar]
- [3].Blaukat A. Structure and signalling pathways of kinin receptors. Andrologia. 2003;35:17–23. doi: 10.1046/j.1439-0272.2003.00533.x. [DOI] [PubMed] [Google Scholar]
- [4].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- [5].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [6].Chen Z, Deddish PA, Minshall RD, Becker RP, Erdos EG, Tan F. Human ACE and bradykinin B2 receptors form a complex at the plasma membrane. Faseb J. 2006;20:2261–2270. doi: 10.1096/fj.06-6113com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crack PJ, Wu TJ, Cummins PM, Ferro ES, Tullai JW, Glucksman MJ, Roberts JL. The association of metalloendopeptidase EC 3.4.24.15 at the extracellular surface of the AtT-20 cell plasma membrane. Brain Res. 1999;835:113–124. doi: 10.1016/s0006-8993(99)01494-8. [DOI] [PubMed] [Google Scholar]
- [8].Dray A, Bevan S. Inflammation and hyperalgesia: highlighting the team effort. Trends Pharmacol Sci. 1993;14:287–290. doi: 10.1016/0165-6147(93)90041-H. [DOI] [PubMed] [Google Scholar]
- [9].Erdos EG, Tan F, Skidgel RA. Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function. Hypertension. 2010;55:214–220. doi: 10.1161/HYPERTENSIONAHA.109.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ferreira J, da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fontenele-Neto JD, Massarelli EE, Gurgel Garrido PA, Beaudet A, Ferro ES. Comparative fine structural distribution of endopeptidase 24.15 (EC3.4.24.15) and 24.16 (EC3.4.24.16) in rat brain. J Comp Neurol. 2001;438:399–410. doi: 10.1002/cne.1323. [DOI] [PubMed] [Google Scholar]
- [12].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- [13].Horsthemke B, Bauer K. Characterization of a nonchymotrypsin-like endopeptidase from anterior pituitary that hydrolyzes luteining hormone-releasing hormone at the tyrosyl-glycine and histidyl-tryptophan bonds. Biochemistry. 1980;19:2867–2873. doi: 10.1021/bi00554a008. [DOI] [PubMed] [Google Scholar]
- [14].Jeske NA, Berg KA, Cousins JC, Ferro ES, Clarke WP, Glucksman MJ, Roberts JL. Modulation of bradykinin signaling by EP24.15 and EP24.16 in cultured trigeminal ganglia. J Neurochem. 2006;97:13–21. doi: 10.1111/j.1471-4159.2006.03706.x. [DOI] [PubMed] [Google Scholar]
- [15].Jeske NA, Glucksman MJ, Roberts JL. EP24.15 is Associated with Lipid Rafts. Journal of Neuroscience Research. 2003;74:468–473. doi: 10.1002/jnr.10778. [DOI] [PubMed] [Google Scholar]
- [16].Jeske NA, Glucksman MJ, Roberts JL. Metalloendopeptidase EC3.4.24.15 is constitutively released from the exofacial leaflet of lipid rafts in GT1-7 cells. J Neurochem. 2004;90:819–828. doi: 10.1111/j.1471-4159.2004.02557.x. [DOI] [PubMed] [Google Scholar]
- [17].Kest B, Orlowski M, Bodnar RJ. Endopeptidase 24.15 inhibition and opioid antinociception. Psychopharmacology (Berl) 1992;106:408–416. doi: 10.1007/BF02245427. [DOI] [PubMed] [Google Scholar]
- [18].Kest B, Orlowski M, Molineaux CJ, Bodnar RJ. Antinociceptive properties of inhibitors of endopeptidase 24.15. Int J Neurosci. 1991;56:141–149. doi: 10.3109/00207459108985410. [DOI] [PubMed] [Google Scholar]
- [19].Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- [20].Lew RA, Tochon-Danguy N, Hamilton CA, Stewart KM, Aguilar MI, Smith AI. Quenched fluorescent substrate-based peptidase assays. Methods Mol Biol. 2005;298:143–150. doi: 10.1385/1-59259-877-3:143. [DOI] [PubMed] [Google Scholar]
- [21].Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium. 2004;35:471–478. doi: 10.1016/j.ceca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [22].Manning DC, Raja SN, Meyer RA, Campbell JN. Pain and hyperalgesia after intradermal injection of bradykinin in humans. Clin Pharmacol Ther. 1991;50:721–729. doi: 10.1038/clpt.1991.212. [DOI] [PubMed] [Google Scholar]
- [23].Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- [24].Noble F, Roques BP. Association of aminopeptidase N and endopeptidase 24.15 inhibitors potentiate behavioral effects mediated by nociceptin/orphanin FQ in mice. FEBS Lett. 1997;401:227–229. doi: 10.1016/s0014-5793(96)01476-7. [DOI] [PubMed] [Google Scholar]
- [25].Norman MU, Lew RA, Smith AI, Hickey MJ. Metalloendopeptidases EC 3.4.24.15/16 regulate bradykinin activity in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2003;284:H1942–1948. doi: 10.1152/ajpheart.00948.2002. [DOI] [PubMed] [Google Scholar]
- [26].Norman MU, Reeve SB, Dive V, Smith AI, Lew RA. Endopeptidases 3.4.24.15 and 24.16 in endothelial cells: potential role in vasoactive peptide metabolism. Am J Physiol Heart Circ Physiol. 2003;284:H1978–1984. doi: 10.1152/ajpheart.01116.2002. [DOI] [PubMed] [Google Scholar]
- [27].Oliveira V, Garrido PA, Rodrigues CC, Colquhoun A, Castro LM, Almeida PC, Shida CS, Juliano MA, Juliano L, Camargo AC, Hyslop S, Roberts JL, Grum-Tokars V, Glucksman MJ, Ferro ES. Calcium modulates endopeptidase 24.15 (EC 3.4.24.15) membrane association, secondary structure and substrate specificity. FEBS J. 2005;272:2978–2992. doi: 10.1111/j.1742-4658.2005.04692.x. [DOI] [PubMed] [Google Scholar]
- [28].Orlowski M, Michaud C, Chu TG. A soluble metalloendopeptidase from rat brain. Purification of the enzyme and determination of specificity with synthetic and natural peptides. Eur J Biochem. 1983;135:81–88. doi: 10.1111/j.1432-1033.1983.tb07620.x. [DOI] [PubMed] [Google Scholar]
- [29].Rioli V, Prezoto BC, Konno K, Melo RL, Klitzke CF, Ferro ES, Ferreira-Lopes M, Camargo AC, Portaro FC. A novel bradykinin potentiating peptide isolated from Bothrops jararacussu venom using catallytically inactive oligopeptidase EP24.15. FEBS J. 2008;275:2442–2454. doi: 10.1111/j.1742-4658.2008.06389.x. [DOI] [PubMed] [Google Scholar]
- [30].Sanden C, Enquist J, Bengtson SH, Herwald H, Leeb-Lundberg LM. Kinin B2 receptor-mediated bradykinin internalization and metalloendopeptidase EP24.15-dependent intracellular bradykinin degradation. J Pharmacol Exp Ther. 2008;326:24–32. doi: 10.1124/jpet.108.136911. [DOI] [PubMed] [Google Scholar]
- [31].Shivakumar BR, Wang Z, Hammond TG, Harris RC. EP24.15 interacts with the angiotensin II type I receptor and bradykinin B2 receptor. Cell Biochem Funct. 2005;23:195–204. doi: 10.1002/cbf.1176. [DOI] [PubMed] [Google Scholar]
- [32].Shrimpton CN, Abbenante G, Lew RA, Smith I. Development and characterization of novel potent and stable inhibitors of endopeptidase EC 3.4.24.15. Biochem J. 2000;345(Pt 2):351–356. [PMC free article] [PubMed] [Google Scholar]
- [33].Skidgel RA. Bradykinin-degrading enzymes: structure, function, distribution, and potential roles in cardiovascular pharmacology. J Cardiovasc Pharmacol. 1992;20(Suppl 9):S4–9. doi: 10.1097/00005344-199200209-00003. [DOI] [PubMed] [Google Scholar]
- [34].Smith AI, Lew RA, Shrimpton CN, Evans RG, Abbenante G. A novel stable inhibitor of endopeptidases EC 3.4.24.15 and 3.4.24.16 potentiates bradykinin-induced hypotension. Hypertension. 2000;35:626–630. doi: 10.1161/01.hyp.35.2.626. [DOI] [PubMed] [Google Scholar]
- [35].Steranka LR, Manning DC, DeHaas CJ, Ferkany JW, Borosky SA, Connor JR, Vavrek RJ, Stewart JM, Snyder SH. Bradykinin as a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc Natl Acad Sci U S A. 1988;85:3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Telford SE, Smith AI, Lew RA, Perich RB, Madden AC, Evans RG. Role of angiotensin converting enzyme in the vascular effects of an endopeptidase 24.15 inhibitor. Br J Pharmacol. 1995;114:1185–1192. doi: 10.1111/j.1476-5381.1995.tb13332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- [38].Vincent B, Beaudet A, Dauch P, Vincent JP, Checler F. Distinct properties of neuronal and astrocytic endopeptidase 3.4.24.16: a study on differentiation, subcellular distribution, and secretion processes. J Neurosci. 1996;16:5049–5059. doi: 10.1523/JNEUROSCI.16-16-05049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vincent B, Dauch P, Vincent JP, Checler F. Stably transfected human cells overexpressing rat brain endopeptidase 3.4.24.16: biochemical characterization of the activity and expression of soluble and membrane-associated counterparts. J Neurochem. 1997;68:837–845. doi: 10.1046/j.1471-4159.1997.68020837.x. [DOI] [PubMed] [Google Scholar]
- [40].Zhang YH, Chen Y, Zhao ZQ. Resiniferatoxin reversibly blocks adjuvant-induced thermal hyperalgesia in the rat. Eur J Pharmacol. 2003;481:301–304. doi: 10.1016/j.ejphar.2003.09.053. [DOI] [PubMed] [Google Scholar]