Abstract
Abnormal lung function is a known risk factor for poor outcomes in the allogeneic hematopoietic stem cell transplant (HSCT) population, although the specific causes of these abnormalities have not been well explored. There is limited data on the effect of cigarette smoking on transplant outcomes. We conducted a retrospective observational cohort study of 845 consecutive patients aged ≥ 18 years who underwent allogeneic HSCT at the Seattle Cancer Care Alliance/Fred Hutchinson Cancer Research Center. Smoking exposure was defined by quit time, smoking status (never, former, and current) and log2-transformed pack-years. The main outcomes were time to respiratory failure within 100 days of transplant, relapse, and non-relapse mortality. In multivariable analyses, a two-fold increase in pack-years smoked was associated with an increased risk of early respiratory failure (HR 1.33, 95% CI 1.09 to 1.64, p = 0.006). This association was observed independent of pre-transplant lung function. A two-fold increase in pack-years smoked was associated with an increased risk of relapse, but this finding was not statistically significant (HR 1.16, 95% CI 0.92 to 1.46, p = 0.21). An association was not observed between cigarette smoking and non-relapse mortality. Cigarette smoking is associated with an increased risk of respiratory failure and relapse within 100 days of allogeneic HSCT. The association with respiratory failure is mediated in part by abnormal lung function prior to transplant and likely through other mechanisms as well. Given the adverse effects associated with cigarette smoking prior to transplant, future studies should focus on obtaining accurate smoking histories, tracking prospective changes in smoking status, and assessing the benefits of tobacco cessation on outcomes in this population.
Keywords: smoking, hematopoietic cell transplant, outcomes, respiratory failure, relapse, mortality
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) has become standard therapy to treat a variety of malignant and other hematologic diseases, with over 20,000 allogeneic transplants performed annually worldwide (1). Despite its widespread use, transplant-related mortality and morbidity remain high. The 100-day mortality rate for allogeneic transplants ranges from 10 to 40% (2). In addition, 15 to 40% of all allogeneic HSCT recipients require intensive care admission after transplant (2, 3). Pulmonary complications account for most of the life-threatening conditions that develop post-transplant, and, traditionally, are classified as early or late depending on whether they occur before or after day 100.
Prior studies have suggested that abnormal pre-transplant pulmonary function tests (PFTs) increase the risk of complications, including early respiratory failure and mortality (4–7). Causes for abnormal pre-transplant lung function, however, have not been well explored. Cigarette smoking is known to be a primary cause of abnormal PFTs (8, 9), and previous studies have reported that 14% to 62% of transplant candidates have a former or current history of smoking (10–13). Cigarette smoking, therefore, may account for some of the abnormal PFTs observed before transplant, but there are limited data on the direct effects of cigarette smoking on early pulmonary complications, relapse, and mortality post-transplant. Marks et al. reported that “high dose” smoking, defined by ≥ 10 pack-year history and smoking ≥ 1 pack per day, was associated with an increased risk of treatment-related mortality and disease relapse (12). Some studies have reported an increased risk of early pulmonary complications among smokers, while others have not demonstrated such associations (11, 13). In general, these studies have been limited by small numbers of patients or have focused on only certain transplant populations. Furthermore, despite growing evidence that cigarette smoking impairs immune function, exacerbates cancer treatment-related toxicities, and increases risk of recurrence and secondary malignancies, few clinical oncology studies collect information on smoking history unless the malignancy is primarily smoking-related (14–17). Given these limitations, we conducted a retrospective observational cohort study to assess the relationship between pre-transplant cigarette smoking and early respiratory failure, relapse, and post-transplant non-relapse mortality among adult allogeneic HSCT recipients.
METHODS
Patient Selection
All adult patients (age ≥ 18 years) who received an allogeneic HSCT at Fred Hutchinson Cancer Research Center between June 20, 2005 and June 30, 2009 were eligible for the study (n = 846). On June 20, 2005, routine tracking of smoking history began during pre-transplant pulmonary function testing.
Cigarette Smoking
Cigarette smoking was the primary exposure. Smoking history was obtained via self-report during pre-transplant pulmonary function testing and categorized as never, former, or current. For this study, we defined a former smoker as an individual who quit smoking for at least 1 year prior to the interview. Patients who were currently smoking or had quit for less than 1 year prior to the interview were considered current smokers. Smoking dose was defined in pack-years (number of cigarettes smoked per day/20 multiplied by years smoked), and log2-transformed to achieve a normal distribution.
Outcomes Assessment
The primary outcomes were respiratory failure within 100 days, disease relapse, and non-relapse mortality. Early respiratory failure was defined as mechanical ventilation for a non-elective reason within 100 days after transplant. Since the majority of our patients are discharged from our center after 100 days, this time period provides the most complete and accurate respiratory failure data (7). For patients who had multiple episodes of respiratory failure within 100 days, only the time to the first respiratory failure episode was analyzed. Non-relapse mortality was defined as mortality without evidence of disease relapse.
Covariates
Data were collected on patient demographics, including age, gender, and race. Disease risk was defined as low, intermediate, or high risk for mortality as described by Parimon and colleagues (18). Low-risk diseases include chronic myelogenous leukemia (CML) in chronic phase, refractory anemia, and aplastic anemia. Intermediate-risk diseases include chronic myelogenous leukemia in accelerated phase or chronic phase after blast phase, acute leukemia or lymphoma in remission, refractory anemia with excess blasts, and chronic lymphocytic leukemia. High-risk diseases include chronic myelogenous leukemia in blast phase, acute leukemia or lymphoma in relapse, myeloma, solid tumor, and non-hematologic diseases.
Stem cell sources were classified as bone marrow, peripheral blood stem cell (PBSC), cord blood, or a combination of bone marrow and PBSC. Donor match status was determined according to donor-recipient human leukocyte antigen (HLA) compatibility. Conditioning regimens were categorized as myeloablative or non-myeloablative. Myeloablative regimens were further grouped according to the dose of total-body irradiation used: none, ≤ 12 Gy, or > 12 Gy. Cytomegalovirus (CMV) serologic status was assessed in both recipient and donor. Acute graft-versus-host disease (GVHD) was graded I–IV, and categorized as “no” (grades 0–II) or “yes” (grades III–V) based on stages of organ involvement using standard criteria (19, 20). Chronic GVDH was defined by the National Institutes of Health (NIH) 2005 criteria (21).
Pulmonary function testing was obtained routinely prior to transplant and again approximately 80–120 days post-transplant. All pulmonary function tests were performed at the Fred Hutchinson Cancer Research Center according to American Thoracic Society guidelines (22). All pulmonary function values, except the ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC), were expressed as a percentage of predicted values according to published equations (23, 24), unless otherwise specified. Diffusing capacity for carbon monoxide (DLCO) measurements were corrected for hemoglobin levels obtained at the closest time to the DLCO measurement (25).
Statistical Methods
All statistical analyses were performed using STATA 11.0 (StataCorp, College Station, TX). Baseline variables were compared between smoking groups using Pearson χ2 testing or t-tests for continuous variables. Time-to-event analyses were conducted using Cox proportional hazards regression models to estimate the hazard ratios (HRs) for associations between pre-transplant smoking and transplant outcomes. Huber-White standard errors were used to relax traditional proportional hazards assumptions. In secondary analyses, we used linear regression to examine the association between smoking dose and day 80–120 pulmonary function. The multivariable model included smoking status as a categorical variable and log2-transformed pack-years. We decided a priori to also include age, gender, and conditioning regimen as potential confounders. Additional covariates, including patient race, disease risk, CMV status, stem cell source, donor HLA match, and acute and chronic GVHD modeled as time-varying covariates, were evaluated independently in the models and included in the final adjusted models based on likelihood ratio testing. Measures of pulmonary function were then added to the multivariable model. Only FEV1 and DLCO were included in these models because of the high correlation between FEV1, FVC, total lung capacity (TLC), and the lack of correlation between DLCO and the other pulmonary function parameters (7). Two-sided p-values less than 0.05 were considered statistically significant.
Data for each patient were censored at the time of the event of interest, death, or date of last contact, whichever came first, with the exception of analyses involving early respiratory failure, where patients were censored at 100 days if they did not have the outcome or die. In analyses involving non-relapse mortality and relapse, these outcomes were considered competing events and censored accordingly. Data for 1 patient was censored at 83 days at the time of a second transplant.
RESULTS
From July 20, 2005 to June 30, 2009, 846 patients underwent allogeneic HSCT. One patient was excluded for missing smoking data. Clinical characteristics are summarized in Table 1. There were 230 former smokers (27%) and 85 current smokers (10%). The overall median number of pack-years smoked was 10 (IQR 4–25). The median number of days before transplant that a smoking history was obtained was 24 days (IQR 20–35 days). The median age was 58 years (IQR 51–63 years) in former smokers and 46 years (IQR 32–57 years) in current smokers compared to 50 years (IQR 38–59 years) in never smokers. This is slightly older than in previous studies and reflects the trend of increasing numbers of allogeneic transplants among individuals older than 50 years of age (10–13, 26). Former smokers were more likely to receive a non-myeloablative conditioning regimen, consistent with older age in this group. Although the majority of transplant patients at our institution undergo bronchoscopy for evaluation of acute radiographic abnormalities, specific pulmonary infections are difficult to identify in this cohort because the majority of patients are already on broad-spectrum antibiotic and antifungal therapy prior to bronchoscopy. Nevertheless, there was no significant difference in the rate of bronchoscopies performed in never smokers vs. former or current smokers. There were no significant differences with regard to patient gender, ethnicity, underlying disease severity, donor HLA match, CMV status, and stem cell source between the groups.
Table 1.
Patient characteristics
| Never Smokers | Former Smokers | Current Smokers | ||
|---|---|---|---|---|
| Characteristic | (N=530) (%) | (N=230) (%) | (N=85) (%) | p-value |
| Age (years) | <0.0005 | |||
| median (IQR) | 50 (38–59) | 58 (51–63) | 46 (32–57) | |
| Male | 302 (57) | 138 (60) | 47 (55) | 0.67 |
| Caucasian race | 456 (89) | 205 (92) | 77 (93) | 0.81 |
| Pack-years | ||||
| median (IQR) | - | 10 (4–20) | 15 (6–30) | 0.05 |
| Donor HLA status | 0.68 | |||
| related/matched | 191 (36) | 86 (37) | 30 (35) | |
| related/mismatched | 27 (5) | 13 (6) | 8 (9) | |
| unrelated/matched | 204 (39) | 80 (35) | 27 (32) | |
| unrelated/mismatched | 107 (20) | 51 (22) | 20 (24) | |
| Disease risk | 0.35 | |||
| Low | 41 (8) | 11 (5) | 7 (8) | |
| Intermediate | 299 (56) | 146 (63) | 48 (57) | |
| High | 190 (36) | 73 (32) | 30 (35) | |
| Conditioning regimen | 0.001 | |||
| Non-myeloablative | 204 (38) | 123 (53) | 25 (29) | |
| Myeloablative | ||||
| Non-TBI | 170 (32) | 55 (24) | 37 (44) | |
| TBI ≤ 12 Gy | 132 (25) | 47 (20) | 19 (22) | |
| TBI > 12 Gy | 20 (4) | 5 (2) | 3 (3) | |
| Stem cell source | 0.75 | |||
| Bone marrow | 75 (14) | 30 (13) | 14 (16) | |
| PBSC | 420 (79) | 190 (83) | 67 (79) | |
| BM, PBSC | 3 (1) | 0 | 0 | |
| Cord | 32 (6) | 10 (4) | 4 (5) | |
| CMV (recipient/donor) | 0.27 | |||
| neg/neg | 149 (30) | 67 (30) | 27 (33) | |
| neg/pos | 57 (12) | 19 (9) | 4 (5) | |
| pos/neg | 140 (28) | 77 (35) | 27 (33) | |
| pos/pos | 151 (30) | 56 (26) | 23 (29) | |
| Acute GVHD | 73 (14) | 27 (12) | 11 (13) | 0.74 |
| Chronic GVHD | 218 (52) | 93 (54) | 22 (32) | 0.005 |
| Bronchoscopy performed | 99 (19) | 52 (23) | 17 (20) | 0.46 |
PFTs, including spirometry and DLCO, were obtained on 96% of patients prior to transplant. Over 75% of patients in all 3 smoking groups had normal FEV1, FVC, and TLC percent predicted values (Table 2). Both former and current smokers had slightly lower FEV1 and FEV1/FVC compared to never smokers. A greater proportion of current smokers had DLCO values that were < 80%.
Table 2.
Distribution of pre-transplant pulmonary function tests
| Never Smokers | Former Smokers | Current Smokers | ||
|---|---|---|---|---|
| PFT parameter | (N=530) (%) | (N=230) (%) | (N=85) (%) | p-value |
| Percent predicted FEV1 | 0.04 | |||
| ≥ 80% | 434 (82) | 180 (78) | 66 (78) | |
| 70–79% | 52 (10) | 27 (12) | 9 (11) | |
| 60–69% | 16 (3) | 10 (4) | 3 (4) | |
| < 60% | 9 (2) | 7 (3) | 4 (5) | |
| Percent predicted FVC | 0.57 | |||
| ≥ 80% | 456 (86) | 202 (88) | 74 (87) | |
| 70–79% | 39 (7) | 17 (7) | 7 (8) | |
| 60–69% | 9 (2) | 4 (2) | 0 | |
| < 60% | 7 (1) | 1 (0.5) | 1 (1) | |
| FEV1/FVC | ||||
| median (IQR) | 0.77 (0.73–0.82) | 0.75 (0.70–0.79) | 0.73 (0.68–0.79) | <0.0005 |
| Percent predicted TLC | 0.21 | |||
| ≥ 80% | 477 (90) | 213 (93) | 77 (91) | |
| 70–79% | 20 (4) | 8 (3) | 4 (5) | |
| 60–69% | 5 (1) | 0 | 0 | |
| < 60% | 7 (1) | 1 (0.5) | 0 | |
| Percent predicted DLCO | 0.0009 | |||
| ≥ 80% | 216 (41) | 95 (41) | 23 (27) | |
| 70–79% | 169 (32) | 67 (29) | 24 (28) | |
| 60–69% | 92 (17) | 38 (16) | 21 (25) | |
| < 60% | 37 (7) | 25 (11) | 14 (16) | |
Smoking and Early Respiratory Failure
Early respiratory failure occurred in 32 (6%) never smokers, 14 (6%) former smokers, and 2 (2%) current smokers. The median number of days after transplant to respiratory failure was 17.5 days (IQR 11–29.5 days). Forty-four of the 48 patients (92%) died after receiving mechanical ventilation. The main reasons for respiratory failure included acute respiratory distress syndrome (ARDS)/acute lung injury (ALI) (n = 15, 31%), aspiration/mucositis (n = 11, 23%), diffuse alveolar hemorrhage (n = 8, 17%), and idiopathic pneumonia syndrome (n = 7, 14%), as defined by previous criteria (27–29). In multivariable analyses adjusting for age, sex, conditioning regimen, smoking status, and acute GVHD, smoking dose, defined in pack-years, was associated with a higher risk of early respiratory failure. For each doubling of pack-years, the hazard ratio for early respiratory failure increased 33% (95% CI 1.09 to 1.64, p = 0.006) (Figure 1).
Figure 1.
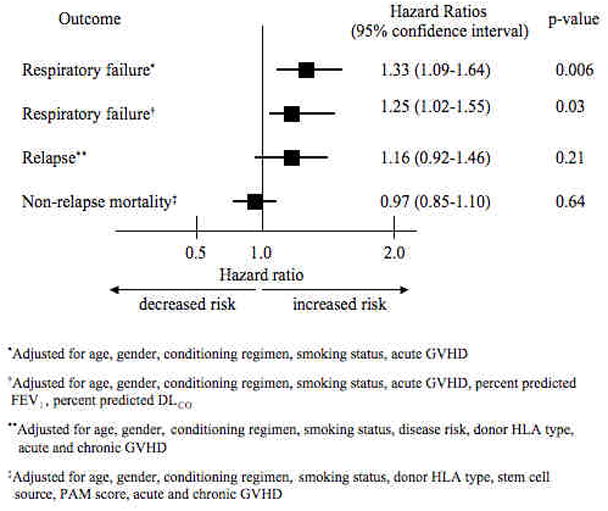
Multivariable adjusted hazards ratios for the association between cigarette smoking and early respiratory failure, relapse, non-relapse mortality.
Figure 2 shows the projected cumulative incidence curves, using our model for early respiratory failure, with all HSCT predictors being equal except smoking dose. In this projection, we combined former and current smokers into one group and compared a never-smoking patient to a smoker with a 20 pack-year history and another smoker with a 40 pack-year history.
Figure 2.
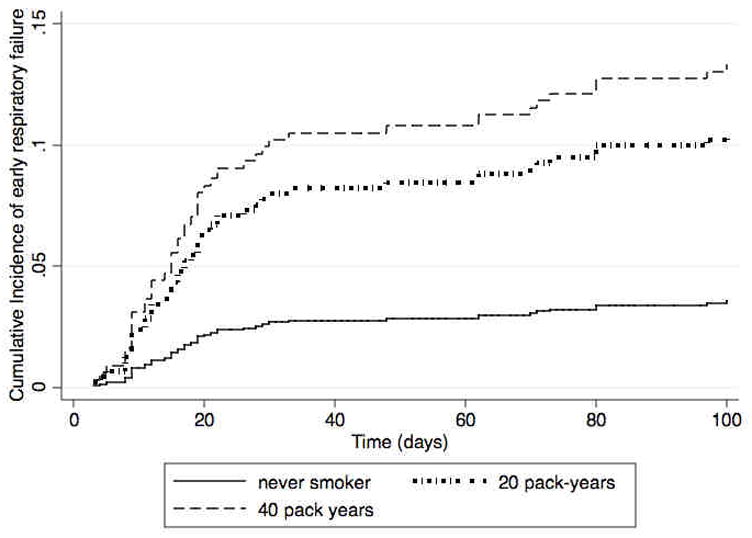
Projected cumulative incidence curves for early respiratory failure associated with never smoking versus 20 pack-year smoking history versus 40 pack-year smoking history.
To better understand how abnormal pulmonary function may have accounted for the association between pre-transplant smoking and early respiratory failure, we then included pre-transplant pulmonary function in the multivariable model. As expected, the addition of variables for pulmonary function attenuated our hazards ratio for early respiratory failure, but an association between the two-fold increase in pack-years and risk of early respiratory failure remained (HR 1.25, 95% CI 1.02 to 1.55, p = 0.03) (Figure 1).
Smoking and Relapse
One hundred and sixty-two of the patients relapsed, including 105 (20%) never smokers, 42 (18%) former smokers, and 15 (18%) current smokers, with a median number of days after transplant to relapse of 103 (IQR 71 – 239 days). Over three quarters of those who relapsed (78%) died. In multivariable analyses adjusting for age, gender, conditioning regimen, smoking status, disease risk, donor HLA type, and acute and chronic GVHD (as time-varying covariates), a two-fold increase in pack-years was associated with a higher risk of relapse, but this finding was not statistically significant (HR 1.16, 95% CI 0.92 to 1.46, p = 0.21) (Figure 1).
Smoking and Non-Relapse Mortality
There were 386 (46%) deaths from all causes in this cohort. Of these, 127 had relapsed, leaving 259 (31%) individuals (156 [37%] never smokers, 79 [42%] former smokers, 24 [34%] current smokers) with non-relapse mortality. The median number of days after transplant to non-relapse mortality was 163 days (IQR 62 – 298 days). In multivariable analyses adjusting for age, gender, conditioning regimen, smoking status, donor HLA type, PAM score, stem cell source, and acute and chronic GVHD, smoking dose was not associated with a higher risk of non-relapse mortality (HR 0.97, 95% CI 0.85 to 1.10, p = 0.64) (Figure 1).
Smoking and Day 100 Pulmonary Function
We performed secondary analyses of the relationship between smoking dose and pulmonary function obtained routinely 80–120 days post-transplant. After adjustment for patient age, gender, conditioning regimen, and smoking status, smoking dose was not significantly associated with changes in FEV1, FVC, TLC, or DLCO from baseline (data not shown).
Stratification By Donor Type
Because prior studies have cited differential transplant outcomes with HLA-identical sibling transplants versus unrelated matched transplants, we analyzed the association between cigarette smoking and transplant outcomes separately for these subgroups. After adjustment, there was still an increased risk of early respiratory failure with a two-fold increase in pack-years in both groups. Patients who received a HLA-identical related transplant had an 83% increased risk of early respiratory failure (95% CI 1.18 to 2.84, p = 0.007) compared to a 46% increased risk among patients receiving an unrelated matched transplant (95% CI 1.02 to 2.09, p = 0.04) (Table 3). Smoking dose was associated with an increased risk of relapse in patients receiving an unrelated matched transplant (HR 1.44, 95% CI 1.04 to 2.01, p = 0.03). There was a weak association between pack-years and relapse in related matched donor transplants that was not significant (HR 1.21, 95% CI 0.92 to 1.60, p = 0.17). Smoking dose was again not associated with non-relapse mortality when stratified by donor type (Table 3).
Table 3.
Multivariable adjusted hazards ratios for the association between smoking and early respiratory failure, relapse, or non-relapse mortality, stratified by donor HLA type
| Outcome | N (%) | HR | 95% CI | p-value |
|---|---|---|---|---|
| Related matched donor | 307 (36%) | |||
| Early respiratory failure* | 1.83 | 1.18 to 2.84 | 0.007 | |
| Relapse** | 1.21 | 0.92 to 1.60 | 0.17 | |
| Non-relapse mortality‡ | 0.99 | 0.77 to 1.29 | 0.97 | |
| Unrelated matched donor | 311 (37%) | |||
| Early respiratory failure* | 1.46 | 1.02 to 2.09 | 0.04 | |
| Relapse** | 1.44 | 1.04 to 2.01 | 0.03 | |
| Non-relapse mortality‡ | 0.91 | 0.74 to 1.12 | 0.38 |
Adjusted for age, gender, smoking status, conditioning regimen, acute GVHD
Adjusted for age, gender, smoking status, conditioning regimen, disease risk, acute and chronic GVHD
Adjusted for age, gender, smoking status, conditioning regimen, stem cell source, PAM score, acute and chronic GVHD
DISCUSSION
The major finding of our study is that lifetime smoking dose is associated with an increased risk of respiratory failure within 100 days of transplant. This relationship appears to be only partially mediated by pre-transplant lung function, suggesting that other biologic mechanisms may be involved. We did not observe significant effects of smoking on overall relapse or non-relapse mortality, although the direction of the association between smoking dose and relapse warrants further investigation.
Few studies have examined whether cigarette smoking is a risk factor for poor transplant outcomes, and those have had conflicting results. Ho et al. found that smoking history, measured by smoking status (never, current, quit >1 year) was not associated with increased risk for early severe pulmonary complications, defined as diffuse alveolar hemorrhage, need for mechanical ventilation, or death from respiratory failure within the first 60 days post-transplant (11). In contrast, a study done by Savani and colleagues reported that a history of smoking was associated with a 5-fold increase in risk of transplant-related mortality from pulmonary causes (13). The latter, however, had a smaller cohort (n = 146), included patients diagnosed with idiopathic pneumonia syndrome, and followed patients for a median of 3.6 years (13). The largest study by Marks et al., with 2,818 patients who received allogeneic transplants for CML in first chronic phase, found that the risk of treatment-related mortality was 57% higher among ≥ 10 pack-year smokers compared to never smokers (12). In addition, such a smoking history was associated with increased risk of disease relapse, a finding consistent with a prior study by Chang et al. (10, 12). These findings, however, were observed only in recipients of HLA-identical sibling donor transplants (12).
We also found an increased risk of relapse associated with smoking, although mainly among patients receiving an unrelated matched HSCT. These results should be considered preliminary, although in light of studies demonstrating that smoking increases the risk of hematologic malignancies, this may be biologically plausible (30, 31). The greater than two-fold increase in relapse risk among recipients of unrelated matched donors in our study may be partly attributable to our cohort having a higher underlying disease risk; we included a variety of underlying diseases that warranted allogeneic transplant as opposed to the cohorts in the Chang and Marks studies, which focused on CML in stable phase. Unlike the study by Marks et al., we did not find an association between smoking and non-relapse mortality, despite observing that 92% of patients who were placed on mechanical ventilation in the first 100 days after transplant ultimately died. It is possible that some patients with respiratory failure chose to forego mechanical ventilation prior to death. Alternatively, it is possible that smoking is not a risk factor for other common causes of non-relapse mortality such as acute or chronic GVHD and non-pulmonary organ toxicity or failure. There are several possible mechanisms for the association between smoking dose and early respiratory failure. Pre-transplant PFTs have been shown to be important predictors of early post-transplant pulmonary complications and mortality (5–7). These parameters likely represent markers of previous lung injury and worse health status prior to transplant. In addition, cigarette smoking has immunomodulatory effects that have been linked to increased susceptibility to infection. The alveolar macrophages found in the lungs of smokers, for example, have a reduced ability to phagocytose bacteria and are known to secrete lower levels of inflammatory cytokines necessary for upregulation of host defenses (32, 33). Cigarette smoke may also have independent effects on mucociliary clearance and alterations in surfactant proteins (34–38).
The strengths of our study include a large cohort, availability of detailed smoking history, and availability of PFTs near transplant. There are, however, important limitations to consider when interpreting our results. First, social desirability, especially prior to upcoming transplant, may have led to under-reporting of smoking. Although we do not have reason to believe this bias is differential among those who experienced respiratory failure or died, the effect would tend to bias our hazard ratios toward the null. Second, we could not assess whether smoking was continued or resumed during the post-transplant period, which may have affected our outcomes. Third, although there are no firm exclusion criteria with regard to PFTs, smoking status, or pulmonary disease for transplant eligibility at our center, patients with severe pulmonary and other co-morbidities may never have made it to transplant; thus, there could be selection bias. Despite these limitations, the findings that: 1) the vast majority of patients undergoing transplant have normal lung function, including former and current smokers (Table 2), and 2) an association remains between smoking dose and early respiratory failure independent of lung function, lends credence to our conclusion that cigarette smoking, even in patients without serious co-morbidities or compromised lung function, has independent deleterious effects after transplant.
In summary, our study found a significant association between cigarette smoking and respiratory failure within 100 days of transplant. This finding appears to be only partially mediated by abnormal lung function prior to transplant. While we found no significant association between smoking and relapse or non-relapse mortality after allogeneic HSCT, a trend was apparent, and future prospective studies with longer-term outcomes and ongoing monitoring of smoking status are warranted to explore this issue. We are not suggesting that HSCT should be withheld or delayed in patients with a smoking history in need of transplant; however, since cigarette smoking, identifies individuals at increased risk of early morbidity post-transplant, they should be counseled accordingly. Given the prevalence of smoking among HSCT cohorts, further studies should focus on tobacco cessation efforts in this patient population. In addition, they should evaluate the potential impact of length of quit time among former smokers and recent quitters on allogeneic transplant outcomes, as this could identify a window of opportunity for aggressive tobacco cessation interventions. This study also emphasizes the need for routine inclusion of smoking status and more detailed smoking history both prior to and after transplant in future HSCT studies.
Acknowledgments
Funding/Support: Dr. Chien was supported by R01 HL 088201/HL/NHLBI NIH HHS/United States and Dr. Tran by T32 HL 007287/HL/NHLBI NIH HHS/United States. The authors have nothing to disclose.
Footnotes
Author Contributions: Dr. Tran was involved in the concept and design of the study, performed the statistical analysis, analyzed and interpreted the data, and was responsible for drafting the manuscript. Dr. Halperin was involved in the concept and design of the study and critical revision of the manuscript for important intellectual content. Dr. Chien was involved in the concept and design of the study, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and overall study supervision.
Financial Disclosure Statement
All work was performed at Fred Hutchinson Cancer Research Center, Seattle, WA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Marrow Donor Program. [Accessed April 1, 2010];Trends in allogeneic transplants. http://www.marrow.org/PHYSICIAN/URD_Search_and_Tx/Number_of_Allogeneic_Tx_Perfor/index.html.
- 2.Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med. 2006;27(3):297–309. doi: 10.1055/s-2006-945530. [DOI] [PubMed] [Google Scholar]
- 3.Huynh TN, Weigt SS, Belperio JA, Territo M, Keane MP. Outcome and prognostic indicators of patients with hematopoietic stem cell transplants admitted to the intensive care unit. J Transplant. 2009;2009:917294. doi: 10.1155/2009/917294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest. 1992;101(5):1257–64. doi: 10.1378/chest.101.5.1257. [DOI] [PubMed] [Google Scholar]
- 5.Ghalie R, Szidon JP, Thompson L, Nawas YN, Dolce A, Kaizer H. Evaluation of pulmonary complications after bone marrow transplantation: the role of pretransplant pulmonary function tests. Bone Marrow Transplant. 1992;10(4):359–65. [PubMed] [Google Scholar]
- 6.Goldberg SL, Klumpp TR, Magdalinski AJ, Mangan KF. Value of the pretransplant evaluation in predicting toxic day-100 mortality among blood stem-cell and bone marrow transplant recipients. J Clin Oncol. 1998;16(12):3796–802. doi: 10.1200/JCO.1998.16.12.3796. [DOI] [PubMed] [Google Scholar]
- 7.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172(3):384–90. doi: 10.1164/rccm.200502-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dockery DW, Speizer FE, Ferris BG, Jr, Ware JH, Louis TA, Spiro A., 3rd Cumulative and reversible effects of lifetime smoking on simple tests of lung function in adults. Am Rev Respir Dis. 1988;137(2):286–92. doi: 10.1164/ajrccm/137.2.286. [DOI] [PubMed] [Google Scholar]
- 9.Higgins MW, Enright PL, Kronmal RA, Schenker MB, Anton-Culver H, Lyles M. Smoking and lung function in elderly men and women. The Cardiovascular Health Study. JAMA. 1993;269(21):2741–8. [PubMed] [Google Scholar]
- 10.Chang G, Orav EJ, McNamara T, Tong MY, Antin JH. Depression, cigarette smoking, and hematopoietic stem cell transplantation outcome. Cancer. 2004;101(4):782–9. doi: 10.1002/cncr.20431. [DOI] [PubMed] [Google Scholar]
- 11.Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7(4):223–9. doi: 10.1053/bbmt.2001.v7.pm11349809. [DOI] [PubMed] [Google Scholar]
- 12.Marks DI, Ballen K, Logan BR, Wang Z, Sobocinski KA, Bacigalupo A, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant. 2009;15(10):1277–87. doi: 10.1016/j.bbmt.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savani BN, Montero A, Wu C, Nlonda N, Read E, Dunbar C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(3):223–30. doi: 10.1016/j.bbmt.2004.12.328. [DOI] [PubMed] [Google Scholar]
- 14.Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328(3):159–63. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 15.Ford MB, Sigurdson AJ, Petrulis ES, Ng CS, Kemp B, Cooksley C, et al. Effects of smoking and radiotherapy on lung carcinoma in breast carcinoma survivors. Cancer. 2003;98(7):1457–64. doi: 10.1002/cncr.11669. [DOI] [PubMed] [Google Scholar]
- 16.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2287–93. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 17.Pickles T, Liu M, Berthelet E, Kim-Sing C, Kwan W, Tyldesley S. The effect of smoking on outcome following external radiation for localized prostate cancer. J Urol. 2004;171(4):1543–6. doi: 10.1097/01.ju.0000118292.25214.a4. [DOI] [PubMed] [Google Scholar]
- 18.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144(6):407–14. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 19.Leisenring WM, Martin PJ, Petersdorf EW, Regan AE, Aboulhosn N, Stern JM, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108(2):749–55. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan KM. Graft-versus-host disease. In: Thomas E, Blume K, Forman S, editors. Hematopoietic stem cell transplantation. 2. Malden, MA: Blackwell Science; 1999. pp. 518–519. [Google Scholar]
- 21.Walter EC, Orozco-Levi M, Ramirez-Sarmiento A, Vigorito A, Campregher PV, Martin PJ, et al. Lung function and long-term complications after allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. 16(1):53–61. doi: 10.1016/j.bbmt.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 23.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123(2):185–9. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 24.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 25.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique--1995 update. Am J Respir Crit Care Med. 1995;152(6 Pt 1):2185–98. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 26.Center for International Blood and Marrow Transplant Research. [Accessed April 14, 2010];HCT trends and survival data. http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/index.html#SupplementalUpdated January 20, 2010.
- 27.Afessa B, Tefferi A, Litzow MR, Krowka MJ, Wylam ME, Peters SG. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166(5):641–5. doi: 10.1164/rccm.200112-141cc. [DOI] [PubMed] [Google Scholar]
- 28.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 29.Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis. 1993;147(6 Pt 1):1601–6. doi: 10.1164/ajrccm/147.6_Pt_1.1601. [DOI] [PubMed] [Google Scholar]
- 30.Brownson RC, Novotny TE, Perry MC. Cigarette smoking and adult leukemia. A meta-analysis. Arch Intern Med. 1993;153(4):469–75. [PubMed] [Google Scholar]
- 31.Sandler RS, Lyles CM, McAuliffe C, Woosley JT, Kupper LL. Cigarette smoking, alcohol, and the risk of colorectal adenomas. Gastroenterology. 1993;104(5):1445–51. doi: 10.1016/0016-5085(93)90354-f. [DOI] [PubMed] [Google Scholar]
- 32.McCrea KA, Ensor JE, Nall K, Bleecker ER, Hasday JD. Altered cytokine regulation in the lungs of cigarette smokers. Am J Respir Crit Care Med. 1994;150(3):696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 33.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5):372–7. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 34.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Cremoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151(3 Pt 1):630–4. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 35.Subramaniam S, Whitsett JA, Hull W, Gairola CG. Alteration of pulmonary surfactant proteins in rats chronically exposed to cigarette smoke. Toxicol Appl Pharmacol. 1996;140(2):274–80. doi: 10.1006/taap.1996.0222. [DOI] [PubMed] [Google Scholar]
- 36.Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS One. 2009;4(12):e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest. 1996;109(4):1006–9. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- 38.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stampfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2004;170(11):1164–71. doi: 10.1164/rccm.200311-1521OC. [DOI] [PubMed] [Google Scholar]


