Abstract
The neurohypophyseal peptide [Arg8]-vasopressin (AVP) exerts major physiological actions through three distinct receptor isoforms designated V1a, V1b, and V2. Among these three subtypes, the vasopressin V1b receptor is specifically expressed in pituitary corticotrophs and mediates the stimulatory effect of vasopressin on ACTH release. To investigate the functional roles of V1b receptor subtypes in vivo, gene targeting was used to create a mouse model lacking the V1b receptor gene (V1bR–/–). Under resting conditions, circulating concentrations of ACTH and corticosterone were lower in V1bR–/– mice compared with WT mice (V1bR+/+). The normal increase in circulating ACTH levels in response to exogenous administration of AVP was impaired in V1bR–/– mice, while corticotropin-releasing hormone–stimulated ACTH release in the V1bR–/– mice was not significantly different from that in the V1bR+/+ mice. AVP-induced ACTH release from primary cultured pituitary cells in V1bR–/– mice was also blunted. Furthermore, the increase in ACTH after a forced swim stress was significantly suppressed in V1bR–/– mice. Our results clearly demonstrate that the V1b receptor plays a crucial role in regulating hypothalamic-pituitary-adrenal axis activity. It does this by maintaining ACTH and corticosterone levels, not only under stress but also under basal conditions.
Introduction
The neurohypophyseal peptide [Arg8]-vasopressin (AVP) is involved in diverse functions, including the contraction of smooth muscle, stimulation of liver glycogenolysis, modulation of corticotropin release from the pituitary, and inhibition of diuresis (1). These physiological effects are mediated through the binding of AVP to specific membrane receptors of the target cells. AVP receptors are G protein–coupled and have been divided into at least three types: V1a, V1b, and V2. The V1a (vascular/hepatic) and V1b (anterior pituitary) receptors act through phosphatidylinositol hydrolysis to mobilize intracellular Ca2+ (2). The V1a receptor mediates physiological effects such as cell contraction and proliferation, platelet aggregation, coagulation factor release, and glycogenolysis. The V1b receptor exists in the anterior pituitary, where it stimulates corticotropin release. The V2 receptors are found primarily in the kidney. They are linked to adenylate cyclase and the production of cAMP, and are associated with antidiuresis (3). All of these receptors have been cloned (4–6) and belong to the family of “seven membrane–spanning” receptors, which signal through G proteins (7).
AVP is synthesized primarily in the magnocellular neurons of the hypothalamic paraventricular nuclei and in the supraoptic nuclei that project to the posterior pituitary. In addition, parvocellular neurons of the paraventricular nuclei coexpressing AVP and corticotropin-releasing hormone (CRH) coordinate hypothalamic-pituitary-adrenal (HPA) system activity and project to the external layer of the median eminence, where AVP and CRH are released into the portal blood (8). Numerous investigations have shown that AVP synergizes potently with CRH to stimulate pituitary ACTH release both in vitro and in vivo (8). A recent study using mice lacking the type 1 CRH receptor gene (Crhr1–/– mice) further provided indirect evidence that this vasopressinergic system can work as a compensatory mechanism to maintain HPA activity when CRH/CRHR1 signaling is impaired (9, 10). Thus, AVP appears to regulate HPA axis activity in modulating the effect of CRH; however, its role is not fully understood.
Highly selective peptide and nonpeptide vasopressin receptor antagonists have been developed (11). Nonpeptide “V1a receptor–selective” antagonists such as OPC-21268 have been developed for potential therapeutic use in treating hypertension and congestive heart failure (12). Also, a “V1b receptor–selective” antagonist, SSR149415, has been recently developed, and pharmacological studies showed that it inhibits the exogenous AVP-induced increase in circulating ACTH and possesses anxiolytic- and antidepressant-like effects (13, 14). More recently, mice lacking the V1b receptor have been produced, and an initial behavioral characterization showed that these mutant mice displayed reduced aggressive behavior; however, the effect of the V1b receptor deficiency on HPA activity has not yet been fully assessed (15). In order to better understand the physiological roles of AVP/V1b receptor signaling in regulation of the HPA axis, we have tried to characterize HPA axis activity in mice lacking the V1b receptor by using receptor-selective pharmacological agents.
Methods
Targeting of the mouse V1b receptor gene.
The mouse V1b receptor gene consists of two exons and one intron, and spans 10 kb (16). DNA fragments of 2.5 kb and 6.0 kb were subcloned from the mouse V1b receptor genomic clone (Figure 1) into pBluescript (Stratagene, La Jolla, California, USA). These two fragments were inserted into a plasmid with a 1.6-kb cassette containing the neomycin resistance gene (Neo) under the control of the phosphoglycerate kinase promoter, as described (17). As a result, the 3.5-kb region including the first exon and part of the first intron of the V1b receptor gene was replaced with the Neo cassette. The diphtheria toxin A fragment gene was used as a negative selection marker (18). The 1.8-kb diphtheria toxin (DT) cassette was inserted into the plasmid to obtain the targeting vector (Figure 1). After its linearization, the targeting vector contained two regions having homology with the V1b receptor gene: a 2.5-kb fragment of the 5′ untranslated sequences and a 6.0-kb fragment containing part of the first intron. The linearized targeting vector was inserted by electroporation into 129Sv ES cells, which were then subjected to selection with G418. Southern blot analysis was performed on 288 neomycin-resistant ES clones. Genomic DNA was digested with EcoRV, electrophoresed on a 0.8% agarose gel, transferred to a membrane, and hybridized with a 5′ probe that was derived from the V1b receptor locus (Figure 1). Digestion of genomic DNA with EcoRV generated 14.0-kb and 2.8-kb restriction fragments for the WT and disrupted alleles, respectively. Five clones positive for the 5′ probe were expanded and subjected to further Southern blot analysis with 3′ and Neo probes, revealing that two of these clones were positive for the correct targeting event. The two positive ES clones were independently microinjected into C57BL/6J mouse blastocysts, which were then transferred into pseudopregnant NMRI females. This generated 12 chimeric mice, as indicated by their coat color. Male chimeras were then mated to C57BL/6J mice, and evidence of germ-line transmission was monitored by agouti coat color contributed from the 129Sv-derived ES cell genome.
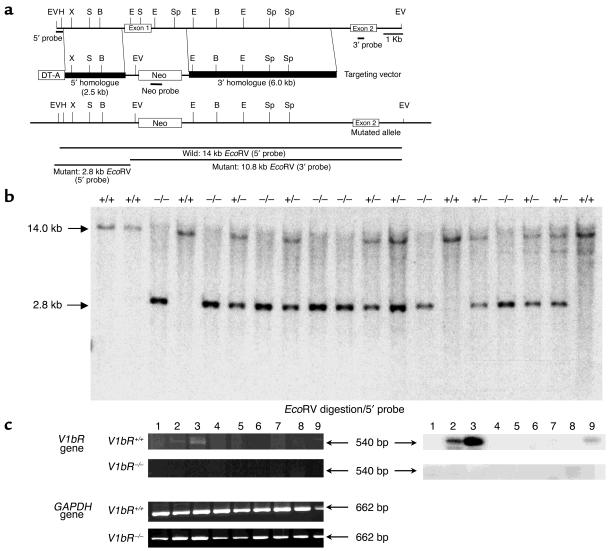
Figure 1.
Generation of V1b receptor–deficient mice. (a) Simplified restriction map of the V1b receptor gene and structure of the targeting vector. The coding region of the exon is boxed. Neo, neo cassette; DT-A, diphtheria toxin A fragment gene; B, BamHI; E, EcoRI; EV, EcoRV; H, HindIII; S, SacI; Sp, SpeI; X, XhoI. (b) Southern blot analysis of tail DNA. DNA was digested with EcoRV and the blot was hybridized with the 5′ probe shown in a. The 14.0-kb band is derived from the WT allele (Wild) and the 2.8-kb band from the targeted allele (Mutant). (c) RT-PCR analysis of the RNA from tissues of V1bR+/+ and V1bR –/– mice. Ethidium bromide stainings of RT-PCR fragments are shown at left. The V1b receptor mRNA transcripts were detected and are shown as 540-bp fragments. The control for RT-PCR analysis was provided by detection of the 662-bp fragment of the GAPDH message. Southern blots of the RT-PCR fragments are shown at right. The specificities of the amplified fragments were assessed using 32P-labeled probes specific for each receptor subtype. Lane 1, brain; lane 2, hippocampus; lane 3, pituitary; lane 4, heart; lane 5, lung; lane 6, liver; lane 7, kidney; lane 8, spleen; lane 9, aorta.
F1 heterozygotes were generated by mating chimeric mice to C57BL/6J mice and homozygotes (F2) were obtained by mating between the F1 heterozygotes. Mice were screened by genotyping using Southern blot analysis and PCR to detect the V1b receptor gene. The knockouts used in this analysis were F3, F4, and F5, which carried the genetic background of 129Sv and C57BL/6J strains. V1bR+/+ littermates were used for the analysis as the WT mice.
Animal housing and manipulations.
Animals were housed in micro-isolator cages in a pathogen-free barrier facility. V1bR+/+ and V1bR–/– mice were maintained under conditions of 12 hours light/12 hours darkness and were fed ad libitum. Mice used for all studies were 8–12 weeks old and were of a C57BL/6 × 129Sv genetic background maintained by breeding within our population. All experimentation was performed according to approved institutional guidelines.
RT-PCR analysis.
RNA preparation and RT-PCR were performed as described previously (17). Thermal cycling was performed for 30 seconds at 94°C, 30 seconds at 57°C, and 2 minutes at 72°C for 30 cycles. The upstream and downstream primers for the V1b receptor (540 bp) (5′ → 3′) were TCTGGCCACAGGAGGCAACCT upstream and ATCTCGTGGCAGATGAGGCCA downstream. The upstream and downstream primers for the V1b receptor gene were located within the first exon for the receptor (16). The GAPDH primers (5′ → 3′) were GGTCATCATCTCCGCCCCTTC upstream and CCACCACCCTGTTGCTGTAG downstream, and the PCR product was 662 bp. Control PCR reactions also were performed on non–reverse-transcribed RNA to exclude any contamination by genomic DNA. The specificity of the amplified DNA fragments was determined by Southern blot using a receptor-specific 32P-labeled cDNA probe.
Histological analysis.
Tissues from V1bR+/+ or V1bR–/– mice (8–12 weeks old) were perfusion fixed in PBS plus 10% formalin. Several sections were obtained for gross morphological analysis, then paraffin embedded for thin sectioning followed by hematoxylin and eosin staining.
Anterior pituitary cultures and ACTH measurements.
Anterior pituitary cells from V1bR+/+ or V1bR–/– male mice (8–9 weeks old) were cultured and ACTH-radioimmunoassays were performed as described (19). Briefly, V1bR+/+ and V1bR–/– male mice were killed by decapitation, and then their anterior pituitary glands were rapidly removed. The anterior pituitary glands were enzymatically dispersed by the method of Oki et al. (20) with a slight modification. The glands were incubated for 1 hour at 37°C with HEPES-dispersion buffer (pH 7.3; 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 10 mM glucose, 0.1% BSA, and 25 mM HEPES) containing 0.4% collagenase and 190 U/ml DNase type 1). The resulting suspension of cells was centrifuged at 400 g for 10 minutes. The pellets were suspended with DMEM containing 100 U/ml penicillin G potassium, 1 mg/ml streptomycin sulfate, and 10% FCS, and then were sieved through a 100-μm nylon mesh. The anterior pituitary cells thus obtained were resuspended with culture medium and then seeded into 24-well plates (2 × 105 to 3 × 105 cells/well). The cells were cultured in a humidified incubator at 37°C in 5% CO2 and 95% air. After 7 days of culture, confluent cells (7 × 105 to 8 × 105 cells/well) were washed with incubation medium. After preincubation for 1 hour with incubation medium, the cells were incubated without or with AVP (Peptide Institute Inc., Osaka, Japan) or CRH (Peptide Institute Inc.) for an additional 3 hours. In experiments with SSR149415 or OPC-21268, the effect of AVP antagonists on AVP-induced ACTH release was examined in these cultured cells. OPC-21268 (Otsuka Pharmaceutical Co.) or SSR149415 (Sanofi-Synthelabo, Montpellier, France) was added 10 minutes prior to the administration of AVP (100 nM). At the end of the incubation period, the medium was collected and stored at –20°C until the ACTH assay could be performed. The ACTH released into the incubation medium was measured by an ACTH immunoradiometric assay (Mitsubishi Chemical Co., Tokyo, Japan). The ACTH concentration was expressed as the amount released per 5 × 105 cells.
A preliminary series of radioligand binding studies measuring [3H]AVP binding to cloned mouse V1a and V1b receptors was performed as described previously (21). Competition binding experiments showed that the affinity of OPC-21268 for the mouse V1a receptor (Ki approximately 500 nM) was more than 200 times higher than its affinity for V1b and V2 receptors. SSR149415 exhibited a higher affinity for the mouse V1b receptor (Ki approximately 100 nM) than for the V1a (Ki approximately 6 μM) and V2 (Ki approximately 6 μM) receptors.
Measurement of ACTH and corticosterone.
To determine basal levels of HPA hormones, individually housed male mice were left undisturbed throughout the night before the experiment and were killed in either the light phase (the third hour of the light phase) or the dark phase (the third hour of the dark phase of the light cycle) by decapitation within 20 seconds of initial cage disturbance. Blood samples were collected in prechilled tubes containing EDTA and a protease inhibitor (10 μl aprotinin; Trasylol, Bayer Corp., Leverkusen, Germany) and then centrifuged for 10 minutes at 3,000 g and 4°C. Plasma samples were stored at –80°C until assay. Plasma ACTH and corticosterone levels were measured from 50 μl and 10 μl plasma, respectively, using commercially available kits (Biochem Immunosystems, Freiburg, Germany) according to the manufacturer’s protocols.
After 1 week of daily handling, V1bR+/+ and V1bR–/– male mice weighing 23–29 g (25 ± 2.6 g for V1bR+/+, n = 8, and 25 ± 2.5 g for V1bR–/–, n = 8, respectively) were injected intraperitoneally with either vehicle (PBS plus 0.3% BSA), CRH (0.5 μg/mouse), or AVP (0.05 or 0.5 μg/kg). Our preliminary time-course study showed that circulating ACTH levels peaked 10 minutes after AVP intraperitoneal injection, and this procedure was chosen to monitor the effects of various agents in the following experiments. Our time-course results are in agreement with previous reports (22). The mice were killed by rapid decapitation 10 minutes after AVP injection or 30 minutes after CRH injection, and trunk blood was collected for determination of plasma ACTH and corticosterone levels.
Forced swim stress test.
Male mice were subjected to forced swim stress as a predominantly physical stressor (23). Between 8:00 am and 10:00 am on the day of testing, each male mouse was placed for 0.5–10 minutes in a glass beaker (diameter, 12.5 cm; height, 14 cm) filled with tap water (23°C). Subsequently, the animals were returned to their home cages, and blood collection was performed by decapitation immediately after the end of stress exposure. Plasma ACTH and corticosterone concentrations were determined.
Statistics.
All values are expressed as mean ± SEM. Statistical analysis was performed using the Student’s t test. A P value below 0.05 was considered statistically significant.
Results
Targeted disruption of the mouse V1b receptor gene.
The strategy for inactivating one copy of the V1b receptor gene in ES cells is shown in Figure 1a. Homologous recombinants were identified by Southern blot analysis of genomic DNA. Two of the positive ES clones confirmed by Southern blot analysis with the 5′, 3′, and Neo probe were independently microinjected into C57BL/6J blastocyst-stage embryos. Six of fourteen chimeric mice were mated to C57BL/6J mice, and germline transmission of the mutant allele was confirmed by genomic Southern analysis of tail DNA from F1 progeny. Mating between heterozygous male and female mice generated F2 progeny with all three genotypes: homozygous mutant (V1bR–/–), heterozygous mutant (V1bR+/–), and WT mice (V1bR+/+) (Figure 1b). The WT allele generates a 14.0-kb EcoRV fragment, and the mutant allele generates a 2.8-kb EcoRV fragment. Analysis of the V1b receptor genotype frequencies after intercrosses of heterozygous mutant mice did not reveal any deviation from mendelian expectations (data not shown). No significant difference in body weight was observed between age-matched V1bR–/–, V1bR+/–, and WT males or females up to 4 months old. In addition, homozygous V1bR–/– mutants did not show any significant difference in their total amount of water and food intake (data not shown). Thus, disruption of the V1b receptor gene does not seem to have any major effect on mouse development, fertility, growth, or feeding behavior under standard breeding conditions.
Tissue weight and histological analysis.
The ratio of adrenal gland weight to body weight was significantly (P < 0.05) lower in the V1bR–/– female mice than in the age-matched V1bR+/+ female mice, but these differences were not observed in male mice (male V1bR+/+: 0.017 ± 0.003, n = 8; male V1bR–/–: 0.019 ± 0.002, n = 12; female V1bR+/+: 0.038 ± 0.005, n = 8; female V1bR–/–: 0.029 ± 0.003, n = 12). The weights of other tissues examined, including brain, liver, kidney, and pituitary, were comparable between V1bR+/+ and V1bR–/– male or female mice (data not shown).
No major morphological differences were apparent in the cerebral cortex, hippocampus, amygdala, hypothalamus, or pituitary in 10-week-old V1bR–/– males. We studied the anterior pituitary in more detail using specific antibodies to ACTH, and found no marked differences in cell densities throughout the entire pituitary, or in the cell densities of corticotrophs and melanotrophs (data not shown). Histological analysis of the adrenal glands did not reveal any apparent changes of the adrenal cortex, including the zona fasciculata (the major site of corticosterone production), in V1bR–/– mice. There was no significant difference in the diameter of the adrenal medulla between homozygous mutants and controls, as determined by evaluating the diameters of serial sections of adrenal glands (data not shown). The absolute densities of chromaffin cells were indicated by tyrosine hydroxylase and phenylethanolamine N-methyltransferase immunostaining, and these also were not significantly altered (data not shown).
Expression of mRNA.
RT-PCR was used to assess the expression of the V1b receptor subtype in various tissues from male V1bR+/+ and V1bR–/– mice. As shown in Figure 1c, in V1bR+/+ mice, the V1b receptor transcript was expressed most abundantly in the pituitary and could be faintly detected in the hippocampus. On the other hand, no V1b receptor transcript was detectable in any tissue examined from the V1bR–/– mice, while expression of GAPDH in V1bR–/– mice was comparable to that in V1bR+/+ mice (Figure 1c), confirming the absence of V1b receptor mRNA expression in V1bR–/– mice. To investigate potential compensatory changes in expression of other vasopressin receptor subtypes (V1a, V2) and of oxytocin receptor after loss of V1b receptor in V1bR–/– mice, we further examined changes in their transcriptions in various tissues using RT-PCR analysis. Expression of the V1a, V2, and oxytocin receptors in V1bR–/– mice was similar to that in V1bR+/+ mice (data not shown), suggesting that inactivation of the V1b receptor gene does not lead to any dramatic compensatory change in expression of the other subtypes.
ACTH release from primary cultured pituitary cells.
First, ACTH release from primary cultured pituitary cells from mice was characterized pharmacologically. As shown in Figure 2a, 10–7 M AVP potently stimulates ACTH release from these primary cultured pituitary cells. Although the V1a receptor–selective antagonist OPC-21268 was ineffective at the concentration of 1 μM, the V1b receptor–selective antagonist SSR149415 potently inhibited AVP-induced ACTH release in a dose-dependent manner (Figure 2a). Around 40% and 50% inhibition was observed at 1 μM and 10 μM SSR149415, respectively. Application of the antagonists alone did not change ACTH release at any of the concentrations used (data not shown).
Figure 2.
ACTH levels in anterior pituitary cell cultures from V1bR+/+ and V1bR–/– mice. (a) Effects of AVP antagonists on ACTH release from primary cultured pituitary cells. SSR149415 or OPC-21268 was added 10 minutes prior to the administration of AVP (10–7 M) in the cultured pituitary cells from V1aR+/+ male mice. The ACTH levels were expressed as the amount secreted over a period of 3 hours per 5 × 105 cells. The values are represented as the mean ± SEM of four experiments. *P < 0.05, antagonists vs. no antagonists. (b) Effects of AVP or CRH on ACTH release in V1bR–/– mice. The cells from V1bR+/+ (white bars) or V1bR –/– (black bars) male mice were incubated without or with AVP or CRH. The values are represented as the mean ± SEM of five or six experiments. *P < 0.05 between genotypes.
To confirm the functional deletion of the V1b receptor in the V1bR–/– mice, we cultured pituitary cells from V1bR–/– and V1bR+/+ males, stimulated the cells with AVP or CRH, and determined the level of ACTH release. Basal ACTH release from pituitary cells was not significantly different between V1bR–/– males and V1bR+/+ males (Figure 2b). AVP significantly increased ACTH release from cells of V1bR+/+ mice (2.7-fold at 100 nM AVP), while there was no change in ACTH release after AVP stimulation in cells from the V1bR–/– mouse. In both groups, the addition of 10 nM CRH to pituitary cells increased the ACTH release by sixfold and fivefold in V1bR+/+ and V1bR–/– mice, respectively, and there was no significant difference in response between the two genotypes.
ACTH and corticosterone.
To investigate the physiological role of the V1b receptor in regulation of the HPA axis, we monitored plasma ACTH and corticosterone concentrations under basal conditions and also after AVP stimulation. The basal plasma ACTH concentration at the light phase was lower in the V1bR–/– mice than in the WT mice (59 ± 13 pg/ml in V1bR+/+ mice, n = 21; and 29 ± 5 pg/ml in V1bR–/– mice, n = 29; P = 0.04). Corresponding to the reduced ACTH, the corticosterone concentration in the light phase was also reduced in V1bR–/– mice compared with V1bR+/+ mice (19 ± 1 ng/ml in V1bR+/+ mice, n = 21; 15 ± 1 ng/ml in V1bR–/– mice, n = 29; P = 0.0004) (Figure 3). Plasma ACTH was elevated in V1bR+/+ mice at the third hour of the dark phase of the light cycle, whereas it was not elevated in V1bR–/– mice (141 ± 81 pg/ml in V1bR+/+ mice, n = 11; 11 ± 4 pg/ml in V1bR–/– mice, n = 7) (data not shown), showing that the circadian variation in ACTH observed in V1bR+/+ mice was blunted in V1bR–/– mice. In contrast to ACTH, the corticosterone level was elevated at the dark phase compared with that at the light phase even in V1bR–/– mice. Circadian variation in corticosterone secretion, compared with that in ACTH secretion, appears to be not much influenced by the absence of the V1b receptor (data not shown).
Figure 3.
Plasma ACTH (a) and corticosterone (b) concentrations under basal conditions and after stimulation with AVP or CRH. The mice were killed by rapid decapitation 10 minutes after intraperitoneal AVP injection or 30 minutes after intraperitoneal CRH injection, and trunk blood was collected for determination of plasma ACTH and corticosterone levels. Data are expressed as mean ± SEM (basal level, n = 21 in V1bR+/+ and n = 29 in V1bR–/– mice; vehicle, AVP, and CRH stimulation, n = 8 for each genotype). *Statistically significant difference between genotypes (P < 0.05). White bars, V1bR+/+ male; black bars, V1bR–/– male.
The HPA axis was examined after AVP stimulation. Exogenous administration of AVP increased ACTH secretion in a dose-dependent manner in the V1bR+/+ male mice (Figure 3). On the other hand, stimulation with AVP (0.05 or 0.5 μg/kg, given intraperitoneally) did not significantly increase the circulating ACTH level in the V1bR–/– male mice (Figure 3). Similarly, administration of AVP increased corticosterone secretion in a dose-dependent manner in the V1bR+/+ male mice, whereas the response stimulated by AVP at 0.5 μg/kg was significantly attenuated in the V1bR–/– male mice (Figure 3). On the other hand, exogenous CRH-induced ACTH or corticosterone secretion levels were comparable between both groups.
Stress-induced ACTH and corticosterone secretions.
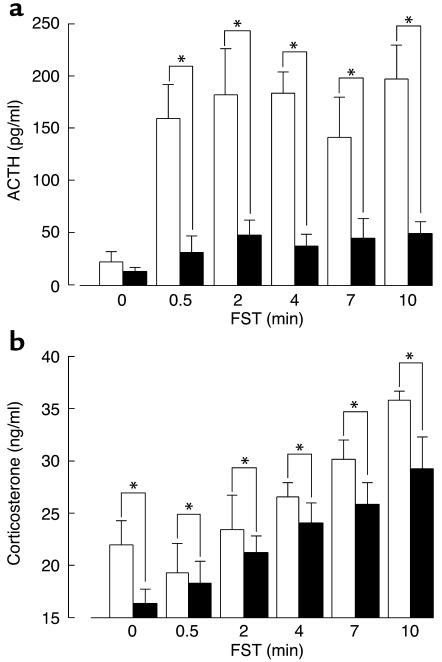
As shown in Figure 4, a significant increase in plasma ACTH after the forced swim test was observed in the V1bR+/+ male mice, but this response was markedly impaired in the V1bR–/– male mice. The corticosterone level after the forced swim test was significantly lower in the V1bR–/– male mice than in the V1bR+/+ mice, but the increase in corticosterone above the basal level was comparable between the two groups of mice.
Figure 4.
Plasma ACTH (a) and corticosterone (b) concentrations after the forced swim test (x). Each mouse was placed for 0.5, 2, 4, 7, or 10 minutes in a glass beaker (12.5 cm in diameter, 14 cm in height) filled with tap water (23°C). Subsequently, the animals were returned to their home cages, and blood collection was performed by decapitation immediately after the end of stress exposure. Plasma ACTH and corticosterone concentrations were determined. Data are expressed as mean ± SEM (n = 8–10). *Statistically significant difference between genotypes (P < 0.05). White bars, V1bR+/+ male; black bars, V1bR–/– male.
V1a receptor– and V2 receptor–mediated responses.
We monitored vasopressor and antidiuretic effects as V1a receptor–mediated and V2 receptor–mediated responses, respectively. Increases in mean arterial pressure monitored after AVP stimulation (0.1–10 μg/kg) were not different between V1bR+/+ and V1bR–/– mice (data not shown). To assess the V2 receptor–mediated antidiuretic effect, we monitored urine output for 24 hours and the urine output during water loading with or without 1-deamino, 8 D-arginine vasopressin (DDAVP). The basal urine outputs for 24 hours were not different between V1bR+/+ and V1bR–/– mice (1.35 ± 0.13 ml, n = 10; and 1.01 ± 0.13 ml, n = 9, respectively). The urine outputs during water loading (5% of body weight) were 1.16 ± 0.07 and 1.04 ± 0.11 ml/4 hours in V1bR+/+ (n = 10) and V1bR–/– mice (n = 10), respectively. In addition, the urine outputs in response to DDAVP (100 ng/kg) during water loading were also not different between V1bR+/+ and V1bR–/– mice: 0.08 ± 0.02 and 0.12 ± 0.06 ml/4 hours in V1bR+/+ (n = 10) and V1bR–/– mice (n = 10), respectively. These studies show that V1a receptor– and V2 receptor–mediated responses are intact in V1b-deficient mice.
Discussion
Using mice lacking the vasopressin V1b receptor and recently developed subtype-selective pharmacological ligands, we investigated the functional roles of the vasopressin V1b receptor subtype, with a particular focus on the HPA axis system. V1bR–/– mice showed lower HPA axis activity under resting conditions, and also displayed reduced responses to AVP administration and to a forced swim stress compared with V1bR+/+ mice. The present study clearly demonstrates that AVP exerts critical regulation of HPA axis activity not only during stress conditions but also in resting conditions, indicating that vasopressinergic regulation of HPA axis activity is of equal importance to the CRH/CRHR1 system.
Basal level of ACTH and corticosterone.
After the discovery of CRH by Vale et al. (24), it was rapidly established that AVP potently synergizes with CRH to stimulate pituitary ACTH release; thus, when CRH and AVP are given together, ACTH output is well above the sum of that resulting from the two peptides alone in both rodents and humans (25, 26). This CRH/AVP synergism is known to be functionally relevant under both physiological (27) and pathophysiological conditions, such as stress (28, 29) or glucocorticoid deficiency (30, 31). A previous study of Crhr1–/– mutant mice suggested that there was a selective compensatory activation of the hypothalamic vasopressinergic system to maintain basal ACTH secretion and HPA system activity in these mice (10). To see whether activation of the vasopressinergic system was functionally relevant, we examined HPA axis activity in V1b receptor–deficient mice. Basal plasma ACTH and corticosterone concentrations in homozygous V1bR–/– mutants were found to be significantly lower than those found in WT controls, although basal plasma ACTH concentrations in homozygous Crhr1–/– mutants were similar to those found in WT controls (32). These observations suggest that basal ACTH secretion might be primarily maintained by signaling pathways involving the AVP/V1b receptor, and these may not be fully compensated for by CRH/CRHR1 signaling pathways.
HPA axis activity under stress conditions.
Regulation of ACTH secretion during stress is multifactorial, with CRH and AVP being the most physiologically important stimulators of its release (8). However, the individual functional roles of AVP and CRH involving the HPA axis under basal or stress conditions have not been fully elucidated. Therefore, we investigated the HPA axis under stress conditions. Stress-induced increases in plasma ACTH and corticosterone levels were significantly decreased in homozygous V1bR–/– mutants compared with those in control mice. The final concentrations of ACTH and corticosterone were also lower in V1bR–/– mutants than in V1bR+/+ mice, and increases in ACTH after a forced swim stress were smaller in V1bR–/– mice than in V1bR+/+ mice. Unlike V1bR–/– mutants, mutant mice lacking the Crhr1 gene showed normal ACTH levels under basal conditions but had impaired stress-induced ACTH responses (9). Taken together, these results show that under the stress condition, both the hypothalamic AVP/V1bR and the CRH/CRHR1 system are required and indispensible to maintain normal HPA system regulation.
Our study showed that the impact of V1b receptor deletion was much greater on ACTH release than on corticosterone. This may be due to the differential regulatory role of vasopressin in ACTH release from the pituitary and corticosterone release from the adrenals. ACTH release is predominantly regulated by two major stimulatory factors of CRH and AVP via CRHR1 and V1b receptor, respectively. Our present study and a previous study (33) using mice lacking the Crhr1 gene clearly showed that AVP and CRH are potent direct stimulators of ACTH and predominantly, if not exclusively, regulate ACTH release from the pituitary. On the other hand, corticosterone secretion from adrenal glands is complexly regulated not only by ACTH, but also by other factors, including direct and indirect vasopressin effects (33). Our data obtained from V1bR–/– mutants show that ACTH release is more closely related to AVP stimulation than the corticosterone release.
In conclusion, our vasopressin V1b receptor knockout mouse study has suggested that AVP/V1b receptor signaling plays a crucial role in maintaining basal ACTH secretion, and that both AVP/V1b receptor signaling and CRH/CRHR1 signaling appear to play a crucial role in modulating HPA activity under stress conditions. Clinical implications include the possibility that drugs that regulate V1b receptor activity may exhibit a therapeutic profile in the fields of stress, anxiety, and depression. Several neuroendocrine studies strongly suggest that dysregulation of the HPA system plays a causal role in the development and course of diseases such as generalized anxiety, depression, and addiction. In addition, many clinical conditions are accompanied by an exaggerated response to stress (34). Since these disorders have been associated with excessive HPA activity in both humans and animals, one can speculate that V1b receptor antagonists have potential for use in all such situations. In fact, it was shown that the V1b receptor antagonist SSR149415 displayed anxiolytic-like activity in mice (13). Also, in keeping with the anxiolytic-like properties of SSR149415, knockout mice displayed behavioral alterations such as reduced aggression (15), confirming the role of V1b receptors in anxiety. Moreover, recent immunohistochemistry analysis showed that the V1b receptor protein has a wide distribution in the rat brain, in particular in the hypothalamus, amygdala, cerebellum, and in areas close to circumventricular organs devoid of a blood-brain barrier (35). This localization in key brain structures that are associated with specific central functions also strongly supports the idea that there are roles for central V1b receptors in learning, memory, and various emotional and behavioral situations. Extensive studies are clearly required to further explore the specific activities of V1b receptors in various models of CNS disorders. V1b receptor knockout mice will continue to be of value not only to investigate regulatory mechanisms in a variety of physiological responses to AVP (15), including control of the HPA axis and its dysregulation, but also for the development of new therapeutic agents.
Acknowledgments
We thank Claudine Serradeil-Le Gal (Sanofi-Synthelabo Recherche, Toulouse, France) for kindly providing SSR149415. This work was supported in part by research grants from the Scientific Fund of the Ministry of Education, Science, and Culture of Japan; the Japan Health Science Foundation; and the Ministry of Human Health and Welfare of Japan.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: [Arg8]-vasopressin (AVP); corticotropin-releasing hormone (CRH); hypothalamic-pituitary-adrenal (HPA); CRH receptor (CRHR).
References
- 1.Michell RH, Kirk CJ, Billah MM. Hormonal stimulation of phosphatidylinositol breakdown, with particular reference to the hepatic effect of vasopressin. Biochem. Soc. Trans. 1979;7:861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- 2.Jard S, et al. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Mol. Pharmacol. 1986;30:171–177. [PubMed] [Google Scholar]
- 3.Thibonnier M. Vasopressin and blood pressure. Kidney Int. 1988;34:S52–S56. [PubMed] [Google Scholar]
- 4.Morel A, O’Carroll AM, Brownstein MJ, Lolait SJ. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356:523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- 5.Lolait SJ, et al. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 6.Keyzer Y, et al. Cloning and characterization of the human V3 pituitary vasopressin receptor. FEBS Lett. 1994;356:215–220. doi: 10.1016/0014-5793(94)01268-7. [DOI] [PubMed] [Google Scholar]
- 7.Thibonnier M, Coles P, Thibonnier A, Shoham M. Molecular pharmacology and modeling of vasopressin receptors. Prog. Brain Res. 2002;139:179–196. doi: 10.1016/s0079-6123(02)39016-2. [DOI] [PubMed] [Google Scholar]
- 8.Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front. Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull AV, et al. CRF type 1 receptor-deficient mice exhibit a pronounced pituitary-adrenal response to local inflammation. Endocrinology. 1999;140:1013–1017. doi: 10.1210/endo.140.2.6675. [DOI] [PubMed] [Google Scholar]
- 10.Muller MB, et al. Selective activation of the hypothalamic vasopressinergic system in mice deficient for the corticotropin-releasing hormone receptor 1 is dependent on glucocorticoids. Endocrinology. 2000;141:4262–4269. doi: 10.1210/endo.141.11.7767. [DOI] [PubMed] [Google Scholar]
- 11.Thibonnier M, Coles P, Thibonnier A, Shoham M. The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu. Rev. Pharmacol. Toxicol. 2001;41:175–202. doi: 10.1146/annurev.pharmtox.41.1.175. [DOI] [PubMed] [Google Scholar]
- 12.Jackson, E.K. 2001. Vasopressin and other agents affecting the renal conservation of water. In The pharmacological basis of therapeutics. J.G. Hardman and L.E. Limbird, editors. McGraw-Hill Inc. New York, New York, USA. 789–808.
- 13.Serradeil-Le Gal C, et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J. Pharmacol. Exp. Ther. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- 14.Griebel G, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi S, Tanoue A, Goda N, Matsuo N, Tsujimoto G. Structure and sequence of the mouse V1a and V1b vasopressin receptor genes. Jpn. J. Pharmacol. 1999;81:388–392. doi: 10.1254/jjp.81.388. [DOI] [PubMed] [Google Scholar]
- 17.Tanoue A, et al. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 2002;109:765–775. doi:10.1172/JCI200214001. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagi T, et al. A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal. Biochem. 1993;214:77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]
- 19.Gohshi A, et al. Changes in adrenocorticotropic hormone (ACTH) release from the cultured anterior pituitary cells of streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 1998;21:795–799. doi: 10.1248/bpb.21.795. [DOI] [PubMed] [Google Scholar]
- 20.Oki Y, Nicholson WE, Orth DN. Role of protein kinase-C in the adrenocorticotropin secretory response to arginine vasopressin (AVP) and the synergistic response to AVP and corticotropin-releasing factor by perifused rat anterior pituitary cells. Endocrinology. 1990;127:350–357. doi: 10.1210/endo-127-1-350. [DOI] [PubMed] [Google Scholar]
- 21.Hirasawa A, Shibata K, Kotosai K, Tsujimoto G. Cloning, functional expression and tissue distribution of human cDNA for the vascular-type vasopressin receptor. Biochem. Biophys. Res. Commun. 1994;30:72–79. doi: 10.1006/bbrc.1994.2150. [DOI] [PubMed] [Google Scholar]
- 22.Bernardini R, et al. In vivo and in vitro effects of arginine-vasopressin receptor antagonists on the hypothalamic-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994;60:503–508. doi: 10.1159/000126787. [DOI] [PubMed] [Google Scholar]
- 23.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 24.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 25.Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- 26.von Bardeleben U, Holsboer F, Stalla GK, Muller OA. Combined administration of human corticotropin-releasing factor and lysine vasopressin induces cortisol escape from dexamethasone suppression in healthy subjects. Life Sci. 1985;37:1613–1618. doi: 10.1016/0024-3205(85)90480-1. [DOI] [PubMed] [Google Scholar]
- 27.Rivier C, Vale W. Interaction of corticotropin-releasing factor and arginine-vasopressin in adrenocorticotropin secretion in vivo. Endocrinology. 1983;113:939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- 28.De Goeij DC, Jezova D, Tilders FJ. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res. 1992;577:165–168. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- 29.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 30.Kiss JZ, Mezey E, Skirboll L. Corticotropin-releasing factor immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy. Proc. Natl. Acad. Sci. U. S. A. 1984;84:1854–1858. doi: 10.1073/pnas.81.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J. Neurosci. 2000;20:3843–3852. doi: 10.1523/JNEUROSCI.20-10-03843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timpl P, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Gen. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 33.Gallo-Payet N, Guillon G. Regulation of adrenocortical function by vasopressin. Horm. Metab. Res. 1998;30:360–367. doi: 10.1055/s-2007-978899. [DOI] [PubMed] [Google Scholar]
- 34.Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J. Psychiatr. Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 35.Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]