Abstract
Given the success of cue exposure (extinction) therapy combined with a cognitive enhancer for reducing anxiety, it is anticipated that this approach will prove more efficacious than exposure therapy alone in preventing relapse in individuals with substance use disorders. Several factors may undermine the efficacy of exposure therapy for substance use disorders, but we suspect that neurocognitive impairments associated with chronic drug use are an important contributing factor. Numerous insights on these issues are gained from research using animal models of addiction. In this review, the relationship between brain sites whose learning, memory and executive functions are impaired by chronic drug use and brain sites that are important for effective drug cue extinction learning is explored first. This is followed by an overview of animal research showing improved treatment outcome for drug addiction (e.g. alcohol, amphetamine, cocaine, heroin) when explicit extinction training is conducted in combination with acute dosing of a cognitive-enhancing drug. The mechanism by which cognitive enhancers are thought to exert their benefits is by facilitating consolidation of drug cue extinction memory after activation of glutamatergic receptors. Based on the encouraging work in animals, factors that may be important for the treatment of drug addiction are considered.
Keywords: addiction, animal models, cognitive enhancement, drugs of abuse, extinction
In the anxiety disorders field, numerous studies have shown that exposure therapy, a procedure involving repeated confrontation with feared stimuli in a controlled setting, is highly effective as a stand-alone treatment for reducing anxiety and preventing its return (Hofmann et al. 2009; Otto et al. 2004). Exposure therapy for substance use disorders is conceptually similar in that in a controlled setting, individuals addicted to drugs are confronted repeatedly with drug cues. This approach, however, is not consistently effective in reducing reactivity to drug cues and for preventing drug relapse (Conklin and Tiffany 2002). Several factors may undermine the efficacy of exposure therapy for substance use disorders, but we suspect that neurocognitive impairments associated with chronic drug use, particularly in individuals who are most severely dependent, are an important contributing factor. Exposure therapy is a form of extinction learning, and it is noteworthy that the brain sites needed for effective extinction learning may become dysfunctional after chronic drug use (Fowler et al. 2007; Stephens and Duka 2008; Liu et al. 2009; for review, see Kantak and Nic Dhonnchadha, in press). One focus of the current review is on animal research models that explore the relationship between brain sites whose learning, memory and executive functions are impaired by chronic drug use and brain sites that are important for effective drug cue extinction learning. While neurocognitive impairments may undermine extinction learning, new hope is afforded by preclinical research, reviewed below, showing improved treatment outcome for drug addiction when explicit extinction training is conducted in combination with acute (single injection) or subacute (two or more injections) dosing with a cognitive-enhancing drug. It is hoped that employment of this dosing regimen will avoid potential confounds such as sensitization of the gluatamatergic system due to repeated administration over short intervals (Boje et al. 1993; Parnas et al. 2005; Botreau et al. 2006; Werner-Seidler and Richardson 2007) that may diminish the efficacy of the particular cognitive enhancer in use. In this respect, the use of cognitive enhancers for the treatment of substance use disorders differs conceptually from their use in the treatment of other neuropsychiatric disorders (e.g., Alzheimer’s, Schizophrenia, and Attention Deficit/Hyperactivity Disorder) where a chronic rather than an acute dosing regimen would be employed.
1. Neurocognitive Deficits Associated with Abused Substances
The close correspondence in the neurocognitive deficits produced by abused substances in humans and animals suggests that meaningful insights can be obtained from animal models of drug-related learning and modification by pharmacological agents. One advantage of conducting animal studies is that they allow systematic assessment of the effects of drugs of abuse on neurocognitive function throughout the lifespan. Studies in animals include work on attention, working memory and impulsivity (prefrontal cortex–related functions) as well as work on associative learning and memory (amygdala- and hippocampus-related functions) following exposure to several drugs of abuse (e.g., cocaine, amphetamine, opiates, ethanol and nicotine). Below, distinctions are made as to whether drugs were administered acutely or chronically, whether drugs were administered contingently (self-administered) or non-contingently (experimenter-delivered injections or passively yoked delivery), and whether animals were tested in the drug-free state or while under the influence of drug. The mode of drug delivery may be an important factor for observing neurocognitive changes because numerous animal studies report a variety of physiological and neurochemical distinctions between contingent and noncontingent drug exposure (Kantak et al. 2005; Udo et al. 2004).
1.1. Attention
Chronic cocaine injection during the prenatal period in rats has been shown to disrupt both selective and sustained attention during adulthood (Garavan et al. 2000; Gendle et al. 2003). Likewise, adolescent rats given repeated injections of cocaine were shown to display abnormally rapid shifts in selective attention during adulthood (Black et al. 2006). When cocaine and other drugs of abuse such as amphetamine and heroin are contingently self-administered by adult rats and then withdrawn, deficits in sustained attention have been found as well (Dalley et al. 2005; 2007). Chronic amphetamine injection additionally produces deficits in selective and sustained attention in adult rats (Crider et al. 1982; Fletcher et al. 2007). Interestingly, acute cocaine or amphetamine injection in adult rats was found to improve selective and sustained attention (Bizarro et al. 2004; Grilly et al. 1989; Koffarnus and Katz 2010) and to reduce variance in the amplitudes of auditory evoked potentials (Robledo et al. 1993). These effects are consistent with the masking of attention deficits after recent cocaine use in dependent individuals (Pace-Schott et al. 2008; Woicik et al. 2009). In a study examining the effects of acute nicotine, acute ethanol and their combination on sustained attention in adult rats, it was demonstrated that nicotine alone improved attention and that ethanol alone slightly disrupted attention, but that both drugs combined produced large decrements in attention (Bizarro et al. 2003). In other studies of sustained attention, it was shown that acute ethanol injection at a dose that did not impair attention was able to block the improvement in attention induced by an acute injection of nicotine (Rezvani and Levin 2003). As nicotine and ethanol often are taken together by humans (Hughes 1995), their combined use may result in suboptimal attention. Interestingly, daily exposure to ethanol vapor for 14 days was shown to improve the accuracy of sustained attention in adolescent and adult rats, which may have been due to central nervous system arousal induced by the ethanol vapor (Slawecki 2006). Collectively, these studies suggest that while acute exposure to certain drugs may improve attention, chronic exposure to drugs such as cocaine, amphetamine and opiates disrupts attention. These disruptions in attention appear to be related to the direct pharmacological effects of these drugs of abuse as there are similar effects of contingent and non-contingent drug exposure.
1.2. Working Memory
In rat models, chronic nicotine infusion was shown to improve working memory (Levin et al. 1996). However, during the two weeks after withdrawal, nicotine-induced improvements in working memory were no longer evident. Regarding other drugs of abuse, working memory deficits are reported in rats trained to self-administer cocaine (Kantak et al. 2005) and trained to self-administer cocaine and then withdrawn (Harvey et al. 2009; George et al. 2008). Interestingly, passively yoked cocaine delivery did not impact working memory (Harvey et al. 2009; Kantak et al. 2005), suggesting that the contingency of cocaine delivery is important for altering the working memory function of the prefrontal cortex. Although acute injection of amphetamine improves working memory (Meneses et al. 2011), chronic injection of amphetamine neither improves nor disrupts working memory (Shoblock et al. 2003), suggesting that contingency of amphetamine delivery may be a factor as well with repeated exposure. Regarding opiates, rats made dependent on morphine displayed deficits in working memory if i.p. injections were given (Braida et al. 1994), but not if oral solutions were provided (Miladi et al. 2008). These findings suggest that non-contingent morphine exposure produces inconsistent effects on working memory. How working memory in rats may be impacted by contingent morphine exposure is not yet known. In contrast, before and after withdrawal from chronic ethanol injection or its oral consumption, working memory deficits are apparent (Santin et al. 2000; Santucci et al. 2004; White et al. 2000). Thus, ethanol may be disruptive to working memory due to its direct pharmacological action. Interestingly, nicotine plus ethanol co-injection in rats produces pronounced deficits in working memory at doses of each that do not alter working memory when injected alone (Rezvani and Levin 2003).
1.3. Impulsivity
While impulsivity is a risk factor that predicts vulnerability for drug abuse, it also is a consequence of chronic drug use (Carroll et al. 2009; Winstanley et al. 2010). Impulsivity is associated with a number of drugs of abuse. In animal studies, chronic cocaine injection (Paine et al. 2003) and acute morphine injection (Kieres et al. 2004; Pattij et al. 2009; Pitts and McKinney 2005) have been shown to increase impulsivity in a delayed discounting task. Notably, chronic cocaine self-administration in rats prescreened for low impulsivity can cause these rats to become more impulsive on a delayed discounting task for food after cocaine is withdrawn (Anker et al. 2009). Rats with low impulsivity also are more impulsive after acute amphetamine injection (Perry et al. 2008) and withdrawal from chronic amphetamine self-administration additionally increases impulsivity in rats (Dalley et al. 2007). In rats chronically injected with nicotine during adolescence or adulthood and then withdrawn for 5 weeks, impulsive choice for immediate small food rewards over delayed large food rewards was not observed (Counotte et al. 2009). However, in another study of chronic nicotine injection, adult rats responded more impulsively in a delayed discounting task for up to 30 days after nicotine was withdrawn (Dallery and Locey 2005). These findings suggest that the nicotine deprivation effect on impulsive choice is associated mainly with the early stages of nicotine withdrawal.
Rats and mice selectively bred for high ethanol-preference were shown to be more impulsive than their counterparts selectively bred for low ethanol-preference, consistent with the idea that impulsivity is a trait characteristic of alcoholism (Oberlin and Grahame 2009; Wilhelm and Mitchell 2008). However, acute ethanol injection in an outbred rat strain was shown to produce increased impulsivity. Rats chose immediate rewards over delayed rewards, suggesting induction of impulsivity by ethanol exposure (Olmstead et al. 2006). Overall, impulsivity appears to be associated with exposure to several drugs of abuse and is particularly apparent when drug is withdrawn following chronic contingent or non-contingent administration.
1.4. Amygdala-Related Learning and Memory
Stimulus-reward learning occurs via a Pavlovian associative mechanism that is regulated by the amygdala (McDonald and White 1993; Kantak et al. 2001). In adult rats trained to self-administer cocaine or receiving yoked-cocaine passively, stimulus-reward learning was disrupted as assessed by preference for a cue paired with a highly palatable food reward (Udo et al. 2004; Kerstetter and Kantak 2007). Chronic amphetamine injection also has been shown to impair amygdala-dependent appetitive cue learning (Ito and Canseliet 2010).
Pavlovian cued fear conditioning also measures amygdala-related learning, but in this case, learning is induced by negative rather than positive affect (Maren et al. 1996; Campeau and Davis 1995). Acute or chronic injection of morphine (Good and Westbrook 1995; Gu et al. 2008) and cocaine (Wood et al. 2007; Burke et al. 2006) have been shown to impair acquisition and extinction of cue-conditioned fear in rats. Acute ethanol injection (Lattal 2007; Land and Spear 2004) and withdrawal from its chronic oral consumption (Bergstrom et al. 2006) also impair acquisition and extinction of cue-conditioned fear in rats. Chronic nicotine injection on the other hand, can impair extinction but not acquisition of cue-conditioned fear (Tian et al. 2008) whereas chronic amphetamine injection can enhance acquisition but not extinction of cue-conditioned fear (Carmack et al. 2010). Collectively, these studies demonstrate mainly impairment in amygdala-related learning following contingent and non-contingent exposure to various drugs of abuse in animal subjects.
1.5. Hippocampus-Related Learning and Memory
The hippocampus is involved in the processing of spatial, contextual and episodic associations (Smith and Mizumori 2006). It is important for the acquisition of new learning and for the strengthening of learned associations for later retrieval (Zola-Morgan and Squire 1990; Morris et al. 2006). Using either a water maze task of spatial learning (Del Olmo et al. 2007) or a radial-arm maze task of spatial learning (Kantak et al. 2005), adult rats self-administering cocaine or receiving it passively in a yoked fashion were shown to reach their goal (finding a hidden platform or retrieving all eight rewards) more quickly than saline controls when tested 0.5 to 3 hr after cocaine sessions ended. It is possible that these findings are explained by the psychomotor stimulant effects of cocaine and do not reflect an actual improvement in spatial learning. Alternatively, cocaine-induced deficits in the functioning of the prefrontal cortex and amygdala could cause other memory systems, such as the hippocampus, to gain greater control over behavior (White and McDonald 2002; Poldrack and Packard 2003). Whether or not this abnormally rapid processing of spatial information in cocaine-exposed rats is maladaptive remains to be determined. It should be noted, however, that one study using experimenter-delivered, high-dose injection of cocaine (40 mg/kg/day, s.c.) found increased escape latencies (worse performance) in the water maze task (Quirk et al. 2001). This is consistent with studies in rats exposed to experimenter-delivered, high-dose injection of cocaine (50 mg/kg/day, s.c.) during the preweaning period and then tested on a radial-arm maze task during adulthood in the drug-free state (Melnick et al. 2001). These findings argue against simple psychomotor activation as an explanation for improved performance in spatial learning tasks following i.v. cocaine exposure. Dose may be a critical factor for observing cocaine-induced improvements or deficits in spatial learning in rats because in the i.v. cocaine studies mentioned above (Del Olmo et al. 2007; Kantak et al. 2005), the cumulative dose of cocaine was approximately 10 to 15 mg/kg/day, with its i.v. delivery spaced over a 2-hr period. The rats receiving a single 40 mg/kg/day s.c. injection of cocaine (Quirk et al. 2001) would have had higher sustained blood levels of cocaine at the time of testing relative to the rats in the self- and passive-administration studies.
Concerning other drugs of abuse, chronic heroin injection in prenatal and adult mice (Tramullas et al. 2008; Wang and Han 2009), chronic high dose nicotine infusion via minipump in adult rats (Scerri et al. 2006), and acute ethanol injection in adolescent and adult rats (Silvers et al. 2003) also were shown to produce deficits in spatial learning. Similar to cocaine, one recent study has shown that while chronic amphetamine injection impaired amygdala-dependent appetitive cue learning, it enhanced hippocampus-dependent spatial learning (Ito and Canseliet 2010).
Like spatial learning, contextual learning is impaired by drugs of abuse in animal models of contextual fear conditioning, which requires the hippocampus (Rudy et al. 2004). Acute and chronic injection of cocaine (Wood et al. 2007; Morrow et al. 1995) has been shown to attenuate acquisition of contextual fear conditioning. Chronic morphine injection also attenuates acquisition of contextual fear conditioning when tested early but not later in withdrawal (Gu et al. 2008; McNally and Westbrook 2003). Whereas acute nicotine injection in low doses has been shown to enhance acquisition of contextual fear conditioning (Wehner et al. 2004), its is disrupted following chronic nicotine withdrawal (Gulick and Gould 2008). Acute ethanol injection has unique effects on contextual fear conditioning; high doses impair and low doses enhance its acquisition (Gulick and Gould 2007; Wehner et al. 2004). Given that high doses of ethanol have anxiolytic actions (Aston-Jones et al. 1984), it is possible that the reduction in freezing behavior in a fear-related context by high dose ethanol is mediated by an anxiolytic effect rather than by a disruption in contextual learning. Nicotine, which also has anxiolytic effects (Cohen et al. 2009), interacts with ethanol in such a way to suggest that high dose ethanol reduces freezing behavior by disrupting contextual learning. Specifically, high dose ethanol-induced deficits in contextual fear conditioning are reversed by acute low dose nicotine injection (Gulick and Gould 2008). Moreover, acute low dose ethanol injection can cause a reversal of high dose nicotine withdrawal-induced deficits in contextual fear conditioning (Gulick and Gould 2008). Collectively, these studies demonstrate that various aspects of hippocampus-related learning are altered following contingent and non-contingent exposure to drugs of abuse or their withdrawal in animal subjects. Dose may be a critical factor for observing deficits or improvements in hippocampus-related learning.
Given the above changes in attention, working memory, impulsivity and associative learning, it appears that functioning of the prefrontal cortex, amygdala and hippocampus is altered by chronic exposure to drugs of abuse. Whether these changes described above are associated with development of the addicted state or are related simply to long-term contingent or non-continent drug exposure remains a question for future investigations. The value of these animal studies is that they help us understand how functioning of key structures important for extinction learning (see below), may be impacted by chronic exposure to drugs of abuse
2. Neurobiological Substrates of Drug Cue Extinction Learning
In order to develop effective pharmacotherapies for use in combination with exposure therapy in the treatment of drug addiction, it is crucial to understand the neurobiological underpinnings of drug cue extinction learning. While research on this topic in the addiction field is still in its infancy, evidence indicates that drug cue extinction may involve circuits and use mechanisms of synaptic plasticity similar to those of conditioned fear learning (Myers and Carlezon 2010b for review). Two animal paradigms are routinely employed to assess addiction-related extinction learning at the preclinical level: the conditioned place preference and drug self-administration procedures.
Conditioned place preference is used to assess the ability of non-contingent or passive administration of drugs of abuse to establish learnt contextual associations and provides a measure of conditioned drug reward (Tzschentke 2007). Using this procedure, a drug is repeatedly paired with a unique contextual environment, and over time the animal exhibits a preference for the drug-paired environment over an environment that has been paired with a neutral pharmacological stimulus (i.e., saline; Carlezon 2003). Subsequently, place conditioning can be reduced or eliminated by conducting repeated preference tests in the drug-free state (extinction training; Bardo et al. 1986; Calcagnetti and Schechter 1993; Mueller and Stewart 2000; Schroeder and Packard 2004). Thus, this procedure measures extinction to background environmental cues associated with drug exposure.
The self-administration model uses operant responding for drug delivery and measures the reinforcing effects of a drug. In this paradigm, subjects typically are trained to perform an operant task (nose poke or lever press) in order to receive an intravenous infusion of drug, serving as the unconditioned stimulus (US). Drug delivery often is paired with the presentation of a conditioned stimulus (CS), a discrete tone and/or light, which allows for the formation of Pavlovian CS-US associations. One form of extinction training involves removal of drug and discrete CSs in the self-administration environment (sometimes referred to as response extinction training). This procedure measures extinction to environmental cues associated with drug exposure and is the most widely used method of extinction training in animal self-administration studies. Response extinction training typically precedes reinstatement tests in which animals are reintroduced to the discrete cues. When the discrete cues are reintroduced, the conditioned response, i.e., operant responding, is reinstated and this behavioral output is designated as drug-seeking behavior (Spealman et al. 1999). Drug-seeking behavior is analogous to cue reactivity in humans and is conceptualized as the sensitivity to drug-associated cues. These reinstatement sessions may be viewed as drug cue extinction sessions, whereby animals learn that the CS associated with the response no longer predicts delivery of primary reinforcement, resulting in a decline in drug-seeking behavior. However, it is important to consider that the use of reinstatement tests to model drug cue extinction involve the animal first undergoing response extinction training, which in itself produces marked neurobiological changes in the brain (Schmidt et al. 2001; Sutton et al. 2003; Self et al. 2004). In some instances, reinstatement tests follow a period of abstinence (removal of both the drug and the drug-paired environment) that also produces neurobiological changes (Lu et al., 2004b; Schmidt and Pierce 2010). Both processes are not necessarily direct contributors to the learning mechanisms at work during drug cue extinction. An animal model that explicitly extinguishes responses only in the presence of discrete drug-paired cues would more closely approximate exposure therapy in drug addicts (e.g., Nic Dhonnchadha et al. 2010b). Nonetheless, a review of research using these three different methods of extinction training in self-administration studies (response extinction training, reinstatement testing, drug cue extinction training), as well as extinction training associated with the conditioned place preference procedure, reveals an overlap between brain sites whose learning, memory, and executive functions are impaired by chronic drug use (see Section 1 above) and brain sites that are important for effective addiction-related extinction learning.
2.1. Basolateral Amygdala
Several lines of research have extensively implicated the basolateral amygdala (BLA) in the initial formation of cocaine-cue associations, as well as expression of cocaine-seeking behavior (e.g., Brown and Fibiger 1993; Whitelaw et al. 1996; Ciccocioppo et al. 2001; Kruzich and See 2001; Mashhoon et al. 2009). The use of c-Fos as a marker of neuronal activation indicates involvement of this area following cue-elicited drug-seeking behavior. Increased c-Fos expression was observed in the BLA following cue-elicited cocaine-seeking behavior to both an extinguished and non-extinguished cocaine-paired cue (Neisewander et al. 2000; Ciccocioppo et al. 2001; Kufahl et al. 2009). Additionally a correlation between lever pressing and c-Fos expression in the BLA was evident (Kufahl et al. 2009). Using the conditioned place preference paradigm, Miller and Marshall (2005) showed that cocaine associated environmental stimuli activate BLA neurons, as shown by increases in c-Fos expression. In addition, increased levels of c-Fos were observed in BLA during reinstatement of alcohol-seeking behavior (Millan et al. 2010).
Disruption to BLA activity via lesions or inactivation blocks the ability of cocaine associated stimuli to reinstate extinguished responding (Meil and See 1997; Grimm and See 2000; Kantak et al. 2002; Yun and Fields 2003; McLaughlin and See 2003; Peters et al. 2008b; Mashhoon et al. 2010). Conversely, electrical stimulation of the BLA reinstates conditioned response in rats subsequent to response extinction training (Hayes et al. 2003). Disruption of BLA functioning following cue-induced reinstatement sessions results in impaired consolidation of this cue extinction memory, as evidenced by poor retrieval during a subsequent cue extinction retention session (Fuchs et al. 2006b).
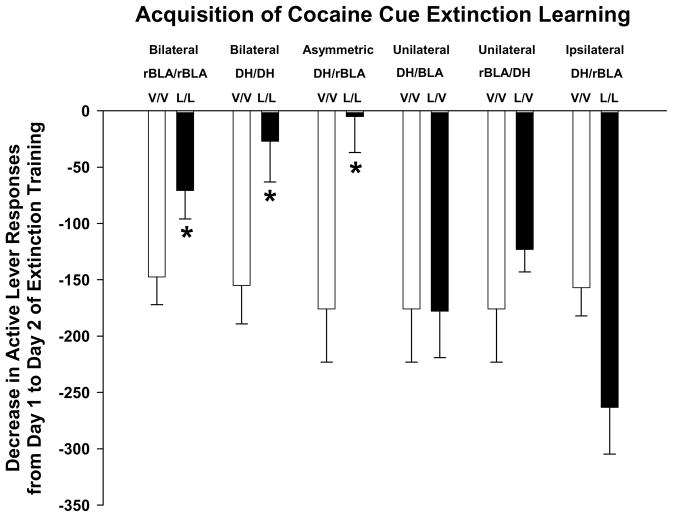
Using an animal model that more closely approximates cue exposure therapy in drug addicts, we recently demonstrated the importance of the BLA for cocaine cue extinction learning (Szalay et al., in press). In this study, rats were trained to self-administer cocaine and then underwent two 1 hr extinction sessions (no cocaine, but cues present). Rats received infusions of lidocaine (a neuronal inactivating agent) or vehicle bilaterally into the rostral BLA (rBLA) prior to extinction sessions to determine if this site was important for acquisition of cocaine cue extinction learning. Additional controls examined the effect of lidocaine or vehicle infused unilaterally into the rBLA. Results (Figure 1) show that bilateral inactivation of rBLA with lidocaine slowed acquisition of cocaine cue extinction learning. The decreases in active lever responses from day 1 to day 2 of extinction training were significantly smaller after lidocaine than after vehicle. Lidocaine was ineffective in altering acquisition of cocaine cue extinction learning when unilateral rBLA manipulation was implemented. Collectively, data from a variety of studies suggest that the BLA may be important for the learning and consolidation of drug cue extinction.
Figure 1. Decrease in active lever responses from day 1 to day 2 of extinction training during acquisition of cocaine cue extinction learning.
Rats were trained to self-administer 1.0 mg/kg cocaine under an FI 5 min (FR5:S) second-order schedule before undergoing two 1 hr extinction training sessions on consecutive days for which cocaine delivery was suspended, but the cocaine-paired discrete light cue was presented upon completion of each FR5. Rats received infusion of vehicle or lidocaine into the rBLA of both hemispheres (bilateral rBLA/rBLA); infusion of vehicle or lidocaine into the DH of both hemispheres (bilateral DH/DH); infusion of vehicle or lidocaine into the DH of one hemisphere and the rBLA of the contralateral hemisphere (asymmetric DH/rBLA); infusion of vehicle or lidocaine into the DH of one hemisphere with infusion of only vehicle into the contralateral rBLA (unilateral DH/rBLA); infusion of vehicle or lidocaine into the rBLA of one hemisphere with infusion of only vehicle into the contralateral DH (unilateral rBLA/DH); infusion of vehicle or lidocaine into the DH and rBLA of the same hemisphere (ipsilateral DH/rBLA). n=4–8 rats per treatment group. * p<0.05 compared to the corresponding vehicle/vehicle (V/V) control treatment. The figure is adapted from Table 1, reported in Szalay et al. in press.
2.2. Hippocampus
Several studies have shown that the dorsal hippocampus (DH) has an important role in encoding contextual information to label and retrieve memories (Rudy et al. 2002; Sanders et al. 2003) in addition to its involvement in the extinction of fear-associated memories (Wilson et al. 1995; Hartley and Phelps 2010). Inactivation or blockade of glutamatergic neurotransmission of the DH can inhibit reinstatement of cocaine-seeking behavior (Fuchs et al. 2005; Fuchs et al. 2007; Xie et al. 2010).
In the same study reported above to examine the role of the BLA in cocaine cue extinction learning, the DH also was investigated (Szalay et al, in press). In addition to evaluating bilateral inactivation of the DH, inactivation of the DH in one hemisphere and the rBLA in the contralateral hemisphere (asymmetric inactivation) was evaluated to determine if the serial connection between these sites on both sides of the brain was important for acquisition of cocaine cue extinction learning. Unilateral DH and ipsilateral DH/rBLA controls were used. Results (Figure 1) show that bilateral inactivation of DH and asymmetric inactivation of DH/rBLA with lidocaine slowed acquisition of cocaine cue extinction learning. The decreases in active lever responses from day 1 to day 2 of extinction training were significantly smaller after lidocaine than after vehicle. Lidocaine was ineffective in altering acquisition of cocaine cue extinction learning after unilateral DH or ipsilateral DH/rBLA manipulations. Collectively, these findings suggest that the BLA and DH need to be functionally active simultaneously in both brain hemispheres to extinguish drug-seeking behavior.
2.3. Ventral and Dorsal Striatum
The ventral striatum consists of the nucleus accumbens core (NAc core) and shell (NAc shell) and is involved in the control of goal-directed behaviors (Kelley et al. 1997; Parkinson et al. 2000; Di Ciano and Everitt 2001) and instrumental learning (Smith-Roe and Kelley 2000). In contrast, the dorsal striatum is involved in habit learning (Wickens et al. 2007). The core region has been implicated primarily in motivated behavior that has become conditioned to particular cues, consistent with its anatomical relationships with the amygdala (Ito et al. 2004). Importantly, a distinct pattern of firing is observed in NAc cells during presentation of conditioned stimuli (Carelli et al. 2000; Ghitza et al. 2003; Nicola et al. 2004; Yun et al. 2004), an effect that persists after an extended period of cocaine abstinence (Hollander and Carelli 2007). With respect to extinction, inactivation of the NAc core suppressed cocaine-seeking on the first day of response extinction training, and appeared to inhibit the formation of extinction memory (Sutton et al. 2003). NAc shell inactivation by contrast did not alter responding during the first extinction training session. Similar results are reported during cue-reinstatement tests whereby inactivation of the NAc core, but not NAc shell, attenuated reinstatement of cocaine-seeking behavior (Fuchs et al. 2004). However, inactivation of either the NAc core or shell failed to alter cue-induced drug-seeking behavior following a period of abstinence (See et al. 2007). These results suggest that different NAc circuitry is engaged during tests for cocaine-seeking behavior following response extinction training vs. abstinence from cocaine self-administration. In contrast to the NAc, inactivation of the dorsal striatum disrupts cocaine-seeking behavior following either response extinction training or abstinence from cocaine self-administration (Fuchs et al. 2006a; See et al. 2007). Collectively, these findings suggest that in the absence of drug reinforcement, the ventral striatum may be engaged to maintain goal-directed responses only in the presence of salient cues and the dorsal striatum may be engaged to maintain habitual responses even in the absence of salient cues to impact the rate of extinction. However, the role of the ventral and dorsal striatum remains unexplored in an animal model that more closely approximates cue exposure therapy in drug addicts.
2.4. Medial Prefrontal Cortex
A role for the medial prefrontal cortex (mPFC) in extinction has been demonstrated during cocaine cue reinstatement tests that follow abstinence, i.e., when the animal is no longer exposed to cocaine or the cocaine-associated environment for a certain period of time. Using c-Fos activation methods to reveal neurosubstrates of extinction, an increase in the expression of c-Fos protein in the ventral mPFC (infralimbic and ventral prelimbic cortices) was observed during reinstatement testing in rats initially trained to self-administer cocaine before undergoing abstinence (Zavala et al. 2007). In mice trained in the conditioned place preference paradigm, cocaine associated environmental stimuli activated c-Fos in interneurons of the prelimbic cortex (Miller and Marshall 2005). Similar changes in the expression of c-Fos in the mPFC are reported after re-exposure to environments previously paired with morphine, nicotine and ethanol (Schroeder et al. 2000; Schroeder et al. 2001; Wedzony et al. 2003).
Following a period of abstinence from cocaine self-administration, inactivation of the ventral mPFC was shown to decrease responses during a cue extinction session, while local stimulation increased responses (Koya et al. 2009). These findings are in contrast to those reported during cue-reinstatement tests conducted following response extinction training. Inactivation of dorsal mPFC (anterior cingulate and dorsal prelimbic cortices), but not ventral mPFC, was shown to attenuate cue-induced reinstatement of cocaine-seeking behavior (McLaughlin and See 2003; Di Pietro et al. 2006; Di Ciano et al. 2007). Furthermore, Peters and colleagues (Peters et al. 2008b) reported that inactivation of the ventral mPFC potentiated spontaneous recovery of cocaine-seeking four weeks after termination of response extinction training. Spontaneous recovery refers to the restoration of the extinguished response that occurs in a test session performed following a delay (Rescorla 2004). As previously mentioned, it has been suggested that the neurocircuitry of cue-elicited responding after response extinction training is different from that after abstinence (Fuchs et al. 2006a; Peters et al. 2008a; See et al. 2007), which may explain the discrepancy in the observed results. These findings underscore the necessity of examining the role of the ventral mPFC in an animal model that more closely approximates cue exposure therapy in drug addicts.
3. Animal Studies with Cognitive Enhancers
The fact that the potential effects of exposure therapy may be hampered by drug-induced deficits in cognitive functioning in drug addicts has led to the study of alternative approaches to compensate for these shortcomings (e.g., Vocci 2008). It is hoped that exposure therapy combined with a cognitive enhancer will prove efficacious in preventing relapse in individuals with substance use disorders. This strategy differs significantly from other approaches that attempt to generally overcome the cognitive deficits associated with drug addiction by administering cognitive enhancers to improve treatment retention and outcome (for review see Sofuoglu 2010). There are several promising candidate cognitive enhancers for use in combination with exposure therapy, as assessed in the four preclinical models employed to study drug cue extinction (see Section 2 above).
Recent studies have shown that consolidation of drug cue extinction learning in rats and monkeys can be facilitated with systemically applied drugs targeting numerous systems. To assess the effects of putative treatment strategies, subsequent tests of cue- or drug-elicited drug-seeking behavior (to mimic reactivity to cues or drug in humans) or reacquisition (when the drug is onboard again in the presence of cues) are evaluated in animals. A common pathway of extinction-facilitating compounds may be the glutamatergic system, modulation of which can regulate synaptic plasticity and hence learning and memory processes (Martin et al. 2000). Activation of N-methyl-D-aspartate (NMDA) receptors leads to long-term potentiation and long-term depression, which are mechanisms of synaptic plasticity associated with learning and memory formation (Kemp and Manahan-Vaughan 2007), as well as its extinction (Quirk 2006; Dalton et al. 2008). Thus, modulation of glutamate activity during extinction training may facilitate the process by which drug-paired cues lose salience and their control over behavior.
3.1. Glycine Site Agonists
To date, the glycine-binding site of the NMDA receptor has been proposed as a putative target for enhancing extinction learning. Since glutamate and direct-acting NMDA receptor agonists may be neurotoxic and are known to cause excitotoxicity (Olney 1994; Svensson 2000), the strategy used in the last decade has relied on drugs that enhance NMDA neurotransmission indirectly through modulatory sites on the NMDA receptor complex (Millan 2005; Stahl 2007). The strychnine-insensitive glycine site on the NMDA receptor complex is one such modulatory site where glycine in the presence of glutamate facilitates ion channel opening and excitatory neurotransmission without directly increasing extracellular levels of glutamate. Much success has been reported with D-cycloserine (DCS), a partial agonist at the glycine site of the NMDA receptor (Hood et al. 1989). In the animal literature, DCS has been shown to improve learning and memory in rats (Land and Riccio 1999; Pussinen and Sirvio 1999; Lelong et al, 2001) and monkeys (Matsuoka and Aigner 1996; Schneider et al. 2000), as well as facilitating fear extinction learning (Davis et al. 2006; Vervliet 2008).
Several studies have investigated the ability of DCS treatment to enhance extinction of drug-induced conditioned place preference. Systemic administration of DCS at doses of 15 and 30 mg/kg either before or immediately following 1 to 3 extinction training sessions has been shown to enhance extinction of a cocaine-associated contextual memory when testing occurs in the same context in rats (Botreau et al. 2006; Paolone et al. 2009) and mice (Kelley et al. 2007; Thanos et al. 2009). The facilitative effect of DCS administered systemically in rats could be replicated by local injections made directly into the BLA, indicating the involvement of this brain region for the acquisition and consolidation of new associations that are formed during cocaine cue extinction training (Botreau et al. 2006). Moreover, the effects of DCS were specific for extinction memory, as the magnitude of cocaine conditioned place preference (original learning) was not affected when DCS was injected during the conditioning phase rather than the extinction phase (Botreau et al. 2006). Additionally, a time-dependent facilitative effect was observed with DCS. Specifically, a 4 hr lapse between termination of extinction training and DCS administration led to reduced effectiveness of the cognitive enhancer, coinciding with the theoretical time-window of NMDA-dependent memory consolidation. Notably, long-lasting effects of intra-amygdalar infusion of DCS (10μg/μl/site) in rats and low dose systemic DCS (15 mg/kg) in mice on extinction of cocaine-conditioned place preference were evident 2 weeks after the end of extinction training sessions (Botreau et al. 2006; Thanos et al. 2009). This was not the case, however when mice were tested 1–2 weeks after termination of extinction training in combination with high dose of DCS (30 mg/kg), and actually resulted in the renewal of the conditioned place preference (Thanos et al. 2009). While these results may indicate divergent dose-dependent effects of DCS, they also highlight the importance of controlling the number of extinction and DCS treatment sessions, as it may be possible that DCS fails to provide additional benefits to extinction when the training protocols are intensive and effective in control animals (i.e., longer sessions and repeated extinction training). The combination of three DCS administration and extinction training sessions prevented cocaine-primed reinstatement of the cocaine conditioned place preference in rats (Paolone et al., 2009), however, a reinstatement effect was observed in mice, with a restoration of the cocaine conditioned place preference regardless of prior DCS treatment and enhanced facilitation of extinction learning (Kelley et al. 2007).
A handful of studies have examined the effects of DCS with other drugs of abuse. Intra-hippocampal administration of DCS (10μg/μl/site) prior to extinction training sessions facilitated the rate of extinction of amphetamine-produced place preference in rats (Sakurai et al. 2007). These results indicate the involvement of NMDA receptors in the hippocampus in amphetamine place preference extinction learning. However, when DCS was administered prior to amphetamine and context re-exposure, the extinction of the conditioned place preference was impeded, possibly due to enhancement of reconsolidation memory process (see below). In another study, administration of DCS (30 mg/kg) prior to extinction trials failed to enhance the rate of extinction of ethanol conditioned place preference in mice (Groblewski et al. 2009). The lack of effects during the extinction phase may be related to the apparent strain-dependent cognitive-enhancing effects of DCS in mice (Sunyer et al. 2008). Thus, the extinction-facilitating effects of DCS may not be evident in the DBA/2J strain used in this study in comparison to the C57bl/c mice used in the cocaine conditioned place preference study (Thanos et al., 2009). While repeated exposure of DCS and extinction sessions (12 in total) failed to directly enhance the extinction learning process itself, this dosing regimen did however enhance the consolidation of extinction learning to impair the subsequent reacquisition (i.e., when ethanol and the cues were re-introduced) of the ethanol-associated contextual memory (Groblewski et al., 2009). The finding that exposure to multiple doses of DCS before conditioning had no effect on the initial development and learning that occurs during ethanol place preference conditioning supports this result.
The conditioned place aversion paradigm in which cues are paired with drug abstinence can be used to study the withdrawal component of the conditioned response in animals. In humans, drug-paired cues elicit not only drug craving but also conditioned withdrawal, which may trigger relapse (Robbins et al. 1997). An opiate receptor antagonist such as naloxone is used to precipitate withdrawal in opiate-dependent animals, thus establishing an aversion to the withdrawal-paired compartment. Administration of DCS immediately before extinction training dramatically increases the rate of extinction of the naloxone-induced place aversion in morphine-dependent rats suggesting that extinction of conditioned drug withdrawal involves mechanisms similar to those involved in other types of drug-related extinction learning (Myers and Carlezon 2010a).
Using an animal model that explicitly extinguishes responses only in the presence of discrete drug-paired cues and more closely approximates exposure therapy in drug addicts, administration of DCS (30 mg/kg) either before or immediately after a single extinction training session of cocaine-associated cues resulted in facilitation of extinction learning and subsequent delay in reacquisition of cocaine self-administration in rats (Nic Dhonnchadha et al. 2010b). The effects of DCS were dose-dependent, time-dependent and specific to its coupling with explicit extinction training. Employing similar conditions, pre-treatment with DCS (10mg/kg) failed to alter cocaine cue extinction learning in monkeys; however, subsequent reacquisition of cocaine self-administration was deterred. This effect of DCS was dose-dependent and specific for reacquisition of cocaine self-administration following extinction training as pretreatment with DCS prior to a self-administration control session did not reduce cocaine self-administration during the session or alter subsequent reacquisition. These results suggest that DCS augmented consolidation of extinction learning to deter reacquisition of cocaine self-administration in rats and monkeys.
In the aforementioned studies, either conditioned place preference procedure or self-administration experiments, all phases of the study (conditioning, extinction and reinstatement or reacquisition) were measured in the same context. A major drawback to exposure therapy is the context specificity of the extinction therapy normally provided in a location that is distinct from the location where drugs are typically consumed (i.e., in a clinic or laboratory). This results in the restoration of cue reactivity in the natural environment (i.e., renewal effect, see Section 4). To address this issue experimentally, Torregrossa and colleagues (2010) extinguished lever responses in the cocaine self-administration conditioning environment (context A) and exposed the rats to two Pavlovian cue extinction sessions (60 non-contingent cue presentations were presented in the absence of levers on two consecutive days) in context B. This models the common forms of cue exposure therapy conducted in humans that involves viewing cues without overt instrumental actions. DCS (15 mg/kg) was administered on completion of each of the Pavlovian cue extinction sessions. When rats were tested in the drug-taking context, DCS-treated rats demonstrated reduced cue-reinstatement. This effect seems to be mediated by the NAc core, and reinstatement is only reduced when DCS is given in conjunction with explicit extinction learning. This study illustrates the ability of DCS to enhance the context-independent consolidation of cocaine cue extinction learning and inhibit the renewal effect of re-exposure to cocaine-associated cues.
Finally, low dose administration of DCS (5 mg/kg) prior to 2 extinction sessions in ethanol self-administration studies facilitated extinction learning in rats (Vengeliene et al. 2008). Repeated administration of DCS in combination with the extinction sessions for a total of 12 sessions did not supplement the initial benefits of DCS on extinction learning. This regimen did reduce alcohol-primed reinstatement when tested on completion of the extinction regime. Taken together, these studies in mice, rats and monkeys suggest that DCS administration reduces the conditioned reinforcing properties of drug-associated stimuli through facilitation of the consolidation of extinction learning and deters relapse to drug-seeking behavior.
Based on this success, analogs of DCS or other systemically effective glycine site modulators also are under investigation. D-serine, which is an agonist at the glycine site has been found to rescue impaired long-term potentiation and NMDA-mediated synaptic potentials in aged rats ex vivo (Mothet et al. 2006) as well as attenuate memory deficits induced by phencyclidine or by lesions of the perirhinal cortex in vivo (Andersen et al. 2003; Andersen and Pouzet 2004). D-serine has been shown to facilitate response extinction learning at relatively low doses (100 mg/kg) that subsequently reduced cocaine-primed reinstatement of drug-seeking behavior in rats trained to self-administer cocaine (Kelamangalath et al. 2009). However, in many cases the doses required to improve memory deficits in vivo are quite high (500–1000 mg/kg s.c.) and are well within the range that induces nephrotoxicity in rats (Maekawa et al. 2005). The nephrotoxic effects of D-serine were not observed in mice, guinea pigs, rabbits, dogs, and gerbils (Kaltenbach et al. 1979) and analysis of kidney function parameters did not reveal any abnormalities in the majority of clinical trials (Tsai et al. 1998; Lane et al. 2005; Heresco-Levy et al. 2005), although see Kantrowitz et al. (2010). Administration of D-serine may be of therapeutic value as a pharmacological adjunct to exposure therapy, however, in humans large gram-level doses of ~2g/day must be employed in order to significantly elevate central nervous system levels and penetrate the blood–brain-barrier (Javitt 2008). Additionally, efficacy and side effect profile of higher doses has not been systematically explored (Kantrowitz et al. 2010), consequently agents targeting other means of selectively modulating the NMDA receptor glycine site may be a more appropriate route to follow.
3.2. Glycine-Transporter Inhibition
Another strategy is to increase glycine levels and hence NMDA functioning via the use of a glycine transporter-1 (Gly-T1) inhibitor. Gly-T1 is located on glial cells and its reuptake pump is the main route of inactivation of synaptic glycine. Therefore, the inhibition of Gly-T1 reuptake can increase glycine levels in glutamatergic synapses and consequently augment NMDA-receptor transmission (Stahl 2007). Rodent studies have shown amelioration of phencyclidine-induced cognitive deficits after treatment with the Gly-T1 inhibitor NFPS (Hashimoto et al. 2008), a synthetic derivative of sarcosine (Nmethylglycine), the endogenous inhibitor of GlyT1 (Bergeron et al. 1998; Herdon et al. 2001). Similarly, MK-801-induced impairments in long term potentiation, reference memory (Manahan-Vaughan et al. 2008) and novel object recognition (Karasawa et al. 2008) is reversed by NFPS treatment. Moreover, Gly-T1 inhibitors (ALX-5704 and Org 24598) ameliorate deficits in prepulse inhibition of the acoustical startle response in mice and reverse phencyclidine induced hypermotility, stereotypy and ataxia (Brown et al. 2001; Kinney et al. 2003). In nonhuman primates, pretreatment with the Gly-T1 inhibitor, PF-3463275 alleviated spatial working memory deficits in an acute ketamine model of cognitive dysfunction (Roberts et al. 2010). These findings indicate that targeting Gly-T1 may be beneficial for improving the cognitive function in hypoglutamatergic states, resulting from impaired NMDA receptor transmission.
To assess potential benefits of a Gly-T1 inhibitor for facilitating exposure therapy targeting drug-related cues, it was shown that administration of RO 4543338 (30 and 45 mg/kg) in combination with 3 weekly 1 hr extinction training sessions facilitated cocaine cue extinction learning and deterred subsequent reacquisition of cocaine self-administration in rats (Nic Dhonnchadha et al. 2010a). The multiple doses of RO 4543338 were well tolerated and failed to produce any non-specific behavioral deficits. In this experiment, RO 4543338 facilitated the rate of extinction, as reflected in rapid loss of responding after a single extinction trial. The persistence of extinction eliminated reacquisition of cocaine self-administration. The use of multiple extinction sessions in conjunction with repeated dosing of RO 4543338 may underlie the longer lasting attenuation of reacquisition observed with the GlyT1 inhibitor relative to the effects observed with DCS (Nic Dhonnchadha et al. 2010b). These studies support the validity of the concept that enhancing NMDA receptor activity by increasing synaptic glycine levels serves to enhance drug cue extinction learning.
3.3. Cystine-Glutamate Exchanger Activation
The cystine-glutamate exchanger is another target for potential pharmacotherapy for enhancement of drug cue extinction learning. The cystine-glutamate exchanger, which exchanges extracellular cystine for intracellular glutamate, is downregulated after chronic cocaine, resulting in reduced extracellular levels of glutamate (Baker et al. 2003a; Madayag et al. 2007; Knackstedt et al. 2009). Acute administration of the nutritional supplement N-acetylcysteine or NAC (60 and 600 mg/kg, i.p.) restored the function of the cystine-glutamate exchanger and increased the basal levels of extracellular glutamate in the nucleus accumbens after withdrawal from cocaine self-administration in rats (Baker et al. 2003b). Administration of NAC has been shown to reverse memory impairment in rats exposed to cadmium, as measured in the inhibitory avoidance task (Goncalves et al. 2010) and improve cognitive functioning in dementia patients (Adair et al. 2001).
In a study examining heroin self-administration (Zhou and Kalivas 2008), daily NAC (100 mg/kg) facilitated extinction learning, an effect most apparent during the first 5 days of response extinction training. Fifteen days of NAC pretreatment in combination with daily response extinction training reduced cue-and heroin-elicited reinstatement. The reduction in cue-elicited reinstatement was long lasting, as a reduction was still evident after 40 days of abstinence without further NAC or extinction training. These effects may be due to the up-regulation of the cysteine-glutamate exchanger and restoration of glutamate transmission (Haugeto et al. 1996) to enhance, in this instance, heroin cue extinction learning.
Thus, use of this compound as a potential for treatment in addicts is supported by these preclinical studies in conjunction with a recent pilot study examining cue-induced cocaine craving (LaRowe et al. 2006; 2007). Following four doses of NAC (600 mg), administered at 12-hour intervals, a reduction in the subjective reports of the desire to use and interest in cocaine was reported without effecting cocaine craving, following exposure to cocaine-related cues. While this study did not specifically use the strategy of NAC in combination with exposure therapy, these results are promising and support further investigation of the effects of NAC in combination with extinction training in the clinical population.
3.4. Metabotropic Glutamate Receptor Activation
Other strategies aimed at pharmacologically enhancing NMDA receptor function involve targeting the metabotropic glutamate (mGlu) receptors. mGlu receptors are structurally and biochemically coupled to NMDA receptors to influence NMDA receptor function and NMDA-dependent synaptic plasticity and learning and memory processes (Anwyl 2009; Niswender and Conn 2010; Rosenbrock et al. 2010). Of particular interest have been drugs which act on the mGlu5 receptors, which are highly expressed in the mescorticolimbic regions of the brain (Abe et al. 1992; Bell et al. 2002). These compounds do not activate the mGlu5 receptor directly, but act at an allosteric site to potentiate activation by glutamate (Conn et al. 2009). Systemic administration of the mGlu5 receptor positive allosteric modulators 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) and ADX47273 improved performance in a model of hippocampus-dependent spatial learning (Ayala et al. 2009). CDPPB has been shown to reverse MK-801-induced impairments in performance in behavioral flexibility tasks (Darrah et al. 2008), and improve cognition as measured by novel object recognition (Uslaner et al. 2009).
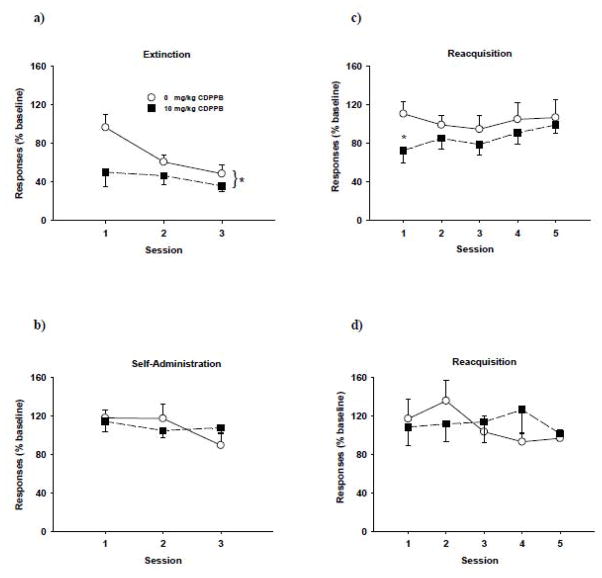
Systemic administration of CDPPB (3 and 30 mg/kg) dose-dependently facilitated extinction of cocaine-conditioned place preference (Gass and Olive 2009). The effect was most pronounced with the highest dose of CDPPB (30 mg/kg) and was blocked by co-administration of the mGlu5 receptor antagonist MTEP or the NMDA receptor antagonist MK-801, highlighting the functional interactions between mGlu5 receptors and NMDA receptors in extinction-related learning. In a study involving cocaine self-administration (Olive 2010), CDPPB (30 mg/kg) was administered prior to 3 daily consecutive extinction sessions, whereby cocaine was no longer available but lever pressing resulted in presentation of the cocaine-paired CS. CDBBP facilitated cocaine cue extinction learning on days 1 and 2 of extinction training. In a preliminary study from our laboratory that was designed to mimic the weekly exposure therapy sessions typically used in people, a facilitation of cocaine cue extinction learning was observed in rats trained to self-administer cocaine when CDPPB (10 mg/kg) was administered in conjunction with 3 weekly 1 hr extinction training sessions (Figure 2, panel a). Additionally, a reduction in responding during the first cocaine reacquisition session was observed (Figure 2, panel c), with responses returning to baseline levels over the next four reacquisition sessions. This effect was observed only when CDBBB was administered in combination with explicit cue extinction training, as CDPPB did not alter responding when administered prior to cocaine self-administration sessions (Figure 2, panel b) and did not alter subsequent reacquisition of cocaine self-administration under these control test conditions (Figure 2, panel d). Testing with a higher dose of CDPPB (30 mg/kg) may produce more robust effects on facilitating extinction and deterring reacquisition (Gass and Olive, 2009; Olive 2010). These studies suggest that positive allosteric modulation of the mGlu5 receptor may be a novel avenue to facilitate extinction of drug-associated memories.
Figure 2. Cocaine cue extinction and reacquisition of cocaine self-administration after treatment with CDPPB.
Rats were trained to self-administer 0.3 mg/kg cocaine under an FI 5 min (FR5:S) second-order schedule paired with a 2-sec light cue before undergoing three 1 hour weekly extinction training sessions. Lever responses were extinguished by substituting saline for cocaine delivery while maintaining response contingent presentation of the cocaine-paired discrete light cue upon completion of each FR5. Rats received i.p. injections of either 0 mg/kg (n=6) or 10 mg/kg CDPPB (n=6) 15 min prior to the weekly extinction (a) or self-administration sessions (b). Reacquisition of cocaine self-administration began 7 days after the last extinction or self-administration session (c and d, respectively) under conditions identical to self-administration training. Values are the mean ± SEM percent of baseline lever responses (last five cocaine self-administration sessions). * p<0.03 compared to the vehicle control.
4. Translational Issues – Lessons Learned from Animal Studies
Animal research using combined treatment with a cognitive-enhancer and extinction training to reduce relapse to drug-seeking behavior is highly encouraging, particularly in light of the fact that the beneficial effects observed in rodents extend to non-human primates. A next step is to translate these preclinical findings to the treatment of substance use disorders. However, there are several challenges we face due to a multitude of issues that are necessary to consider for this approach to be successful (for discussion of additional translational issues, see Kantak and Nic Dhonnchadha, in press).
4.1. Beware of Memory Reactivation and Reconsolidation
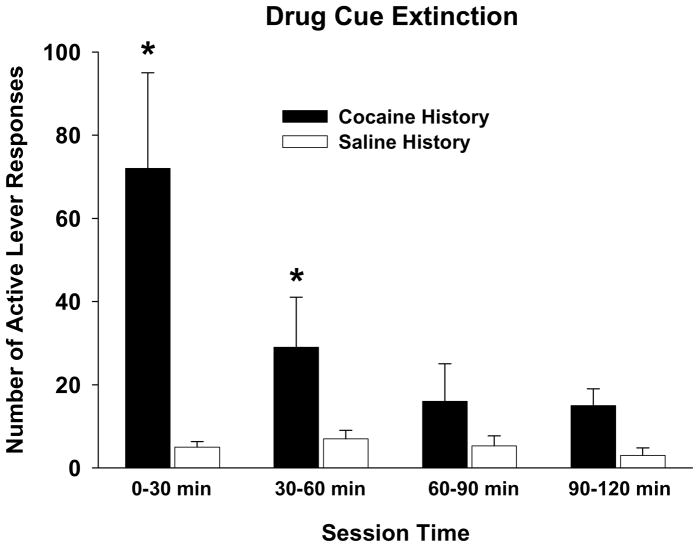
The timing of treatment with a cognitive enhancer and length of the exposure therapy sessions need to be considered carefully in clinical studies. Investigators agree that the general mechanism by which DCS in combination with extinction training reduces drug relapse is through enhanced consolidation of the newly formed extinction memory that competes with retrieval of the previously established drug memory. The theoretical time window for NMDA-dependent memory consolidation is up to 4 hr post-training (Dash et al. 2004). Thus, if DCS is administered more than 4 hr after extinction training, drug-seeking behavior is not attenuated (Nic Dhonnchadha et al. 2010b). A more critical concern is if the length of the extinction training session is too short. Early in extinction training, a memory reconsolidation process is initiated, which serves to restabilize and strengthen old memories following their reactivation through cue exposure (Nader 2003). It has been demonstrated that when DCS is administered prior to a single 30 min session of non-contingent drug cue exposure in rats trained to self-administer cocaine, lever responses are elevated during a subsequent test for drug-seeking behavior (Lee et al. 2009). These findings indicate that the previously established drug memory can be enhanced if DCS is administered in combination with too brief a period of cue exposure in rats. The formation of extinction memory and its facilitation by DCS or other cognitive-enhancer may require a longer period of non-reinforced cue exposure (Pedreira and Maldonado 2003). Preliminary findings from our laboratory suggest that greater than 60 min of non-reinforced drug cue exposure is necessary to stabilize cocaine cue extinction responses to saline-like levels in rats (Figure 3).
Figure 3. Time course of drug cue extinction.
Rats were trained to self-administer 0.3 mg/kg cocaine (n=8) or passively receive yoked saline (n=4) under an FR5 schedule before undergoing a single 2 hr extinction training session for which cocaine delivery was suspended, but the cocaine-paired discrete light cue was presented upon completion of each FR5. The number of active lever responses during the extinction session was divided into 30 min bins. * p<0.05 compared to the corresponding saline control.
In studies in which an augmentation of exposure therapy was reported for anxiety disorders (Ressler et al. 2004; Hofmann et al. 2006; Guastella et al. 2008; Kushner et al. 2007; Wilhelm et al. 2008; Otto et al. 2010), the length of exposure therapy sessions varied from 35 to 90 min. Is 35 to 90 min of drug cue exposure in addicts sufficient to avoid enhancing reconsolidation of drug memory after treatment with a cognitive-enhancing drug? It is important to note that in animal studies with DCS, the length of extinction training sessions is shorter for extinguishing fear-conditioned responses (15 to 24 min) than drug-conditioned responses (60 min or more). Unclear is the time course of the transition from memory reconsolidation to extinction consolidation upon cue exposure in people, especially those who are addicted to drugs and are drug cue reactive. We suggest that human laboratory studies are needed that manipulate length of the exposure sessions to ascertain optimal therapeutic conditions for enhancing consolidation of drug cue extinction and avoiding reconsolidation of drug memory after treatment with a cognitive enhancer. An additional strategy that has been proposed is to use a combined approach whereby a cognitive enhancer is used to facilitate consolidation of drug cue extinction and an amnesic agent is used to interfere with reconsolidation of drug memory during cue exposure (Taylor et al. 2009). While this concept is very appealing, navigating the temporal complexities inherent in this approach requires careful consideration and systematic evaluation.
4.2. Navigating Spontaneous Recovery, Renewal, Reinstatement and Incubation of Craving
Extinction is not unlearning, but is a form of new learning that competes with the original memory for retrieval. Consequently, after extinction training the original memory can spontaneously recover or can be renewed or reinstated. Another point to consider is incubation of craving, which may influence the long-term efficacy of exposure therapy.
Spontaneous recovery of the extinguished response occurs with the passage of time, and can be viewed as a renewal effect that occurs when the CS is tested outside its temporal context (Bouton 2004). This situation results in a failure to retrieve an extinction memory, which would be detrimental to a drug addict who has completed exposure therapy sessions and is later confronted with stimuli that can trigger a drug memory and cause relapse. Research in rats has shown, though, that when a cue is presented intermittently during extinction training, spontaneous recovery is attenuated (Brooks 2000). Thus, just as too short a length of cue exposure during extinction training is counterproductive (leading to memory reconsolidation); too frequent the rate of cue exposure during extinction training may be equally counterproductive (leading to spontaneous recovery at later time points). It has been shown that in rats trained to self-administer cocaine under a second-order schedule before undergoing drug cue extinction, spontaneous recovery of cocaine-seeking behavior was significantly greater after 21 days than 1 day of cocaine and cocaine cue abstinence (Di Ciano and Everitt 2002). It is important to note that the schedule of contingent cue presentation during extinction training in this study was quite frequent, which may have undermined retrieval of the extinction memory at a later time point. If exposure therapy targeting drug-related cues is to be successful, attention to the frequency of cue presentation may be an important factor for reducing spontaneous recovery. Of great interest is the fact that when DCS is combined with fear extinction training in rats, spontaneous recovery is reduced (Vervliet 2008).
Renewal refers to the robust return of conditioned responding when there is a change of context after extinction (Bouton, 2004). The renewal effect is observed, for example, when conditioning takes place in one context (context A) and extinction training in a second context (context B) prior to testing taking place in the original conditioning context (context A). In other words, renewal is context-specific. This situation is similar to what may be faced by individuals who become addicted to drugs in one environment, undergo exposure therapy in a therapeutic setting, and then return to their original environment. Renewal may be an obstacle to successful treatment, even if exposure therapy is combined with a cognitive enhancer. For example, DCS administration during extinction training in rats did not prevent a renewal effect from occurring when the fear-associated CS was tested in the original conditioning context (Woods and Bouton 2006). However, in the first test for context-specificity of drug cue extinction in rats trained to self-administer cocaine (Torregrossa et al. 2010), the renewal effect was not observed in DCS-treated rats. These findings demonstrate that although DCS does not reduce context-specificity of fear extinction, it can prevent context-specificity of drug cue extinction. Further research examining the degree to which DCS and other cognitive-enhancing drugs may prevent the renewal effect for extinguished drug cues may assist in determining medication choices in individuals addicted to drugs and undergoing exposure therapy.
Reinstatement refers to the return of an extinguished response after re-exposure to the US or the CS-US complex (Bouton, 2004). In many studies of fear extinction, animals are tested for reinstatement 24 hr after footshock re-exposure. For drugs of abuse, animals are tested for reinstatement immediately after drug or drug + cue re-exposure. Drug prime-induced reinstatement is thought to model relapse in abstinent addicts following drug re-exposure (de Wit and Stewart 1981; Jaffe et al. 1989). It is of interest that reinstatement of fear following footshock re-exposure is not evident in rats that received DCS during fear extinction training (Ledgerwood et al. 2004). A lessening of the impact of reinstating stimuli by treatment with DCS and other cognitive enhancers during extinction training also has been demonstrated in cocaine-trained rats and monkeys (Kelamangalath et al. 2009; Paolone et al. 2009; Nic Dhonnchadha et al. 2010b). Collectively, these findings suggest that when exposure therapy targeting drug-related cues is provided as a stand alone treatment, addicts would remain vulnerable to relapse via spontaneous recovery, renewal and reinstatement processes. If a cognitive enhancer is combined with exposure therapy, concern for spontaneous recovery, renewal and reinstatement may be mitigated. As DCS and other cognitive enhancers also facilitate neuroplasticity in memory systems required for effective extinction learning (Rouaud and Billard 2003; Richter-Levin and Maroun 2010), neurocognitive impairments that may undermine exposure therapy in drug addicts may be mitigated as well.
A key factor in determining the efficacy of cue exposure therapy in combination with a cognitive enhancer may be the duration period of withdrawal or abstinence the addict has undergone prior to treatment. Numerous studies in rats, non-human primates and humans indicate that the salience of drug-related cues and hence their ability to induce drug-seeking behavior, increases in a time-dependent manner (Grimm et al. 2001; Weerts et al. 2006; Kerstetter et al. 2008; Bedi et al. 2010). This phenomenon, termed “incubation of craving” is believed to occur when most of the neuroadaptations that accompany withdrawal from chronic drug use are in progressive decline (Lu et al. 2004b). Re-exposure to drug-related cues during abstinence induces exaggerated cue reactivity, as evidenced by with an increase in extinction responding in the rat. Incubation of craving has been demonstrated to follow an inverted U-shaped curve in rats trained to self-administer cocaine, heroin, nicotine and methamphetamine (Grimm et al. 2001; Shalev et al. 2001; Lu et al. 2004a; Abdolahi et al. 2010; Shepard et al. 2004) with levels of extinction responding remaining elevated over the course of the first 3 months of withdrawal. In cigarette smokers, cue-induced craving in response to smoking cues was greater in subjects abstinent for 35 days in comparison to those that underwent 1 or 14 days or abstinence (Bedi et al. 2010). These studies indicate that the risk of relapse may persist or increase with abstinence and that the timing of extinction therapy will be an important consideration to its efficacy.
4.3. Generalizing from Cocaine Treatment to Other Drugs of Abuse
As reviewed in Section 3, the majority of preclinical studies investigating the effects of cognitive enhancers on drug cue extinction have focused on cocaine as the drug of abuse. These studies have shown positive effects in that cognitive enhancers facilitated consolidation of cocaine cue extinction and attenuated relapse to cocaine-seeking and cocaine-taking behavior. Use of this strategy as a potential treatment in individuals addicted to cocaine is clearly warranted, and one group of investigators has begun to explore the effect of DCS on exposure therapy targeting cocaine-related cues in a preliminary fashion (Price et al. 2009). As these studies progress, an important point to consider is whether or not the benefits observed for DCS and other cognitive enhancers on cocaine cue extinction in preclinical studies will extend to other drugs of abuse, and in the process, provide a framework for drug abuse treatment in general.
One argument suggests this may be so, insofar as a broad spectrum of drugs of abuse (serving as USs) produces a strong associative link with discrete and contextual cues (serving as CSs) that are present in the environment at the time of drug-taking (Everitt et al. 2001). Through repeated CS-US pairings, the CS is conditioned to predict the availability the US, and forms the basis for drug memory and drug-seeking behavior. During extinction training, an organism learns that a CS no longer predicts the US, which forms the basis for extinction memory (Bouton et al. 2006). Thus, if the purpose of treatment with a cognitive enhancer is to facilitate the process by which a CS no longer predicts the US, then whichever drug of abuse is represented by the US is irrelevant. The few preclinical studies that have examined drug cue extinction and its facilitation by a cognitive enhancer support this view for drugs of abuse other than cocaine (e.g., amphetamine, heroin, nicotine and ethanol).
4.4. From Anxiety to Addiction and Back: A Translational Pathway for Identifying New Treatments
The idea that DCS might augment drug cue extinction originated from reports showing a facilitation of fear extinction after treatment with DCS in rats (Walker et al. 2002; Ledgerwood et al. 2003). These findings led to the first studies evaluating the effects of DCS combined with exposure therapy for the treatment of anxiety disorders (Ressler et al., 2004; Hofmann et al. 2006). A dual translational approach may serve as a pathway for identifying new cognitive-enhancing drugs to use in combination with exposure therapy for individuals with substance use disorders. Treatments appropriate for enhancing extinction of fear and anxiety also may be appropriate for enhancing drug cue extinction. The emergence of GlyT-1 inhibitors as treatments to enhance drug cue extinction follows this translational pathway.
One possible new treatment lead suggested by fear conditioning studies is 4-[2-(phenyl-sulfonylamino)ethylthio]-2,6-difluorophenoxyacetamide (PEPA), which is an allosteric potentiator of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors via an enhanced expression of GluR3/4 subunits preferentially in mPFC vs. amygdala or hippocampus (Zushida et al. 2007). Past work has demonstrated that chronic administration of PEPA improves Morris water maze test performance in rats made ischemic by occlusion of the middle cerebral artery (Sekiguchi et al. 2001), suggesting action as a cognitive enhancer. In fear conditioning studies in mice, PEPA administered prior to extinction training has been shown to facilitate fear extinction by reducing the duration of the freezing response during the post-extinction retrieval test (Zushida et al., 2007). These investigators additionally demonstrated that, unlike DCS, PEPA does not facilitate reconsolidation of fear memory following brief (3 min) exposure to the fear-inducing context (Yamada et al. 2009). Recently, infusion of PEPA into the infralimbic cortex following brief (15 or 30 min) exposure to a cocaine self-administration environment was shown to enhance extinction retention (LaLumiere et al. 2010). These findings support the idea that PEPA does not facilitate reconsolidation of the drug memory even when context exposure is relatively brief during response extinction training sessions. How PEPA influences drug cue extinction learning in an animal model that more closely approximates cue exposure therapy in drug addicts remains unexplored.
A second new treatment lead concerns activation of the cannabinoid CB1 receptor. While synthetic and endogenous cannabinoids impair performance on standard tests for memory in animals (Lichtman et al. 1995; Riedel and Davies 2005), research has shown that CB1 receptor agonists facilitate rather than impair extinction learning. Pioneering work by Marsicano et al. (2002) illustrated the importance of the CB1 receptor for extinction learning by showing impaired fear extinction in mutant mice lacking CB1 receptors. Subsequent studies in rats demonstrated that systemic administration of AM404 (an inhibitor of cannabinoid breakdown and reuptake) and WIN55212-2 (a CB1 receptor agonist) enhanced fear extinction (Chhatwal et al. 2005; Pamplona et al. 2006). Recently, both compounds were shown to not only facilitate within-session extinction of fear, but also produce long-term retention of fear extinction (Pamplona et al. 2008). Findings also support the use of CB1 receptor agonists to facilitate drug cue extinction learning. Using the conditioned place preference model in rats, administration of low doses of Δ9-THC was shown to facilitate extinction of environmental cues associated with cocaine or amphetamine exposure (Parker et al. 2004). The use of this class of compounds with exposure therapy is made even more intriguing by findings in rats showing that intra-amygdala infusion of CB1 receptor agonists after a memory reactivation session actually blocks reconsolidation of fear memory, as well as reinstatement and spontaneous recovery of fear (Lin et al. 2006).
Another agent that has been tested in studies of fear and anxiety is the α-2 adrenergic autoreceptor antagonist yohimbine. Systemic administration of yohimbine has been shown to facilitate fear extinction in rats and mice (Cain et al. 2004; Morris and Bouton 2007; Mueller et al. 2009) and to augment exposure therapy in individuals with claustrophobia (Powers et al. 2009). The mechanism by which yohimbine is thought to produce these effects is via noradrenergic stimulation of the mPFC. Yohimbine, though, was not able to prevent the renewal of fear when rats were tested outside the extinction context and was not able to strengthen retention of fear extinction. Preliminary evidence in rats and mice suggests that yohimbine may actually impair extinction of responses maintained by environmental cues associated with cocaine (Davis et al. 2008; Kupferschmidt et al. 2009). These findings suggest that yohimbine may not be a promising lead for augmenting drug cue extinction. Thus, treatments appropriate for enhancing extinction of fear and anxiety may not always translate into treatments appropriate for enhancing drug cue extinction.
5. Conclusions
The trajectory from drug use to addiction progresses as neural plasticity in key brain circuits plays upon the added pharmacological impact of the abused substance. The means to reverse drug-induced neural plasticity and therapeutically improve cognitive function in the addicted brain is an important quest. Preclinical studies showing the strengthening of drug cue extinction memory with DCS (summarized in Table 1) provide translational support for evaluating adjunct DCS treatment with exposure therapy in individuals addicted to drugs.
Table 1.
Consequences of the Effects of DCS Combined with Drug Cue Extinction Training in Animals
| Measure | Drug | Effect | Reference |
|---|---|---|---|
| Extinction Consolidation | Alcohol | ↑ |
Groblewski et al., 2009 Vengeliene et al., 2008 |
| Amphetamine | ↑ | Sakurai et al., 2007 | |
| Cocaine | ↑ |
Botreau et al., 2006 Kelly et al., 2007 Paolone et al., 2009 Nic Dhonnchadha et al., 2010b Thanos et al., 2009 Torregossa et al., 2010 |
|
| Morphine WD | ↑ | Myers & Carlezon, 2010a | |
| Reconsolidation | Amphetamine | ↑ | Sakurai et al., 2007 |
| Cocaine | ↑ | Lee et al., 2009 | |
| Reacquisition | Alcohol | ↓ | Groblewski et al., 2009 |
| Cocaine | ↓ | Nic Dhonnchadha et al., 2010b | |
| Spontaneous Recovery | ND | ||
| Renewal | Cocaine | ↑ | Thanos et al., 2009 |
| ↓ |
Botreau et al., 2006 Thanos et al., 2009 Torregossa et al., 2010 |
||
| Reinstatement | Alcohol | ↓ | Vengeliene et al., 2008 |
| Cocaine | ↓ | Paolone et al., 2009 | |
| ↑ | Kelly et al., 2007 | ||
↑: Facilitation; ↓: Blockade; ND: Not determined; WD: Withdrawal
Further exploration of neurobehavioral mechanisms by which cognitive enhancers facilitate drug cue extinction is warranted. Important aspects of drug action to delineate include identifying target effector substrates, specifying anatomical localization, and revealing interactions with other neural systems. Such studies can help improve the understanding of the neurobiology of drug cue-related extinction memory and aid in the development of therapeutic agents geared to ultimately cure addiction or vastly improve the chances for recovery.
Acknowledgments
This work was supported by NIH DA024315. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse.
Footnotes
Conflicts of interest
Dr. Nic Dhonnchadha declares no conflicts of interest. Over the past 3 years, Dr. Kantak reports consulting fees and stock options from Yaupon Therapeutics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology. 2001;57:1515–1517. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- Andersen JD, Pouzet B. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacol. 2004;29:1080–1090. doi: 10.1038/sj.npp.1300394. [DOI] [PubMed] [Google Scholar]
- Andersen JM, Fonnum F, Myhrer T. D-Serine alleviates retrograde amnesia of a visual discrimination task in rats with a lesion of the perirhinal cortex. Brain Res. 2003;979:240–244. doi: 10.1016/s0006-8993(03)02894-4. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Aston-Jones S, Aston-Jones G, Koob GF. Cocaine antagonizes anxiolytic effects of ethanol. Psychopharmacology (Berl) 1984;84:28–31. doi: 10.1007/BF00432019. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacol. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003a;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003b;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Miller JS. Repeated testing attenuates conditioned place preference with cocaine. Psychopharmacology. 1986;89:239–243. doi: 10.1007/BF00310636. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, deWit H. Incubation of cue-Induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2010 Sep 2; doi: 10.1016/j.biopsych.2010.07.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MI, Richardson PJ, Lee K. Functional and molecular characterization of metabotropic glutamate receptors expressed in rat striatal cholinergic interneurones. J Neurochem. 2002;81:142–149. doi: 10.1046/j.1471-4159.2002.00815.x. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG, Smith RF. Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiol Behav. 2006;88:466–472. doi: 10.1016/j.physbeh.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Bizarro L, Patel S, Stolerman IP. Comprehensive deficits in performance of an attentional task produced by co-administering alcohol and nicotine to rats. Drug Alcohol Depend. 2003;72:287–295. doi: 10.1016/j.drugalcdep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boje KM, Wong G, Skolnick P. Desensitization of the NMDA receptor complex by glycinergic ligands in cerebellar granule cell cultures. Brain Res. 1993;603:207–214. doi: 10.1016/0006-8993(93)91239-o. [DOI] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Braida D, Gori E, Sala M. Relationship between morphine and etonitazene-induced working memory impairment and analgesia. Eur J Pharmacol. 1994;271:497–504. doi: 10.1016/0014-2999(94)90811-7. [DOI] [PubMed] [Google Scholar]
- Brooks DC. Recent and remote extinction cues reduce spontaneous recovery. Q J Exp Psychol B. 2000;53:25–58. doi: 10.1080/027249900392986. [DOI] [PubMed] [Google Scholar]
- Brown A, Carlyle I, Clark J, Hamilton W, Gibson S, McGarry G, McEachen S, Rae D, Thorn S, Walker G. Discovery and SAR of org 24598-a selective glycine uptake inhibitor. Bioorg Med Chem Lett. 2001;11:2007–2009. doi: 10.1016/s0960-894x(01)00355-9. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 1993;113:123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Burke KA, Franz TM, Gugsa N, Schoenbaum G. Prior cocaine exposure disrupts extinction of fear conditioning. Learn Mem. 2006;13:416–421. doi: 10.1101/lm.216206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Extinction of cocaine-induced place approach in rats: A validation of the “biased” conditioning procedure. Brain Res Bull. 1993;30:695–700. doi: 10.1016/0361-9230(93)90102-h. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Carmack SA, Wood SC, Anagnostaras SG. Amphetamine and extinction of cued fear. Neurosci Lett. 2010;468:18–22. doi: 10.1016/j.neulet.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104 (Suppl 1):S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacol. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, Ettenberg A. Anxiolytic effects of nicotine in a rodent test of approach-avoidance conflict. Psychopharmacology (Berl) 2009;204:541–549. doi: 10.1007/s00213-009-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacol. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Crider A, Solomon PR, McMahon MA. Disruption of selective attention in the rat following chronic d-amphetamine administration: relationship to schizophrenic attention disorder. Biol Psychiatry. 1982;17:351–361. [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacol. 2007;32:1195–1206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wang YT, Floresco SB, Phillips AG. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacol. 2008;33:2416–2426. doi: 10.1038/sj.npp.1301642. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hebert AE, Runyan JD. A unified theory for systems and cellular memory consolidation. Brain Res Brain Res Rev. 2004;45:30–37. doi: 10.1016/j.brainresrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Davis AR, Shields AD, Brigman JL, Norcross M, McElligott ZA, Holmes A, Winder DG. Yohimbine impairs extinction of cocaine-conditioned place preference in an alpha2-adrenergic receptor independent process. Learn Mem. 2008;15:667–676. doi: 10.1101/lm.1079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Del Olmo N, Higuera-Matas A, Miguens M, Garcia-Lecumberri C, Ambrosio E. Cocaine self-administration improves performance in a highly demanding water maze task. Psychopharmacology (Berl) 2007;195:19–25. doi: 10.1007/s00213-007-0873-1. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacol. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13:397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, ham-Hermetz J, Fogg AP, Osborne GE. Role of the prelimbic cortex in the acquisition, re-acquisition or persistence of responding for a drug-paired conditioned reinforcer. Neuroscience. 2007;150:291–298. doi: 10.1016/j.neuroscience.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacol. 2007;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007;3:4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006a;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacol. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006b;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Morgan RE, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Prenatal cocaine exposure impairs selective attention: evidence from serial reversal and extradimensional shift tasks. Behav Neurosci. 2000;114:725–738. [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Impaired sustained attention and altered reactivity to errors in an animal model of prenatal cocaine exposure. Brain Res Dev Brain Res. 2003;147:85–96. doi: 10.1016/j.devbrainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacol. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler VL, Morsch VM, Schetinger MR. N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact. 2010;186:53–60. doi: 10.1016/j.cbi.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Good AJ, Westbrook RF. Effects of a microinjection of morphine into the amygdala on the acquisition and expression of conditioned fear and hypoalgesia in rats. Behav Neurosci. 1995;109:631–641. doi: 10.1037//0735-7044.109.4.631. [DOI] [PubMed] [Google Scholar]
- Grilly DM, Gowans GC, McCann DS, Grogan TW. Effects of Cocaine and d-Amphetamine on Sustained and Selective Attention in Rats. Pharmacol Biochem Behav. 1989;33:733–739. doi: 10.1016/0091-3057(89)90463-2. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacol. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Lattal KM, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Li P, Hu B, Ouyang X, Fu J, Gao J, Song Z, Han L, Ma Y, Tian S, Hu X. Chronic morphine selectively impairs cued fear extinction in rats: implications for anxiety disorders associated with opiate use. Neuropsychopharmacol. 2008;33:666–673. doi: 10.1038/sj.npp.1301441. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short- and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 2008;196:483–495. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacol. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology (Berl) 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Ishima T, Chaki S, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the glycine transporter-1 inhibitor NFPS and D-serine. Eur Neuropsychopharmacol. 2008;18:414–421. doi: 10.1016/j.euroneuro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology (Berl) 2003;168:75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- Herdon HJ, Godfrey FM, Brown AM, Coulton S, Evans JR, Cairns WJ. Pharmacological assessment of the role of the glycine transporter GlyT-1 in mediating high-affinity glycine uptake by rat cerebral cortex and cerebellum synaptosomes. Neuropharmacology. 2001;41:88–96. doi: 10.1016/s0028-3908(01)00043-0. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, Catinari S, Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Korte KJ, Smits JA. Is it beneficial to add pharmacotherapy to cognitive-behavioral therapy when treating anxiety disorders? A meta-analytic review. Int J Cogn Ther. 2009;2:160–175. doi: 10.1521/ijct.2009.2.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Combining behavioral therapy and pharmacotherapy for smoking cessation: an update. NIDA Res Monogr. 1995;150:92–109. [PubMed] [Google Scholar]
- Ito R, Canseliet M. Amphetamine exposure selectively enhances hippocampus-dependent spatial learning and attenuates amygdala-dependent cue learning. Neuropsychopharmacol. 2010;35:1440–1452. doi: 10.1038/npp.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glycine transport inhibitors and the treatment of schizophrenia. Biol Psychiatry. 2008;63:6–8. doi: 10.1016/j.biopsych.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JP, Ganote CE, Carone FA. Renal tubular necrosis induced by compounds structurally related to D-serine. Exp Mol Pathol. 1979;30:209–214. doi: 10.1016/0014-4800(79)90054-6. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance after lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Nic Dhonnchadha BÁ. Pharmacological enhancement of drug cue extinction learning: translational challenges. In: Uhl G, editor. Addiction Reviews. New York Academy of Sciences; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Udo T, Ugalde F, Luzzo C, Di PN, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology (Berl) 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF, D’Souza C, Saksa J, Woods SW, Javitt DC. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121:125–130. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kelamangalath L, Seymour CM, Wagner JJ. D-serine facilitates the effects of extinction to reduce cocaine-primed reinstatement of drug-seeking behavior. Neurobiol Learn Mem. 2009;92:544–551. doi: 10.1016/j.nlm.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci U S A. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neurorep. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de WH, Richards JB. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology (Berl) 2004;173:167–174. doi: 10.1007/s00213-003-1697-2. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Sur C, Burno M, Mallorga PJ, Williams JB, Figueroa DJ, Wittmann M, Lemaire W, Conn PJ. The glycine transporter type 1 inhibitor N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]sarcosine potentiates NMDA receptor-mediated responses in vivo and produces an antipsychotic profile in rodent behavior. J Neurosci. 2003;23:7586–7591. doi: 10.1523/JNEUROSCI.23-20-07586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Katz JL. Response requirement and increases in accuracy produced by stimulant drugs in a 5-choice serial reaction-time task in rats. Psychopharmacology (Berl) 2010 Oct 6; doi: 10.1007/s00213-010-2027-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56 (Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav. 2009;91:473–480. doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land C, Riccio DC. d-Cycloserine: effects on long-term retention of a conditioned response and on memory for contextual attributes. Neurobiol Learn Mem. 1999;72:158–168. doi: 10.1006/nlme.1998.3897. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Fear conditioning is impaired in adult rats by ethanol doses that do not affect periadolescents. Int J Dev Neurosci. 2004;22:355–362. doi: 10.1016/j.ijdevneu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:1196–1204. doi: 10.1001/archpsyc.62.11.1196. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-acetylcysteine? Am. J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- Lattal KM. Effects of ethanol on encoding, consolidation, and expression of extinction following contextual fear conditioning. Behav Neurosci. 2007;121:1280–1292. doi: 10.1037/0735-7044.121.6.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Lee JL, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem. 2009;16:82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Kim P, Meray R. Chronic nicotine working and reference memory effects in the 16-arm radial maze: interactions with D1 agonist and antagonist drugs. Psychopharmacology (Berl) 1996;127:25–30. doi: 10.1007/BF02805971. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13:316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, Zhang Y, Wang W, Wang Y, Li Q, Zhao L, Lu L, von Deneen KM, Liu Y, Gold MS. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460:72–77. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004a;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47 (Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Okamura T, Kasai N, Hori Y, Summer KH, Konno R. D-aminoacid oxidase is involved in D-serine-induced nephrotoxicity. Chem Res Toxicol. 2005;18:1678–1682. doi: 10.1021/tx0500326. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Wildforster V, Thomsen C. Rescue of hippocampal LTP and learning deficits in a rat model of psychosis by inhibition of glycine transporter-1 (GlyT1) Eur J Neurosci. 2008;28:1342–1350. doi: 10.1111/j.1460-9568.2008.06433.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Tsikitas LA, Kantak KM. Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci. 2009;29:1641–1653. doi: 10.1111/j.1460-9568.2009.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Wells AM, Kantak KM. Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2010;96:347–353. doi: 10.1016/j.pbb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka N, Aigner TG. D-cycloserine, a partial agonist at the glycine site coupled to N-methyl-D-aspartate receptors, improves visual recognition memory in rhesus monkeys. J Pharmacol Exp Ther. 1996;278:891–897. [PubMed] [Google Scholar]
- McDonald AJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Melnick SM, Kubie JL, Laungani R, Dow-Edwards DL. Impairment of spatial learning following preweaning cocaine exposure in the adult rat. Neurotoxicol Teratol. 2001;23:445–451. doi: 10.1016/s0892-0362(01)00157-x. [DOI] [PubMed] [Google Scholar]
- Meneses A, Ponce-Lopez T, Tellez R, Gonzalez R, Castillo C, Gasbarri A. Effects of d-amphetamine on short- and long-term memory in spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Behav Brain Res. 2011;216:472–476. doi: 10.1016/j.bbr.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Miladi GH, Rashidy-Pour A, Fathollahi Y. Effects of morphine dependence on the performance of rats in reference and working versions of the water maze. Physiol Behav. 2008;93:622–627. doi: 10.1016/j.physbeh.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. N-Methyl-D-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology (Berl) 2005;179:30–53. doi: 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kelly PA. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Taylor JR, Roth RH. Prior exposure to cocaine diminishes behavioral and biochemical responses to aversive conditioning: reversal by glycine/N-methyl-D-aspartate antagonist co-treatment. Neuroscience. 1995;69:233–240. doi: 10.1016/0306-4522(95)00184-k. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Rouaud E, Sinet PM, Potier B, Jouvenceau A, Dutar P, Videau C, Epelbaum J, Billard JM. A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell. 2006;5:267–274. doi: 10.1111/j.1474-9726.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- Mueller D, Olivera-Figueroa LA, Pine DS, Quirk GJ. The effects of yohimbine and amphetamine on fear expression and extinction in rats. Psychopharmacology (Berl) 2009;204:599–606. doi: 10.1007/s00213-009-1491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: Reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr D-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol Psychiatry. 2010a;67:85–87. doi: 10.1016/j.biopsych.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr Extinction of drug- and withdrawal-paired cues in animal models: relevance to the treatment of addiction. Neurosci Biobehav Rev. 2010b;35:285–302. doi: 10.1016/j.neubiorev.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BÁ, Achat-Mendes C, Platt DM, Pinard E, Alberati D, Wettstein JG, Spealman RD, Kantak KM. Inhibiting glycine transporter-1: effects on extinction and reacquisition of cocaine self-administration. College on Problems of Drug Dependence 72nd Annual Meeting; 2010a. p. 121. Abstract #484. [Google Scholar]
- Nic Dhonnchadha BÁ, Szalay JJ, chat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacol. 2010b;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Hellemans KG, Paine TA. Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology (Berl) 2006;184:221–228. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- Olney JW. Neurotoxicity of NMDA receptor antagonists: an overview. Psychopharmacol Bull. 1994;30:533–540. [PubMed] [Google Scholar]
- Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65 (Suppl 5):34–41. [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, Hofmann SG, Eisenmenger K, Krystal JH, Pollack MH. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. Am J Drug Alcohol Abuse. 2008;34:109–121. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–147. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem. 2008;90:290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212–2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D: -cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R. Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology (Berl) 2004;175:360–366. doi: 10.1007/s00213-004-1825-7. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res. 2000;126:263–285. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen MC, Wiskerke J, Schoffelmeer AN. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl) 2009;205:489–502. doi: 10.1007/s00213-009-1558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008a;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008b;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP. Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JA, Otto MW, Sanders C, Emmelkamp PM. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. J Anxiety Disord. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Price KL, Rae-Clark AL, Saladin ME, Maria MM, DeSantis SM, Back SE, Brady KT. D-cycloserine and cocaine cue reactivity: preliminary findings. Am J Drug Alcohol Abuse. 2009;35:434–438. doi: 10.3109/00952990903384332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pussinen R, Sirvio J. Effects of D-cycloserine, a positive modulator of N-methyl-D-aspartate receptors, and ST 587, a putative alpha-1 adrenergic agonist, individually and in combination, on the non-delayed and delayed foraging behaviour of rats assessed in the radial arm maze. J Psychopharmacol. 1999;13:171–179. doi: 10.1177/026988119901300210. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Extinction: new excitement for an old phenomenon. Biol Psychiatry. 2006;60:317–318. doi: 10.1016/j.biopsych.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Quirk PL, Richards RW, Avery DD. Subchronic cocaine produces training paradigm-dependent learning deficits in laboratory rats. Pharmacol Biochem Behav. 2001;68:545–553. doi: 10.1016/s0091-3057(01)00462-2. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacol Biochem Behav. 2003;76:75–83. doi: 10.1016/s0091-3057(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Maroun M. Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex. Cereb Cortex. 2010;20:2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handb Exp Pharmacol. 2005:445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Roberts BM, Shaffer CL, Seymour PA, Schmidt CJ, Williams GV, Castner SA. Glycine transporter inhibition reverses ketamine-induced working memory deficits. Neurorep. 2010;21:390–394. doi: 10.1097/WNR.0b013e3283381a4e. [DOI] [PubMed] [Google Scholar]
- Robledo P, Kaneko WM, Ehlers CL. The effects of acute cocaine administration on auditory event-related potentials in rats. Neurosci Lett. 1993;160:4–8. doi: 10.1016/0304-3940(93)90903-x. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Kramer G, Hobson S, Koros E, Grundl M, Grauert M, Reymann KG, Schroder UH. Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. Eur J Pharmacol. 2010;639:40–46. doi: 10.1016/j.ejphar.2010.02.057. [DOI] [PubMed] [Google Scholar]
- Rouaud E, Billard JM. D-cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. Br J Pharmacol. 2003;140:1051–1056. doi: 10.1038/sj.bjp.0705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Yu L, Tan SE. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav Pharmacol. 2007;18:497–506. doi: 10.1097/FBP.0b013e3282ee7b62. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Rubio S, Begega A, Arias JL. Effects of chronic alcohol consumption on spatial reference and working memory tasks. Alcohol. 2000;20:149–159. doi: 10.1016/s0741-8329(99)00070-1. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Mercado M, Bettica A, Cortes C, York D, Moody E. Residual behavioral and neuroanatomical effects of short-term chronic ethanol consumption in rats. Brain Res Cogn Brain Res. 2004;20:449–461. doi: 10.1016/j.cogbrainres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Scerri C, Stewart CA, Breen KC, Balfour DJ. The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacology (Berl) 2006;184:540–546. doi: 10.1007/s00213-005-0086-4. [DOI] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Tinker JP, Van VM, Giardiniere M. Effects of the partial glycine agonist D-cycloserine on cognitive functioning in chronic low dose MPTP-treated monkeys. Brain Res. 2000;860:190–194. doi: 10.1016/s0006-8993(00)02036-9. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Holahan MR, Landry CF, Kelley AE. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37:146–158. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Facilitation of memory for extinction of drug-induced conditioned reward: role of amygdala and acetylcholine. Learn Mem. 2004;11:641–647. doi: 10.1101/lm.78504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Yamada K, Jin J, Hachitanda M, Murata Y, Namura S, Kamichi S, Kimura I, Wada K. The AMPA receptor allosteric potentiator PEPA ameliorates post-ischemic memory impairment. Neurorep. 2001;12:2947–2950. doi: 10.1097/00001756-200109170-00038. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. Differences between d-methamphetamine and d-amphetamine in rats: working memory, tolerance, and extinction. Psychopharmacology (Berl) 2003;170:150–156. doi: 10.1007/s00213-003-1522-y. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Berry RB, White AM, Matthews DB. Impairments in spatial learning and memory: ethanol, allopregnanolone, and the hippocampus. Brain Res Brain Res Rev. 2003;43:275–284. doi: 10.1016/j.brainresrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Two-choice reaction time performance in Sprague-Dawley rats exposed to alcohol during adolescence or adulthood. Behav Pharmacol. 2006;17:605–614. doi: 10.1097/01.fbp.0000236272.10418.62. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Novel therapeutics for schizophrenia: targeting glycine modulation of NMDA glutamate receptors. CNS Spectr. 2007;12:423–427. doi: 10.1017/s1092852900015297. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T. Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2008;363:3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer B, Patil S, Frischer C, Hoeger H, Lubec G. Strain-dependent effects of cognitive enhancers in the mouse. Amino Acids. 2008;34:485–495. doi: 10.1007/s00726-007-0511-6. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Svensson TH. Dysfunctional brain dopamine systems induced by psychotomimetic NMDA-receptor antagonists and the effects of antipsychotic drugs. Brain Res Brain Res Rev. 2000;31:320–329. doi: 10.1016/s0165-0173(99)00048-x. [DOI] [PubMed] [Google Scholar]
- Szalay JJ, Morin ND, Kantak KM. Involvement of the dorsal subiculum and rostral basolateral amygdala in cocaine cue extinction learning in rats. Eur J Neurosci. doi: 10.1111/j.1460-9568.2010.07581.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56 (Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Gao J, Han L, Fu J, Li C, Li Z. Prior chronic nicotine impairs cued fear extinction but enhances contextual fear conditioning in rats. Neuroscience. 2008;153:935–943. doi: 10.1016/j.neuroscience.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramullas M, Martinez-Cue C, Hurle MA. Chronic administration of heroin to mice produces up-regulation of brain apoptosis-related proteins and impairs spatial learning and memory. Neuropharmacology. 2008;54:640–652. doi: 10.1016/j.neuropharm.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Tsai G, Yang P, Chung LC, Lange N, Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998;44:1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Udo T, Ugalde F, DiPietro N, Eichenbaum HB, Kantak KM. Effects of persistent cocaine self-administration on amygdala-dependent and dorsal striatum-dependent learning in rats. Psychopharmacology (Berl) 2004;174:237–245. doi: 10.1007/s00213-003-1734-1. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008;43:626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: effects of D-cycloserine. Acta Psychol (Amst) 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Vocci FJ. Cognitive remediation in the treatment of stimulant abuse disorders: a research agenda. Exp Clin Psychopharmacol. 2008;16:484–497. doi: 10.1037/a0014101. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Han TZ. Prenatal exposure to heroin in mice elicits memory deficits that can be attributed to neuronal apoptosis. Neuroscience. 2009;160:330–338. doi: 10.1016/j.neuroscience.2009.02.058. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, Mackowiak M, Rogowski A, Kostowski W, Bienkowski P. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:331–341. doi: 10.1007/s00210-003-0811-7. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30:2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Werner-Seidler A, Richardson R. Effects of D-cycloserine on extinction: consequences of prior exposure to imipramine. Biol Psychiatry. 2007;62:1195–1197. doi: 10.1016/j.biopsych.2007.04.010. [DOI] [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology. 1996;127:213–224. [PubMed] [Google Scholar]
- Wickens JR, Budd CS, Hyland BI, Arbuthnott GW. Striatal contributions to reward and decision making: making sense of regional variations in a reiterated processing matrix. Ann N Y Acad Sci. 2007;1104:192–212. doi: 10.1196/annals.1390.016. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behav Neurosci. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacol. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC, Fay J, Sage JR, Anagnostaras SG. Cocaine and Pavlovian fear conditioning: dose-effect analysis. Behav Brain Res. 2007;176:244–250. doi: 10.1016/j.bbr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Zushida K, Wada K, Sekiguchi M. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacol. 2009;34:2574–2584. doi: 10.1038/npp.2009.86. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. The neuropsychology of memory. Parallel findings in humans and nonhuman primates. Ann N Y Acad Sci. 1990;608:434–450. doi: 10.1111/j.1749-6632.1990.tb48905.x. [DOI] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]