Abstract
Evidence that aromatase expression in tumor-associated breast stroma is elevated, provides a rationale for use of aromatase inhibitors (AIs) in breast cancer treatment. However, regulation of local aromatase expression in cancer-free breast stroma is poorly understood. Recent clinical work indicates that stromal cells in dense breast tissue tend to express higher levels of aromatase than their counterpart from non-dense tissue. Consistent with the clinical observation, our cell culture-based study indicated that cell density, cell shape, and extracellular matrix (ECM) significantly induced stromal aromatase expression via a distinct signal transduction pathway. In addition, we identified a number of cell surface markers that are commonly associated with aromatase-expressing stromal cells. As mammographic density is one of the strongest and most prevalent risk factors for breast cancer, these findings provide a potential mechanistic link between alterations in tissue composition of dense breast tissue and increased stromal aromatase expression. Further exploration of the in vitro model system may advance understanding of an important problem in breast cancer biology.
Keywords: aromatase, mechanical phenotype, cell shape, cell density, ECM, mammographic density
INTRODUCTION
Estrogen biosynthesis in breast stroma is a significant source of estrogens in the tumor microenvironment [1–3]. For example, concentrations of local estradiol in breast tumors were similarly high in pre- and post-menopausal women [4, 5]. Furthermore, the efficacy of aromatase inhibitors (AIs) for ER+ post-menopausal breast cancer clearly demonstrates the importance of aberrant extra-ovarian estrogen biosynthesis in breast cancer development and progression [6–8].
It has been well documented that tumor cell-derived factors such as cytokines and prostaglandin E2 (PGE2) stimulate stromal expression of aromatase (Cyp19), the rate-limiting enzyme in estrogen biosynthesis, in adipose stromal cells (ASCs) [9–12]. Subsequent promotion of the growth of estrogen-dependent tumor cells by elevated estrogen levels completes a “vicious cycle” for ER+ breast cancer progression [5, 13]. Despite extensive studies of intratumoral stromal aromatase expression, relatively little is known about dysregulation of aromatase expression and local estrogen production in cancer-free microenvironment. However, there is a substantial individual-based variation in stromal aromatase expression [5, 14, 15]. Furthermore, forced expression of aromatase in mouse mammary gland results in hyperplasia [16] and increased incidence of chemical-induced tumor growth [17], supporting the notion that prolonged exposure to aberrant local estrogen biosynthesis can promote breast cancer.
Mammographic density refers to the extent of radiologically dense regions on a mammogram. Dense regions are enriched with epithelium and stroma [18]. The latter consists of various stromal cells and extracellular matrix (ECM) proteins [19, 20]. In contrast, non-dense tissue is enriched with adipocytes. High mammographic density is a strong and prevalent risk factor for breast cancer [21–23]. Compared with those women with little or no mammographic density, women with mammographic density in more than 75% of the breast have 3–5 times higher risk of breast cancer [21, 24–29].
The molecular cause for mammographic density-associated breast cancer risk remains poorly understood. Studies indicate that increased collagen density can promote cancer cell proliferation and invasiveness [30, 31]. It has also been proposed that increased breast density could lead to aberrant production of hormones and growth factors, which in turn may stimulate proliferation of mammary epithelial cells [32–34]. Consistent with this notion, Vachon and coworkers recently showed that dense breast tissue expressed higher aromatase protein than non-dense tissue [35]. In the current study, we used cell culture models to investigate the potential impact of mechanical phenotype including cell number, cell shape, and ECM proteins on the aromatase-expressing capability of ASCs isolated from cancer-free individuals. Findings of our in vitro work offer some potential molecular and cellular explanations for the elevated aromatase expression in dense breast tissue.
EXPERIMENTAL
Cell culture
Primary human adipose stromal cells (ASCs) were isolated from patients undergoing abdominal lyposuction and reduction mammoplasty at the University of Virginia and University of Texas Health Science Center at San Antonio, respectively. The methods for tissue procurement and cell isolation were previously published and approved by the institutional Human Investigation Committees [36]. ASCs were cultured in DMEM/F-12 medium with 10% FBS and 1% antibiotic-antimycotic solution [36]. Sub-confluent and confluent ASC cultures were plated at 0.75×105 and 2×105/well in 6 well plates, respectively. To assess the effect of shape change, cells were cultured in ultra-low attachment plate (Corning) at 2×105/well in 6 well plates. For culturing ASCs in ECM, 2×105 cells were suspended in 125 μl of growth medium, mixed with the same volume of Matrigel (with low growth factor, Invitrogen) and cultured in 24 well plates for 2 days.
RNA isolation, cDNA preparation, and quantitative RT-PCR
Total RNA was isolated using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. RNA was reverse-transcribed using the ImPrompII kit (Promega). Real-time PCR was carried out using the fluorescent dye SYBR-Green and an ABI 7900 Real-Time PCR System (Applied Biosystems). Primers used for GAPDH and aromatase were previously reported [37].
Gene knockdown with siRNA
Gene-specific knockdown by siRNA oligonucleotides were conducted using Lipofectamine reagent RNAiMAX (Invitrogen). The knockdown experiment was performed as previously described [38]. Briefly, cells at an approximate density of 60% were transfected with siRNA oligos at a final concentration of 10 nM and incubated for 72 h before they were harvested for RNA isolation.
Flow cytometry
For flow cytometry study, 5X105 cells were trypsinized and centrifuged for 3 min at 4,000 rpm at 40°C. They were subsequently resuspended in 100 μl of suspension buffer (1X PBS with 2% FBS), and incubated on ice with respective fluorescence-conjugated antibodies for 20 min. All antibodies were purchased from eBioscience. Samples were centrifuged, washed once with 1 ml suspension buffer, and suspended in 250 μl of suspension buffer. The cell surface marker analysis was performed by using a FACScalibur flow cytometer (Becton Dickinson).
RESULTS
Primary ASCs used in published studies are usually isolated from reduction mammoplasty or lyposuction, and therefore are subject to individual-based sample variation. The lack of defined cell surface marker-based verification in standard protocols for ASC isolation could further contribute to inconsistency in laboratory findings. To address this technical caveat, we conducted an extensive marker-based characterization of primary ASCs from ten donors. As shown in Table 1, we found that aromatase-expressing ASCs isolated per our established protocol [36] reached at least 80% enrichment for the following cell surface markers: CD31−, CD45−, CD61−, CD140a−, CD29+, and CD90+. These markers provide useful tools for verifying the purity of primary aromatase-expressing ASCs.
Table 1. Characterization of cell surface marker expression in ASCs.
| CD34 | CD45 | CD49d | CD90 | CD140a | CD31 | CD61 | CD29 | |
|---|---|---|---|---|---|---|---|---|
| 07L | 71.39 | 1.48 | 0.58 | 98 | 0.04 | 0.26 | 1.51 | 96.69 |
| 08L | 13.69 | 0.48 | 8.73 | 96.78 | 2 | 0.18 | 0.17 | 78.68 |
| h6-17 | 12.9 | 0.73 | 3.98 | 96.22 | 1.68 | 0.64 | ||
| 40504 | 13.31 | 0.87 | 23.68 | 98 | 0.38 | 0.71 | 80.64 | |
| h6-11 | 27 | 0.44 | 2.47 | 95.34 | 1.51 | 0.41 | ||
| 41229 | 33.37 | 0.11 | 1.85 | 98.99 | 0.95 | |||
| BSC-5-10 | 14.97 | 0.56 | 0.12 | |||||
| BSC5 | 84.56 | 5 | 12.45 | 96.98 | 0.27 | 7.44 | 79.4 | |
| BSC28 | 71.3 | 0.3 | 43.9 | 99.5 | 13.5 | 1.2 | 85.3 | |
| BSC29 | 2.4 | 0.5 | 1.8 | 98.2 | 3.7 | 3.1 | 50.4 |
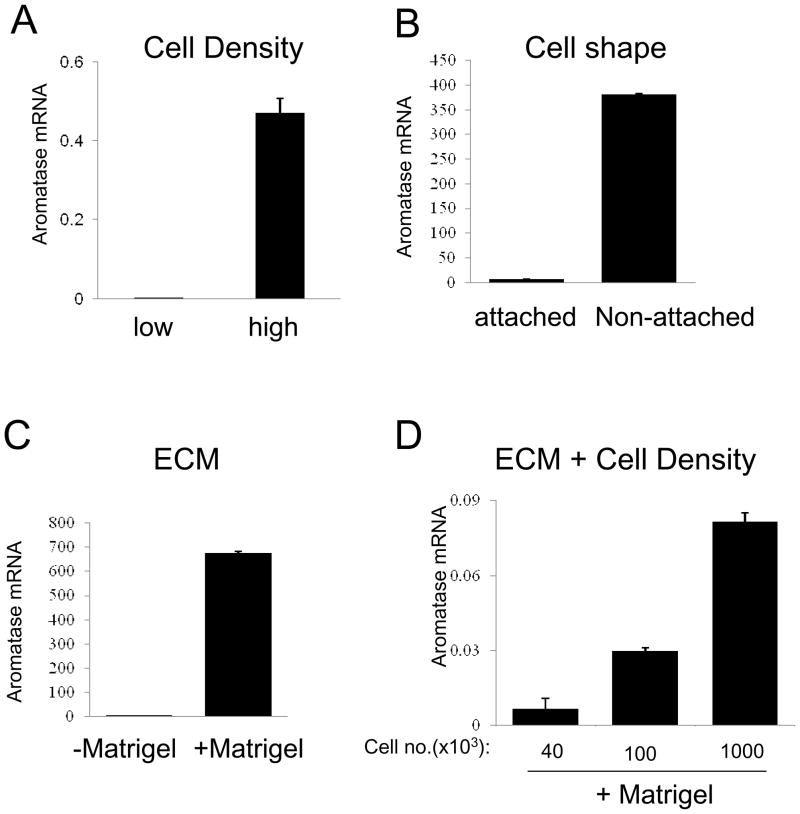
Given that dense breast tissues are enriched with stromal cells and ECM proteins, we hypothesized that increased density of stromal cells and matrix proteins might alter the stromal expression of aromatase. Using primary ASCs from cancer-free individuals, we made the initial observation that high cell density increased aromatase mRNA level up to 500 fold (Fig. 1A) [15]. This was at least partly due to confluency-induced changes in cell shape, as physical alterations of cell shape by plating cells in low-attachment plates can also stimulate aromatase gene expression (Fig. 1B) [15]. The striking elevation in aromatase transcript upon cell density increase correlated with increases in aromatase protein and enzymatic activity [15]. Because the ASCs used in our study had not been exposed to any tumor milieu or changes in culture medium, our findings indicate that mechanical phenotype such as cell density and change in cell shape alone can markedly induce aromatase expression in ASCs. This can apparently occur in the absence of any tumor-associated factors or other exogenous stimuli. Using ASC-conditioned medium, we further showed that cell density-induced estrogen production in ASCs significantly stimulated estrogen-dependent transcription in ER-positive breast cancer cells [15].
Figure 1. Stromal aromatase expression in response to changes in mechanical phenotype.
ASCs were cultured with different cell density (A), on a plastic surface with different cell-attaching features (B), and with or without lrECM (C and D). Aromatase mRNA levels were measured by real-time RT-PCR.
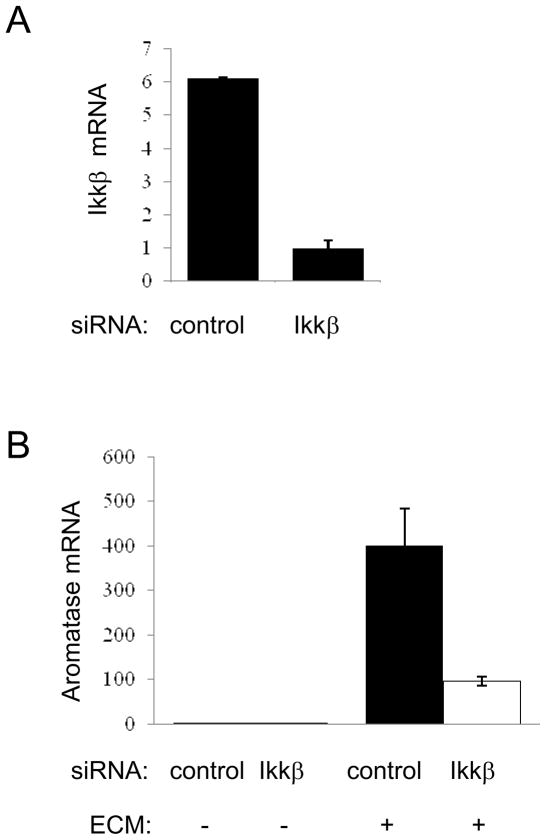
Our published work on cell density and cell shape-induced aromatase expression was conducted in a 2-D system where ASCs were cultured on a plastic surface [15]. To explore the impact of ECM on stromal aromatase expression, we embedded ASCs in laminin-rich ECM (lrECM). When seeded with the same number, ECM-embedded ASCs express several orders of magnitude more aromatase mRNA than their counterparts cultured in 2-D (Fig. 1C). Interestingly, increasing cell density with a constant ECM concentration resulted in further elevation of aromatase transcription (Fig. 1D), indicating that the stimulatory effects of cell density and matrix are additive. Our published work identified IkB kinase β (IKKβ) as a key player in the cell density/shape-triggered aromatase induction [15]. IKKβ is a kinase that plays a critical role in chronic inflammation and cancer development [39, 40]. Importantly, ECM-stimulated aromatase expression in rlECM-embedded ASCs is also dependent on IKKβ, as IKKβ knockdown substantially reduced the aromatase mRNA level in the 3D system (Fig. 2). Taken together, our findings support the notion that densities of stromal cells and ECM proteins both contribute to activation of stromal aromatase expression.
Figure 2. IKKβ is important for matrix-stimulated aromatase transcription.
IKKβ (A) and aromatase mRNA (B) in control and IKKβ-knockdown cells was assessed by real-time PCR.
DISCUSSION
Changes in mechanical phenotype in tumor microenvironment have long been associated with cancer progression [41]. For example, breast tumors are often detected initially based on the stiffness of the affected tissue. From the mechanistic perspective, dysregulation of actomyosin contractility and actin dynamics alters cell shape and facilitates aggressive tumor cell growth, survival, and invasion via the RhoGTPase-dependent pathway [42–46]. Likewise, increased mechanical force from ECM due to changes in its density, crosslinking, and topology can affect behaviors of tumor cells via the integrin-dependent pathways [47, 48]. However, much less is known about the relationship between mechanical homeostasis and hormone metabolism in breast stroma. As altered mechanical homeostasis in breast tumor affects both epithelial and stromal compartments of the same tumor microenvironment, there is a need to integrate the studies of the impact of mechanical forces on tumor and the surrounding stromal cells.
High mammographic density, which correlates with increased cell/ECM density, is a well-recognized and prevalent risk factor for breast cancer [22, 23]. By recapitulating the microenvironment of dense breast tissue in vitro, the cell culture systems used in our initial studies provide an excellent in vitro model to delineate the impact of dense tissue on regulation of aromatase gene expression. Our data suggest that cell density/shape changes significantly influence aromatase gene expression and estrogen biosynthesis in ASCs [15]. Furthermore, we show in the current study that matrix proteins also substantially stimulate stromal aromatase transcription. Importantly, our study identifies IKKβ as an important signaling molecule in mediating the mechanical phenotype-induced aromatase transcription. More extensive investigation in future will uncover additional regulatory molecules that transduce the mechanical signals to the transcriptional machinery responsible for aromatase gene activation.
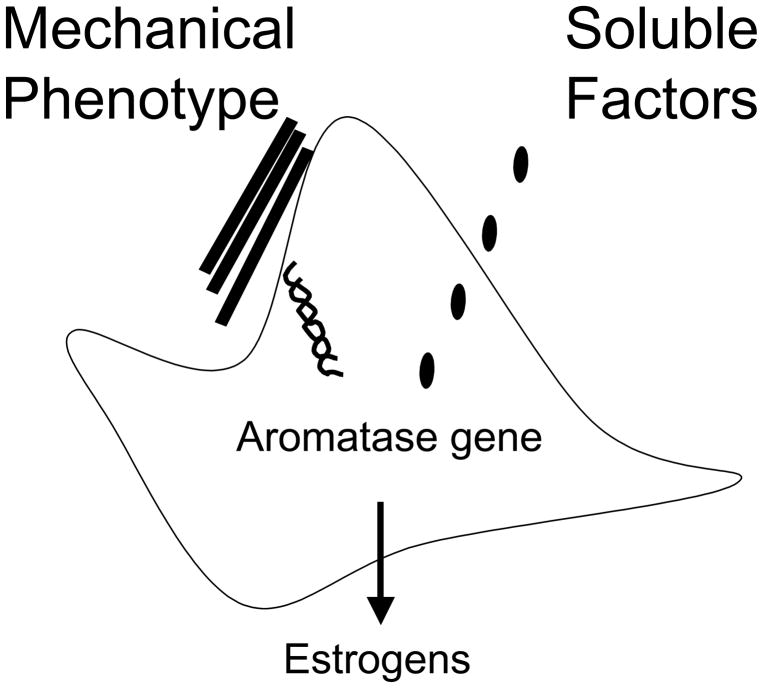
Previously published work from several groups has clearly demonstrated that tumor-secreted soluble factors such as prostaglandin E2 (PGE2) can stimulate aromatase transcription in ASCs [9, 11, 12]. Furthermore, PGE2, via its membrane-associated receptors [49, 50] activate both protein kinase C (PKC) and cAMP-dependent protein kinase A (PKA) [51, 52]. In light of the current finding, we propose that mechanical phenotype-induced signaling pathway is distinct from, and perhaps synergistic with, the previously characterized PGE2 signaling pathway that mediates tumor factor-stimulated aromatase transcription (Fig. 3). A comprehensive understanding of the mechanical phenotype-mediated signaling pathway and its potential crosstalk with the PGE2 signaling may provide new information instrumental to cancer etiology and novel therapeutics. Quantifiable metrics for assessment of tissue stiffness may also be useful in assessment of cancer risk. In addition, combinatorial targeting of both aromatase-activating signaling pathways may represent a novel concept in breast cancer prevention and treatment.
Figure 3. A model illustrating the mechanical phenotype and PGE2-stimulated signaling pathways for activating stromal aromatase transcription.
Acknowledgments
We thank Dr. Adam Katz for some of the ASC samples used in the current study. The work was supported by grants to R.L. (CA93506) and Y.H. (CA118578) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen S, Zhou D, Yang C, Okubo T, Kinoshita Y, Yu B, Kao Y-C, Itoh T. Modulation of aromatase expression in human breast tissue. J Steroid Bioc & Mol Biol. 2001;79:35–40. doi: 10.1016/s0960-0760(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 2.Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen--the good, the bad, and the unexpected. Endocr Rev. 2005;26:332–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 3.Simpson ER, Clyne C, Rubin G, Boo WC, Robertson K, Britt K, Speed C, Jones M. Aromatase-a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 4.Sasano H, Harada N. Intratumoral aromatase in human breast, endometrial, and ovarian malignacies. Endocrine Rev. 1998;19:583–607. doi: 10.1210/edrv.19.5.0342. [DOI] [PubMed] [Google Scholar]
- 5.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 6.Brodie A, Lu Q, Long B. Aromatase and its inhibitors. J Steroid Biochem Mol Biol. 1999;69:205–10. doi: 10.1016/s0960-0760(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 7.Morales L, Neven P, Paridaens R. Choosing between an aromatase inhibitor and tamoxifen in the adjuvant setting. Curr Opin Oncol. 2005;17:559–565. doi: 10.1097/01.cco.0000180434.31991.bf. [DOI] [PubMed] [Google Scholar]
- 8.van Landeghem AA, Poortman J, Nabuurs M, Thijssen JH. Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res. 1985;45:2900–6. [PubMed] [Google Scholar]
- 9.Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE. Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: mechanism of desmoplastic reaction. Cancer Res. 2001;61:2250–2255. [PubMed] [Google Scholar]
- 10.Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo. J Endocrinol. 2005;187:225–35. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 11.Brueggemeier RW, Richards JA, Petrel TA. Aromatase and cyclooxygenases: enzymes in breast cancer. J Steroid Biochem Mol Biol. 2003;86:501–507. doi: 10.1016/s0960-0760(03)00380-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Suzuki T, Kovacic A, Saito R, Miki Y, Ishida T, Moriya T, Simpson ER, Sasano H, Clyne CD. Interactions between prostaglandin E(2), liver receptor homologue-1, and aromatase in breast cancer. Cancer Res. 2005;65:657–663. [PubMed] [Google Scholar]
- 13.Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE. Malignant Breast Epithelial Cells Stimulate Aromatase Expression via Promoter II in Human Adipose Fibroblasts: An Epithelial-Stromal Interaction in Breast Tumors Mediated by CCAAT/Enhancer Binding Protein beta. Cancer Research. 2001;61:2328–2334. [PubMed] [Google Scholar]
- 14.Quinn AL, Burak WE, Jr, Brueggemeier RW. Effects of matrix components on aromatase activity in breast stromal cells in culture. J Steroid Biochem Mol Biol. 1999;70:249–56. doi: 10.1016/s0960-0760(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Choudary A, Ghosh S, Musi N, Hu Y, Li R. IKK{beta} Mediates Cell Shape-Induced Aromatase Expression and Estrogen Biosynthesis in Adipose Stromal Cells. Mol Endocrinol. 2009 doi: 10.1210/me.2008-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandava U, Kirma N, Tekmal RR. Aromatase overexpression transgenic mice model: cell type specific expression and use of letrozole to abrogate mammary hyperplasia without affecting normal physiology. Steroid Biochem Molec Biol. 2001;79:27–34. doi: 10.1016/s0960-0760(01)00133-9. [DOI] [PubMed] [Google Scholar]
- 17.Keshava N, Mandava U, Kirma N, Tekmal RR. Acceleration of mammary neoplasia in aromatase transgenic mice by 7,12-dimethylbenz[a]anthracene. Cancer Letters. 2001;167:125–33. doi: 10.1016/s0304-3835(01)00478-5. [DOI] [PubMed] [Google Scholar]
- 18.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW, Yaffe MJ, Tritchler DL. Mammographic densities and breast cancer risk. Breast Dis. 1998;10:113–26. doi: 10.3233/bd-1998-103-412. [DOI] [PubMed] [Google Scholar]
- 19.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–35. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Bowerman S, Heber D. Health ramifications of the obesity epidemic. Surg Clin North Am. 2005;85:681–701. doi: 10.1016/j.suc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 21.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 22.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 23.Vachon CM, van Gils CH, Sellers TA, Ghosh K, Pruthi S, Brandt KR, Pankratz VS. Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res. 2007;9:217. doi: 10.1186/bcr1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 25.Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1662–1669. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 26.Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000;60:3744–3748. [PubMed] [Google Scholar]
- 27.Ursin G. Mammographic density as indicator of breast cancer risk. Tidsskr Nor Laegeforen. 2003;123:3373–6. [PubMed] [Google Scholar]
- 28.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230:29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 29.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 30.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–43. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–248. [PubMed] [Google Scholar]
- 33.Diorio C, Pollak M, Byrne C, Masse B, Hebert-Croteau N, Yaffe M, Cote G, Berube S, Morin C, Brisson J. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14:1065–1073. doi: 10.1158/1055-9965.EPI-04-0706. [DOI] [PubMed] [Google Scholar]
- 34.Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT. Endogenous sex hormones, prolactin and mammographic density in postmenopausal Norwegian women. Int J Cancer. 2007;121:2506–11. doi: 10.1002/ijc.22971. [DOI] [PubMed] [Google Scholar]
- 35.Vachon CM, Sasano H, Ghosh K, Brandt KR, Watson DA, Reynolds C, Lingle WL, Goss PE, Li R, Aiyar SE, Scott CG, Pankratz VS, Santen RJ, Ingle JN. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res Treat. doi: 10.1007/s10549-010-0944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–23. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh S, Lu Y, Katz A, Hu Y, Li R. Tumor Suppressor BRCA1 Inhibits a Breast Cancer-Associated Promoter of the Aromatase Gene (Cyp19) in Human Adipose Stromal Cells. Am J Physiol Endocrinol Metab. 2007;292:246–252. doi: 10.1152/ajpendo.00242.2006. [DOI] [PubMed] [Google Scholar]
- 38.Hu YF, Ghosh S, Amleh A, Yue W, Lu Y, Katz A, Li R. Modulation of aromatase expression by BRCA1: a possible link to tissue-specific tumor suppression. Oncogene. 2005;24:8343–8348. doi: 10.1038/sj.onc.1208985. [DOI] [PubMed] [Google Scholar]
- 39.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 40.Karin M. The IkappaB kinase- a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 41.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964–87. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 43.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 45.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 46.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 50.Richards JA, Brueggemeier RW. Prostaglandin E2 regulates aromatase activity and expression in human adipose stromal cells via two distinct receptor subtypes. J Clin Endocrinol Metab. 2003;88:2810–6. doi: 10.1210/jc.2002-021475. [DOI] [PubMed] [Google Scholar]
- 51.Mendelson CR, Corbin CJ, Smith ME, Smith J, Simpson ER. Growth factors suppress and phorbol esters potentiate the action of dibutyryl adenosine 3′,5′-monophosphate to stimulate aromatase activity of human adipose stromal cells. Endocrinology. 1986;118:968–973. doi: 10.1210/endo-118-3-968. [DOI] [PubMed] [Google Scholar]
- 52.Evans CT, Corbin CJ, Saunders CT, Merrill JC, Simpson ER, Mendelson CR. Regulation of estrogen biosynthesis in human adipose stromal cells. Effects of dibutyryl cyclic AMP, epidermal growth factor, and phorbol esters on the synthesis of aromatase cytochrome P-450. J Biol Chem. 1987;262:6914–20. [PubMed] [Google Scholar]