Abstract
Introduction:
Each year, the US Antarctic Program rapidly transports scientists and support personnel from sea level (SL) to the South Pole (SP, 2835 m) providing a unique natural laboratory to quantify the incidence of acute mountain sickness (AMS), patterns of altitude related symptoms and the field effectiveness of acetazolamide in a highly controlled setting. We hypothesized that the combination of rapid ascent (3 hr), accentuated hypobarism (relative to altitude), cold, and immediate exertion would increase altitude illness risk.
Methods:
Medically screened adults (N = 246, age = 37 ± 11 yr, 30% female, BMI = 26 ± 4 kg/m2) were recruited. All underwent SL and SP physiological evaluation, completed Lake Louise symptom questionnaires (LLSQ, to define AMS), and answered additional symptom related questions (eg, exertional dyspnea, mental status, cough, edema and general health), during the 1st week at altitude. Acetazolamide, while not mandatory, was used by 40% of participants.
Results:
At SP, the barometric pressure resulted in physiological altitudes that approached 3400 m, while T °C averaged −42, humidity 0.03%. Arterial oxygen saturation averaged 89% ± 3%. Overall, 52% developed LLSQ defined AMS. The most common symptoms reported were exertional dyspnea-(87%), sleeping difficulty-(74%), headache-(66%), fatigue-(65%), and dizziness/lightheadedness-(46%). Symptom severity peaked on days 1–2, yet in >20% exertional dyspnea, fatigue and sleep problems persisted through day 7. AMS incidence was similar between those using acetazolamide and those abstaining (51 vs. 52%, P = 0.87). Those who used acetazolamide tended to be older, have less altitude experience, worse symptoms on previous exposures, and less SP experience.
Conclusion:
The incidence of AMS at SP tended to be higher than previously reports in other geographic locations at similar altitudes. Thus, the SP constitutes a more intense altitude exposure than might be expected considering physical altitude alone. Many symptoms persist, possibly due to extremely cold, arid conditions and the benefits of acetazolamide appeared negligible, though it may have prevented more severe symptoms in higher risk subjects.
Keywords: hypoxia, acute mountain sickness, rapid transport, Antarctica
Introduction
The physical altitude of the geographic South Pole (2835 m, 9300 ft) makes high altitude illness likely in polar workers, but the disease incidence and symptom patterns in this population are unknown. Polar workers undergo rapid air transport from McMurdo station (sea level) to the South Pole in 3–4 hours and often report directly to jobs that require physical exertion in extreme cold. Rapid exposure to high-altitude coupled with immediate physical exertion, low barometric pressure, and the extremely cold, arid climate at the South Pole produce an intensified altitude exposure risk for polar workers.1
In the context of acute exposure to hypobaric hypoxia, one or more well described high altitude syndromes may occur during the initial days at a new elevation. Acute mountain sickness (AMS) is a syndrome typically arising in the first 1–2 days at a new altitude and is diagnosed by the presence of a headache along with sleep disturbance, gastrointestinal symptoms, fatigue, and dizziness or lightheadedness. High altitude cerebral edema, referred to as HACE, is a syndrome often diagnosed in the presence of acute mountain sickness when mental status changes or ataxia emerge. High altitude pulmonary edema, also known as HAPE, usually occurs after 2–4 days at altitude and may present rapidly in individuals with no signs of AMS. Each of these syndromes presents a health risk to polar workers and may significantly impair judgment or disrupt work productivity.
To mitigate these and other health risks, all workers in the United States Antarctic Program are medically screened prior to deployment. This screening marks polar workers as a unique, generally healthy cross section of the population in which to observe the physical effects of hypobaric hypoxia.
Antarctic workers have been the subject of occupational health research, but the health effects encountered by workers at the South Pole have not been studied in detail.2,3 In 2001, John B. West speculated that workers at the Amundsen-Scott South Pole Station in Antarctica might experience altitude illnesses due to low barometric pressure, extreme cold, and rapid transport and that these effects would be worse during the Antarctic winter when barometric pressures linger around 508 torr.4
West’s comments came after decades of intensive research had revealed reliable preventive measures for the most commonly encountered high altitude syndromes.5 It is clear that hypoxia is the main physiologic precursor of altitude illnesses, yet the precise physiologic mechanisms for most high altitude illnesses remain a subject of investigation with an increased focus on genetic susceptibility.6,7 Field studies remain a favored approach for investigating the effects of hypobaric hypoxia but present unique technical challenges in subject selection, exposure measurement, and interpretation of results. Consequently, field studies have generally been small, recruited physically fit subjects, and have utilized variable rates of ascent, final elevations, and global latitudes. To overcome these challenges, increased understanding of the physiologic interrelationships producing high altitude illnesses will be advanced by larger, well-controlled field studies of the general population involving rapid transport to high altitude.8,9
We conducted the Antarctic Study of Altitude Physiology (ASAP), a large multi-year field study of United States Antarctic Program participants to quantify the incidence and patterns of high altitude illnesses in polar workers. The study was conducted during the 50th anniversary of the United States Antarctic Program and concluded during the International Polar Year (2007–2008) capitalizing on the construction of the new Amundsen-Scott Station dedicated on January 1, 2009. We hypothesized that the incidence of high altitude illness would be greater than that observed in other studies of rapid transport at similar elevations, and that there would be symptom pattern variability due to the low barometric pressures, cold working environments, and arid atmospheric conditions at the South Pole. We also suspected that the observed field effectiveness of acetazolamide in this population would differ from its demonstrated efficacy in placebo controlled clinical trials.
Methods
Two summer research expeditions to Antarctica were conducted during the 2006–07 and 2007–08 seasons with support from the National Science Foundation (NSF). The study was approved by the IRB at the Mayo Clinic in Rochester, Minnesota and all subjects gave written informed consent after reviewing the study protocol.
Study participants
Each October, United States Antarctic Program participants destined for summer work at the Amundsen-Scott South Pole Station were recruited. Most jobs at SP require physical activity and a significant portion of personnel work outdoors. Typical activities included construction, heavy equipment operation, transport of supplies, science support and fuel delivery. After passing an extensive medical screen at home, these individuals arrived at McMurdo Station from destinations around the United States. During both 2006 and 2007, South Pole workers waited nearly two weeks at McMurdo station prior to the opening of the South Pole station.
Upon arrival at McMurdo station, polar workers received a flight schedule update and a medical briefing regarding high altitude illnesses. Symptoms and risks were explained by medical staff and prophylactic acetazolamide packets were made available. The study was then explained to the group and individuals were invited to enroll. For subjects that chose to take acetazolamide; they were given a packet with 10–250 mg tablets and instructed to begin taking the medication 24–48 hr before departure, twice daily. If they had any side effects from the medication, they were further instructed to reduce the dose to 125 mg BID.
Baseline questionnaire and symptom reporting
Participants answered a baseline medical questionnaire and received physiological testing (heart rate, blood pressure, arterial oxygen saturation-SaO2, blood draw for hemoglobin and hematocrit) at sea level and after their second night at altitude. Lake Louise Symptom Score questionnaires were completed at baseline, during rapid ascent and for 7 days after arrival at the South Pole. Subjects also answered additional symptom questions (not part of the Lake Louise Scoring System) related to 1) shortness of breath at rest and on exertion, 2) general health limitation, 3) mental status changes, 4) cough and 5) peripheral edema.
The baseline medical questionnaire explored chronic medical conditions, current medications, previous altitude experience, lifestyle assessments (eg, smoking, alcohol use, exercise tendencies) and previous Antarctic experience. Job classification, activity levels, and medications were also coded for analysis.
Participants submitted nine separate symptom reports labeled Baseline, PLANE, Day 1, Day 2, and Day 3, through Day 7. These symptom reports included the standard Lake Louise Symptom Score format plus the additional symptom questions noted. For each symptom, subjects could report none = 0, mild = 1, moderate = 2, or severe = 3. The sheet labeled BASELINE was completed soon after arrival to McMurdo, while the PLANE was completed on the polar flight, and Day 1 was completed prior to sleep on the day of arrival. Subjects were instructed to complete Days 2–7 immediately upon rising from sleep each day. The case definition for AMS utilized the standard Lake Louise Symptom Scoring System which requires the presence of a headache and an overall symptom score of 3 or greater.10
Workers were included in the study if they had assignments longer than one week at the South Pole. During 2007 we only included subjects who had not participated in our study during 2006.
Statistical analysis
Data were described as mean ± standard deviation, or count (percent). Chi-square and two sample t-tests were used to compare participant characteristics and symptoms among those who took acetazolamide and those abstaining. All tests were two-sided, P < 0.05 was considered statistically significant. Paired t-tests were used for the change in physiology measurements from sea level to altitude. SAS v9.1 (SAS Institute Inc, Cary, NC) was used.
Results
Overall, 246 participants submitted symptom questionnaires and completed baseline medical evaluations. Since, some symptom reports were incomplete; results are reported based only upon complete survey results for each question.
Environmental conditions
Temperatures at the South Pole averaged −42 ± 1.9 °C (−43 °F) during 2006 and −43.3 ± 4.6 °C (−45 °F) during 2007 with an average relative humidity of 0.03% both years. Average barometric pressures during 2006 and 2007 were 512.7 ± 1.4 mmHg and 513 ± 4.6 mmHg respectively corresponding to equivalent altitudes of 3197.4 ± 21.4 meters (10,490 feet), and 3183.7 ± 70.1 meters (10,445 feet). The lowest average daily barometric pressure recordings were 509.8 mmHg (∼3,241.8 m, 10,636 feet) during 2006 and 501.8 mmHg (∼3,365.2 m, 11,040 feet) during 2007.
Baseline subject characteristics
Baseline subject characteristics (Table 1) revealed that this group was 70% male with a mean age of 36.8 ± 10.6 years. Mean BMI was 26.1 ± 4.2 kg/m2 (women: 25.1 ± 4.8, men: 26.6 ± 3.9). Mean body fat was 16.5% ± 6.2% for males and 26.3% ± 7.1% for females. While 89% of the group reported previous experience above 10,000 feet, the vast majority of this group (95%) had lived below 7,500 feet during the last three months. Of those with previous high altitude experience, 86% had their most recent exposure above 10,000 feet more than 4 weeks prior to arrival in Antarctica, 11% 2–4 weeks ago, and only 2% in the last 2 weeks. In this same group with prior altitude exposure, 39% reported no prior altitude related health problems while 53% reported mild problems and 8% reported moderate problems. About half of this group (46%) had previously traveled to the South Pole for work (Table 1).
Table 1.
Baseline subject characteristics.
| Whole group | No acetazolamide | Acetazolamide | P | |
|---|---|---|---|---|
| Age (years) | 36.8 ± 10.6 | 35.7 ± 9.7 | 39.1 ± 11.5 | 0.019 |
| Gender, M (%) | 168 (70) | 94 (69) | 63 (69) | 0.92 |
| BMI (kg/m2) | 26.1 ± 4.2 | 25.6 ± 3.7 | 26.7 ± 4.7 | 0.07 |
| Body fat (%) | ||||
| Males | 16.5 ± 6.2 | 15.5 ± 6.0 | 17.9 ± 6.1 | 0.015 |
| Females | 26.3 ± 7.1 | 25.7 ± 7.3 | 27.5 ± 6.8 | 0.32 |
| Elevation of residence (last 3 months), no. (%) | 0.13 | |||
| <5,000 ft above sea level | 152 (64) | 78 (58) | 66 (73) | |
| 5,000–7,500 ft above sea level | 74 (31) | 49 (36) | 21 (23) | |
| 7,500–10,000 ft above sea level | 11 (5) | 7 (5) | 4 (4) | |
| >10,000 ft above sea level | 1 (0) | 1 (1) | 0 (0) | |
| Previous altitude exposure (≥10,000 ft), no. (%) | 213 (89) | 128 (94) | 75 (82) | 0.005 |
| Last time at an altitude ≥10,000 ft, no. (%) | ||||
| <2 weeks ago | 5 (2) | 4 (3) | 1 (1) | 0.027 |
| 2 to 4 weeks ago | 24 (11) | 20 (16) | 3 (4) | |
| >4 weeks ago | 184 (86) | 104 (81) | 71 (95) | |
| Previous altitude illness (among those with previous exposure), no. (%) | 0.006 | |||
| No problem | 84 (39) | 56 (44) | 23 (31) | |
| Mild problem | 112 (53) | 67 (52) | 40 (53) | |
| Moderate problem | 17 (8) | 5 (4) | 12 (16) | |
| Previously at south pole before, no. (%) | 110 (46) | 75 (56) | 32 (35) | 0.003 |
General physiology
Physiology test results at McMurdo (sea level) and the South Pole revealed increased resting heart rate (71.5 ± 12.3 bpm vs. 82.8 ± 13.1 bpm, P < 0.001) decreased oxygen saturation (97.3% ± 1.3% vs. 89.1% ± 3.2%, P < 0.001) and decreased average systolic blood pressure (111.3 ± 12.5 mmHg vs. 105.8 ± 14.8 mmHg, P < 0.001). Hematocrit and hemoglobin increased slightly with a small estimated reduction in blood and plasma volumes consistent with dehydration.11
General symptom patterns and timing
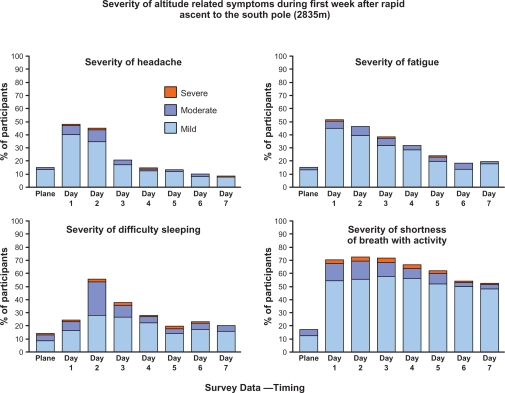
For each of the eleven symptoms recorded, the number of participants reporting mild, moderate or severe values are reported in Table 2. Shortness of breath with activity was the most commonly reported symptom (87%), followed by sleeping difficulty (74%), headache (66%), fatigue (65%), dizziness/lightheadedness (46%) and general health limitation (41%). Trends were analyzed for overall symptom reporting (Fig. 1, Lake Louise Symptoms plus additional questions) which were consistent with the Lake Louise Symptom Score (LLSS) peak on Day 2 (LLSS = 2.5 ± 2.1). The trends for the four most prominent symptoms reveal persistent reporting of shortness of breath with activity and generalized fatigue and to a lesser extent difficulty sleeping and headache (Fig. 2).
Table 2.
Maximum symptom score.
| Symptom |
Whole group symptomatic |
No acetazolamide symptomatic |
Acetazolamide symptomatic |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Shortness of breath with activity | 213 | 87 | 125 | 89 | 84 | 91 | 0.61 |
| Sleeping difficulty | 182 | 74 | 109 | 78 | 69 | 75 | 0.61 |
| Headache | 162 | 66 | 99 | 71 | 61 | 66 | 0.48 |
| Fatigue | 160 | 65 | 94 | 67 | 64 | 70 | 0.70 |
| Dizzy/lightheaded | 113 | 46 | 68 | 49 | 43 | 47 | 0.78 |
| General health limitation | 99 | 41 | 53 | 38 | 44 | 48 | 0.13 |
| Mental status changes | 90 | 37 | 48 | 34 | 41 | 45 | 0.12 |
| Shortness of breath at rest | 77 | 31 | 43 | 31 | 32 | 35 | 0.52 |
| Cough | 74 | 30 | 34 | 24 | 37 | 41 | 0.008 |
| GI upset | 59 | 24 | 26 | 19 | 31 | 34 | 0.009 |
| Peripheral edema | 23 | 9 | 17 | 12 | 6 | 7 | 0.16 |
| AMS by Lake Louise criteria | 116 | 52 | 70 | 52 | 46 | 51 | 0.87 |
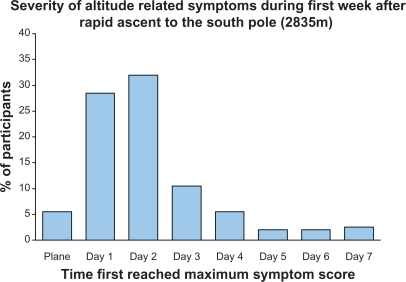
Figure 1.
Percentage of participants that reached their maximum symptoms score for a given day at South Pole. Most participants reached their maximum symptom score on the first 2 days at altitude, however, >20% of subjects, maximum symptoms were reached in days 3–7.
Figure 2.
Severity of most commonly reported symptoms over the first week of exposure in personnel rapidly transported to the South Pole (2835 m, 9300 ft).
Acute mountain sickness
Using strict Lake Louise Criteria, 52% of subjects satisfied acute mountain sickness criteria at some point during their visit to altitude. On Day 1, 24% met acute mountain sickness criteria, and 33% met criteria on Day 2, with steadily decreasing values on Day 3 (15%), Day 4 (9%), Day 5 (6%), Day 6 (4%), and Day 7 (4%).
Acetazolamide
Overall, 40% of subjects reported using Acetazolamide during the study. Of those reporting Acetazolamide usage, 51% scored positive for acute mountain sickness versus 52% of those not using Acetazolamide. Gender distribution was similar for both groups (69% male). In general, those who used acetazolamide tended to be older (39.1 ± 11.5 years vs. 35.7 ± 9.7 years, P = 0.019), report less previous experience at altitudes >10,000 ft. (82% vs. 94%, P = 0.005), less South Pole experience (35% vs. 56%, P = 0.003) and a greater amount of previous mild or moderate problems with altitude P = 0.006, Table 1).
Discussion
The Antarctic Study of Altitude Physiology examined a large group of working individuals rapidly transported the South Pole. The value of this study resides in the unique environment, the large, medically screened working population, and the uniform rapid ascent of participants to high altitude.
Incidence of acute mountain sickness
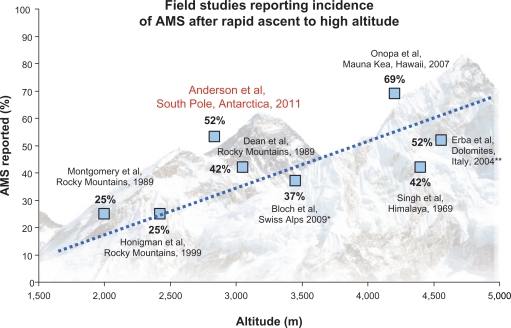
Overall, 52% of individuals satisfied Lake Louise criteria for acute mountain sickness, which represents an incidence of acute mountain sickness higher than rapid ascent studies conducted at similar elevations. For example, Honigman’s convenience sampling of 3158 tourists engaging rapid transport to elevations between 6700–9200 feet found that 25% of subjects reported symptoms consistent with a diagnosis of acute mountain sickness.9 The same results were obtained when Montgomery et al studied 454 men and women at a ski conference at 6,560 ft.12 In 1990, Dean et al observed incidence of acute mountain sickness of 42% in 105 conference attendees rapidly ascending to 10,000 feet.13 The rapid transport experienced by polar workers clearly produces incidence of acute mountain sickness higher than observations from these other studies of individuals rapidly transported to similar altitudes elsewhere on the planet (Fig. 3).14
Figure 3.
Acute mountain sickness (AMS) incidence in the present study (Anderson 2011) relative to previous investigations examining the incidence of AMS.9,12–17
Notes: *Bloch’s study included children and adolescents,**Erba’s study required mountaineering. All others were passive transport.
The incidence of altitude illness during the Antarctic Study of Altitude Physiology more closely resembles studies conducted at higher elevations such as the summit of Mauna Kea (4020 m/13,796 ft).15 Onopa et al conducted a survey of 198 day visitors and 46 working astronomers rapidly transported to the summit of Mauna Kea observing that 69% of astronomers and 30% of day visitors reported acute mountain sickness symptoms during their sojourn. Similarly, Erba et al found that 52% of mountaineers reported acute mountain sickness after a 4–5 hour ascent to Capanna Regina Margherita (4559 m/14,957 ft) in the Italian Dolomites.16 Previous studies by Singh indicate that 47% of those rapidly air transported to elevations between 3000–5200 m in the Himalayas will be diagnosed with acute mountain sickness.17 Van Patot et al diagnosed acute mountain sickness in 45% of subjects taking placebo after rapid transport to 4300 m (14,107 ft).18 These data provide quantitative support for the conclusion that the unique features of the Antarctic physical environment (hypobarism, extreme cold, and low humidity coupled with physical exertion) contribute to an intensified high altitude exposure for polar workers when compared with similar elevations at different geographic locations.
Time course of symptom development
Symptom reports at the South Pole were mild to moderate in severity with symptom prevalence peaking on the day after arrival at high altitude. The Day 2 questionnaire in our study corresponded to approximately 12–18 hours after arrival for most participants reflecting conventional knowledge that symptoms do not appear until 6–48 hours after arrival in most people and resolve within the first 2–3 days.19 Figure 1 shows the peak of symptoms on Day 2 with declining values thereafter.
Rank order of symptoms
Rank order of symptoms during this study follow other reports with exertional dyspnea, sleep disruption, headache, and fatigue as the most common symptoms. Headache predominates many rank lists from higher elevations,20–22 while exertional dyspnea, insomnia, and fatigue predominate rank lists at lower elevations.23
Exertional dyspnea was persistently reported throughout our 7 day questionnaire series, while most other symptoms followed a steady pattern of decline. Exertional dyspnea at altitude most likely represents a consequence of exercise in conditions of reduced partial pressure of oxygen rather than a maladaptive symptom. Since many South Pole workers regularly perform heavy physical work, the persistent reporting of this symptom may represent their daily experience of breathing difficulty as they executed tasks in the cold and arid environment of the polar plateau.
Acetazolamide
Acetazolamide did not appear to reduce the incidence of acute mountain sickness during this study which may indicate difficulties with prophylaxis along with self selection of individuals more prone to altitude symptoms. Prescription guidelines for acetazolamide prophylaxis presuppose administration of the medication 24–48 hours prior to a predicted exposure to altitude.24 The uncertainty of flight schedules in Antarctica forces many individuals to begin the medication on the same day as exposure or after arrival, possibly reducing the effectiveness of the treatment. In addition, data suggest that those who used acetazolamide were older, had less experience at the South Pole, had experienced more moderate illness at altitude on previous visits, and had less experience at the South Pole (Table 1). This self selection process may have identified individuals who felt they had a higher risk of illness based on previous experience. If this is true, the use of acetazolamide in this group may have reduced their incidence of illness to levels experienced by those who declined the use of medication.25
Relationship of symptoms to physiological measures
A number of physiological measures have been thought to predict the onset of AMS. Examples include a high resting heart rate (HR), low resting and post-exercise arterial oxygen saturation (SaO2), elevated BMI, and altered blood pressure (BP).26–28 We assessed the relationship of resting HR, SaO2, BMI and BP at SP with day 3 LLSS. Only HR was significantly, albeit mildly associated with LLSS (R2 = 0.035), indicating only 3.5% of the score could be explained by HR (P = 0.01). Based on the parameter estimate, a +0.24 increase in LLSS would be associated with a +10 bpm change in HR. Oxygen saturation did not differ among those with LLSS ≤2 vs. >2 (P = 0.82), while there was a tendency for a lower BP to be associated with increased LLSS (P = 0.10).
Polar environment
Physiological data from this study indicate that the altitude exposure to workers at the South Pole is more intense than would be expected at the same physical elevation at more moderate latitudes. Immediate heavy exertion,29 extremely low humidity, and cold air30 are all suspected to increase the risk of acute mountain sickness and HAPE, though the exact mechanisms remain unclear. During the 2006 season, 7 workers were evacuated from the South Pole for symptoms consistent with HAPE. The following season, more aggressive intervention with medications and oxygen therapy for the most symptomatic individuals at SP was pursued.
Limitations
While this study was carefully designed to quantify symptoms in a large, healthy, non-mountaineering cohort, symptom reporting was subjective and may reflect environmental factors that are difficult to control. For example, jet lag and Antarctica’s perpetual daylight may have increased the tendency to report poor sleep quality at altitude. However, two weeks of circadian adjustment at McMurdo Station probably minimized the effect of jet-lag from air travel. At the South Pole station, darkened sleeping quarters also reduced the effect of light induced circadian disruption. In a similar way uncontrollable environmental factors may have exerted minor influence on other subjective symptom reports (e.g. headache, shortness of breath).
Conclusion
This study at the Amundsen Scott South Pole station reveals that over half of polar workers experience modest altitude related symptoms for at least 2 days after arrival with 20% of individuals having persistent symptoms beyond day 3. Shortness of breath with activity, sleep difficulties, headache, and fatigue were the most prominent symptoms reported, with shortness of breath and fatigue being particularly common over the course of the initial week. Life threatening symptoms occurred in approximately 2% of subjects studied at SP. The benefits of acetazolamide appeared negligible, though it may have prevented more severe symptoms in higher risk subjects. As predicted by John West in 2001, the SP constitutes a more intense altitude challenge than would be expected based on the physical altitude. This suggests that equivalent air altitude (EAA) alone is an inadequate predictor of hypoxic stress.
Acknowledgments
We would like to thank the hard working employees of the US Antarctic Program who each year make their way to the South Pole to support the many scientific research agendas in Antarctica. This includes general science support, station management and cargo crew at South Pole, Crary Lab, medical team and transportation/shipping at both McMurdo and South Pole. We would also like to thank Jay O’Brien for his help and support in this project in Antarctica as well as Kent Bailey for statistical support. This study was supported by a grant from the National Science Foundation, B-179-M. J Mueller was supported by funding through the Mayo Clinic Center for Translational Science Activity (CTSA), Clinical Research Unit, Grant Number 1 UL1 RR024150 from the National Center for Research Resources.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Gallagher SA, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22:329–55. viii. doi: 10.1016/j.emc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya M, Pal MS, Sharma YK, Majumdar D. Changes in sleep patterns during prolonged stays in antarctica. Int J Biometeorol. 2008;52:869–79. doi: 10.1007/s00484-008-0183-2. [DOI] [PubMed] [Google Scholar]
- 3.Francis G, Bishop L, Luke C, Middleton B, Williams P, Arendt J. Sleep during the antarctic winter: Preliminary observations on changing the spectral composition of artificial light. J Sleep Res. 2008;17:354–60. doi: 10.1111/j.1365-2869.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 4.West JB. Acute mountain sickness at the south pole? High Alt Med Biol. 2001;2:559. doi: 10.1089/152702901753397153. [DOI] [PubMed] [Google Scholar]
- 5.West JB. High life: A history of high-altitude physiology and medicine. New York: Oxford University Press; 1998. [Google Scholar]
- 6.Koehle MS, Wang P, Guenette JA, Rupert JL. No association between variants in the ace and angiotensin ii receptor 1 genes and acute mountain sickness in nepalese pilgrims to the janai purnima festival at 4380 m. High Alt Med Biol. 2006;7:281–9. doi: 10.1089/ham.2006.7.281. [DOI] [PubMed] [Google Scholar]
- 7.Thompson J, Raitt J, Hutchings L, et al. Angiotensin-converting enzyme genotype and successful ascent to extreme high altitude. High Alt Med Biol. 2007;8:278–85. doi: 10.1089/ham.2007.1044. [DOI] [PubMed] [Google Scholar]
- 8.Grocott M, Richardson A, Montgomery H, Mythen M. Caudwell xtreme everest: A field study of human adaptation to hypoxia. Crit Care. 2007;11:151. doi: 10.1186/cc5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honigman B, Theis MK, Koziol-McLain J, et al. Acute mountain sickness in a general tourist population at moderate altitudes. Ann Intern Med. 1993;118:587–92. doi: 10.7326/0003-4819-118-8-199304150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Sutton JRCG, Houston CS, editors. Hypoxia: Mountain medicine. Burlington, Vermont: Queen City Press; 1992. Lake louise consensus on definition and quantification of altitude illness; pp. 327–30. [Google Scholar]
- 11.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. Journal of applied physiology. 1974;37:247–8. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery AB, Mills J, Luce JM. Incidence of acute mountain sickness at intermediate altitude. JAMA. 1989;261:732–4. [PubMed] [Google Scholar]
- 13.Dean A, Yip R, Hoffmann R. High incidence of mild acute mountain sickness in conference attendees at 10000 foot altitude. Journal of Wilderness Medicine. 1990;1:86–92. [Google Scholar]
- 14.Bloch J, Duplain H, Rimoldi SF, et al. Prevalence and time course of acute mountain sickness in older children and adolescents after rapid ascent to 3450 meters. Pediatrics. 2009;123:1–5. doi: 10.1542/peds.2008-0200. [DOI] [PubMed] [Google Scholar]
- 15.Onopa J, Haley A, Yeow ME. Survey of acute mountain sickness on mauna kea. High Alt Med Biol. 2007;8:200–5. doi: 10.1089/ham.2007.8307. [DOI] [PubMed] [Google Scholar]
- 16.Erba P, Anastasi S, Senn O, Maggiorirni M, Bloch KE. Acute mountain sickness is related to nocturnal hypoxemia but not to hypoventilation. Eur Respir J. 2004;24:303–8. doi: 10.1183/09031936.04.00006504. [DOI] [PubMed] [Google Scholar]
- 17.Singh I, Khanna PK, Srivastava MC, Lal M, Roy SB, Subramanyam CS. Acute mountain sickness. The New England Journal of Medicine. 1969;280:175–84. doi: 10.1056/NEJM196901232800402. [DOI] [PubMed] [Google Scholar]
- 18.van Patot MC, Leadbetter G, 3rd, Keyes LE, Maakestad KM, Olson S, Hackett PH. Prophylactic low-dose acetazolamide reduces the incidence and severity of acute mountain sickness. High Alt Med Biol. 2008;9:289–93. doi: 10.1089/ham.2008.1029. [DOI] [PubMed] [Google Scholar]
- 19.Hultgren H. High Altitude Medicine. Hultgren Publications; 1997. Acute and subacute mountain sickness; p. 216. [Google Scholar]
- 20.Billings C, Brashear R, Bason R, Mathews D. Medical observations during prolonged residence at 3800 m.252
- 21.Hackett P, Rennie D. Seminars in Respiratory Medicine. New York: Theime-Stratton; 1983. Acute mountain sickness; pp. 132–40. [Google Scholar]
- 22.Hall WH, Barila TG, Metzger EC, Gupta KK. A clinical study of acute mountain sickness. Arch Environ Health. 1965;10:747–53. doi: 10.1080/00039896.1965.10664085. [DOI] [PubMed] [Google Scholar]
- 23.Hansen JE, Harris CW, Evans WO. Influence of elevation of origin, rate of ascent and a physical conditioning program on symptoms of acute mountain sickness. Mil Med. 1967;132:585–92. [PubMed] [Google Scholar]
- 24.Dickinson JG. Acetazolamide in acute mountain sickness. British medical journal (Clinical Research ed.) 1987;295:1161–2. doi: 10.1136/bmj.295.6607.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsten C, Swenson ER, Ruoss S. A dose-response study of acetazolamide for acute mountain sickness prophylaxis in vacationing tourists at 12,000 feet (3630 m) High Altitude Medicine and Biology. 2004;5:33–9. doi: 10.1089/152702904322963672. [DOI] [PubMed] [Google Scholar]
- 26.Karinen HM, Peltonen JE, Kahonen M, Tikkanen HO. Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent. High Alt Med Biol. 2010;11:325–32. doi: 10.1089/ham.2009.1060. [DOI] [PubMed] [Google Scholar]
- 27.Wu TY, Ding SQ, Liu JL, et al. Reduced incidence and severity of acute mountain sickness in qinghai-tibet railroad construction workers after repeated 7-month exposures despite 5-month low altitude periods. High Alt Med Biol. 2009;10:221–32. doi: 10.1089/ham.2009.1012. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland A, Morris D, Owen C, Bron A, Roach R. Optic nerve sheath diameter, intracranial pressure and acute mountain sickness on mount everest: A longitudinal cohort study. Br J Sports Med. 2008;42:183–8. doi: 10.1136/bjsm.2007.045286. [DOI] [PubMed] [Google Scholar]
- 29.Roach RC, Maes D, Sandoval D, et al. Exercise exacerbates acute mountain sickness at simulated high altitude. J Appl Physiol. 2000;88:581–5. doi: 10.1152/jappl.2000.88.2.581. [DOI] [PubMed] [Google Scholar]
- 30.Reeves J, Wagner J, Zafren K, et al. Seasonal variatoin in barometric pressure and temperature in summit county: Effect on altitude medicine. In: Sutton JRCG, Houston CS, editors. Hypoxia and Molecular Medicine. Burlington, Vermont: Queen City Press; 1993. [Google Scholar]