Abstract
OBJECTIVE
The best method to estimate glomerular filtration rate (GFR) in diabetic patients is still largely debated. We compared the performance of creatinine-based formulas in a European diabetic population.
RESEARCH DESIGN AND METHODS
We compared the performance of Cockcroft and Gault, simplified Modification of Diet in Renal Disease (MDRD), and Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equations in 246 diabetic patients by calculating the mean bias and the interquartile range (IQR) of the bias, 10% (P10) and 30% (P30) accuracies, and Bland-Altman plots. GFR was measured by inulin clearance.
RESULTS
For the whole population, the IQR was slightly lower for CKD-EPI, but the mean bias was lower and P10 and P30 were higher for MDRD. Similar results were observed in specific subgroups, including patients with mild renal insufficiency, obese patients, or type 2 diabetic patients.
CONCLUSIONS
In our population, the CKD-EPI formula does not exhibit better performance than the simplified MDRD formula for estimating GFR.
Using a creatinine-based formula is the most common way to evaluate the glomerular filtration rate (GFR) in clinical practice. However, it can lead to an inaccurate evaluation, especially in patients with normal renal function (1). A new GFR formula, the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equation, has recently been developed and has exhibited better performance than the other creatinine-based formulas in the general population (2). Therefore, we compared the performance of the CKD-EPI equation to Cockcroft and Gault (CG) and simplified Modification of Diet in Renal Disease (MDRD) equations in a population of diabetic patients.
RESEARCH DESIGN AND METHODS
The study included 246 nondialyzed diabetic adult patients (59% men, 95.1% white, 85.8% type 2 diabetic patients). Mean age was 62.5 ± 13.0 years, and mean BMI was 28.8 ± 5.0 kg/m2 (39% of patients had a BMI >30 kg/m2). Mean plasma creatinine (PCr) was 137 ± 69 μmol/L, and 60.6% of the patients had measured GFR (mGFR) <60 mL/min/1.73 m2.
GFR was measured by inulin clearance (Inutest, Fresenius Kabi, Graz, Austria) using a continuous infusion of inulin after a loading dose and urine collections. The clearance value was calculated by the standard (urinary inulin × urine flow)/plasma inulin (UV/P) formula, and was normalized to 1.73 m2 of body surface area (BSA), calculated according to the Du Bois formula (3). PCr was assayed with a kinetic colorimetric-compensated Jaffe technique (Roche Modular, Meylan, France).
The following equations were used to determine estimated GFR (eGFR):
CG = 1.73/BSA × [(140 − age {years}) × body weight (kg) × k/PCr (μmol/L)] (4).
MDRD = 186.3 × [(PCr in μmol/L)/88.5)]−1.154 × age in years−0.203 × 0.742 (if female) × 1.21 (if black) (5,6).
CKD-EPI = k1 × [(PCr/88.5)/k2)]−k3 × 0.993age, with
–k1 = 141, 143, 163, 166 for white male and female and black male and female, respectively;
–k2 = 0.7 or 0.9 for female and male, respectively;
–k3 = 1.209, 0.411, 0.329 for male with PCr >80 μmol/L, female with PCr >62 μmol/L, male with PCr ≤80 μmol/L, and female with PCr ≤62 μmol/L, respectively (2).
To assess the performance of formulas, the correlation coefficient (R2), the mean absolute bias (eGFR − mGFR), the interquartile range of the bias (IQR), and 10% (P10) and 30% (P30) accuracies were calculated. Bland-Altman plots were used to show the agreement between mGFR and eGFR (7). P values < 0.05 were considered significant.
RESULTS
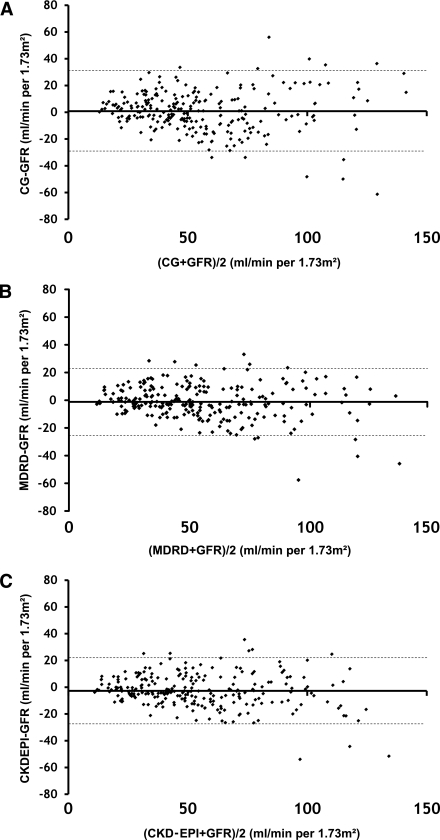
For the whole population of diabetic patients, mean mGFR was 55.4 ± 29 mL/min/1.73 m2. Correlation between mGFR and eGFR was significant for the three formulas, with R2 values of 0.728, 0.818, and 0.814 for CG, MDRD, and CKD-EPI, respectively. Mean absolute bias was 0.8 ± 15, −1.2 ± 12, and −12.7 ± 12 mL/min/1.73 m2, and IQR was 16.4, 15.8, and 16 mL/min/1.73 m2 for CG, MDRD, and CKD-EPI, respectively. Figure 1 shows the graphic representation of agreement for each formula according to the Bland-Altman method. P10 and P30 were, respectively, 25.6 and 72.8% for CG, 37.4 and 82.1% for MDRD, and 28 and 80.1% CKD-EPI.
Figure 1.
Bland-Altman graph shows the agreement between GFR measured by inulin clearance and GFR estimated by normalized CG (A), simplified MDRD (B), and CKD-EPI (C) equations. The solid line shows the mean value and the dotted line shows the range of 95% of the values of the bias.
The mean mGFR was 61.2 ± 31 and 54.4 ± 28 mL/min/1.73 m2 in type 1 and 2 diabetic patients, respectively. In both groups of patients, MDRD exhibited the highest P10 (31.4 and 38.4%) and P30 (85.7 and 81.5%), respectively, compared with CG and CKD-EPI.
The mean mGFR was 36.4 ± 13 and 84.6 ± 21 mL/min/1.73 m2 in patients with GFR <60 and >60 mL/min/1.73 m2, respectively. MDRD exhibited the highest P10 (36.2 and 39.2%) and P30 (75.3 and 91.7%) in both groups compared with CG and CKD-EPI.
Finally, MDRD exhibited the highest accuracy in nonobese (BMI <30 kg/m2, mGFR = 59.8 ± 29) and obese patients (mGFR = 48.5 ± 26), with P10 at 35.3 and 40.6% and P30 at 81.3 and 83.3%, respectively.
CONCLUSIONS
Our data showed that the CKD-EPI equation exhibited similar (or worse) performance than the simplified MDRD equation in our population of diabetic patients, as well as in specific subgroups according to the type of diabetes, GFR, or presence or not of obesity. We confirm that the CG formula is less accurate than the MDRD equation and should not be used to evaluate GFR in diabetic patients (8,9). Several authors have demonstrated better performance of CKD-EPI compared with MDRD in the general population and in diabetic patients (2,10). We are unable to confirm those results in our population of European diabetic patients. This discrepancy could be attributed to differences between American and European diabetic patients, including a greater proportion of black patients, a smaller proportion of type 1 diabetic patients, and higher BMIs in North America (11,12).
The use of a nonenzymatic assay of PCr and, therefore, the non–re-expressed MDRD formula, comparatively with the CKD-EPI study, could be another factor to explain the difference (2,13). However, values obtained with our compensated Jaffe method were very similar to those of an enzymatic method (14). In conclusion, our data suggest that the non–re-expressed simplified MDRD formula can be used in European diabetic patients to evaluate GFR because the CKD-EPI equation does not seem to exhibit better performance and is less convenient to use in clinical practice. However, these results should be confirmed in larger studies.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
N.R. contributed to every aspect of this article. S.L. contributed to data research, discussion, and review. M.L. contributed to discussion and review. A.H.-A. contributed to data research, discussion, and review. L.D. contributed to every aspect of this article.
Parts of this work were presented in abstract form as posted communication at the 2010 annual meeting of La Société de Néphrologie, Brussels, Belgium, 28 September–1 October 2010.
The authors thank Miss Lynn Richardson, a freelance copy editor in English translation, for help in English language editing.
References
- 1.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473–2483 [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–311; discussion: 312–303 [PubMed] [Google Scholar]
- 4.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine (Abstract). J Am Soc Nephrol 2000;11:155A [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 7.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310 [PubMed] [Google Scholar]
- 8.Rigalleau V, Lasseur C, Perlemoine C, et al. Cockcroft-Gault formula is biased by body weight in diabetic patients with renal impairment. Metabolism 2006;55:108–112 [DOI] [PubMed] [Google Scholar]
- 9.Rigalleau V, Lasseur C, Perlemoine C, et al. Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or Modification of Diet in Renal Disease study equation? Diabetes Care 2005;28:838–843 [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 2010;56:486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KDOQI KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007;49(2 Suppl. 2):S12–S154 [DOI] [PubMed] [Google Scholar]
- 12.Whaley-Connell AT, Sowers JR, McFarlane SI, et al. ; Kidney Early Evaluation Program Investigators Diabetes mellitus in CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition and Examination Survey (NHANES) 1999-2004. Am J Kidney Dis 2008;51(Suppl. 2):S21–S29 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–772 [DOI] [PubMed] [Google Scholar]
- 14.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol 2011;6:552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]